Abstract
Objective
Recent studies have shown women experience an acceleration of cognitive problems after menopause, and that estrogen treatment can improve or at least maintain current levels of cognitive functioning in postmenopausal women. However, we have previously shown that the negative emotional effects of psychosocial stress are magnified in normal postmenopausal women after estrogen treatment. This study examined whether estradiol administration can modify cognitive performance after exposure to psychological stress and monoamine depletion.
Methods
Participants consisted of 22 postmenopausal women placed on either oral placebo or 17β-estradiol (E2) (1 mg/day for 1 month, then 2 mg/day for 2 months). At the end of the 3 month treatment phase, participants underwent three depletion challenges in which they ingested one of three amino acid mixtures: deficient in tryptophan, deficient in phenylalanine/tyrosine, or balanced. Five hours later, participants performed the Trier Social Stress Test (TSST), followed by mood and anxiety ratings and cognitive testing. Cognitive measures included tests of attention, psychomotor function, and verbal episodic memory.
Results
E2-treated compared to placebo-treated participants exhibited significant worsening of cognitive performance on tasks measuring attentional performance and psychomotor speed. Similar trends for impairment were seen in measures of long-term episodic memory compared to placebo-treated postmenopausal women. E2-treated participants also showed a significant increase in negative mood and anxiety compared to placebo-treated women after but not before the TSST, though the worsening of both cognitive and behavioral functioning were not correlated. These effects were independent of tryptophan or tyrosine/phenylalanine depletion and were not manifest before the TSST or at baseline.
Conclusions
These data suggest that the relationship between estrogen administration and cognitive/behavioral performance in postmenopausal women may be more complex than initially appreciated and that effects of psychosocial stress may influence whether hormone effects are beneficial.
Keywords: estrogen, menopause, monoamines, stress, cognition
INTRODUCTION
Studies of the cognitive effects of estrogen or of women following menopause have strongly suggested that estrogen levels are directly relevant to cognitive function. Experimental studies of postmenopausal estrogen or estrogen treatment have in general tended to show positive effects on cognitive functioning1. Beneficial effects of hormone therapy on cognition after menopause have been confirmed in a number of studies showing that administration of estrogen to healthy postmenopausal women (PMW) improved visuospatial abilities, memory, and frontal lobe function 2–7 8–10, although not all studies have not shown positive effects 11–15 and studies examining estrogen therapy specifically in older postmenopausal women have not shown significant benefit, including the large Women's Health Initiative (WHI) study 16–20. Meta-analyses21, 22 demonstrated that hormone therapy shows cognitive benefit in younger women but older women show less evidence of benefit or small negative effects.
Overall, studies support the hypothesis that estrogen helps to maintain aspects of attention, verbal and visual memory23–25, and may have positive effects on tasks mediated by the prefrontal cortex26 and hippocampus27 especially in younger PMW, although in the recent SWAN study, perimenopausal women did not show the expected improvement with estradiol treatment28. Certain estrogen receptor polymorphisms appear to be associated with the risk of developing cognitive impairment29 and estrogen reduces neuronal generation of β-amyloid peptides which may be relevant to the onset of Alzheimer's disease (AD)30. PMW appear to be at higher risk for AD, particularly if they carry the APOE4 allele31, and there is considerable epidemiologic evidence from both prospective and case-control studies that E2 use in PMW may decrease the risk of the development and/or expression of AD32–36, with an overall odds ratio of 0.6624. In memory clinics, hormone users showed lower rates of dementia diagnoses versus mild cognitive impairment than nonusers, who deteriorated more rapidly than hormone users37.
In contrast to cognitive functioning, the increased vulnerability for depression seen in reproductive-age women in women declines after the menopause38, 39 although the perimenopause may be associated with increased vulnerability for both depressive symptoms and a diagnosis of new onset depression40, 41. While some studies have supported positive mood effects of estrogen or hormonal therapy in postmenopausal women42–45, others have not46–48.
However, there are few studies regarding the interaction between mood effects and cognitive performance in postmenopausal women. A strong candidate for explaining the cognitive and mood alterations after menopause is the influence of declining levels of gonadal steroids on neurotransmitter systems and mood regulatory systems39, perhaps interacting with genetic vulnerability and life stress49. A potential hypothesis for how estrogen or its loss after menopause exerts effects on cognition and mood is through interactions with modulatory neurotransmitter systems. For example, significant work has been done on examining how estrogen interacts with cholinergic system activity to alter cognitive functioning in both animal models and humans (see Gibbs50 and Dumas et al,51 for review). Recently, this laboratory has shown that estradiol (E2) appears to improve cognitive performance related to cholinergic function as measured by increased cognitive resistance to anti-cholinergic blockade in normal PMW52. This improvement may be dose and domain specific, i.e. lower doses improves primarily attentional functioning, higher doses may influence episodic memory53. Effects of E2 on cholinergic function related to episodic memory may be age-specific with younger women showing benefit but older women showing no benefit or impairment (providing direct experimental support for the "critical period hypothesis"53 of estrogen benefit after menopause). However, other monoamine neurotransmitters appear to have substantial modulatory roles on mood, anxiety, and on cognitive performance and behavior. For example, estrogen shows effects on modulation of serotonin and dopamine receptor density54, dopamine release55, and potentiation of serotonin function56–59. Postmenopausal women respond more briskly to serotoninergic antidepressants if taking estrogens60–63.
Catecholamine and indolamine systems can be investigated in humans utilizing conceptually similar treatment-challenge models to those that have been utilized to investigate cholinergic system-hormone interactions. We have previously reported the effects of estrogen and monoamine depletion on mood following psychosocial stress64. As an extension of this study we now report the effects of the same manipulation on cognitive functioning. The primary goal of the overall project was to test whether short-term administration of E2 to normal PMW would alter mood reactivity and cognitive performance to experimental psychosocial stress and quantitatively change the behavioral responses to CNS catecholamine and/or serotonin depletion, pharmacological challenges that directly interact with central monoamine systems.
Estrogen levels vary both within individuals and compared to premenopausal levels during the perimenopause and postmenopause and this variability is associated with physical and behavioral symptomatology65, 66. We reasoned that fluctuations in estrogen levels may lead to alterations in levels of monoamine neurotransmitters, which may influence mood reactivity and cognitive performance to external events. Thus, to probe the interaction of estrogen and monoamine neurotransmitters on cognition and mood, we used the technique of monoamine depletion and experimentally-induced psychosocial stress. Acute tryptophan depletion (ATD) is a well-established technique for examining the role of serotonin systems in mood 67, 68. Acute phenylalanine/tyrosine depletion (APTD) is a newer technique designed to examine the effects of reduced catecholamine synthesis and transmission on behavior and performance 69. Tryptophan depletion can, in some circumstances, produce adverse effects on mood and behavior that are considered relevant to understanding the causes of affective illness 68, 70. Further, central catecholamine depletion has been examined in normal premenopausal women and has been found to produce negative effects on mood under stress 69.
In this study, women who were postmenopausal (> 50 years) took a fixed dose of 17β-estradiol (E2) or placebo for three months and then participated in three challenges using monoamine depletion to briefly change the relative amounts of monoamine neurotransmitters in the brain (serotonin, dopamine, and norepinephrine). Participants then participated in a psychosocial stress paradigm to potentiate negative mood (Trier Social Stress Test, TSST)71 that involves public speaking and has been shown previously to reliably produce mild-moderate psychosocial stress. We have reported previously on the mood effects of this manipulation64, in which we showed significant enhancement of negative mood effects after the psychosocial stress maneuver in the E2-treated participants. Here we report on the cognitive results from that study with additional participants.
We hypothesized that the psychosocial stress manipulation (TSST) would enhance any negative mood and cognitive effects of monoamine depletion and that estrogen administration would blunt or buffer potential negative effects produced by the combination of the monoamine depletion and the stress test in a measurable and quantifiable way. Since estrogen has been noted to interact with both serotonergic and catecholaminergic systems, it was hypothesized that depletion of either monoamine system would interact with estrogen treatment to alter cognitive performance.
METHODS
The basic design consisted of a double-blind parallel group design (each subject was randomly assigned to receive either 3 months of E2 or placebo) with each treatment group then undergoing acute depletion and social stress challenges. All participants signed fully informed consent after an explanation of all procedures, risks, and benefits. Participants received $100 as compensation for their time and a small gift pack after each study session. The study was approved by the University of Vermont Committee for Human Research in the Medical Sciences (IRB).
Participants
Participants were recruited through newspaper advertisement and health newsletters published by our Medical Center, public information sessions, newspaper advertisements, and random mailings. Study participants were first screened by phone for eligibility. Participants consisted of 22 normal PMW ages 52–83 (M=64.3, SD=10.6). Subject demographics are described in Table 1.
Table 1.
Subject Demographic Data (n=22)
| Estrogen Treatment (n=11) | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Age (years) | 65.18 ± 11.81 | 52 | 83 |
| Body Mass Index | 25.83 ± 3.70 | 18.71 | 31.3 |
| Years Since Menopause | 14.57 ± 11.30 | 1.0 | 31.0 |
| Baseline FSH | 61.87 ± 16.11 | 42.8 | 99.0 |
| Education (years) | 14.36 ± 2.58 | 9 | 18 |
| Placebo Treatment (n=11) | Mean ± SD | Minimum | Maximum |
| Age (years) | 63.45 ± 9.80 | 52 | 80 |
| Body Mass Index | 26.63 ± 5.29 | 20.44 | 33.27 |
| Years Since Menopause | 14.03 ± 10.24 | 1.3 | 37.0 |
| Baseline FSH | 69.60 ± 17.76 | 38.6 | 86.5 |
| Education (years) | 15.54 ± 2.87 | 12 | 20 |
Medical Screening
Participants were without menses for at least 1 year, had an FSH level greater than 30 mIU/ml, nonsmokers, had a normal mammogram within the last year, and were without surgically-induced menopause (bilateral oophorectomy). They were not taking HT, or oral contraceptives, and were at least one year without such treatment. Participants were physically healthy, had a body mass index ≤34 kg/m2, and had no cardiovascular disease other than mild hypertension. Participants with major concomitant illnesses were excluded on the basis of history, physical exam, and laboratory tests assessing hematopoietic, renal, hepatic and hormonal function. (CBC, Chem 20, TSH, U/A, ECG). Participants were physically examined by a gynecologic nurse-practitioner to establish general physical health and for specific physical contraindications for E2 therapy (e.g. adnexal mass, large uterine fibroids, etc.)
Participants were excluded if they had specific contraindications for E2 treatment, or current or any past Axis I psychiatric disorders. Specific criteria for exclusion for the E2 treatment included contraindications for hormone replacement including history of breast cancer or E2-dependent neoplasia; blood pressure > 160/100 (untreated); history of deep vein thrombosis or other thromboembolic disease; hepatoma; severe migraines or stroke on oral contraceptives; concurrent use of barbiturates, rifampin, insulin, carbamezepine, oral hypoglycemics, antidepressants, or lipid-lowering drugs; known intolerance to conjugated E2s; diabetes; untreated thyroid disease; clinical osteoporosis; severe menopausal symptoms. All participants were taking no centrally active drugs. No participants were taking selective estrogen receptor modulators (SERMs) or herbal menopause preparations. A minimum of 14 days elapsed between discontinuing centrally active or psychoactive agents and participation in this study.
Cognitive/Behavioral Screening
All participants were cognitively and behaviorally assessed using standard tests designed to exclude participants with cognitive or behavioral impairment. Participants were evaluated using the Mini Mental State Exam (MMS)72, Brief Cognitive Rating Scale73, the Mattis Dementia Rating Scale74, to establish a Global Deterioration Scale score (GDS) which rates the degree of cognitive impairment75. Participants were required to have a GDS score of 1–2 and a MMS score of greater than or equal to 27. Participants were excluded if they scored below 123 on the Dementia Rating Scale74 scale and were matched across the two groups in terms of educational background.
Behavioral screening consisted of a partial Structured Clinical Interview for DSM-IV-TR (SCID)76 to establish the presence/absence of present or past Axis I major psychiatric disorders, particularly any present or past history of mood disorders. In addition, participants completed the Beck Depression Rating Scale77 and a menopause symptom checklist modified from Sherwin78 to detect subclinical depressive symptoms. An exclusion cut off score of 10 was used for the Beck Depression Rating Scale.
Estrogen/Placebo Treatment
After screening and acceptance into the study, each subject was placed randomly and blindly on either oral placebo or 17-β estradiol (E2) (using identical pink capsules) for 3 months. There were 11 women in the E2 group and 11 women in the placebo group. Women were initially placed on E2 1 mg per day for 30 days, and then were increased to 2 mg per day. This was done because early pilot trials revealed that estrogen-related side effects (e.g. breast tenderness or spotting) tended to be noticed by participants if the participant was begun on 2 mg of E2 from the beginning. Using 1 mg of E2 for the first 30 days helped to protect the blind.. At the end of the 3 month treatment period, participants participated in a series of challenge studies designed to examine differences in sensitivity to acute transmitter depletion and psychosocial stress. E2 or placebo treatment continued throughout the challenge/stress studies. Twelve days of medroxyprogesterone acetate (MPA) (Provera) was given at the end of the study to produce shedding of the endometrial lining.
Acute Depletion Challenges
All studies took place on the University of Vermont General Clinical Research Center (GCRC). Each participant underwent three test days, at least seven days apart, in which they received the each of the two amino acid depletion mixtures and the nutritionally-balanced control mixture. The depletion sequence was determined by a random order procedure.
The procedure for the administration of the amino acid mixtures was the same as we have used previously69. Participants were placed on a low-protein diet for the evening meal prior to each study day. Following an overnight fast, the study began at 0800 with baseline testing and evaluation. Participants then ingested one of three amino acid (AA) mixtures: (1) a nutritionally balanced AA mixture, (2) a mixture deficient in tryptophan (ATD), or (3) a mixture deficient in phenylalanine and tyrosine (APTD). The composition of the AA mixtures was that used in prior studies, adjusted for the generally lower weight of women. Mixtures consisted of amino acid suspended and water, with the worst tasting amino acids (L- methionine, L- cysteine and L- arginine) in capsules. The liquid suspensions were flavored with noncaloric, no-protein flavoring of orange, grapefruit, lemon, chocolate, or cranberry-lemon (subject’s choice) to disguise the unpleasant taste. We have previously demonstrated the feasibility of administering 3 AA mixtures to female participants with acceptable tolerability79. Testing concluded with a high protein snack for repletion of amino acid levels.
Social Stress Test
Five hours after amino acid ingestion, participants performed a mildly stressful psychological task, the Trier Social Stress Test (TSST)71. The TSST consisted of three parts, a brief Instruction Period, a 10-min Anticipation Period, and a 10-min Test Period. For the brief Instruction Period, participants were taken to the TSST room where 3 persons were already sitting at a table and a visible video camera was set up. The subject was asked to stand on an “X” on the floor in front of the panel of people. The Instructor presented the subject with one of three scenarios and asked the subject to prepare a 5-min speech about the topic. Participants were told that the panel was specially trained to monitor nonverbal behavior and that a voice-frequency analysis of the speech would be performed. Following the instructions, the subject returned to her room.
During the Anticipation Period, participants were asked to prepare the 5-min speech. They were given 10 minutes to prepare and take notes in a separate room, but were not allowed to use them during their speech. Participants presented their 5-min speech followed by 5 minutes of arithmetic problems. The TSST was originally designed to be conducted one time per subject, utilizing only the first speech scenario and arithmetic problem. In order to repeatedly confront the subject with the TSST on each of the three study visit days, the two additional scenarios and arithmetic problems were created. For each of the three scenarios, the subject was asked to take on a role within a given context and had to convince a panel to grant her a specific request: 1) Role of a job applicant for the position of manager at a banking firm. 2) Role of the director of a rehabilitation program for prisoners requesting a donation of a large sum of money to support the program. 3) Role of a building developer requesting a building permit to build a strip mall in a rural New England town. For the arithmetic problem portion of the Test Period, the problems consisted of serial subtractions of a two digit number from a four digit number, and upon every mistake, the subject was asked to begin again at the first number. Repeated exposure to the TSST has been shown to induce an equal physiologic stress response 80, 81. Consultation with the creators of the TSST and their review of our scenarios produced general agreement that the repeated use of the TSST with our scenarios had precedent and would produce repeated equivalent stress (Schommer, personal communication).
Participants were briefed before the study began about the general nature of the TSST and what was expected of them. This was done so as to equalize the anticipation of the TSST across the three study days. It should be noted that the actual performance of the subject during the TSST was not evaluated. The psychological stress induced was a product of the actual event of standing in front of a panel of strangers and delivering a speech; thus, the topic of the speech was less important. Regardless, the speech scenarios and arithmetic problems were judged to be equal in difficulty and equivalently controversial topics for the population being studied. Further, the order of scenarios was randomized across participants, decreasing the possibility that differences in scenarios would produce different stress outcomes. Participants were debriefed at the end of the study regarding the mild deception in the stress test (i.e., no actual monitoring of test performance).
OUTCOME MEASURES
COGNITIVE
A cognitive testing battery was constructed to evaluate a number of cognitive domains potentially sensitive to monoamine depletion and psychosocial stress as well as affected by loss of and subsequent treatment with estrogen. These cognitive domains included tests of simple attention, complex attention and verbal episodic memory. Each task is described below. The cognitive battery was performed once each study day, after the psychosocial stress maneuver. Participants were pre-trained on the entire cognitive battery prior to study initiation to ensure stable asymptotic performance to ensure equivalent cognitive performance at baseline between the groups.
Simple Attention
The Critical Flicker Fusion (CFF) task82 and the Choice Reaction Time (CRT) task83 from the Milford Test Battery were used as the measures of simple attention and were performed using the Leeds Psychomotor Device. During the CFF task there were two different types of trials. In an ascending trial, the participant pressed a button that indicated when the frequency of flashing lights had increased to the point that the lights appear to be no longer flashing but rather appear continuously on (“fused”). The lights began flashing at a rate of 12 Hz and the frequency was increased to 50 Hz. In a descending trial, the participant pressed a button when the frequency of apparently fused lights was decreased such that lights began to appear to be flashing. The lights began flashing at 50 Hz and decreased to 12 Hz. The participant needed to respond before the frequency hit the upper or lower limit in each trial. The participant was presented with three of each trial type. Dependent measures for this task were the median detection frequency across all trials, as well as the median detection frequency on the ascending and descending trials separately. Lower frequency values are generally understood to reflect impaired attention and/or arousal.
The CRT task was a reaction time task in which participants kept their index finger on a “home” light sensitive diode (LSD) until one of six LCD lights arrayed in a semicircle, approximately 25 cm from the “home” key, was lit on the response box. The subject lifted her index finger and moved it to cover the LSD corresponding to the illuminated LCD. She then returned her finger to the “home” LSD. Three performance measures were obtained from the CRT. The first was the median total reaction time (RT) per trial. The second was the median recognition RT, the amount of time it took the subject to lift her finger off of the home LSD once the signal to respond appeared. The third measure was the median motor RT, the time it took the participant to move her finger and to cover the LSD corresponding to the illuminated LCD.
Complex Attention
The measures of complex attention were the Digit Symbol Substitution Test (DSST84), and the Connors Continuous Performance Test (CPT85). In the DSST, participants were presented with nine numbers that corresponded to nine symbols. On the answer form the participant was instructed to write the symbol that corresponded to each number and to complete as many as possible in 90 seconds. The dependent measure was the total correct completions.
In the computerized CPT task, individual letters appeared on the computer screen for 300 ms with a response period of two seconds for 120 trials. Participants were instructed to press a button when they saw an A followed by an X. The dependent measures were hits, errors of omission and commission, and hit reaction time.
Verbal Episodic Memory
The Buschke Selective Reminding Task (SRT; Buschke, 1973) and the Verbal Paired Associates Test (VPA; Wechsler Memory Scale III) were used as measures of verbal episodic memory. In the SRT, participants were read a list of 14 words, followed by an immediate recall trial. The experimenter then reminded the participant of any words she did not recall and she was instructed to recall all 14 words again. This process was repeated for eight trials. Three measures were obtained from this task: the total number of words recalled across all lists, the recall consistency from one trial to the next, and the recall failure from one trial to the next.
In the VPA, participants were read a list of eight pairs of words. Then they were read the first word in each pair and asked the recall the associate. The list was read and recalled a maximum of 6 times. If the participant recalled all words on the list within the first three trials, the tests was discontinued after three trials. Four of the word pairs were strong associates (easy pairs) while the other four were weak associates (hard pairs). Dependent measures were number correct for the strong and weak associates after three and six trials.
The final test of verbal memory was a Paragraph Recall test86. Participants were read a short paragraph and then asked to retell the story from memory. The dependent measure was the number of information units correctly recalled from memory.
Cognitive task were performed in the following order: CFF, SRT, CRT, VPA, CPT, DSST, Paragraph Recall. This administration order was the same for all participants on all study days. A minimum of 10 equivalent versions of the testing forms were created so that a new version of each test was available for each of the testing days. These forms were counterbalanced across study days for all participants.
Behavior
The primary mood and anxiety measure was the subject-completed Profile of Mood States (POMS)87. This scale is a 65 item adjective checklist that generates 6 bipolar factor-analytically derived factors, (elated-depressed, composed-anxious, energetic-tired, agreeable-hostile, confident-unsure, and clearheaded-confused) or 12 unipolar factors, plus total score. This scale has been used extensively in challenge study paradigms and is sensitive to the effects of psychotropic drugs and CNS state manipulations. It was administered 3 times during the experimental session: pre-depletion, post-depletion prior to TSST, and post TSST. Participants completed a Beck Depression Index (BDI) 77 twice during the day: pre-depletion and post-depletion but prior to the TSST.
Neuroendocrine/physiologic
Measures of estradiol and FSH were collected to assess compliance and the effectiveness of E2 therapy. Estradiol and FSH were measured with an ADVIA Centaur chemiluminescence competitive immunoassay (estradiol) and an ADVIA Centaur two-site sandwich immunoassay (FSH) both utilizing a labeled acridinium ester. Samples were collected at screening and on the first day of each challenge sequence. Blood was collected on each study day for measures of plasma total tryptophan, phenylalanine, and tyrosine to assess the adequacy of depletion. Samples were collected pre-depletion (−45’) and end of session (+400’). Plasma phenylalanine and tyrosine concentrations were determined using a Beckman System Gold amino acid analyzer using gradient HPLC with precolumn derivatization and fluorometric detection. Tryptophan was measured by isocratic HPLC with fluorometric detection. Cortisol was measured by radioimmunoassay (Diagnostic Products Corporation).
Vital signs were recorded pre-depletion at −45’, pre-TSST at +300’, and post-TSST at +400’
DATA ANALYSIS
The general approach was that of mixed model repeated measures analysis of variance (ANOVA) utilizing SAS PROC MIXED. Initial analysis of cognitive measures was a 2×3 treatment; 2 (E2 vs PLC) × depletion 3 (ATD, ATPD, Mock) mixed model ANOVA as an overall test of the effect of estradiol treatment and monoamine depletion effects on cognition following psychosocial stress. Treatment (E2 vs. placebo) was the between-participants factor and depletion (ATD, APTD, and mock) were the within-subject factors. For the mood measures only, time was an additional factor as there was a pre-depletion, pre-stress maneuver mood assessment. Cognitive testing was performed once on each of the three experimental days and thus each subject performed cognitive testing under each monoamine depletion-psychosocial stress condition. As the primary effect of interest in this study was the impact of E2 treatment on cognitive performance following monoamine depletion and psychosocial stress, if no treatment-by-depletion effect was found, results were collapsed across depletions and the analyses were redone as an independent samples t-test. When there was a significant interaction (e.g. treatment × depletion), non-orthogonal a-priori contrasts were used to test for differences between treatment across depletions. Correlations between cognitive and mood measures were performed using Pearson product-moment correlations adjusted for multiple comparisons. The alpha level for rejection of the null hypothesis was set at p<.05.
RESULTS
Participants
Participants were matched for age, education, weight, and years since menopause (Table 1). The mean age of participants was 64.3± 10.6. BMI averaged 26.23 ± 4.47 kg/m2 and participants were an average of 14.3 ± 10.5 years post menopause. This was a highly educated group with an average of 14.9 years of education. Fifteen participants had had previous experience with hormone replacement therapy (>1 year previously) and 7 did not. For those women who had previously used hormone therapy, the average duration of hormone use was 3.9 ± 4.9 years.
FSH, Estradiol and Cortisol Levels
Pretreatment FSH showed a mean level of 65.73 mIU/ml (menopausal level is considered above 30–35 mIU/ml) and was not significantly different between treatment groups (t(18) =.98, p > .34. After three months of treatment, the E2-treated participants showed a significantly reduced mean FSH level of 29.6 compared to the placebo-treated participants who had a mean level of 69.0 (t(17)=5.39, p < .001). Mean plasma estradiol levels after three months of treatment were significantly elevated at 135.36 pg/ml for the E2-treated group compared to 18.3 pg/ml for the placebo-treated group (t(19)=5.14, p<.001). The levels of estradiol seen in the E2-treated women are comparable to late follicular phase levels in premenopausal women, whereas the level seen in the placebo-treated women is comparable to the early follicular phase. Cortisol levels were measured at baseline and +420 minutes (post-TSST). Baseline levels (pre-depletion, pre-TSST) were higher, (p< .01) in the E2-treated participants but declined similarly across the experimental day in both treatment groups.
Amino Acid Levels
Plasma concentrations of total tryptophan, phenylalanine, and tyrosine were measured at baseline (pre-depletion) and at +400 minutes (post-depletion) (Table 2). After tryptophan depletion, plasma tryptophan levels declined 76%. After tyrosine/phenylalanine depletion, both tyrosine and phenylalanine levels declined by 60% suggesting that an adequate depletion was achieved 69, 88.
Table 2.
Amino Acid Levels (n=22)
| Pre-depletion Mean ± SD |
Post-depletion Mean ± SD |
Percent Change Mean |
||
|---|---|---|---|---|
| Total Plasma Tryptophan µmol/L |
||||
| ATD | 10.03 ± 1.46 | 2.49 ± 3.40 | −76.061 | |
| ATPD | 9.90 ± 1.82 | 12.46 ± 4.37 | 33.562 | |
| MOCK | 9.90 ± 1.71 | 12.53 ± 3.78 | 31.091 | |
| Plasma Phenylalanine µmol/L |
||||
| ATD | 5.10 ± 12.6 | 85.34 ± 34.21 | 71.421 | |
| ATPD | 46.95 ± 5.82 | 19.51 ± 20.9 | −58.691 | |
| MOCK | 48.70 ± 4.70 | 70.15 ± 34.34 | 42.421 | |
| Plasma Tyrosine* µmol/L |
||||
| ATD | 61.42 ± 23.09 | 177.41 ± 53.09 | 203.571 | |
| ATPD | 56.02 ± 10.52 | 21.6 ± 33.55 | −59.981 | |
| MOCK | 58.72 ± 9.70 | 161.86 ± 64.31 | 184.541 | |
Pre-depletion time-point is −45 min.; depletion time-point is 0 min.; post-depletion time-point is +420 min.
Tyrosine n=21.
p<.01;
p<.05 for pre-post difference.
ATD: Acute Tryptophan Depletion
ATPD: Acute Tyrosine/Phenylalanine Depletion
MOCK: Mock (Placebo) Depletion
Clinical Assessment of Mood across Treatment Phase
A comparison of the clinical depression ratings (BDI) from screening to the end of the treatment phase for each subject revealed no significant time-by-treatment interaction (F(1,18)=.58, p > .45). Furthermore, a comparison of the end of treatment BDI scores (the baseline BDI score on the first depletion challenge day) between treatment groups showed a small numerical difference (PLC: 2.91 ± 3.6; E2: 4.55 ± 2.9) that was not significantly different between treatments (t(20)=1.19, p >. 25). These data demonstrate that the treatment alone (E2 or placebo) did not produce significant or clinically manifest negative changes in mood across the three-month treatment phase nor did the groups differ prior to beginning the monoamine depletion challenges.
Cognitive Performance
Cognitive testing results are presented in Table 3. Cognitive testing was only performed after the TSST. In general, significant impairment was seen across many cognitive measures in the E2 treated group, particularly on attention and psychomotor measures.
Table 3.
Cognitive performance scores by treatment and depletion condition (n = 22; Placebo = 11; E2 = 11).
| Cognitive Construct |
Task | Dependent Variable |
Treatment | Mock Depletion | ATD Depletion |
ATPD Depletion |
|---|---|---|---|---|---|---|
| Attention | ||||||
| CFF | ||||||
| Total (Hz)1 | Placebo | 30.39 (0.87) | 30.90 (0.85) | 31.80 (0.73) | ||
| E2 | 27.90 (0.87) | 28.45 (0.56) | 27.39 (0.72) | |||
| Ascending (Hz)2 | Placebo | 28.71 (1.32) | 29.91 (0.92) | 30.45 (0.76) | ||
| E2 | 27.98 (1.32) | 28.22 (0.94) | 27.37 (0.75) | |||
| Descending (Hz)1 | Placebo | 31.95 (0.87) | 31.78 (0.87) | 31.95 (0.88) | ||
| E2 | 27.98 (0.87) | 28.63 (0.88) | 27.36 (0.87) | |||
| CRT | ||||||
| Total (ms)2 | Placebo | 702.12 (37.52) | 684.70 (37.52) | 734.21 (38.20) | ||
| E2 | 780.77 (37.52) | 774.55 (38.94) | 792.20 (37.50) | |||
| Recognition RT (ms)2 | Placebo | 382.40 (20.7) | 391.84 (20.7) | 412.61 (20.9) | ||
| E2 | 420.50 (20.7) | 419.21 (21.1) | 451.63 (20.7) | |||
| Motor RT (ms) | Placebo | 311.87 (20.8) | 324.31 (20.8) | 311.08 (21.1) | ||
| E2 | 349.37 (20.8) | 340.93 (21.5) | 332.50 (20.8) | |||
| CPT | ||||||
| Hits (proportion correct)2 | Placebo | 0.98 (0.06) | 0.99 (0.05) | 0.95 (0.07) | ||
| E2 | 0.86 (0.06) | 0.89 (0.05) | 0.84 (0.07) | |||
| Errors of Omission (errors)2 | Placebo | 0.64 (2.25) | 0.18 (1.95) | 1.99 (2.88) | ||
| E2 | 5.73 (2.25) | 4.47 (1.98) | 6.36 (2.81) | |||
| Errors of Commission (errors) | Placebo | 0.45 (0.25) | 0.64 (0.39) | 0.57 (0.34) | ||
| E2 | 0.54 (0.25) | 0.73 (0.39) | 0.18 (0.33) | |||
| Hit RT (ms)1 | Placebo | 508.35 (24.79) | 467.32 (24.79) | 497.30 (25.36) | ||
| E2 | 400.67 (24.79) | 407.35 (25.36) | 433.46 (24.79) | |||
| DSST | ||||||
| Number Correct2 | Placebo | 58.82 (2.78) | 60.18 (2.76) | 60.98 (2.81) | ||
| E2 | 52.36 (2.78) | 53.40 (2.81) | 49.73 (2.78) | |||
| Errors | Placebo | 0.09 (0.15) | 0.36 (0.22) | 0.29 (0.14) | ||
| E2 | 0.27 (0.15) | 0.26 (0.23) | 0.18 (0.13) | |||
| Verbal Memory |
||||||
| SRT | ||||||
| Total Recall (number correct) |
Placebo | 83.36 (4.72) | 81.18 (4.72) | 79.31 (4.79) | ||
| E2 | 81.60 (4.95) | 76.10 (4.95) | 76.70 (4.95) | |||
| Recall Consistency (number correct) |
Placebo | 48.90 (5.23) | 43.45 (5.23) | 40.75 (5.32) | ||
| E2 | 48.60 (5.49) | 40.70 (5.43) | 40.90 (5.49) | |||
| Recall Failure (number of failures) |
Placebo | 11.82 (3.73) | 11.82 (3.73) | 13.9 (3.73) | ||
| E2 | 15.1 (3.91) | 18.2 (3.91) | 16.3 (3.91) | |||
| PR | ||||||
| Proportion Recalled (story units) |
Placebo | 0.43 (0.05) | 0.38 (0.05) | 0.37 (0.05) | ||
| E2 | 0.32 (0.05) | 0.35 (0.05) | 0.39 (0.05) | |||
| VPA | ||||||
| Easy Pairs (proportion recalled) |
Placebo | 0.95 (0.07) | 0.95 (0.06) | 0.94 (0.05) | ||
| E2 | 0.83 (0.07) | 0.81 (0.08) | 0.87 (0.05) | |||
| Hard Pairs2 | Placebo | 0.75 (0.08) | 0.80 (0.08) | 0.78 (0.08) | ||
| E2 | 0.59 (0.08) | 0.59 (0.08) | 0.64 (0.08) |
Mock Depletion = balanced amino acid administration, ATD = Acute Tryptophan Depletion, ATPD = Acute Tyrosine/Phenylalanine Depletion. CFF = Critical Flicker Fusion; CRT = Choice Reaction Time; CPT = Continuous Performance Task; DSST = Digit Symbol Substitution Task; SRT = Selective Reminding Task; PR = Paragraph Recall; VPA = Verbal Paired Associates. Values displayed are means (SE) except for CFF and RT values which are medians.
p<.01;
p<.05 for effect of E2 treatment.
Attention/Psychomotor
Critical Flicker Fusion (CFF)
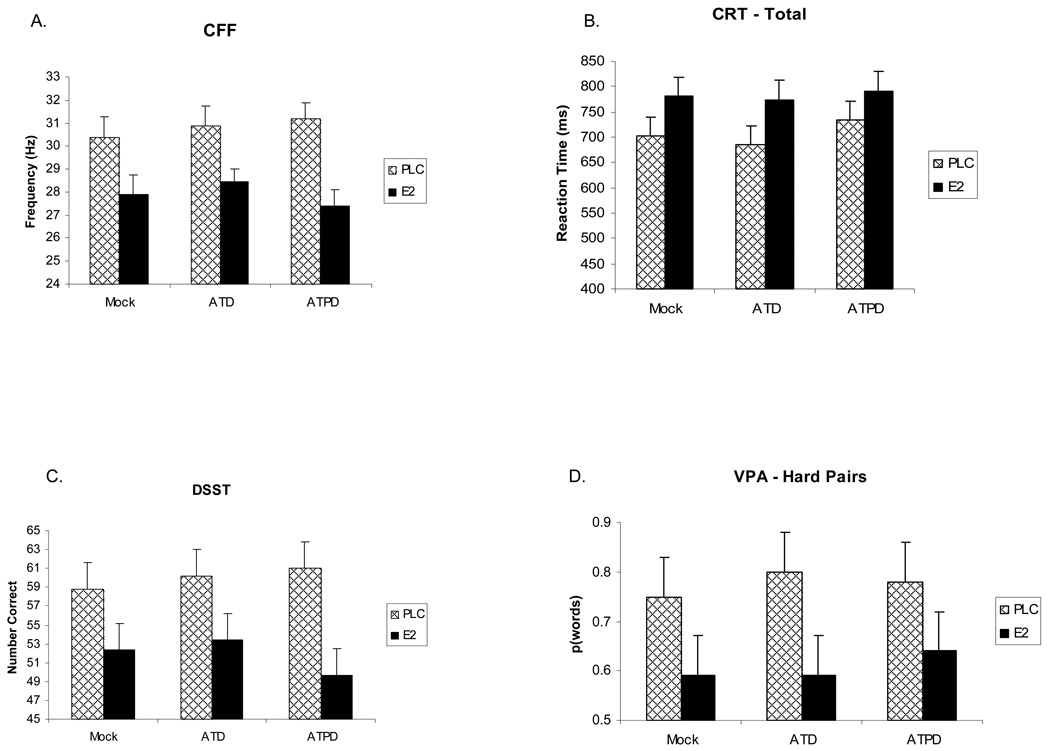
Attentional performance as measured by the median frequency of all trials showed a significant main effect of treatment (F(1,20) = 8.66, p = .008) with E2-treated participants showing a reduced frequency compared to placebo-treated participants (Figure 1), suggesting impaired attention. There was a strong trend for a treatment-by-challenge interaction (F(2,20) = 3.17, p > .06) with the tyrosine/phenylalanine depletion condition showing a slightly poorer performance compared to tryptophan depletion and mock depletion after E2 treatment. Falling trials showed a significant (F(1,20) = 11.61, p = .003) main effect of E2 treatment as well, producing a median frequency reduction, but rising trials did not (p > .11).
Figure 1.
Cognitive performance measures following the Trier Social Stress Test. Data are presented for estradiol (E2) and placebo (PLC) treatment groups for each monoaminergic depletion: Acute Tryptophan Depletion (ATD), Acute Tyrosine/Phenylalanine Depletion (ATPD), Mock Depletion (Mock). Values shown are mean scores ± SEM for accuracy measures and median times ± SEM for reaction times. A. Critical Flicker Fusion (CFF) task median detection frequency for all trials. B. Choice Reaction Time (CRT) task median total reaction time. C. Digit Symbol Substitution Task (DSST) number correct completed items. D. Verbal Paired Associates (VPA) number correct recalled for the hard word pair condition. E2 treatment produced impairment compared to placebo treatment across depletion conditions on all four cognitive measures (all p < .05 for effect of E2 treatment).
Choice Reaction Time (CRT)
For the CRT, total median RT showed no treatment-by-challenge interaction. Collapsing the data across challenge conditions demonstrated a significant (t(20) = 2.68, p < .05) effect of E2 treatment with the pattern of means showing that estrogen treated participants performed slower across all depletion conditions than placebo treated participants (Figure 1). The recognition component of the CRT showed a significant effect of challenge, F(2,37) = 10.04, p = .0003, on median recognition RT with the tyrosine/phenylalanine depletion showing a slower RT then either the mock or tryptophan depletion conditions. However there was no significant treatment or challenge-by-treatment effects. Analyses collapsed across challenge conditions revealed a significant effect of E2 treatment (t(20) = 2.25, p < .05) with RT significantly greater (slower) for E2-treated participants. For the motor component of CRT, there were no significant treatment-by-challenge interaction effects on median RT, but as with the other components, collapsing across challenge conditions revealed a significant (t(20) = 2.68, p < .05) slowing effect of E2 treatment.
Continuous Performance Task (CPT)
As there were no significant challenge-by-treatment interactions, we examined treatment effects by looking at the data collapsed across challenge conditions. The proportion of hits showed a significant (t(20) = 2.65, p < .05) negative effect of estrogen treatment, with E2 treated participants demonstrating a reduced proportion of hits across all conditions. A similar significant pattern (t(20) = 2.65, p < .05) was seen in errors of omission with errors showing increases under all conditions for the E2 treated participants. There were no significant or trend-level effects on commission errors, but the proportion of commission errors was very low.
By contrast, there was a significant F(1,20) = 6.17, p = .02 positive main effect of E2 treatment on hit RT with E2 treated participants showing a faster RT (between 60 and 100 ms) across all depletion conditions. The contrast in these results suggests the possibility that E2 treated participants demonstrated a speed-accuracy trade-off, becoming faster, but less accurate. No other parameters showed significant effects.
Digit Symbol Substitution Task (DSST)
There was a significant, F (1,20) = 4.63, p = .04, main effect of treatment on the number of items correctly completed with E2-treated participants showing a significantly (p = .001) reduced number of items correctly completed compared to the placebo-treated participants (Figure 1). In addition, there was a significant challenge-by-treatment interaction, F(2, 38) = 3.88, p = .03, with E2-treated participants showing significantly t(20) = 2.5, p < .05) fewer correct completions after tyrosine/phenylalanine depletion.
Memory
Selective Reminding Task (SRT)
There were no effects at E2 treatment on this task. There was a trend (p = .12) for 8-trial recall to be reduced under both tryptophan and tyrosine/phenylalanine depletion conditions. In addition, recall consistency showed a significant main effect of depletion challenge, F(2, 37) = 4.93, p = .01), with consistency being significantly reduced under both tryptophan and tyrosine/ phenylalanine depletion conditions. There was no interaction with E2 treatment on this parameter. Recall Failure showed a pattern of increased failure scores under E2 treatment, but the effect of treatment was not significant.
Verbal Paired Associate Task (VPA)
While there was no significant challenge-by-treatment interactions, collapsing across challenge conditions, there was a significant (t(20) = 2.15, p < .05) effect of E2 such that E2-treated participants showed a reduced recall of hard word pairs across all depletion conditions (Figure 1). There was a similar trend (p =.16) for E2-treated participants to show a similarly reduced recall for easy pairs of words.
Paragraph Recall
There were no significant or a trend-level treatment, challenge, or interaction effects on this measure.
Mood Effects
The effects of the hormone-monoamine depletions/psychosocial stress manipulation on mood were previously presented in detail in Newhouse et al. 64. We briefly review and update those findings here, focusing on the POMS results.
An examination of the entire model for the POMS total score and subscales revealed no significant treatment-by-depletion challenge interactions. Thus the analyses were redone collapsing across challenge conditions to examine hormone treatment effects. Total Mood Disturbance score showed a significant interaction of hormone treatment by time, F(2, 20) = 9.40, p = .001, with E2 subject showing a significant increase in Total Mood Disturbance scores following the stress/monoamine depletion manipulation. Examining the subscale scores from the POMS revealed similar hormone treatment-by-time interactions for Depression (F(2,20) = 4.50, p =.02), Confusion (F = 3.93. p = .04), and Vigor (F = 6.67, p = .003), with E2 participants showing significant score changes indicating worsening self-ratings following the stress/monoamine depletion manipulation compared to placebo treatment. In addition, on the Tension subscale, there was a main effect of hormone treatment, F (1, 20) = 6.84, p = .02), with E2-treated participants showing higher scores across time. The Anger/Hostility subscale showed only trivial significant effects of time and did not show any treatment or hormone treatment-stress manipulation effects.
Correlation with Mood Effects of Psychosocial Stress and Monoamine Depletion
We examined whether group differences in cognitive performance correlated with changes in self-rated mood following the psychosocial stress manipulation and monoamine depletion. We compared the POMS total score and subscale scores at the post-depletion rating in relationship to the performance measures that were obtained at the same time. The relationships were inconsistent, with some mood measures correlating with performance under placebo on some tasks and under estrogen on others.
There were small correlations between the Tension subscale of the POMS and impaired performance on the Selective Reminding Task, the CRT, and the DSST, however the pattern of treatment correlations was inconsistent as the Tension subscale correlated with performance on placebo on some tasks and estrogen on others. A similar pattern was seen for the Depression, Fatigue, and Confusion subscales. Furthermore, none of these correlations survived correction for multiple comparisons. Thus it does not appear that the magnitude of the mood changes alone explained the estrogen treatment-related negative effects on cognitive performance.
We also had previously64 examined effects of age, TSST scenario, and the effect of repeated administration of the TSST on mood dependent variables. Neither age, day, nor TSST scenario interacted with hormone treatment or depletion challenge. Moreover, there was no significant effect of repeated administration of the TSST and no significant interaction with hormone treatment or depletion challenge was found.
Vital Signs
Only minor effects of hormone treatment and amino acid depletion were seen on vital signs. There was a trend for a treatment-by-challenge interaction on diastolic blood pressure (p > .07) with diastolic blood pressure showing a slight increase after tyrosine-phenylalanine depletion. No effects were seen on systolic blood pressure. Pulse showed significant main effects of challenge (F(2,33) = 3.77, p < .05) and time (F(2,38) = 8.40, p < .001), but no interactions with hormone treatment was found. The pattern of means showed that the mock depletion was not associated with an increase in pulse across the psychosocial stress maneuver compared to the ATD or ATPD depletions that were associated with a reliable increase in pulse. Temperature showed a small significant F(1,19 = 60.81, p < .001) time-related change as expected but did not show any significant E2 treatment-related effects or any systematic results of monoamine depletion. No clinically relevant changes occurred.
DISCUSSION
Post-menopausal women who were administered estradiol (E2) at a dose of 1 mg of oral 17β-estradiol per day for 1 month, then 2 mg per day for 3 months generally exhibited poorer cognitive performance following a psychologically stressful event compared to placebo-treated women. This response was independent of the effects of monoamine depletion, which appeared to have only a small overall effect on the cognitive and emotional responses and did not interact with the effects of E2. These effects did not appear to be secondary to baseline mood differences prior to depletion or the TSST, as participants’ end of treatment depression scores (Beck), and pre-depletion mood scores (POMS) and depression scales were not significantly different between treatment groups. We expected that monoamine depletion and psychosocial stress together would produce negative cognitive and mood changes, as was seen by Leyton et al.69 but that might be modified by the presence of E2. Monoamine depletion produced only minor negative cognitive changes compared to mock depletion. By comparison, the effects of E2 treatment on cognitive performance following social stress were larger and appeared to be largely independent of the monoamine depletion maneuvers.
The cognitive domains of impairment included both attention and to a lesser extent, memory. Attentional impairment included simple speed measures which have generally been shown to be improved by E23, 89. The current study reliably showed that estradiol treatment after psychosocial stress lengthened reaction time and decreased perceptual discrimination ability. Dumas et al52 showed that these measures were improved by estradiol treatment after cholinergic challenge. However, estradiol had the opposite effect on these measures after psychosocial stress. Additionally, there was also partial impairment on some verbal episodic memory measures for the estradiol group relative to the placebo group. These data contrast with prior data by Maki and colleagues and Sherwin and colleagues9, 90 who showed that E2 improved verbal episodic memory performance, although these studies were not done with a psychosocial stress or neuro-chemical stress maneuver. Thus, the psychosocial stress manipulation in this study appeared to interact with the estradiol treatment to generally impair cognitive performance, which was the opposite of what we originally hypothesized. Below we discuss further the implications for such an interaction.
The one exception to these findings was the hit RT measure during the CPT task, which improved in E2-treated participants after the psychosocial stress/amino acid depletion maneuver, in contrast to the RT for other tasks such as the CRT, which showed significant slowing. On the CPT task participants displayed a speed-accuracy trade off that interacted with the effects of estrogen treatment. The E2 treated participants made fewer hits but had faster hit RTs compared to the placebo treated group. Additionally, differences in task specifics may explain these results. The CRT task is a sensory detection and motor task. By contrast, the CPT test requires a deeper level of stimulus processing to make an appropriate decision on whether to respond to particular stimuli, and thus has greater test demands than the simpler CRT task. Further studies should investigate whether task complexity or depth of processing changes the effect of stress- or hormone-related alterations on cognitive performance.
These results differ from prior published findings from our laboratory showing that three months of E2 administration to PMW enhances cognitive performance following cholinergic blockade52, 53, however, there were significant differences between the present study and our prior published work on E2 and cognitive performance. Although the pattern of E2 administration and the subject population was very similar to our prior studies showing cognitive enhancement, no psychosocial stress manipulation was used in our prior studies, rather partial cholinergic blockade provided the provocative stimulus. These prior studies showed that E2 treatment reduced the sensitivity to cholinergic blockade and reduced the cognitive impairment associated with that blockade. Thus, E2 appears to show evidence of enhancing cholinergic-system related cognitive function. By contrast, no cholinergic manipulation was used in the present study, rather the focus was on emotional stress and monoamine neurotransmitter manipulations. The impact of emotional or psychosocial stress on the ability of the E2 to enhance cholinergic-related cognitive performance remains to be examined.
How Does Psychosocial or Emotional Stress or Stress Hormones Affect Cognitve Performance?
A series of studies have shown that psychosocial stress can induce certain cognitive deficits both working memory and retrieval deficits91.92, 93. Chronic stress appears to have long-term negative effects on memory functioning and brain structure94. However, emotional arousal can result in enhancing as well as impairing effects on long term memory. The directionality of this effect may depend on the baseline state of arousal, the type of emotion present, and the phase of the cognitive or memory process that is exposed to emotional arousal or stress. Significant gender differences exist in the neuronal circuitry involved in emotion-cognition interactions suggesting the possibility that sex steroids may play a role in this process95. Kim and Diamond96 have suggested that excess amygdala input and increased glucocorticoid secretion act to impair hippocampal plasticity and subsequent learning. Since estrogen levels can modulate HPA axis activity in response to psychosocial stress97 as well as the activity of limbic structures such as the amygdala response to negative emotional information98, 99, it is likely that E2 may directly affect the brain circuitry involved in an acute stress response and subsequent emotional learning.
Studies of the effects of E2 on emotional perception are also few. Pearson and Lewis100 have shown that recognition of emotional expressions is reliably altered across the menstrual cycle with the recognition of fear improving when E2 levels were high. Protopopescu and colleagues99 have shown that specific subregions of orbital frontal cortex (OFC) changed their pattern of activity in reaction to negative emotional stimuli across the menstrual cycle. The authors interpreted this data as premenstrual enhancement of top-down modulation of limbic activity, with the accompanying suppression of sensory evaluative function. In a somewhat differently designed menstrual cycle study, Goldstein and colleagues98 showed that brain areas associated with negative emotional responses including the amygdala, anterior cingulate gyrus, and OFC showed lower activity during mid-late follicular phase (when estradiol levels could be expected to be high) than during early follicular phase (when estradiol levels would be lower). No such studies have examined the brain activity associated with emotional stimuli or emotional cognition in postmenopausal women or with HT. Alterations in cortical activity produced by differing circulating levels of hormones such as E2 may thus play a role in regulating how the amygdala and other emotion-related structures respond to emotional stimuli and/or stressful events98, 99, 101. How the processing of emotional stimuli changes after menopause is at this point unknown. Thus, it may be that the steady administration of E2 to postmenopausal women at a plasma level consistent with late follicular phase, as in this study, may have produced alterations in the cortical or subcortical processing of stressful or emotional experiences.
In two recent reviews, Phelps102, 103 has noted that the amygdala is responsible for the emotional contribution to declarative memory. Specifically, she suggests that the amygdala can modulate both the encoding and storage of hippocampal-dependent memories and that bidirectionally the hippocampus, by forming episodic representations of emotional significance, can influence the amygdala response when emotional stimuli are encountered102. Based on our data and neuroimaging studies of premenopausal women, it is not unreasonable to suggest that sex hormone status may have significant impact on cognitive processes after emotional stress in PMW. Our data suggests that exogenously manipulated E2 levels may have significant impact on both emotional reactivity and cognitive performance. It is therefore important in future studies to examine how menopause and postmenopausal HT may affect emotional reactivity and emotional memory.
Postmenopausal women appear to show greater sensitivity than premenopausal women in their physiologic response to cognitive and speech tasks with the difference being ascribed to both age and hormonal status104. Previous studies of the effects of hormones on experimental stressors have found that the various forms of estrogen appear to reduce some of the physiologic effects of mild laboratory-induced stress (e.g. solving arithmetic problems)105, 106. Lindheim and colleagues107 showed that the greater biophysical response of PMW following stress was reduced after six weeks of transdermal estradiol treatment. Estrogen treatment has been shown to enhance parasympathetic responsiveness to experimental stress, suggesting reduced sympathetic activation108 although in one study the TSST was not found to provoke a differential effect on physiological measures in estrogen treated women97. Kajantie and Phillips109 conclude that there is an increase in sympathoadrenal responsiveness after menopause which is attenuated during oral hormone therapy.
The lack of interaction of the E2-induced effect on cognitive impairment following psychosocial stress with monoamine depletion suggests that other neurotransmitter systems may be involved in mediating these effects. While the exact neurochemical mechanisms responsible for the negative cognitive and emotional responses seen here cannot be ascertained from this particular study, the lack of concomitant progesterone administration suggests that the impact of stress-related symptoms that would normally benefit from progesterone-derived neurosteroid-GABAA receptor interactions may have been had impact on the effects seen in this study.
Limitations
While the effects of estrogen on cognition after psychosocial stress in this study were large, caution is indicated in interpreting these results. Concerns regarding the repeatability of the TSST as well as the dose of E2 are similar to our prior study64. The TSST was not originally designed for repeated administration and repeated presentation may diminish the stressful response to the test. We examined this possibility in detail in our prior published work64, and while were small effects of the day and scenario, the magnitude was small suggesting little habituation or sensitization in the current study. In addition, the negative effect of estradiol treatment remained across all depletion challenges. We also had to use a between-subjects design with regard to estradiol treatment because of limitations regarding how often participants can perform the depletion challenges and TSST. The cognitive battery was only performed after the psychosocial stress maneuver, because the primary comparison of interest was between treatments, rather than within-subjects. Additionally, the length and difficulty of the entire experimental procedure was such that adding pre-depletion cognitive testing was felt to be too burdensome for participants. Thus we do not have information about how the participants would have performed prior to the monoamine depletion and psychosocial stress. However, participants were extensively cognitively screened at baseline and trained on the cognitive battery prior to initiation of the overall study and thus we are confident that cognitive performance was essentially equivalent between the two groups prior to estrogen or placebo treatment and amino acid/psychosocial stress challenge. Additionally, the the E2 dose used in this study was somewhat higher than average clinical doses, although not beyond the normal clinical range. Estradiol blood levels were not higher than is typically seen during the late follicular phase of a normal menstrual cycle. We have shown previously that E2 levels in this range are beneficial in a cholinergic challenge model52 however the interaction with psychosocial stress in the current study showed the opposite effects. Additional cortisol sampling beyond the two samples that we obtained would have been helpful to further characterize the magnitude of the stress response, but we were unable to do so in this study design. Finally, although this was the blinded study, we recognize that the subjective effects of estradiol may have been difficult to fully blind.
CONCLUSION
Twenty two postmenopausal women who were administered E2 for 3 months exhibited marked worsening of cognitive performance after a social stress test compared to placebo-treated PMW. These effects were generally independent of Tryptophan or Tyrosine/Phenylalanine depletion and were not significantly correlated with negative mood changes. These data imply that the effect of E2 on cognitive performance after menopause is not straightforward and may interact significantly with psychological stress or especially stressful events. Effects of the hormone-stress interaction on cognitive performance did not appear to be significantly modified via catecholamine or indoleamine mechanisms. Further research will be necessary to confirm and clarify these findings as well as explore underlying mechanisms. Replication without the depletion maneuver, the examination of the effects of different doses of E2, combined E2 and progestin therapy, and the examination of women during different phases of the menstrual cycle will help clarify these findings.
Acknowledgements
This work was supported by an Independent Investigator award from NARSAD and R01 AG021476 to P.N., CIHR grant MOP-150051 to S.N.Y, and GCRC M01-00109.
The authors wish to thank the staff of the University of Vermont General Clinical Research Center for their efforts in the support of this project and our research volunteers for their dedication to clinical research.
Footnotes
Conflicts of Interest/Disclosures: None. In addition, none of the sponsors had any role in the design or conduct of the study, management, analysis, and interpretation of the data, or preparation, review, or approval of the manuscript.
A partial version of this work was previously presented as a poster at the Society for Neuroscience Annual Meeting, Washington, DC, November 19, 2008
Reference List
- 1.Sherwin BB. Estrogen and Cognitive Functioning in Women. Endocrine Reviews. 2003;24(2):133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 2.Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory: a possible protective effect. Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- 3.Smith YR, Giordani B, Lajiness-O'Neill R, Zubieta J. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertility and Sterility. 2001;76(6):1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs DM, Tang MX, Stern Y, et al. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- 5.Shaywitz SE, Naftolin F, Zelterman D, et al. Better oral reading and short-term memory inmidlife, postmenopausal women taking estrogen. Menopause. 2003;10(5):420–426. doi: 10.1097/01.GME.0000060241.02837.29. [DOI] [PubMed] [Google Scholar]
- 6.Krug R, Born J, Rasch B. A 3-day estrogen treatment improves prerontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology. 2006;31:965–975. doi: 10.1016/j.psyneuen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Stevens Clark, Prestwood Low-dose estradiol alters brain activity. Psychiatry Research: Neuroimaging. 2005;139(3):199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- 9.Sherwin BB. Estrogenic effects on memory in women. Annals of the New York Academy of Sciences. 1993;743:213–230. doi: 10.1111/j.1749-6632.1994.tb55794.x. [DOI] [PubMed] [Google Scholar]
- 10.Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Hormones and Behavior. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- 11.Ditkoff EC, Crary WG, Cristo M, Lobo RA. Estrogen improves psychological function in asymptomatic postmenopausal women. Obstetrics and Gynecology. 1991;78:991–995. [PubMed] [Google Scholar]
- 12.Barrett-Connor E, Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. Journal of the American Medical Association. 1993;269(20):2637–2641. [PubMed] [Google Scholar]
- 13.Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM. Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas. 2001;38:137–146. doi: 10.1016/s0378-5122(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 14.Polo-Kantola P, Portin R, Polo O, Helenius H, Irjala K, Erkkola R. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, cross-over trial in postmenopausal women. Obstetrics & Gynecology. 1998;91(3):459–466. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- 15.Alhola P, Polo-Kantola P, Erkkola R, Portin R. Estrogen therapy and cognition: A 6-year single-blind follow-up study in postmenopausal women. Neurology. 2007;67:706–709. doi: 10.1212/01.wnl.0000230135.10179.86. [DOI] [PubMed] [Google Scholar]
- 16.Buckwalter JG, Crooks VC, Robins SB, Petitti DB. Hormone Use and Cognitive Performance in Women of Advanced Age. Journal of the American Geriatric Society. 2004;52:182–186. doi: 10.1111/j.1532-5415.2004.52053.x. [DOI] [PubMed] [Google Scholar]
- 17.Espeland MA, Rapp SR, Shumaker S, et al. Conjugated equine estrogen and global cognitive function in postmenopausal women. Journal of the American Medical Assocation. 2004;291(24):2959–2967. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 18.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition, and quality of life. Neurobiology of Aging. 2006;27(1):141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Resnick SM, Coker LH, Maki PM, Rapp PR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clinical Trials. 2004;1(5):440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 20.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. Journal of the Americam Medical Association. 2004;291(24):2947–2957. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 21.Maki PM. A Systematic Review of Clinical Trials of Hormone Therapy on Cognitive Function: Effects of Age at Initiation and Progestin Use. Annals of the New York Academy of Science. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 22.Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognition function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101(3):485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 23.Sherwin BB. Hormones, mood, and cognitive functioning in postmenopausal women. Obstetrics and Gynecology. 1993;87(2 (supplement)):20S–26S. doi: 10.1016/0029-7844(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 24.LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. Journal of the American Medical Association. 2001;285(11):1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 25.Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen's affect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- 27.Maki PM. Estrogen effects on the hippocampus and frontal lobes. International Journal of Fertility and Women's Medicine. 2005;50(2):67–71. [PubMed] [Google Scholar]
- 28.Greendale GA, Huang M-H, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Lui L, Grady D, Massa S, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biological Psychiatry. 2002;51:677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Gouras GK, Greenfield JP, et al. Estrogen reduces neuronal generation of Alzheimer b-amyloid peptides. Nature Medicine. 1998;4(4):447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 31.Bretsky PM, Buckwalter JG, Seeman TE, et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Disease & Associated Disorders. 1999;13(4):216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. American Journal of Epidemiology. 1994;140(3):256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 33.Tang MX, Jacobs D, Stern Y, et al. Effect of estrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 34.Panidis DK, Matalliotakis IM, Rousso DH, Kourtis AI, Koumantakis EE. The role of estrogen replacement therapy in Alzheimer's disease. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2001;95:86–91. doi: 10.1016/s0301-2115(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 35.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 36.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women. Journal of the American Medical Association. 2002;288(17):2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 37.Costa MM, Reus VI, Wolkowitz OM, Manfredi F, Lieberman M. Estrogen Replacement Therapy and Cognitive Decline in Memory-Impaired Post-Menopausal Women. Biological Psychiatry. 1999;46:182–188. doi: 10.1016/s0006-3223(98)00355-2. [DOI] [PubMed] [Google Scholar]
- 38.Soares CN, Poitras JR, Prouty J. Effect of reproductive hormones and selective estrogen receptor modulators on mood during menopause. Drugs & Aging. 2003;20(2):85–100. doi: 10.2165/00002512-200320020-00001. [DOI] [PubMed] [Google Scholar]
- 39.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of Affective Disorders. 2003;74(1):67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 40.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 41.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status With depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 42.Whooley MA, Grady D, Cauley JA. Postmenopausal estrogen therapy and depressive symptoms in older women. Journal of General Internal Medicine. 2000;15:535–541. doi: 10.1046/j.1525-1497.2000.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palinkas LA, Barrett-Connor E. Estrogen use and depressive symptoms in postmenopausal women. Obstet Gynecol. 1992;80(1):30–36. [PubMed] [Google Scholar]
- 44.Zweifel JE, O'Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22(3):189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: A preliminary report. American Journal of Obstetrics and Gynecology. 2000;183(2):414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 46.Hlatky MA, Boothroyd D, Vittinghoff E, Sharp P, Whooley MA.for the HRGQuality-of-life and depressive symptoms in postmenopausal women After receiving hormone therapy: results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial JAMA 20022875591–597. [DOI] [PubMed] [Google Scholar]
- 47.Hays J, Ockene JK, Brunner R, et al. Effects of estrogen plus progestin on health-related quality of life. The New England Journal of Medicine. 2003;348(19):1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 48.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. Journal of Clinical Endocrinology and Metabolism. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 49.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 50.Gibbs RB. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocr Rev. 2009 doi: 10.1210/er.2009-0036. er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumas J, Edgren C, Newhouse PA. Estrogen replacement after menopause: role in normal cognition and Alzheimer's disease. Aging Health. 2006;2:955–966. [Google Scholar]
- 52.Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse PA. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal post-menopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- 53.Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen interacts with the cholinergic system to affect the verbal memory in postmenopausal women: evidence for the critical period hypothesis. Hormones and Behavior. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fink G, Sumner BEH, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cellular and Molecular Neurobiology. 1996;16(3):325–344. doi: 10.1007/BF02088099. [DOI] [PubMed] [Google Scholar]
- 55.McDermott JL, Kreutzberg JD, Liu B, Dluzen DE. Effects of estrogen treatment on sensorimotor task performance and brain dopamine concentrations in gonadectomized male and female CD-1 mice. Hormones and Behavior. 1994;28(1):16–28. doi: 10.1006/hbeh.1994.1002. [DOI] [PubMed] [Google Scholar]
- 56.Rubinow D, Schmidt P, Roca C. Estrogen-serotonin interactions: implications for affective regulation. Biological Psychiatry. 1993;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 57.McEwen B. Estrogen actions throughout the brain. Recent Progress in Hormone Research. 2002;57(1):357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 58.Bethea CL, Mirkes SJ, Lu NZ, Streicher JM, Cameron JL. Differences in central serotonergic activity in stress-sensitive versus stress-resilient monkeys: tryptophan hydroxylase (TPH), serotonin reuptake transporter (SERT) and serotonin 1A autoreceptor (5HT1A) mRNA expression. Paper presented at: The Society of Biological Psychiatry 2003 Annual Meeting; 2003; 2003; San Francisco, CA. [Google Scholar]
- 59.Schmidt PJ. Mood, depression and reproductive hormones in the menopausal transition. The American Journal of Medicine. 2005;118(12B):54S–58S. doi: 10.1016/j.amjmed.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 60.Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. American Journal of the Geriatric Psychiatry. 1993;5:97–106. [PubMed] [Google Scholar]
- 61.Nagata H, Nozaki M, Nakano H. Short-term combinational therapy of low-dose estrogen with selective serotonin re-uptake inhibitor (fluvoxamine) for oophorectomized women with hot flashes and depressive tendencies. Journal of Obstetrics & Gynaecology Research. 2005;31(2):107–114. doi: 10.1111/j.1447-0756.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 62.Soares CN, Poitras JR, Prouty J, Alexander AB, Shifren JL, Cohen LS. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. Journal of Clinical Psychiatry. 2003;64(4):473–479. doi: 10.4088/jcp.v64n0419. [DOI] [PubMed] [Google Scholar]
- 63.Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: A pilot study. Journal of Psychiatric Research. 2007;41(3–4):338–343. doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Newhouse PA, Dumas J, Hancur-Bucci C, et al. Estrogen Administration Negatively Alters Mood Following Monoaminergic Depletion and Psychosocial Stress in Postmenopausal Women. Neuropsychopharmacology. 2008;33(7):1514–1527. doi: 10.1038/sj.npp.1301530. [DOI] [PubMed] [Google Scholar]
- 65.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19(4):397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 66.Maki PM. Menopause and anxiety: immediate and long-term effects. Menopause. 2008;15(6):1033–1035. doi: 10.1097/gme.0b013e318186d823. 1010.1097/gme.1030b1013e318186d318823. [DOI] [PubMed] [Google Scholar]
- 67.Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology. 1993;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 68.Delgado P. Monoamine depletion studies: Implications for antidepressant discontinuation syndrome. Journal of Clinical Psychiatry. 2006;67 Supplement 4:22–26. (Supplement 4) [PubMed] [Google Scholar]
- 69.Leyton M, Young SN, Pihl RO, et al. Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology. 1999;22(1):52–63. doi: 10.1016/S0893-133X(99)00086-X. [DOI] [PubMed] [Google Scholar]
- 70.Young S, Leyton M. The role of serotonin in human mood and social interaction: insight from altered tryptophan levels. Pharmacology Biochemistry and Behavior. 1993;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 71.Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 72.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 73.Reisberg B, Schenk M, Ferris S, Schwartz G, deLeon M. The Brief Cognitive Rating Scale (BCRS): Findings in primary degenerative dementia (PDD) Psychopharmacology Bulletin. 1993;(19):47–50. [Google Scholar]
- 74.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2. Lutz, FL: Psychological Assessment Resources Inc.; 2001. [Google Scholar]
- 75.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. American Journal of Psychiatry. 1993;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 76.First MBRLS, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition. SCID-I/P, 2/2001 ed. Washington, D.C.: American Psychiatric Press Inc.; 2001. [Google Scholar]
- 77.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 78.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 1991;72(2):336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 79.Ellenbogen MA, Young SN, Dean P, Palmour RM, Benkelfat C. Mood response to acute tryptophan depletion in healthy volunteers: sex differences and temporal stability. Neuropsychopharmacology. 1996;15(5):465–474. doi: 10.1016/S0893-133X(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 80.Kirschbaum C, Pruessner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine. 1995;57(5):468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic Medicine. 2001;63(6):966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 82.Kupke T, Lewis R. Relative influence of subject variables and neurological parameters on neuropsychological performance of adult seizure patients. Archive of Clinical Neuropsychology. 1989;4:351–363. [PubMed] [Google Scholar]
- 83.Hindmarch I. Psychological performance models as indicators of the effects of hypnotic drugs on sleep. Psychopharmacology. 1984;S1:58–68. doi: 10.1007/978-3-642-69659-6_4. [DOI] [PubMed] [Google Scholar]
- 84.Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 85.The Continuous Performance Test [computer program]. Version 3.0. Toronto: Multi-Health Systems; 1995. [Google Scholar]
- 86.Lezak MD. Neuropsychological Assessment. 2nd ed 1995. [Google Scholar]
- 87.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 88.Golightly KL, Lloyd JA, Hobson JE, Gallagher P, Mercer G, Young AH. Acute tryptophan depletion in schizophrenia. Psychological Medicine. 2001;31:75–84. doi: 10.1017/s0033291799003062. [DOI] [PubMed] [Google Scholar]
- 89.Baker LD, Cholerton B, Gleason C, et al. Estrogen favorably affects selective attention for healthy postmenopausal women. Society for Neuroscience, 2002. 2002 [Google Scholar]
- 90.Maki P, Zonderman A, Resnick S. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. American Journal of Psychiatry. 2001;158(2):227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- 91.Bartolic Ei, Basso MR, Schefft BK, Glauser T, Titanic-Schefft M. Effects of experimentally-induced emotional states on frontal lobe cognitive task performance. Neuropsychologia. 1999;37(6):677–683. doi: 10.1016/s0028-3932(98)00123-7. [DOI] [PubMed] [Google Scholar]
- 92.Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33(5):643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. the Journal of Neuroscience. 2005:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Koch K, Pauly K, Kellermann T, et al. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45(12):2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 97.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70(6):422–430. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- 98.Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. The Journal of Neuroscience. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Protopopescu X, Pan H, Altemus M, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. PNAS. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pearson R, Lewis MB. Fear recognition across the menstrual cycle. Hormones and Behavior. 2005:267–271. doi: 10.1016/j.yhbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage. 2006;32(1):457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 103.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 104.Saab PG, Matthews KA, Stoney CM, McDonald RH. Premenopausal and postmenopausal women differ in their cardiovascular and neuroendocrine responses to behavioral stressors. Psychophysiology. 1989;26(3):270–280. doi: 10.1111/j.1469-8986.1989.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 105.Ceresini G, Freddi M, Morganti S, et al. The effects of transdermal estradiol on the response to mental stress in postmenopausal women: a randomized trial. American Journal of Medicine. 2000;109(6):463–468. doi: 10.1016/s0002-9343(00)00523-4. [DOI] [PubMed] [Google Scholar]
- 106.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84(2):606–610. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- 107.Lindheim SR, Legro RS, Bernstein L, et al. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. American Journal of Obstetrics and Gynecology. 1992;167:1831–1836. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 108.Burleson MH, Malarkey WB, Cacioppo JT, et al. Postmenopausal hormone replacement: effects on autonomic, neuroendocrine, and immune reactivity to brief psychological stressors. Psychosomatic Medicine. 1998;60:17–25. doi: 10.1097/00006842-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 109.Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]