Abstract
Rationale
Apolipoprotein A-I (apoA-I) mimetic peptides are a promising type of anti-atherosclerosis therapy, but how the structural features of these peptides relate to the multiple anti-atherogenic functions of HDL is poorly understood.
Objective
To establish structure-function relationships of apoA-I mimetic peptides with their anti-atherogenic functions.
Methods and Results
Twenty two bi-helical apoA-I mimetic peptides were investigated in vitro for the capacity and specificity of cholesterol efflux, inhibition of inflammatory response of monocytes and endothelial cells and inhibition of low density lipoprotein (LDL) oxidation. It was found that mean hydrophobicity, charge, size of hydrophobic face and angle of the link between the helices are the major factors determining the efficiency and specificity of cholesterol efflux. The peptide with optimal parameters was more effective and specific towards cholesterol efflux than human apoA-I. Charge and size of hydrophobic face were also the major factors affecting anti-inflammatory properties and the presence of cysteine and histidine residues was the main factor determining anti-oxidant properties. There was no significant correlation between capacities of the peptides to support individual functions; each function had its own optimal set of features.
Conclusions
None of the peptides was equally effective in all the anti-atherogenic functions tested, suggesting that different functions of HDL may have different mechanisms and different structural requirements. The results do suggest, however, that rationalizing the design of apoA-I mimetic peptides may improve their therapeutic value and may lead to a better understanding of mechanisms of various anti-atherogenic functions of HDL.
Keywords: Mimetic peptides, high density lipoprotein, atherosclerosis
Introduction
Atherosclerosis underlies most cases of cardiovascular disease (CVD), which is now the major cause of morbidity and mortality in developed countries. Accumulation of cholesterol in the arterial wall and vascular inflammation are in the centre of the pathogenesis of atherosclerosis, and treatments controlling delivery of cholesterol and inflammation (statins) reduce the incidence of cardiovascular disease by 30-40% 1. There is, however, an urgent need for further reduction of the unacceptably high remaining risk of CVD. A most promising direction is complementing reduction in levels of the pro-atherogenic lipoproteins with increasing levels of the anti-atherogenic lipoprotein, high density lipoprotein (HDL), “HDL therapy” 2. It is becoming clear that success of HDL therapy critically depends on the mechanism for elevating HDL; a straight forward and so far the most successful approach being direct infusion of exogenous HDL 3, 4. Infusion of reconstituted HDL (rHDL), however, has considerable limitations due to high cost and requirement for intravenous delivery making it suitable mainly for acute treatment. An alternative to rHDL is apolipoprotein A-I (apoA-I) mimetic peptides.
ApoA-I mimetic peptides mimic the secondary structure of the major structural element of apoA-I, 22-mer amphipathic type A α-helix 5. It appears that there is no requirement for a homology between the primary structure of these peptides and apoA-I; these peptides are active as long as the secondary structure of apoA-I is replicated 5, 6. ApoA-I mimetic peptides show remarkable capacity to support cholesterol efflux, share the anti-inflammatory properties of HDL and reduce development of atherosclerosis in animal models 7-11. ApoA-I mimetic peptides cost a fraction of the cost of rHDL; they are safe and well tolerated 12, and approaches for oral delivery are being developed 8, 13. Perhaps the best advantage of these peptides is the ability to modify their structure to better understand the mechanisms of atheroprotective action of HDL, with a view to further improving the atheroprotective capacity of the peptides. Relatively limited research has been done to understand structure-function relationships of apoA-I mimetic peptides. The current understanding of the structure-function relationship of the peptides can be summarized as follows:
The amphipathic α-helix of 18- 22 residues is essential for a peptide to mimic apoA-I and to be atheroprotective 14.
There is no stereospecificity: peptides made of D-amino acids are as effective as those made of L-amino acids 10, 13, 15.
Increasing hydrophobicity by including 2 or 4 phenylalanine residues improves the capacity of peptides to associate with lipids 16 and anti-inflammatory capacity of the peptides 8.
Alignment of negative charges on the polar face increases cholesterol efflux 17.
Two helixes connected through proline residue work better than a single helix in cholesterol efflux and inflammation assays 18, 19.
Introducing asymmetry in bi-helical peptide improves its specificity in cholesterol efflux assay and reduces toxicity 20.
While the atheroprotective functions of HDL are not limited to its role in reverse cholesterol transport and inflammation, only these two functions have been tested in most studies, but never, to our knowledge, simultaneously. In the current study, we undertook a comprehensive analysis of the structure-function relationship of apoA-I mimetic peptides. We used bi-helical peptides not only because they have shown a better anti-atherosclerosis activity, but also because they offer an opportunity to mimic variability in the structure of two adjacent helices, a property increasing their similarity to apoA-I and improving specificity of at least some their functions 18, 20. We investigated the impact of changes in peptide mean hydrophobicity, size of hydrophobic face, charge, type of α-helix, configuration of the bridge between two helices, asymmetry of the helices and inclusion of specific residues. The impact of these changes on the capacity and specificity of cholesterol efflux, inflammatory response of monocytes and endothelium and anti-oxidant properties were studied. We endeavored to determine which structural features listed above are important for the four anti-atherogenic functions of the peptides and to establish optimal combination(s) of these features favoring each or all of these functions.
Methods
Cholesterol efflux
Cellular cholesterol was labeled by incubation in serum-containing medium with [3H]-cholesterol for 48 h in a CO2 incubator. Cells were then washed and incubated for 18 h at 37°C in serum-free medium in the presence or absence of TO-901317 (4 μmol/L). Cells were washed and incubated for another 4 h at 37°C (THP-1 cells) or 18 h (BHK-1 cells) in serum-free medium containing indicated concentrations of the peptides or lipid-free apoA-I. Where indicated, cells were fixed by incubation for 20 min with paraformaldehyde (4%) prior to the efflux experiments.
Expression of CD11b on human monocytes
Resting human monocytes were stimulated with 1μmol/L phorbol-12 myristate 13-acetate (PMA) in the presence or absence of the peptides or apoA-I final (concentration 40 μg/mL) and incubated with the FITC conjugated Ab to the active epitope of CD11b for 15min at 37°C. Cells were then fixed with 4% formaldehyde and CD11b expression was measured by flow cytometry.
Expression of VCAM-1 in mouse endothelial cells
SVEC4/VCAM-1 cells were washed and apoA-I, HDL or apoA-I mimetic peptides were added at the final concentration of 0.75 mg/ml. After 18 h incubation tissue necrosis factor (TNF-α) was added in serum-free medium to the final concentration of 10 ng/ml. Cells were incubated for 5 h and luciferase activity was measured using Bright-Glo Assay.
Oxidation of LDL
Freshly isolated LDL (final concentration 100 μg/ml) was incubated at 25°C for the indicated periods of time with CuSO4 (final concentration 15 μMol/L) in the presence of the peptides or apoA-I (final concentration of 100 μg/ml) and absorption was continually monitored at 234 nm.
Results
Structure of apoA-I mimetic peptides
Twenty two apoA-I mimetic peptides were synthesized; their sequences, physicochemical properties and general features are shown in Table 1. Two peptides were used as prototypes, in order to understand how changes in their structures affect their function. The first prototype peptide was 5A (#1), which was described by us previously; it consists of two type A amphipathic α-helices connected through proline; hydrophobicity of the second helix was reduced by substituting hydrophobic amino acids with alanine 20. Four derivatives of 5A were synthesized (peptides 19-22) to test the impact of the introduction of two aminoacids known to be anti-oxidants, cysteine and histidine, on its properties. The second prototype peptide was ELK (#2), which is made of just three amino acids, glutamic acid, leucine and lysine 21. Itconsists of two identical canonical type A amphipathic α-helices with 180 degree hydrophobic face and neutral net charge; helices are connected with a proline residue. ELK peptide was used to make sixteen modifications (peptides 3-18) testing the impact of modification affecting the following features: i) net charge, as it may affect interaction with cellular receptors and lipids; ii) mean hydrophobicity and size of hydrophobic face, as they may affect the interaction with lipids and cellular receptors; iii) type of helix and configuration of the proline bridge between the two helices, as it may affect interaction with lipoprotein particles and cellular receptors, as well as properties of complexes of peptides with lipids after they acquire the latter from cells; iv) asymmetry, as it was shown to affect specificity of cholesterol efflux. Some of these properties are interdependent (e.g. charge and hydrophobicity) requiring testing several peptides with combinations of these features. Peptides were tested in lipid-free form to mimic the interaction of lipid-free apoA-I with cells and to exclude the confounding effects of lipid-binding properties of the peptides and variations in size of “rHDL” particles.
Table 1.
Sequences and structural features of apoA-I mimetic peptides
| # | Peptide | Sequence | Mean Hydrophobicity |
Charge | Key features |
|---|---|---|---|---|---|
| 1 | 5A | DWLKAFYDKVAEKLKEAF- P- DWAKAAYDKAAEKAKEAA |
−0.57 | 0 | A symmetrical |
| 2 | ELK | EKLKELLEKLLEKLKELL- P- EKLKELLEKLLEKLKELL |
−0.4 | 0 | Canonical Type A helix with 180 degree hydrophobic face and 0 net charge |
| 3 | ELK-3E3LK | EELKEKLEELKEKLEEKL -P- EELKEKLEELKEKLEEKL |
−1.1 | −6 | 3 x (K-E, L-K) substitutions. Decreased hydrophobic face and 3 additional negative charges per helix |
| 4 | ELK- 3E3K3A |
EELKAKLEELKAKLEEKL- P- EELKAKLEELKAKLEEKL |
−0.76 | −2 | 3 x (K-E, E-A, L-K) substitutions. Decreased hydrophobic face and 1 additional negative charges per helix |
| 5 | ELK-2A | EKLKALLEKLLAKLKELL P- EKLKALLEKLLAKLKELL |
0.12 | +4 | 2 x E-A substitutions. Increased hydrophobic face and 2 negative charges per helix less |
| 6 | ELK-1W | EWLKELLEKLLEKLKELL- P- EWLKELLEKLLEKLKELL |
−0.19 | −2 | K-W substitution. 1 positive charges per helix less |
| 7 | ELK-2F | EKFKELLEKFLEKFKELL- P- EKFKELLEKFLEKFKELL |
−0.43 | 0 | 2x (L-F) substitutions. Increased hydrophobic face |
| 8 | ELK-1L1K | EKLKELLEKLLELLKKLL- P- EKLKELLEKLLELLKKLL |
−0.01 | +2 | K-L and E-K substitutions. 1 negative charge per helix less |
| 9 | ELK- 1K1A1E |
EKLKELLEKLKAKLEELL- P- EKLKELLEKLKAKLEELL |
−0.39 | 0 | L-K, E-A and K-E substitutions. Decreased hydrophobic face |
| 10 | ELK-1A | EKLKELLEKLLAKLKELL- P- EKLKELLEKLLAKLKELL |
−0.1 | +2 | E-A substitution. 1 negative charge per helix less |
| 11 | ELK-1F | EKFKELLEKLLEKLKELL- P- EKFKELLEKLLEKLKELL |
−0.35 | 0 | L-F substitution Increased hydrophobic face |
| 12 | ELK- 2A2K2E |
EKLKAKLEELKAKLEELL- P- EKLKAKLEELKAKLEELL |
−0.47 | 0 | 2 x (E-A, L-K and K-E) substitutions. optimal hydrophobicity and charge |
| 13 | ELK-3E3EK | EELKELLKELLKKLEKLL- P- ELKELLKELLKKLEKLL |
−0.31 | 0 | 3 x (K-E, E-K) substitutions. G-helix |
| 14 | ELK-2E2K | EELKKLLEELLKKLKELL- P- EELKKLLEELLKKLKELL |
−0.31 | 0 | 2 x (K-E, E-K) substitutions. Y-Helix |
| 15 | ELK-PA | EKLKELLEKLLEKLKELL- A- EKLKELLEKLLEKLKELL |
−0.2 | 0 | A-P substitution in the link |
| 16 | ELK-P2A | EKLKELLEKLLEKLKELL- AA- EKLKELLEKLLEKLKELL |
−0.16 | 0 | 2A-P substitution in the link |
| 17 | ELKA | EKLKAKLEELKAKLEELL- P- EKAKAALEEAKAKAEELA |
−0.49 | 0 | ELK-2A2K2E peptide with 5A substitution in second helix. |
| 18 | ELKA-CH2 | EKLKAKLEELKAKLEELL- P- EHAKAALEEAKCKAEELA |
−0.46 | 0 | ELKA peptide with C+H substitution in the second helix. |
| 19 | 5A-CH1 | DHLKAFYDKVACKLKEAF- P- DWAKAAYDKAAEKAKEAA |
−0.47 | 0 | C+H substitution in the first helix |
| 20 | 5A-CH2 | DWLKAFYDKVAEKLKEAF- P- DHAKAAYDKAACKAKEAA |
−0.52 | 0 | C+H substitution in the second helix |
| 21 | 5A-C1 | DWLKAFYDKVACKLKEAF- P- DWAKAAYNKAAEKAKEAA |
−0.44 | 0 | C substitution in the first helix |
| 22 | 5A-H1 | DHLKAFYDKVAEKLKEAF- P- DWAKAAYDKAAEKAKEAA |
−0.61 | +1 | H substitution in the first helix |
Efficiency of cholesterol efflux from human monocyte cell line THP-1
To test the capacity of cholesterol efflux to the apoA-I mimetic peptides, human monocytic cells THP-1 were differentiated into macrophages, activated or not with LXR agonist TO-901317, which induces expression of ABC transporters, labeled with [3H]cholesterol and incubated with various concentrations of peptides for 4 h. THP-1 cells not activated with LXR agonist contain low levels of ABC transporters, therefore, the efflux from non-activated cells was considered to represent the component of the efflux that was not mediated by these transporters. The difference between the efflux in the presence and absence of LXR agonist was therefore defined as ABCA1-mediated cholesterol efflux. The dose-dependencies of the efflux from THP-1 cells are presented in Supplementary Figures I-III. Fig. I shows the efflux from the cells activated with TO-901317, Fig. II shows the efflux from cells not-activated with TO-901317, and Fig. III shows a difference between the effluxes from activated and non-activated cells, i.e. ABCA1-dependent efflux. To quantitate cholesterol efflux, the areas under the dose dependence curves (AUC) were calculated, as well as the contribution of ABCA1 for cholesterol efflux at non-saturating concentration of 20 μg/ml. These parameters are shown in Table 2. Analysis of the structure-function relationships, as related to the capacity of the peptides to support ABCA1-dependent cholesterol efflux, allowed for the following conclusions.
Table 2.
Efficiency of cholesterol efflux from THP-1 cellsand contribution of ABC A1 transporter.
| Peptide | Cholesterol efflux efficiency (AUC) |
Contribution of ABCA1- dependent cholesterol efflux (%) |
|---|---|---|
| ELK-2A2K2E | 147.0 | 75 |
| ELK-2F | 140.3 | 58 |
| ApoA-I | 130.3 | 69 |
| 5A-CH2 | 125.3 | 61 |
| ELK-1K1A1E | 105.0 | 36 |
| 5A | 82.0 | 64 |
| ELK-P2A | 72.9 | 65 |
| ELK-1F | 68.3 | 50 |
| ELK-2E2K | 58.4 | 69 |
| ELK-1A | 50.8 | 22 |
| ELK-3E3EK | 44.8 | 84 |
| ELK-1L1K | 44.0 | 36 |
| ELK | 38.9 | 20 |
| ELK-1W | 37.4 | 43 |
| ELK-PA | 28.7 | 38 |
| 5A-C1 | 27.3 | 34 |
| ELK-3E3LK | 26.3 | 60 |
| ELKA | 7.7 | 0 |
| 5A-H1 | 5.6 | 48 |
| ELKA-CH2 | 2.6 | 0 |
| ELK-3E3K3A | 1.3 | 0 |
| ELK-2A | 0 | 0 |
| 5A-CH1 | 0 | 0 |
|
| ||
| ELK-2A2K2E/POPC | 381.6 | ND |
Hydrophobicity and charge
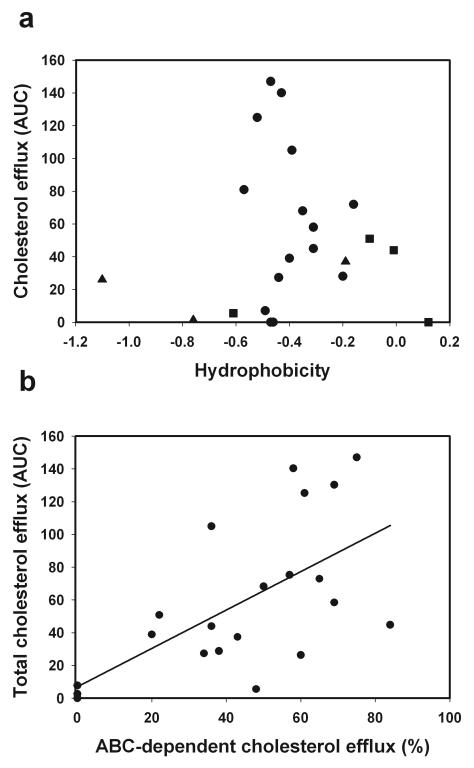
The relationship between mean hydrophobicity and the capacity of the peptides to support cholesterol efflux is shown in Fig. 1 A. It appears that the relationship is characterized by a sharp peak around a mean hydrophobicity value of −0.5. Adding charges inevitably changes hydrophobicity, making it difficult to investigate the effect of the charge independently of hydrophobicity. It appears, however, that peptides carrying positive (squares) and negative (triangles) charges have lesser capacity to support cholesterol efflux and an overall neutral charge is optimal. The peptide ELK-2A2K2E was synthesized after this initial analysis, creating a peptide with neutral charge and optimal hydrophobicity. Indeed this peptide showed exceptional capacity to support cholesterol efflux, even exceeding that of apoA-I, supporting our conclusions. Four neutral charged peptides that had average hydrophobicity around −0.5, but still failed to support cholesterol efflux (Fig. 1 A) all had other features strongly detrimental for the efflux capacity, such as the inclusion of histidine and/or cysteine residues or asymmetry in ELK peptides or both (see below).
Fig. 1. Dependence of the capacity of the peptides to support cholesterol efflux on mean hydrophobicity and charge of the peptides (a) and relationship between cholesterol efflux capacity and contribution of ABC transporters (b).
Data for cholesterol efflux capacity and specificity are taken from Table 2 and data for hydrophobicity and charge are taken from Table 1. Squares denote positively charged peptides; triangles denote negatively charged peptides.
Size of hydrophobic face
Increasing the size of hydrophobic face was beneficial, as long as overall hydrophobicity and charge were maintained (ELK-2F, ELK-1F).
Type of helix
Changing the type of helix from the type A helix found in ELK to type G and Y helices, as was done for peptides ELK-3E3EK and ELK-2E2K, had a small beneficial effect on the capacity of the peptides to support cholesterol efflux.
Proline bridge
Substitution of Ala for Pro in the bridge, as was done for peptide ELK-PA, was detrimental for the efflux capacity, however, substitution of Ala-Ala for Pro (peptide ELK-P2A), which generated half of the angle generated by proline, restored the efflux capacity.
Asymmetry
Asymmetry had a significant beneficial effect for the peptide 5A as compared to the parent symmetrical peptide L37PA 20; however, the same feature tested on ELK peptides had a strong detrimental effect (ELKA versus ELK).
Inclusion of Cys and His
This was tested on the derivatives of asymmetrical peptide 5A. Inclusion of Cys or His and especially Cys+His in the first (hydrophobic) helix (peptides 5A-CH1, 5A-H1 and 5A-C1) was detrimental for the efflux, whereas inclusion of these amino-acids in the second (less hydrophobic) helix (peptide 5A-CH2) was beneficial.
With few exceptions, the contribution of ABCA1 transporter for cholesterol efflux was proportional to the overall capacity of the peptides to support cholesterol efflux (Table 2 and Supplementary Figure IV), resulting in a statistically significant correlation between these two parameters (r=0.66, p<0.001), (Fig. 1 B). This finding confirms that changes in the peptide structure affected specifically the ABCA1-dependent component of the efflux.
Finally, we analyzed the effect of complexing the most active peptide, ELK-2A2K2E with phospholipid on cholesterol efflux. Complex ELK-2A2K2E/POPC was significantly more effective in supporting cholesterol efflux from THP-1 cells (Table 2, last row). Cholesterol efflux to lipidated particles is mediated by several mechanisms, including ABCG1, SR-B1 as well as by aqueous diffusion, and while the former is activated by LXR agonists, the latter are not; thus the contribution of specific transporters could not be tested using this design.
Specificity of cholesterol efflux from human monocyte cell line THP-1
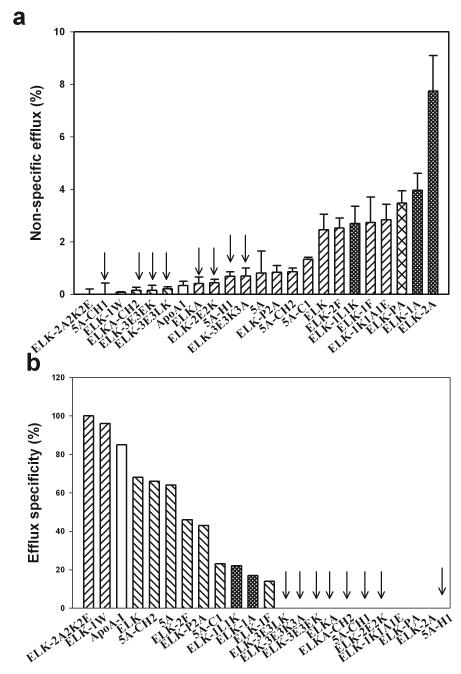
The amphipathic nature of the peptides is essential for their capacity to support cellular cholesterol efflux and form lipoprotein particles; however, it can potentially cause cytotoxicity by damaging the plasma membrane 15. To analyze the contribution of the potentially cytotoxic “non-specific” efflux, we compared cholesterol efflux to the peptides at saturating concentration (80 μg/ml) from live THP-1 cells and cells fixed with paraformaldehyde, a method we used previously to analyze cytotoxic properties of peptides 5A and L37PA 20. The data for cholesterol efflux are shown in Supplementary Figure V, the absolute values of non-specific efflux (i.e. efflux from fixed cells) is shown in Fig. 2 A and the “specificity” of the efflux (i.e. efflux from live cells minus efflux from fixed cells divided by the efflux from live cells x100%) is shown in Fig. 2 B. Analyzing features of the peptides responsible for the high non-specific efflux, we excluded from consideration peptides with low overall capacity to support efflux from live cells (marked with arrows in Fig. 2). The rationale for this exclusion was that analyzing efflux properties of the peptides that do not support total cholesterol efflux would not provide meaningful information about specificity of the efflux. Two features of the peptides associated with the high level of non-specific efflux were the following:
Net positive charge of the peptide (peptides with charge ≥ +2 denoted with fine cross-hatched bars in Fig. 2)
Replacement of the proline a bridge with a single alanine (peptide ELK-PA denoted with coarse cross-hatched bar in Fig. 2)
Fig. 2. Specificity of cholesterol efflux from THP-1 cells.
Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium; concentration of the peptides was 80 μg/ml. Fine cross-hatched bars denote peptides with charge ≥ +2; coarse cross-hatched bar denotes peptides with A for P substitution.
a – Efflux from the fixed cells
b - Contribution of the specific efflux (efflux from live cells to the total efflux (efflux from live cells – efflux from fixed cells/efflux from live cells x100%). Negative values are shown as “0”.
Thus, these two features should be avoided to avert toxicity of the peptides. The analysis also pointed to the two peptides with exceptional specificity, ELK-2A2K2E and ELK-1W; their specificity surpassing that of apoA-I. However, while the former peptide was very active in ABCA1-dependent cholesterol efflux, the latter had a modest capacity for the ABC-dependent efflux, indicating that peptide ELK-1W may interact with alternative transporters or receptors that promote cholesterol efflux.
Cholesterol efflux from BHK and BHK/ABCA1 cells
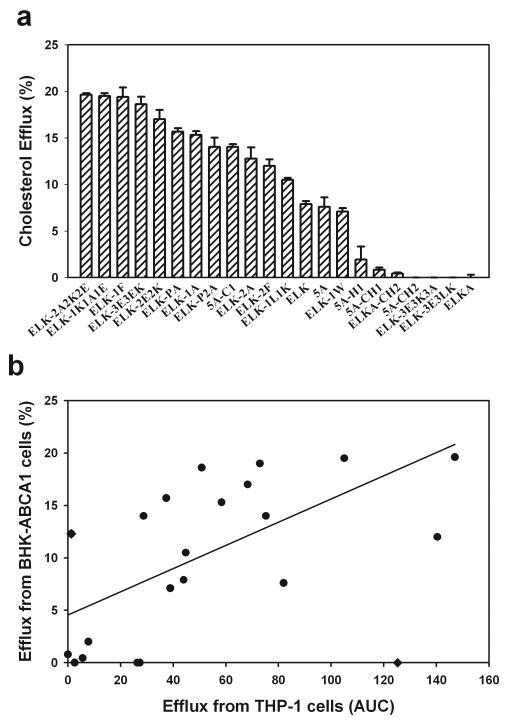
Although it is likely that the cholesterol efflux observed from the THP-1 cells was due to ABCA1, because only lipid-free peptides were used, it is possible that other ABC transporters, such as ABCG1, could also be contributing to the measured cholesterol efflux. An alternative model was, therefore, also used to assess the capacity and specificity of the peptides towards cholesterol efflux. BHK cells stably transfected with ABCA1 were compared to parent BHK cells, which do not have significant levels of endogenous ABCA1 or ABCG122; thus the difference between the two cell lines was defined as ABCA1-dependent cholesterol efflux.
With two exceptions, peptides ELK-2A and 5A-CH2, the efficiency of the peptides toward specifically ABCA1-dependent efflux closely followed the ABCA1-dependent efflux observed from THP-1 cells (Fig. 3 A). There was a significant correlation between the ABCA1-dependent efflux from BHK cells and ABC-dependent efflux from THP-1 cells (rank order correlation: r=0.75, p<0.0001) (Fig. 3 B). The relationships between structural features of the peptides and efficiency of the efflux from BHK-ABCA1 cells was similar to that of THP-1 cells, except that relationship between efflux and mean hydrophobicity peaked at a slightly higher value of −0.4 (not shown). Thus, cholesterol efflux to lipid-free peptides primarily reflects ABCA1-dependent efflux.
Fig. 3. Cholesterol efflux from BHK/ABCA1 cells.
Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium; concentration of the peptides was 20 μMol/ml (or approximately 90 μg/ml).
a – ABCA1-dependent efflux from BHK cells. Data presented are a difference between the efflux from BHK/ABCA1 cells and BHK/mock cells. Means ± SEM are presented.
b - Correlation between the ABC-dependent efflux from THP-1 and ABCA1-dependent efflux from BHK cells. Two peptides, ELK-2A and 5A-CH2, shown as ◆, were excluded from the analysis of correlations.
Anti-inflammatory properties: monocytes
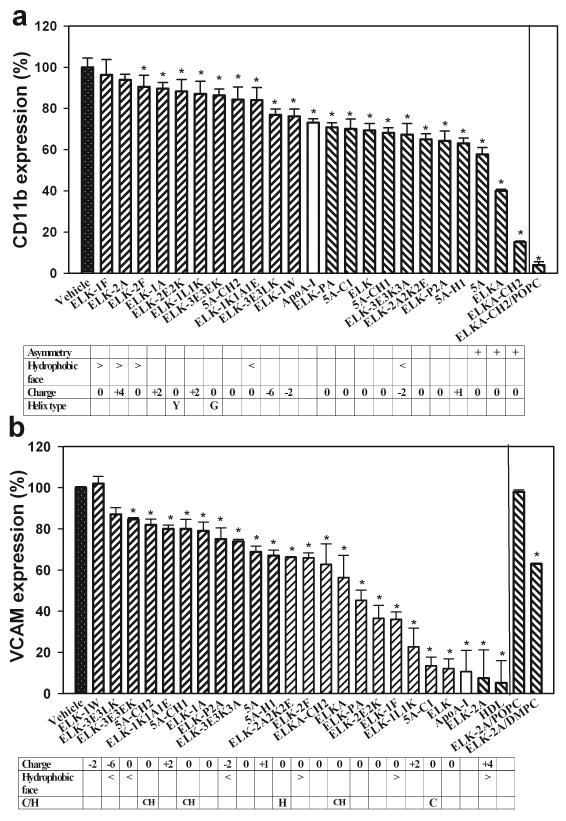
We have previously demonstrated that apoA-I and HDL are capable of reducing the expression of a key adhesion molecule, CD11b, on human monocytes in response to activation with a number of pro-inflammatory stimuli 23. We tested the capacity of the peptides to mimic this property of apoA-I on human monocytes activated with PMA in the presence of the peptides or apoA-I (final concentration 40 μg/ml). The expression of CD11b was assessed by flow cytometry and is shown in Fig. 4 A. All peptides, with the exception of ELK-1F and ELK-2A, inhibited the expression of CD11b on activated human monocytes; there was a 6-fold difference in the magnitude of inhibition between the most and the least efficient peptides. Analysis of structure-function relationships as related to the inhibition of CD11b expression led to the following conclusions:
The structural feature having the most impact on the capacity of peptides to inhibit CD11b expression was asymmetry: all three asymmetrical peptides, 5A, ELKA and ELKA-CH2, were most active in inhibition of CD11b expression on monocytes.
Second feature having considerable impact on the capacity of the peptides to inhibit CD11b expression was the size of the hydrophobic face. An increase of the size of hydrophobic face over 180° was detrimental for the anti-inflammatory property of the peptides (peptides ELK-2F, ELK-1F and ELK-2A).
Third feature affecting this anti-inflammatory property was charge. Two or more additional positive charges were detrimental (peptides ELK-2A, ELK-1A and ELK-1L1K); however, additional negative charges were neither detrimental nor beneficial (peptides ELK-1W, ELK-3E3LK and ELK-3E3K3A).
Changing the type of helix to type G or Y was detrimental for the anti-inflammatory capacity of the peptides (peptides ELK-2E2K and ELK-3E3EK).
Introduction of Cys and His residues or manipulation with proline bridge between the two helices had limited impact on the anti-inflammatory properties of the peptides.
Fig. 4. The effect of peptides on anti-inflammatory properties.
a - CD11b expression in human monocytes. CD11b expression was measured by flow cytometry; results were expressed as percentage of the CD11b expression compared to cells stimulated with PMA in the presence of a vehicle; concentration of peptides was 40 μg/mL. Means ± SEM are presented; *p<0.01 (versus vehicle). Table shows peptide properties that are likely to influence CD11b expression.
b - VCAM-1 expression in mouse endothelial cells, SVEC4/VCAM-1. Data were expressed per milligram of cellular protein and related to the luciferase activity in cells incubated with a vehicle instead of the peptides; concentration of peptides was 0.75 mg/mL. Means ± SEM are presented; *p<0.01 (versus vehicle). Table shows peptide properties that are likely to influence VCAM-1 expression.
Thus, the optimal structural features of an effective anti-inflammatory peptide are an asymmetrical pair of type A α-helices with hydrophobic face less than 180° and with neutral or negative charge.
We also analyzed the effect of complexing of the most active peptide, ELK-CH2, with phospholipid on its capacity to inhibit CD11b expression. Complex ELK-CH2/POPC had a higher capacity to inhibit CD11b expression compared to lipid-free peptide (Fig. 4 A). Similar effects were observed for the peptide, 5A (not shown).
Anti-inflammatory properties: endothelium
HDL and apoA-I affect expression of adhesion molecules on endothelial cells and this function may contribute significantly to the anti-inflammatory properties of HDL 24. To test the capacity of the peptides to mimic anti-inflammatory function of apoA-I towards endothelium we used a mouse endothelial cell line (SVEC4) stably transfected with luciferase under control of human VCAM-1 promoter. It was originally suggested that while apoA-I reconstituted with phospholipid or native HDL were potent inhibitors of VCAM-1 expression, lipid-free apoA-I may not be as effective ascribing endothelial anti-inflammatory property of HDL to its lipid constituencies 25. This was not confirmed in our studies, with mouse endothelial cells: lipid-free apoA-I was just as an effective inhibitor of VCAM-1 expression as HDL, inhibiting 90% of VCAM-1 expression (HDL inhibited VCAM-1 expression by 95%, p>0.05 versus apoA-I) (Fig. 4 B). Peptides were tested in lipid-free form; cells were activated with TNF-α and incubated with apoA-I, HDL or the peptides at the final concentration of 0.75 mg/ml, which was found to be a non-saturating concentration of the peptides for this response. Analysis of structure-function relationships as related to the inhibition of VCAM-1 expression is shown in Fig. 4 B and led to the following conclusions:
Increased size of hydrophobic face (peptides ELK-2A, ELK-1F, ELK-2F) was beneficial for the inhibition of the VCAM-1 expression
Negative charge (peptides ELK-1W, ELK-3E3LK, ELK-3E3K3A) was detrimental for the inhibition of VCAM-1 expression.
Inclusion of a combination of Cys+His residue was detrimental independently of their location (peptides 5A-CH2, 5A-CH1), while inclusion of Cys residue into the first helix of asymmetrical peptide was beneficial (peptide 5A-C1).
Hydrophobicity, changing helix type, disruption of the proline bridge and asymmetry had limited impact on the capacity of the peptides to inhibit VCAM-1 expression.
Thus, to be effective in inhibition of VCAM-1 expression the peptide ideally should have a larger hydrophobic face, positive or neutral charge, and may contain a Cys residue. We also analyzed the effect of complexing of the most active peptide, ELK-2A, with phospholipid on its capacity to inhibit VCAM-1 expression. Unexpectedly, complex ELK-2A/POPC did not inhibit VCAM-1 expression (Fig. 4 B). Complexing ELK-2A with another phospholipid, DMPC, partially restored the capacity of the peptide to inhibit VCAM-1 expression (Fig. 4 B). Similar effects were observed for the peptide, 5A (not shown).
Anti-oxidant properties
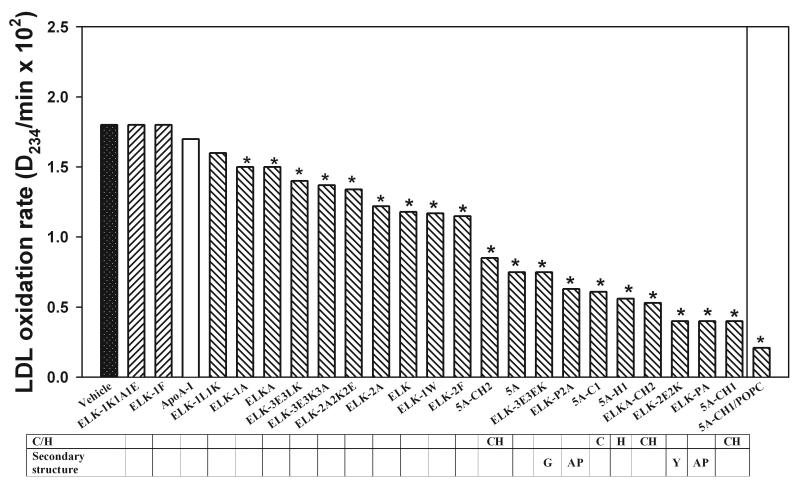
The anti-oxidant properties of the peptides were assessed in an LDL oxidation assay. Human plasma LDL was incubated in the presence of Cu++ and apoA-I mimetic peptides or apoA-I (final concentration 100 μg/ml); time-course of diene formation was monitored by measuring absorption at 234 nm. Duration of lag phase and maximum diene formation were used to quantitate the rate of LDL oxidation as described by Pinchuk et al 26. The time-course curves for LDL oxidation are shown in supplementary Fig. VI, and the rates of LDL oxidation are shown in Fig. 5. All peptides, with the exception of ELK-1K1A1E, ELK-1F and ELK-1L1K inhibited oxidation of LDL by Cu++; there was a 5-fold difference in the magnitude of inhibition between the most and least effective peptides. Analysis of structure-function relationships as related to the inhibition of LDL oxidation led to the following conclusions:
As expected, presence of Cys and/or His residue (peptides 5A-CH1, 5A-C1, 5A-H1, 5A-CH2, ELKA-CH2) significantly increased the anti-oxidant capacity of the peptides. The exact position of the residues had limited impact.
Unexpectedly, changes affecting secondary structure of the peptides had beneficial effect: asymmetrical peptides, peptides comprising type G and type Y helices (peptides ELK-3E3EK and ELK-2E2K), as well as peptides with modification of the proline bridge (peptides ELK-P2A and ELK-PA) were all better anti-oxidants. Most peptides were better anti-oxidants than apoA-I.
Charge, hydrophobicity and size of hydrophobic face had limited impact on anti-oxidant capacity of the peptides.
Fig. 5. The effect of peptides on LDL oxidation.
Concentration of peptides was 100 μg/mL. Rate of oxidation was calculated as maximum absorbance divided to the length of the lag period. *p<0.01 (calculated from comparing the time-dependence curves presented in the Supplementary Figure VI). Table shows peptide properties that are likely to influence anti-oxidant properties. G – G-helix, Y-Y-helix, AP-substitution of A for P.
Thus, an effective anti-oxidant peptide should contain Cys residue and preferably contains a non- type A α-helix, such as type G or type Y.
We also analyzed the effect of complexing of the most active peptide, 5A-CH1, with phospholipid on its capacity to inhibit LDL oxidation. Complex 5A-CH1/POPC had a higher capacity to inhibit LDL oxidation compared to lipid-free peptide (Fig. 5).
Relationships between different anti-atherogenic properties of the peptides
The finding that different apoA-I mimetic peptides have a wide range of efficiencies towards various anti-atheroghenic properties made it possible to investigate if any of these functions are related to each other. We found, however, no significant positive relationship for the various assays. In fact, the analysis of the structural features shows that features beneficial for one function may be detrimental for another (Table 3). For example, increased size of hydrophobic face was beneficial for cholesterol efflux, but detrimental for the monocyte anti-inflammatory function. Maintaining the proline bridge was essential for the efflux, but was detrimental for the anti-oxidant function and did not affect monocyte anti-inflammatory function. While several peptides were superior to apoA-I for some of the individual functional assays, none of them was better than apoA-I in all tested functional assays. These findings are consistent with a suggestion that the various functions of apoA-I may have different structural requirements and are determined by different regions of the protein.
Table 3.
Structural features responsible and individual anti-atherogenic properties of the peptides.
| Function | Hydrophobicity | Size of hydrophobic face |
Charge | Maintaining proline bridge |
Type of helix | Inclusion of Cys/His residues |
Asymmetry |
|---|---|---|---|---|---|---|---|
| Efficiency of cholesterol efflux |
Optimal (−0.5) | Increased size is beneficial |
Neutral | Essential | Limited effect | Detrimental in the first helix, beneficial in the second helix |
Beneficial in 5A, detrimental in ELK |
| Specificity of cholesterol efflux |
Limited effect | Limited effect | Neutral or negative |
Essential | Limited effect | Limited effect | Beneficial in 5A, detrimental in ELK |
| Anti-inflammatory -monocytes |
Limited effect | Increased size is detrimental |
Neutral or negative |
Limited effect | Changing to G or Y is detrimental |
Limited effect | Beneficial |
| Anti-inflammatory -endothelium |
Limited effect | Increased size beneficial |
Neutral or positive |
Limited effect | Limited effect | C+H detrimental, C beneficial |
Limited effect |
| Anti-oxidant | Limited effect | Limited effect | Limited effect | Detrimental | Changing to G or Y is beneficial |
Beneficial | Limited effect |
Discussion
In this study we analyzed the structure-function relationship of 22 bi-helical apoA-I mimetic peptides to identify structural features of these peptides that enable them to better mimic the various anti-atherogenic functions of HDL. We also aimed at establishing if the various anti-atherogenic properties of apoA-I are dependent upon the same structural features, and if so, whether they share the same underlying mechanism.
The critical features of the peptides for cholesterol efflux capacity and ABCA1 specificity were hydrophobicity, size of the hydrophobic face, charge, and angle between two helices. Some of these features, such as requirement for a proline bridge 18, 19 or size of hydrophobic face 8, were previously investigated and our data are consistent with the results of these studies; however, this is the first systematic analysis of multiple structural modifications on cholesterol efflux. Following this analysis, we were able to synthesize a peptide combining all beneficial features required for cholesterol efflux, ELK-2A2K2E. This peptide was more effective than apoA-I in capacity and specificity of cholesterol efflux, thus supporting our findings. As was shown for the experiment with ABCA1 transfected cells, the optimal structural features most likely enable these peptides to interact with ABCA1 and for triggering any downstream events that are necessary for cholesterol efflux. An unresolved issue among factors affecting cholesterol efflux is requirement for an asymmetry of the peptides. We have previously shown that introduction of asymmetry in bi-helical peptide L37PA resulted in dramatic improvement of specificity, with only modest reduction in overall efflux capacity 20. We attempted to introduce such an asymmetry into ELK-2A2K2E peptide (peptide ELKA) expecting a peptide with even better cholesterol efflux specificity. However, this peptide had very low overall capacity to support cholesterol efflux; the reasons for this are yet to be established.
Anti-oxidant property of the peptides strongly depend on the presence of particular amino acids, such as histidine and cysteine, a finding consistent with that of Jia et al 27. Presence of these amino-acids and enhanced anti-oxidant capacity have also been proposed to be behind the anti-atherogenic properties of apoA-IMilano and apoA-IParis 28. As expected, the other physico-chemical properties of the peptides had limited impact on anti-oxidant capacity, but unexpectedly changes disrupting “apoA-I – like” secondary structure, such as changing helix type or removing proline bridge were beneficial. Possibly these changes alter the binding of these peptides to LDL, as was suggested by Getz et al 19.
Anti-inflammatory properties of the peptides were investigated in two models, related to the expression of adhesion molecules on monocytes and endothelium. The anti- inflammatory effect of HDL to these two cell types, however, likely involves different mechanisms. The anti-inflammatory effect of HDL on monocytes is fast, short lived and requires low concentration of apoA-I 23, whereas the response of endothelial cells is slow, long lasting and requires high levels of HDL 29. These differences suggest different mechanisms responsible for the anti-inflammatory effects of HDL in these cell types. It is therefore not surprising that the structural requirements for anti-inflammatory effect of the different peptides in these two models did not overlap and in fact were almost opposite. Peptides active in inhibiting monocyte CD11b were asymmetrical peptides with a smaller hydrophobic face and a negative charge. In contrast, peptides active in inhibiting expression of endothelial VCAM-1 had relatively large hydrophobic faces and were positively charged. As the mechanisms of the anti-inflammatory effects of apoA-I on monocytes and endothelial cells are not known, it is premature to speculate on how these structural features are translated into differences in the expression of adhesion molecules.
Another interesting finding of this study was that different atheroprotective functions of the peptides were determined by different structural features. No consistent correlation was found between the capacities of the peptides to mediate the various functions. No specific structural feature equally benefitted all functions. Furthermore, time and dose dependencies of the effects of the peptides and apoA-I on specific functions varied dramatically from one function to another: it took under 15 min to inhibit the expression of CD11b and almost 24 h to inhibit expression of VCAM-1. The saturating concentration of the peptides significantly differed for the different assays. Approximately 20 μg/ml of the peptides was required for maximum cholesterol efflux, whereas 100 μg/ml was needed for anti-oxidant capacity and over 750 μg/ml for inhibition of VCAM-1 expression in endothelium. These findings suggest that different anti-atherogenic functions of apoA-I have different mechanisms. This is consistent with findings of Wool et al 18, who demonstrated that modification of the peptides that favors HDL remodeling have negative impact on anti-oxidant function. Although a number of peptides were better than apoA-I in supporting individual functions, none of them could match the versatility of apoA-I when all the functions were taken into consideration. Possibly, different parts of apoA-I are responsible for different anti-atherogenic functions and mimicking just one or two structural features of apoA-I is insufficient to create a peptide active in the many anti-atherogenic facets of apoA-I. Although it may, therefore, be difficult to duplicate all of the biological properties of apoA-I in a single peptide of limited length, it may be possible to use a combination of peptides, an option that is currently being tested. The uncoupling, in the peptides, of the different anti-atherogenic properties of HDL, however, creates a unique opportunity to investigate the relative contribution of the different anti-atherogenic activities of HDL by testing them in animal models of atherosclerosis.
It is important to recognize several potential limitations of this study. First, HDL constituents other than apoA-I most likely also contribute to the anti-atherogenic properties of HDL. Size of HDL affects its ability to support cholesterol efflux 30, paraoxonase has a significant contribution to the anti-oxidant function 31, phospholipids may contribute to the anti-inflammatory effects of HDL to endothelium 25 and various pro- and anti-inflammatory factors carried on HDL may contribute to the HDL anti-inflammatory effects 32. Furthermore, a number of HDL functions were not investigated in this study, such as anti-thrombotic activity, suppression of apoptosis, regulation of endothelial function, insulin secretion and glucose oxidation. Although these functions may contribute to the anti-atherogenic properties of HDL, most available data suggest that involvement of HDL in cholesterol efflux, inflammation and oxidation are the major determinants of its atheroprotective potential and therefore are the main targets of “HDL therapy”. Finally, peptides were examined in their lipid-free form. This was a deliberate strategy, as the physico-chemical properties of the peptides will have a significant impact on the lipid content of rHDL particles assembled with the peptides, the size of these particles and on the number of peptide molecules per particle. These factors may have a significant confounding influence on many anti-atherogenic properties of the peptides. The lipid-free peptides may also better simulate the process by which apoA-I interacts with cells and lipid-free peptides were shown to be as effective as their phospholipid complexes in preventing atherosclerosis in vivo 33. It is likely, however, that these peptides, like apoA-I, will readily acquire in vivo various lipids and/or will recombine with endogenous HDL; therefore, future in vivo studies will be needed to fully assess the effect of these peptides on atherosclerosis. Lipidated peptides (rHDL) would also interact with endogenous HDL and undergo remodeling and therefore would not be a better representation of the in vivo situation than lipid-free apoA-I. Acknowledging however that in vivo apoA-I mimetic peptides are likely to be lipidated, we examined the effect of complexing the most active peptides with phospholipid on their activity. As expected, lipidation increased peptide cholesterol efflux capacity, most likely by complementing ABCA1-dependent efflux with the efflux through other pathways. Lipidation further improved peptide capacities to prevent LDL oxidation and to inhibit CD11b expression on monocytes. Surprisingly, lipidation had a profound effect on the capacity of peptides to inhibit VCAM-1 expression in endothelium. While lipidation of apoA-I into HDL did not alter its anti-inflammatory capacity, lipidation of the peptides with DMPC reduced this capacity and lipidation with POPC abolished it. In vivo, however, lipidated peptide 5A did inhibit expression of VCAM-1 in rabbit arteries 34. Clearly, this property of the peptides may be affected by lipid constituency.
In summary, by examining a panel of amphipathic bi-helical peptides, it was found that the different anti-atherogenic features of these peptides requires different structural features. This is relevant to the design of apoA-I mimetic peptides for HDL therapy and may lead to new insights into what properties of HDL are the most relevant for its ability to reduce cardiovascular disease.
Novelty and Significance.
What is Known?
Apolipoprotein A-I (apoA-I) mimetic peptides are active in a number of anti-atherogenic functions of high density lipoprotein (HDL) and can protect against atherosclerosis
Structurally, ApoA-I mimetic peptides reproduce secondary structure of apoA-I, an array of 22-mer amphipathic α-helices, without close homology to the apoA-I primary structure
Knowledge on structure-function relationship of the peptides is limited to the facts that peptides with two helices work better than single helix peptides and that increasing peptide hydrophobicity improves their functionality
What New Information Does This Article Contribute?
Using a panel of related peptides, we established optimal structural requirements for the activity of the peptides in cholesterol efflux, anti-inflammatory and anti-oxidant actions
Different anti-atherogenic properties of apoA-I have different, and sometimes conflicting, structural requirements pointing to independent mechanisms
Different parts of apoA-I may be responsible for different anti-atherogenic functions and it may not be possible to combine all the features in one peptide of limited length
ApoA-I mimetic peptides are a promising therapeutic approach attempting to reproduce the anti-atherogenic properties of HDL. These peptides support cholesterol efflux and have anti-inflammatory and anti-oxidant activities. Structurally, they are comprised of one or two 18-22 mer canonical amphipathic α-helices. ApoA-I, however, is comprised of “almost” canonical, as well as “imperfect” α-helices, and such mixture may be required for the variety of anti-atherogenic functions of apoA-I. By changing the structure of one or both helices in bi-helical peptides, we were able to “fine tune” the peptides to be more active and more specific in individual functions compared with apoA-I. However, structural requirements for the individual functions were often conflicting with each other, and although a number of peptides have high activity in all assays, we were unable to design a peptide that was more active than apoA-I in all its functions. These findings provide a rationale for designing more complex formulations that would combine multiple anti-atherogenic activities. We have also designed a number of peptides that are active in one, but inactive in other anti-atherogenic functions, providing a powerful tool to study the relative contribution of various HDL functions to its overall antiatherogenic capacity.
Supplementary Material
Supplementary Figure I. Dose-dependence of cholesterol efflux from THP-1 cells activated with LXR agonist
Cellular cholesterol was labeled by incubation in serum-containing medium with [3H]-cholesterol. Cells were then washed and incubated for 18 h at 37°C in serum-free medium in the presence of the LXR agonist TO-901317 (4 μmol/L). Cells were washed and incubated for another 4 h at 37°C in serum-free medium containing indicated concentrations of the peptides or lipid-free apoA-I. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Non-specific efflux (i.e. the efflux in the absence of an acceptor) was subtracted. Data from different experiments were normalized to the efflux to the peptide 5A, which was included in all experiments.
Supplementary Figure II. Dose-dependence of cholesterol efflux from THP-1 cells not activated with LXR agonist
Cellular cholesterol was labeled by incubation in serum-containing medium with [3H]-cholesterol. Cells were then washed and incubated for 18 h at 37°C in serum-free medium, washed and incubated for another 4 h at 37°C in serum-free medium containing indicated concentrations of the peptides or lipid-free apoA-I. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Nonspecific efflux (i.e. the efflux in the absence of an acceptor) was subtracted. Data from different experiments were normalized to the efflux to the peptide 5A, which was included in all experiments.
Supplementary Figure III. Dose-dependence of ABCA1-dependent cholesterol efflux from THP-1.
Data presented in this figure are a difference between values presented in Supplementary Figure I and Supplementary Figure II, calculated for each data point. When calculations gave negative values they were interpreted as “0” value.
Supplementary Figure IV: Contribution of ABCA1-dependent efflux
Data presented in this figure show efflux from activated and non-activated THP-1 cells (data taken from Supplementary Figure I and Supplementary Figure II) at peptide concentration 20 μg/ml
Supplementary Figure V. Specificity of cholesterol efflux from THP-1 cells.
Cellular cholesterol was labeled by incubation with [3H]-cholesterol for 48 h in a CO2 incubator. Cells were then washed and incubated for 18 h at 37°C in serum-free medium, and fixed or not fixed by incubation for 20 min with paraformaldehyde (4%). Cells washed and incubated for another 4 h at 37°C in serum-free medium containing 80 μg/ml of the peptides or lipid-free apoA-I. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Non-specific efflux (i.e. the efflux in the absence of an acceptor) was subtracted.
Supplementary Figure IV: Contribution of ABCA1-dependent efflux
Data presented in this figure show efflux from activated and non-activated THP-1 cells (data taken from Supplementary Figure I and Supplementary Figure II) at peptide concentration 20 μg/ml
Acknowledgments
Sources of Funding: This study was supported by a grant from the National Heart Foundation of Australia (G 07M 3165) to DS and JCD and Postgraduate Award from the National Health and Medical Research Council of Australia to WD. DS and JCD are Fellows of the National Health and Medical Research Council of Australia. Research done by ATR was supported by intramural research funds from the NIH. AAS was supported by the Danish Agency for Science, Technology and Innovation.
Abbreviations
- ApoA-I
apolipoprotein A-I
- AUC
area under the curve
- DMPC
dimyrisoylphosphatidyl choline
- CVD
cardiovascular disease
- HDL
high density lipoprotein
- LXR
liver X receptor
- PMA
phorbol-12 myristate 13-acetate
- POPC
palmitoyloleoyl phosphatidyl choline
- TNF-α
tissue necrosis factor
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinberg D. Thematic review series: The Pathogenesis of Atherosclerosis. An interpretive history of the cholesterol controversy, part V: The discovery of the statins and the end of the controversy. J Lipid Res. 2006;47:1339–1351. doi: 10.1194/jlr.R600009-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Remaley AT, Amar M, Sviridov D. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther. 2008;6:1203–1215. doi: 10.1586/14779072.6.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye K-A, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. Reconstituted High-Density Lipoprotein Increases Plasma High-Density Lipoprotein Anti-Inflammatory Properties and Cholesterol Efflux Capacity in Patients With Type 2 Diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 5.Sethi AA, Amar M, Shamburek RD, Remaley AT. Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2007;8:201–212. [PubMed] [Google Scholar]
- 6.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, Fogelman AM. Apolipoprotein A-I Mimetic Peptides. Arterioscler Thromb Vasc Biol. 2005;25:1325–1331. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 7.Navab M, Anantharamaiah GM, Hama S, Hough G, Reddy ST, Frank JS, Garber DW, Handattu S, Fogelman AM. D-4F and Statins Synergize to Render HDL Antiinflammatory in Mice and Monkeys and Cause Lesion Regression in Old Apolipoprotein E-Null Mice. Arterioscler Thromb Vasc Biol. 2005;25:1426–1432. doi: 10.1161/01.ATV.0000167412.98221.1a. [DOI] [PubMed] [Google Scholar]
- 8.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, EK-W Hui, Nayak DP, Fogelman AM. D-4F, an Apolipoprotein A-I Mimetic Peptide, Inhibits the Inflammatory Response Induced by Influenza A Infection of Human Type II Pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM. Oral D-4F Causes Formation of Pre-{beta} High-Density Lipoprotein and Improves High-Density Lipoprotein-Mediated Cholesterol Efflux and Reverse Cholesterol Transport From Macrophages in Apolipoprotein E-Null Mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 10.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral Administration of an Apo A-I Mimetic Peptide Synthesized From D-Amino Acids Dramatically Reduces Atherosclerosis in Mice Independent of Plasma Cholesterol. Circulation. 2002;105:290–292. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 11.Garber DW, Datta G, Chaddha M, Palgunachari MN, Hama SY, Navab M, Fogelman AM, Segrest JP, Anantharamaiah GM. A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J Lipid Res. 2001;42:545–552. [PubMed] [Google Scholar]
- 12.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navab M, Ruchala P, Waring AJ, Lehrer RI, Hama S, Hough G, Palgunachari MN, Anantharamaiah GM, Fogelman AM. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J Lipid Res. 2009;50:1538–1547. doi: 10.1194/jlr.M800539-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendez AJ, Anantharamaiah GM, Segrest JP, Oram JF. Synthetic amphipathic helical peptides that mimic apolipoprotein A-I in clearing cellular cholesterol. J Clin Invest. 1994;94:1698–1705. doi: 10.1172/JCI117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, Santamarina-Fojo S, Brewer HB. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44:828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- 17.Natarajan P, Forte TM, Chu B, Phillips MC, Oram JF, Bielicki JK. Identification of an Apolipoprotein A-I Structural Element That Mediates Cellular Cholesterol Efflux and Stabilizes ATP Binding Cassette Transporter A1. J Biol Chem. 2004;279:24044–24052. doi: 10.1074/jbc.M400561200. [DOI] [PubMed] [Google Scholar]
- 18.Wool GD, Reardon CA, Getz GS. Apolipoprotein A-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J Lipid Res. 2008;49:1268–1283. doi: 10.1194/jlr.M700552-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wool GD, Vaisar T, Reardon CA, Getz GS. An apoA-I mimetic peptide containing a proline residue has greater in vivo HDL binding and anti-inflammatory ability than the 4F peptide. J Lipid Res. 2009;50:1889–1900. doi: 10.1194/jlr.M900151-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D’Souza W, Sviridov D, Remaley AT. Asymmetry in the Lipid Affinity of Bihelical Amphipathic Peptides: A STRUCTURAL DETERMINANT FOR THE SPECIFICITY OF ABCA1-DEPENDENT CHOLESTEROL EFFLUX BY PEPTIDES. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushima D, Yokoyama S, Kroon DJ, Kezdy FJ, Kaiser ET. Chain length-function correlation of amphiphilic peptides. Synthesis and surface properties of a tetratetracontapeptide segment of apolipoprotein A-I. J Biol Chem. 1980;255:10651–10657. [PubMed] [Google Scholar]
- 22.Oram JF, Vaughan AM, Stocker R. ATP-binding Cassette Transporter A1 Mediates Cellular Secretion of alpha -Tocopherol. J Biol Chem. 2001;276:39898–39902. doi: 10.1074/jbc.M106984200. [DOI] [PubMed] [Google Scholar]
- 23.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SPA, Remaley AT, Sviridov D, Chin-Dusting J. High-Density Lipoprotein Reduces the Human Monocyte Inflammatory Response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 24.Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002;13:285–288. doi: 10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Baker PW, Rye KA, Gamble JR, Vadas MA, Barter PJ. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res. 1999;40:345–353. [PubMed] [Google Scholar]
- 26.Pinchuk I, Schnitzer E, Lichtenberg D. Kinetic analysis of copper-induced peroxidation of LDL. Biochim Biophys Acta. 1998;1389:155–172. doi: 10.1016/s0005-2760(97)00139-2. [DOI] [PubMed] [Google Scholar]
- 27.Jia Z, Natarajan P, Forte TM, Bielicki JK. Thiol-bearing synthetic peptides retain the antioxidant activity of apolipoproteinA-I(Milano) Biochem Biophys Res Comm. 2002;297:206–213. doi: 10.1016/s0006-291x(02)02143-5. [DOI] [PubMed] [Google Scholar]
- 28.Bielicki JK, Oda MN. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 2002;41:2089–2096. doi: 10.1021/bi011716p. [DOI] [PubMed] [Google Scholar]
- 29.Clay MA, Pyle DH, Rye K, Vadas MA, Gamble JR, Barter PJ. Time sequence of the inhibition of endothelial adhesion molecule expression by reconstituted high density lipoproteins. Atherosclerosis. 2001;157:23–29. doi: 10.1016/s0021-9150(00)00659-6. [DOI] [PubMed] [Google Scholar]
- 30.Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P. Antiatherogenic Functionality of High Density Lipoprotein: How Much versus How Good. J Atheroscler Thromb. 2008;15:52–62. doi: 10.5551/jat.e571. [DOI] [PubMed] [Google Scholar]
- 31.Moren X, Deakin S, Liu M-L, Taskinen M-R, James RW. HDL subfraction distribution of paraoxonase-1 and its relevance to enzyme activity and resistance to oxidative stress. J Lipid Res. 2008;49:1246–1253. doi: 10.1194/jlr.M700439-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao X-Q, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, Johansson J, Azhar S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res. 2010 doi: 10.1194/jlr.M003665. jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye K-A, Lambert G. The 5A Apolipoprotein A-I Mimetic Peptide Displays Antiinflammatory and Antioxidant Properties In Vivo and In Vitro. Arterioscler Thromb Vasc Biol. 2010;30:246–252. doi: 10.1161/ATVBAHA.109.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure I. Dose-dependence of cholesterol efflux from THP-1 cells activated with LXR agonist
Cellular cholesterol was labeled by incubation in serum-containing medium with [3H]-cholesterol. Cells were then washed and incubated for 18 h at 37°C in serum-free medium in the presence of the LXR agonist TO-901317 (4 μmol/L). Cells were washed and incubated for another 4 h at 37°C in serum-free medium containing indicated concentrations of the peptides or lipid-free apoA-I. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Non-specific efflux (i.e. the efflux in the absence of an acceptor) was subtracted. Data from different experiments were normalized to the efflux to the peptide 5A, which was included in all experiments.
Supplementary Figure II. Dose-dependence of cholesterol efflux from THP-1 cells not activated with LXR agonist
Cellular cholesterol was labeled by incubation in serum-containing medium with [3H]-cholesterol. Cells were then washed and incubated for 18 h at 37°C in serum-free medium, washed and incubated for another 4 h at 37°C in serum-free medium containing indicated concentrations of the peptides or lipid-free apoA-I. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Nonspecific efflux (i.e. the efflux in the absence of an acceptor) was subtracted. Data from different experiments were normalized to the efflux to the peptide 5A, which was included in all experiments.
Supplementary Figure III. Dose-dependence of ABCA1-dependent cholesterol efflux from THP-1.
Data presented in this figure are a difference between values presented in Supplementary Figure I and Supplementary Figure II, calculated for each data point. When calculations gave negative values they were interpreted as “0” value.
Supplementary Figure IV: Contribution of ABCA1-dependent efflux
Data presented in this figure show efflux from activated and non-activated THP-1 cells (data taken from Supplementary Figure I and Supplementary Figure II) at peptide concentration 20 μg/ml
Supplementary Figure V. Specificity of cholesterol efflux from THP-1 cells.
Cellular cholesterol was labeled by incubation with [3H]-cholesterol for 48 h in a CO2 incubator. Cells were then washed and incubated for 18 h at 37°C in serum-free medium, and fixed or not fixed by incubation for 20 min with paraformaldehyde (4%). Cells washed and incubated for another 4 h at 37°C in serum-free medium containing 80 μg/ml of the peptides or lipid-free apoA-I. Cholesterol efflux was expressed as the proportion of [3H]cholesterol transferred from cells to medium. Non-specific efflux (i.e. the efflux in the absence of an acceptor) was subtracted.
Supplementary Figure IV: Contribution of ABCA1-dependent efflux
Data presented in this figure show efflux from activated and non-activated THP-1 cells (data taken from Supplementary Figure I and Supplementary Figure II) at peptide concentration 20 μg/ml