The structure and function of arteries change throughout the lifetime of humans and animals. Epidemiologic studies have found unequivocally that age is the dominant risk factor for hypertension, coronary heart disease, congestive heart failure, and stroke. Specifically, the greatest risk is posed by the most advanced age-associated changes in the arteries, which reflect unsuccessful aging.1 It is reasonable to hypothesize that specific mechanisms that underlie the arterial substrates that have been altered during aging are intimately linked to the aforementioned cardiovascular diseases. The age-associated arterial changes include lumenal dilation, diffuse intimal and medial thickening, increased stiffness, reduced compliance of central arteries, endothelial dysfunction, impaired nitric oxide (NO) availability, increased reactive oxygen species (ROS), inflammation, and angiogenesis. These age-associated alterations impair arterial regulatory mechanisms and the ability of arteries to adapt, repair, and remodel through the integration of multiple signaling mechanisms.
A megacept emerges with the realization that evolution of these age-associated profiles within the arterial wall is strikingly similar to that which develops in arteries of younger animals in response to experimental induction of early atherosclerosis or hypertension. Thus, “aging”-associated arterial changes and those associated with the aforementioned “diseases” are fundamentally intertwined at the cellular and molecular levels. In humans, other well-known risk factors (eg, altered lipid metabolism, smoking, and lack of exercise) interact with this arterial substrate that has been altered during aging and that renders the aging artery a fertile soil facilitating the initiation and progression of arterial diseases. The cellular/molecular proinflammatory alterations that underlie arterial aging thus become novel putative candidates to be targeted by interventions aimed at attenuating arterial aging as a major risk factor for cardiovascular diseases. This approach is similar to those aimed at lifestyle and pharmacologic interventions that already have proved effective in preventing or ameliorating arterial diseases associated with aging.
This review provides a landscape of central arterial aging and age-disease interactions by attempting to integrate perspectives that range from humans to molecules, with the goal that future therapies for cardiovascular diseases, such as hypertension, also will target the prevention or amelioration of unsuccessful arterial aging.
AGE-ASSOCIATED CHANGES IN CENTRAL ARTERIAL STRUCTURE AND FUNCTION
Aortic Macroscopic Structure
The structure and function of the central arteries change throughout the lifetime of humans.2–8 Cross-sectional studies show that central elastic arteries dilate with age, leading to an increase in lumen size (Fig. 1A). The thickness of the arterial wall also increases with advancing age (Fig. 1B). Postmortem studies indicate that in humans, this increase mainly is the result of intimal thickening.6 Studies of experimental animal models have increased understanding of age-associated alterations in arterial structure in humans. Age-associated restructuring of the central arteries of rats, rabbits, and nonhuman primates includes diffuse intimal thickening in the absence of clinical arterial diseases and is similar to that observed in grossly normal arterial segments in humans (Table 1).1,3–8
Fig. 1.
Age-associated macroscopic changes in central arterial structure in humans. Central arterial lumen (A) and intimal-medial wall thickness (B) increase with advancing age in men (red) and women (blue). Best-fitting age regression curves are shown for men (solid lines) and women (dashed lines).
Table.
Arterial Remodeling: Impact of Dietary Sodium, Aging, Hypertension, and Atherosclerosis and Ang II Signaling
| Aging | |||||||
|---|---|---|---|---|---|---|---|
| Humans (>65 yrs) |
Monkeys (15–20 yrs) |
Rats (24–30 mos) |
Rabbits (3–6 yrs) |
Hypertension | Atherosclerosis | Ang II Signaling | |
| Lumenal dilation | + | + | + | + | ? | ? | ? |
| ↑ Stiffness | + | + | + | + | + | + | + |
| Endothelial dysfunction | + | + | + | + | + | + | + |
| Diffuse Intimal Thickening | + | + | + | + | + | + | + |
| Lipid involvement | − | − | − | − | ± | + | + |
| ↑ VSMC number | + | + | + | + | + | + | + |
| Macrophages | + | − | − | + | + | + | + |
| ↑ Matrix | + | + | + | + | + | + | + |
| ↑ Local ACE-ANGII- AT1 | + | + | + | + | + | + | + |
| MMP/Calpain dysregulation | + | + | + | ? | + | + | + |
| ↑ MCP-1/CCR2 | + | + | + | + | + | + | + |
| ↑ ICAM-1 | ? | ? | + | ? | + | + | + |
| ↑ TGF-B | + | + | + | ? | + | + | + |
| ↑ NADPH Oxidase | ? | ? | + | ? | + | + | + |
| ↓ Nitric Oxide Bioavailability | ? | ? | + | + | + | + | + |
| HYPERTENSION | ± | ± | ± | ? | + | ± | + |
| ATHEROSCLEROSIS | ± | − | − | − | ± | + | + |
? =information unknown
Aortic Microscopic Structure
Viewed microscopically, age predominantly alters the intima, which is located between the lumen-endothelial interface and the internal elastic lamina of the artery. A series of studies show that age dramatically alters the volume and contents of this zone in rats, nonhuman primates, and humans.9–16 Small disoriented vascular smooth muscle cells (VSMC) and collagen types I and III all increase markedly within the thickened intima of old FXBN rats. Because nonhuman primate cardiovascular structure and function is similar to those of humans, primates are an ideal model in which to study arterial aging.9 Old monkeys (approximately 20 years) have a thickened intima containing cells and matrix beneath an intact endothelium, and nearly all of these cells stain positively for α-smooth muscle actin (α-SMA), a marker of VSMC (Fig. 2A).10,11 Aging also increases intimal cells in humans (Fig. 2B), which stain positively with an antibodies to α-SMA (see Fig. 2B) and to SMemb, a marker for the fetaltype VSMC.12 SMemb is a non–muscle-type myosin heavy chain that is expressed predominantly in undifferentiated VSMC in the fetal stage and is remarkably reduced in adults17 but reemerges in response to arterial injury.17
Fig. 2.
Age-associated aortic structural remodeling. (A) Arterial immunostaining for α-SMA (brown) from monkeys. Arrowhead indicates internal elastin lamina. (B) Movat staining (upper panels) and immunohistochemical staining (brown color) for α-SMA (middle panels) and for CD68 (brown color) (lower panels) in aorta from humans. (C) Toludine blue staining for mast cells in old human aorta (left panel) and observed under an oil lens (right panel). I, intima; L, lumen; M, media. Up-down arrows indicate thickened intima. (From Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 2003;41(6):1308–16 and Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 2007;50(1):219–7; with permission.)
In nonhuman primates and rats, inflammatory cells have not been detected in the thickened intima.9–11,13–16 In grossly normal specimens from humans over age 65, aortic intimal cell infiltration and matrix deposition are increased dramatically compared with specimens from younger individuals (approximately 20 years) (see Fig. 2B).12 The vast majority of cells within the intima stain positively for a-SMA.12 Unlike aged rats and monkeys, sporadic clusters of macrophages (CD-68 stained cells) are more numerous (see Fig. 2B) and mast cells also are detected occasionally in the older intima of the human aortic walls (Fig. 2C), even in the absence of lipid deposition.12 These mast cells within the older human aorta exhibit signs of activation and release a large number of enzymatic granules, including chymase.11,18 These findings suggest that intimal thickening and VSMC cellularity are characteristic of arterial aging in various species.
The age-associated increase of intimal VSMC cellularity is assumed to be the result of the migration/invasion of VSMC from the tunica media to the intima where they proliferate.1,3–6 This resembles experimental mechanical arterial injury.17,19 Early passage (discussed later) medial VSMC of old rats do exhibit an exaggerated chemotactic response to platelet-derived growth factor-BB (PDGF-BB), whereas medial VSMC from young aorta require several additional passages in culture to generate an equivalent response (Fig. 3A).19 Furthermore, as early as 3 days in culture, VSMC from old rats display a faster growth rate than VSMC from young rats (Fig. 3B).20 Old VSMC have a greater percentage of their population in the S phase of the cell cycle and a reduced number in G2/M or G0G1 compared with young VSMC.21 Thus, early passage cultured VSMC of old aorta do not exhibit the in vitro cell senescence pattern of some other cell types in which proliferative capacity wanes.
Fig. 3.
VSMC during aging. (A) Chemotatic response to a PDGF-BB gradient is increased in early passage VSMC from the aortic media of old rats compared with those from younger rats. VSMC within the older aorta are primed to respond to the growth factor. (From Pauly RR, Passaniti A, Crow M, et al. Experimental models that mimic the differentiation and dedifferentiation of vascular cells. Circulation 1992;86(Suppl):III68–73; with permission.) (B) Growth curves of VSMC cultured from young and old rat aortae. The number of VSMC obtained from old rats was significantly higher at days 3, 7, and 14. (From Li Z, Cheng H, Lederer WJ, et al. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol 1997;64(1):1–11; with permission.)
Human Endothelial Function In Vivo
Endothelial cells (EC) play a pivotal role in regulating several arterial properties, including vascular tone, permeability, and the response to inflammation. Several features of these endothelial properties undergo age-associated alterations in function via endothelial-derived substances (eg, NO) and ROS. In brachial or coronary arteries, endothelial function, as assessed by agonist- or flow-mediated vasoreactivity, has been shown to decline with advancing age22 in the absence of clinical disease (Fig. 4A).
Fig. 4.
Age-associated changes in arterial function in humans. (A) Flow-mediated induced dilation in the brachial artery of apparently healthy women. (Adapted from Celermajer DS, Sorensen KE, Spiegelhalter DJ, et al. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 1994;24(2):471–6; with permission.) Age-associated increase in carotid-femoral PWV (B), an index of central arterial stiffness, and in augmentation index (C) in healthy men (red) and women (blue). Best-fitting age regression curves are shown for men (solid lines) and women (dashed lines).
Molecular and Cellular Studies of Endothelial Function
Studies of endothelia in experimental animal models have increased understanding of age-associated arterial endothelial dysfunction in humans. Many molecular and cellular alterations in arterial EC structure and function that occur with aging likely are implicated in age-associated endothelial dysfunction.23 Morphologically, aortic EC from older donors are flattened and enlarged, and the number of EC with polyploid nuclei increases with advancing age.10,24 Age alters the amount, arrangement, and structural integrity of the EC cytoskeleton, which affects the mobility, migration, proliferation, and secretory proteomic profile of EC.10,23–25 In addition, simple end-to-end inter-EC connections increase, but stronger more complex overlapping or interdigitating connections decrease with advancing age, suggesting an age-associated deficiency in intercellular communication and in endothelial barrier function.26 Advancing age also is associated with decreased EC capacity for replication and repair, which has been linked to increased apoptosis,10 telomere shortening,27,28 proinflammatory state,23 reduced NO bioavailability,29 and decreased number30 and the function31 of endothelial progenitor cells.
As in humans, mechanical vasodilation and agonist-induced NO-dependent endothelial vasodilation are attenuated in older versus younger rats and monkeys.10,32 The impairment of endothelial-mediated vasodilatation with aging in humans can, in part, be reversed by L-arginine administration, suggesting that NO production becomes reduced with aging.33 Plasma levels of asymmetric dimethylarginine, which reduces NO synthase (NOS) activity, also increase with age in humans. In rats, arterial arginase activity increases with age and may deplete local substrates for NOS.32
Some animal studies show that the marked age-associated reduction in arterial NO bioavailability occurs in the context of an increase in endothelial NOS expression, whereas other studies have noted that the expression of endothelial NOS isoform is markedly reduced with aging.29–34 ROS are important modulators of NO bioavailability. Nicotinamide adenine dinucleotide phosphate (NAD[P]H) oxidase is a major source of ROS in vascular cells and within the rat arterial wall, where NADH-driven O2− generation increases with aging.29,34 NAD(P)H oxidase is composed of six subunits: Rho guanosine triphosphatase (GTPase), usually Rac1 or Rac2, and five phagocyte oxidase (phox) units of p90, p22, p40, p47, and p67. Among NAD(P)H subunits, p22 phox is higher in the aortic endothelium of old compared with young rats.35 In addition, arterial wall activities of the ROS scavengers, Cu/Zn superoxide dismutase (SOD), Mg SOD, and extracellular matrix SOD, do not change or decrease with increasing age in rats.29 Thus, an imbalance between oxidase and dismutase results in increased in situ levels of superoxide and hydrogen peroxide and a decrease in NO bioavailability in the coronary and aortic walls of rats with aging.
Noninvasive Evaluation of Arterial Mechanical Properties in Humans
Arterial stiffness and compliance
The increase in arterial wall thickening and reduction in endothelial function with advancing age are accompanied by an increase in arterial stiffening (Fig. 4B).36 Arterial stiffness depends on intrinsic stress/strain relationships that are determined by structural properties of the blood vessel wall and by smooth muscle tone. Among the various indexes of arterial stiffness, carotid-femoral pulse wave velocity (PWV) has emerged as the gold standard for noninvasive assessment of central arterial stiffness.37 PWV is assessed as the distance between the carotid and femoral sampling sites divided by the time delay for the onset of the pressure wave between these two sites. In contrast to central arteries, the stiffness of muscular arteries does not increase with advancing age.38 Thus, the manifestations of arterial aging may vary among the different vascular beds, reflecting differences in the structural compositions of the arteries and, perhaps, differences in the age-associated signaling cascades that modulate the arterial properties (discussed later) or differences in the response to these signals across the arterial tree. Organs with high flow and low resistance, however, such as the heart, kidney, and brain, are particularly vulnerable to the increased pulsatility that is associated with central arterial stiffening, because this pulsatility can be transmitted to the microvasculature where it my cause damage.39 For example, a longitudinal study showed that age-associated accelerated progression of arterial stiffness is an independent predictor of longitudinal decline in some aspects of cognitive function, such as verbal learning, nonverbal memory, and a measure of cognitive screening in nondemented individuals.40
Reflected pulse waves
PWV assesses the velocity of the forward pulse wave that is generated with each cardiac cycle. When this wave reaches an area of impedance mismatch, a reflected wave is generated, which travels back up the arterial tree toward the central aorta. This reflected wave alters the central arterial pressure waveform. The velocity of the reflected flow wave is proportional to the stiffness of the arterial wall. Thus, in young individuals whose vascular wall is compliant, the reflected wave does not reach the large elastic arteries until late systole. With advancing age and increasing arterial stiffening, the velocity of the reflected wave increases, and the wave reaches the central circulation earlier in the cardiac cycle during the early phase of systole (ascending limb of pressure waveform). This reflected wave can be assessed noninvasively from recordings of the carotid or radial arterial pulse waveforms by arterial applanation tonometry and high-fidelity micromanometer probes.41 Inspection of the recorded arterial pulse wave contour often shows an inflection point, which heralds the arrival of the reflected wave (Fig. 4C). The height from the inflection point to the peak of the arterial waveform is the pressure pulse augmentation that is due to the early arrival of the reflected wave. Dividing this augmentation by the height from the peak to the trough of the arterial waveform (corresponding to the pulse pressure) yields the augmentation index. Unlike PWV, which increases quadratically with age in older individuals, the augmentation index increases with age (see Fig. 4C) but seems to plateau at older ages.42
The pressure pulse augmentation provided by the early return of the reflected wave is an added load against which the ventricle must contract. Furthermore, the loss of diastolic augmentation that is observed with the early return of the reflected wave leads is associated with a drop in diastolic blood pressure, which has the potential to compromise coronary blood flow, because the latter occurs almost exclusively during diastole.
Although the augmentation index traditionally has been considered an index of arterial stiffness, it is increasingly recognized that because reflected waves originate, in part, in small arteries, the age-associated changes in the augmentation index also probably are determined, in part, by age-associated changes in the structure and function of these small arteries. Evaluation of the diastolic decay of pulse wave contour may provide insights into the characteristics and the pathology of more distal vessels in which reflected waves originate.43
Arterial Stiffness Under the Microscope
Arterial mechanical properties are influenced by alterations in the arterial extracellular matrix.6 The arrangement and interrelation of the macromolecular matrix and cellular components of the aorta determine the viscoelastic characteristics that account for many of the static and dynamic mechanical features of the aorta. At physiologic blood pressures, aortic medial elastin and collagen fibers and smooth muscle cells form well-defined layers: thick elastin bands form concentric plates or lamellae, between which elastin microfibers form networks; collagen fibers align circumferentially and are dispersed in the interstices of the elastic network; and VSMC extend circumferentially between adjacent elastin lamellae among the finer elastin and collagen fibers.
Elastin, which constitutes approximately 30% of the dry weight of the arteries, decreases with aging. An imbalance between the synthesis and degradation of the precursor tropoelastin leads to a reduction in the formation of mature elastin within the aged arterial wall.44,45 The steady-state level of aortic tropoelastin mRNA decreases dramatically with increasing age.45 Furthermore, because of the age-associated reduction in lysyl oxidase, tropoelastin in older animals is insufficiently crosslinked, thus has a diminished capacity to form a meshwork of mature elastin fibers.46 In addition, with maturation and aging, the glycoprotein component of elastin fibrils decreases and eventually disappears; elastin (in rats) becomes fragile and its Ca2+ content increases.47
In addition to mature collagen fiber deposition (an increase in the distribution of immature collagen occurs with age), it has been proposed that age-associated changes involve a decrease in the coiling and twisting of molecular chains and a reduction in effective chain length.48,49 With increasing age, free amino groups on collagen proteins become more vulnerable to nonenzymatic glycation, oxidation, and nitration to form advanced glycated end products via the Maillard reaction.50 These can lead to covalent cross-linking of adjacent collagen molecules, which further increases their tensile strength.50
Thus, the alterations in mechanical properties of aged vessels are consistent with a relative loss or damage of elastin and an increase in collagen deposition and cross-linking.51
Angiotensin II Signaling Molecules within the Aortic Wall
In addition to the structural alterations (described previously), arterial function is governed by age-associated changes in several signaling cascades, most prominently the renin-angiotensin system (RAS). The classic RAS is composed of angiotensinogen, renin, angiotensin (Ang) I and II, angiotensin-converting enzyme (ACE), chymase, angiotensin, and Ang II receptor (AT1). All of these components have been found to increase within the aged arterial wall in various species.9,11,12,16 The local Ang II concentration is more than 1000-fold that of circulating Ang II, is independently regulated, and plays an important role in vascular pathophysiology with aging.9,16,52,53 Ang II protein abundance increases in the aged aortic wall in rats (Fig. 5A).16 Studies of nonhuman primates also show that Ang II (Fig. 5B), ACE (Fig. 5C), and chymase (Fig. 5D) staining are increased in the thickened intima of older monkey aorta.11 Studies also show that in humans, Ang II (Fig. 6A), AT1 (Fig. 6B), ACE (Fig. 6C), and chymase immunofluorescence increase within the grossly normal aortic wall of samples from older donors.12 Further, double immunolabeling demonstrates that intimal Ang II is co-localized with ACE or chymase staining in older aorta.12 These findings demonstrate that elements of the classic RAS are all up-regulated in the aged arterial wall.
Fig. 5.
Ang II and its converting enzymes increase in the aged aortic wall from rats and nonhuman primates. (A) Immunolabeled Ang II (red) in the en face medial aortic sections from young (left panel) and old rats (right panels). (From Jiang L, Wang M, Zhang J, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE 2008;3:e2231.) (B) The immunofluorescent staining for Ang II (red color) on frozen sections of aortae from monkeys. (C) The immunostaining for ACE (red color) on frozen sections of aortae from monkeys. (D) The immunostaining for chymase in an old monkey aorta. Inset, rectangular region under high power. A, adventitia; L, lumen; M, media. (From Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 2003;41(6): 1308–16; with permission.)
Fig. 6.
Components of the RAS in the human aortic wall. Immunofluorescence staining for (A) Ang II (red color); (B) AT1 (green color) (upper panels); and (C) ACE (green color) (upper panel). L, lumen; M, media. (From Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 2007;50(1):219–7; with permission.)
The molecules linked to the Ang II signaling cascade, including calpain-1, matrix metalloproteinase types 2 and 9 (MMP-2 and MMP-9), monocyte chemotactic protein-1 (MCP-1), and transforming growth factor-beta 1 (TGF-b1) are up-regulated within the aged arterial wall (Fig. 7) (see Table 1).9,11–16
Fig. 7.
Simplified Ang II signaling cascades. (From Wang M, Lakatta EG. Central arterial aging: humans to molecules. In: Safar M, editor. Handbook of hypertension: arterial stiffness in hypertension. Amsterdam: Elsevier; 2006. p. 137–60; with permission.)
Calpain-1
Calpain-1 is a ubiquitous cytosolic Ca2+ activated neutral protease. Arterial calpain-1 transcript levels and protein abundance are enhanced with aging, and increased calpain-1 protein within the old rat aorta is co-localized with VSMC.16 Calpain -1 activity also increases within the aged aorta in rats.16
Matrix metalloproteinase types 2 and 9
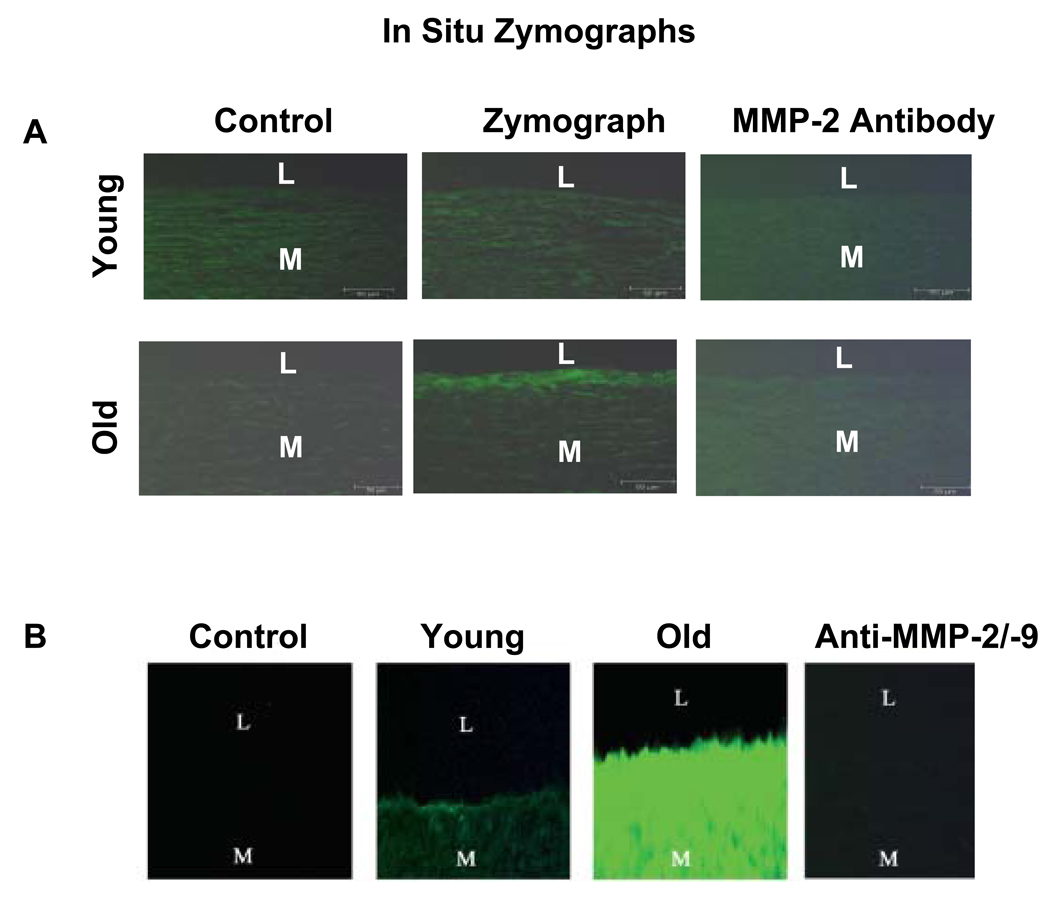
MMP-2 and MMP-9 are members of the zinc-containing endopeptidase family. They degrade native and denatured collagen and elastin, promote matrix protein degradation in vascular disease remodeling, and facilitate VSMC migration. Aortic MMP-2 activation in situ is progressively enhanced with aging, mainly localized to the intima, and co-localized with EC and VSMC in rats9,14 and monkeys (Fig. 8A).11 A recent study shows that in humans, MMP-2 and MMP-9 activity also is enhanced in situ in the grossly normal aortic segments with aging (Fig. 8B).12
Fig. 8.
In situ gelatin zymograms. (A) In situ gelatin zymographs of monkey aorta. Protease activity (green color) is localized mainly in the older aortic intima (middle panel). (From Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 2003;41(6):1308–16.) (B) In situ gelatin zymographs (green color) of humans. I, intimae; L, lumen; M, media. *P<.05, young versus old. (From Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 2007;50(1):219–7; with permission.)
Transforming growth factor-beta 1
TGF-β1 is a pluripotent growth factor implicated in various aspects of vascular development and structural remodeling in health and disease. TGF-β1 is a key regulator of collagen and fibronectin expression.14 TGF-β1, transcription, translation, and activity are increased within the aorta of old rats, particularly within the thickened intima.14
Activated TGF-β1 exerts its biologic effects by binding to its receptor TGF-β type II receptor (TβRII) via SMAD signaling. Aortic TβRII transcription and translation also are increased with aging. TβRII is increased within the wall of the aged aorta. Furthermore, the SMAD proteins, the receptor-regulated phosphorylated p-SMAD2/3, and the common-mediator SMAD4, are increased within the aged aortic wall, whereas the antagonistic or inhibitory SMAD7 protein decreases by 20% with age within the arterial media.14
Monocyte chemotactic protein-1
MCP-1/CCL2 (chemokine [C-C motif] ligand 2), a well-known chemokine, plays multiple roles in proinflammatory responses through the activation of its receptor, CCR2. Aortic MCP-1 and CCR2 transcriptome and protein abundance increase in rats with aging, mainly within the intima.15 As in rats, the increased MCP-1 within human old aorta resides predominantly within the intima, resulting in a markedly increased, age-associated intimal-medial gradient of the MCP-1, which potentially can induce migration of medial VSMC to intima.12
Up-Regulation of Angiotensin II Signaling Mimics Arterial Aging
In young FXBN rats, in vivo chronic infusion of Ang II at concentrations sufficient to elicit an increase in arterial pressure similar to that observed with age increases MMP-2, calpain-1, and TGF-β1 activity and imparts to their central arteries structural and molecular characteristics of arteries of old, untreated rats.9,16 Even a subpressor infusion of Ang II increases MMP-2 expression and activity and increases collagen production within the arterial wall.9 Administration of phenylephrine, an adrenergic receptor agonist, to young rats increases arterial Ang II levels and reproduces Ang II effects on arteries.9 These results demonstrate that Ang II signaling can cause structural, biochemical, and functional alterations of the arterial wall of young rats that resemble those that occur with aging and that the effects of phenylephrine signaling are mediated, in part at least, by Ang II.
In vitro Ang II treatment of VSMC from young rats induces MMP-2 and calpain-1 activity, reaching the levels of untreated VSMC from old rats.9,11,16 These Ang II- and age-associated effects are reduced by losartan, an AT1 receptor blocker.9,11,16 The increased MMP-2 activity driven by Ang II or aging also is abolished by Ci 1, a calpain inhibitor.9,11,16
Other in vitro studies show that the age-associated increase in VSMC invasion/migration could be modulated by concurrent alterations in Ang II signaling molecules. 9,11,15,16 Relative to young VSMC, those isolated from old aorta exhibit increased invasion of a synthetic basement membrane in response to a gradient of PDGF-BB or MCP-1, and this age difference is abolished or substantially reduced by losartan, an AT1 receptor blocker; vCCI, an inhibitor of CCR2 signaling; GM 6001, an inhibitor of MMP; and Ci 1.9,11,15,16 Furthermore, exposure of young aortic medial VSMC to Ang II, MCP-1, or calpain-1, induces an increase in invasion ability in a dose-dependent manner, up to the level of untreated, old medial VSMC.9,11,15,16 These effects are inhibited by losartan, vCCI, GM6001, and Ci 1.9,11,15,16
Thus, these results support the concept that Ang II signaling is a central pathway that mediates the cellular and molecular mechanisms that underlie arterial aging.
Arterial Blood Pressure
Arterial pressure is determined by the interplay of central arterial compliance, peripheral resistance, stroke volume, and the pattern of left ventricular (LV) ejection. A decline in central arterial compliance accompanies the age-associated increase in arterial wall stiffness, but neither peripheral resistance nor stroke volume changes appreciably with advancing age in normotensive individuals,54 although this normotensive status is maintained in only approximately 30% of older persons. In the face of the age-associated increase in arterial wall stiffness and the early return of reflected waves, a higher systolic blood pressure is required to distend the hardened capacitance vessels and maintain stroke volume. Furthermore, diastolic blood pressure is decreased as a result of the reduced storage capacity of the stiffened central aorta. Thus, with advancing age, systolic blood pressure rises, whereas diastolic blood pressure increases until the fifth decade, after which it plateaus and subsequently decreases. As a result, pulse pressure increases with advancing age throughout life, whereas mean arterial pressure increases with age but seems to plateau at older ages.
Most of the literature on blood pressure has focused on brachial blood pressure, which is readily measurable noninvasively. Central arterial pressure (ie, the pressure that is sensed by the heart) may differ from peripheral blood pressure because of central to peripheral arterial blood pressure amplification. This amplification is attributed to central to peripheral alterations in the properties of the arterial tree.55 This amplification is attenuated by the early return of reflected waves. Thus central to peripheral systolic pressure (and pulse pressure) amplification is inversely related to age. Emerging evidence suggests that central pressures may carry more robust prognostic information than peripheral pressures56 and that monitoring of central pressure may provide more accurate reflection of the efficacy of antihypertensive medications and may help explain, in part, the differential impact on outcomes that has been observed with some medications in spite of apparently equivalent changes in peripheral blood pressure.57
EXAGGERATED ARTERIAL AGING AND DISEASE
The age-associated alterations in arterial structure, function, molecules, and local signaling cascades, such as endothelial dysfunction, arterial stiffening, and intimalmedial thickening, and Ang II signaling increasingly are recognized as potent risk factors for arterial diseases, even after accounting for traditional cardiovascular risk factors, such as arterial pressure, plasma lipids, and smoking. For example, increased intimal-medial thickness is associated with silent ischemia among asymptomatic older individuals58 and is an independent predictor of stroke and future myocardial infarction. 59 Several clinical studies recently have shown the adverse cardiovascular effects of accelerated arterial stiffening.37 In hypertensive patients, PWV is a marker of cardiovascular risk and coronary events and is an independent predictor of mortality. In addition, PWV is an independent predictor of mortality in population-based studies and in subjects over 70 years of age. The augmentation index also has been shown to be a predictor of adverse events in end-stage renal disease patients.60 A discussion of the interventions to retard or reverse accelerated arterial aging is beyond the scope of this article. Suffice it to mention that these include lifestyle modifications (diet and exercise) and some traditional (eg, angiotensin antagonists) and promising (eg, the advanced glycation end-product cross-link breaker, Alagebrium) pharmacologic agents.3
Arterial Aging and Hypertension
Patients who have hypertension exhibit increased central arterial wall thickness, greater carotid wall thickness,61 and central pressure augmentation62 than do normotensive subjects, even after adjusting for age. They are believed to have larger central arterial diameters although this is debated.53–65
Systolic hypertension is the dominant form of hypertension in older individuals. As discussed previously, central arterial stiffness is one of the main determinants underlying the increase in systolic and pulse pressure. Longitudinal studies in humans have shown that arterial stiffness is an independent predictor of the rise in systolic blood pressure and of incident hypertension.66 The intimate link and the continuum between measures of arterial aging and hypertension increasingly are being recognized. A recent position article by the American Society of Hypertension Writing Group (HWG)67 proposed that the classification of hypertension be expanded by integrating additional preclinical and clinical cardiovascular manifestations that stress the structural and functional status of the vasculature and target organ damage. Thus, the HWG implicitly recognizes the role of accelerated arterial aging in the continuum of cardiovascular risk leading to hypertension. It is hoped that, in the future, a composite measure of arterial aging can be developed that would modulate, or at best even replace, chronologic age as a risk factor within this continuum.
Endothelial Dysfunction in Hypertension
Endothelial dysfunction occurs early in several cardiovascular disorders, including atherosclerosis, diabetes, and hypertension. Impaired endothelial vasoreactivity, in the coronary and peripheral arterial beds, is an independent predictor of future cardiovascular events.68 Hypertensive individuals exhibit endothelial dysfunction,69 and the mechanisms underlying their endothelial dysfunction are similar to the ones that occur with normotensive aging, although they appear at an earlier age.70 The normotensive offsprings of hypertensive subjects also exhibit endothelial dysfunction, 71 suggesting that endothelial dysfunction may precede the development of clinical hypertension. Among hypertensive subjects, greater endothelial dysfunction is an independent predictor of adverse cardiovascular outcomes.68
Arterial Stiffening in Hypertension
As discussed previously, clinical and epidemiologic studies in several different populations with varying prevalence of cardiovascular diseases have confirmed the prognostic importance of arterial stiffness and of pulse pressure. Furthermore, in several studies, pulse pressure was a stronger predictor of outcomes than systolic or diastolic blood pressures. The implication of this is that the status of the arterial wall, which is a major determinant of pulse pressure, may be an important therapeutic target. In a study of patients who had end-stage renal disease who required dialysis,72 treatment of hypertension had differing effects on PWV, despite having similar blood pressure– lowering effects in patients. Mortality was higher in the group in whom PWV increased in spite of therapy, and progression of vascular stiffening was an independent predictor of mortality. These observations suggest that treating increases in blood pressure is necessary but not sufficient therapy for the syndrome of hypertension. Whether or not arterial stiffness is a risk factor or a risk marker for cardiovascular diseases awaits intervention studies aimed at reducing arterial stiffness, to determine whether or not this improves outcomes independent of the effects on blood pressure.
Arterial-Ventricular Coupling
The interaction of the LV with the arterial system, termed arterial-ventricular coupling,73 can be indexed by the ratio of effective arterial elastance (Ea), a measure of the net arterial load exerted on the LV, to LV end-systolic elastance (ELV), a loadindependent measure of LV chamber performance. Previous studies of healthy individuals have shown that, at rest, Ea/ELV is tightly controlled within a narrow range across a broad age spectrum and even across species. This tight coupling allows the CV system to optimize energetic efficiency.
Arterial-ventricular coupling and age
During exercise, Ea/ELV decreases because of disproportionate increases in ELV versus Ea to ensure that cardiac performance is augmented sufficiently to meet the increased demands for blood flow. The reduction in Ea/ELV (inversely related to EF) during exercise has been shown to differ by age and gender.74 In both genders, Ea/ELV decreases during exercise (because ELV increases more than Ea), but the ratio declines to a lesser extent in older subjects. There are gender differences in the components of Ea/ELV during exercise: Ea is greater in older versus young women but is unaffected by age in men. ELV increases to a greater extent in young versus older subjects. Thus, suboptimal ventricular-vascular coupling helps to explain the age-associated blunting of maximal exercise EF, and its underlying mechanisms seem to differ between men and women.
Arterial-ventricular coupling and hypertension
Hypertension is a major risk factor for heart failure, including heart failure with preserved ejection fraction, particularly burdensome in older individuals. The specific mechanisms that underlie the transition of a hypertensive LV to a failing LV have not been elucidated completely. Hypertension is associated with structural and functional alterations in the central arteries and in the LV that are gender specific and believed to be, at least in their early stages, adaptive in nature. It is well established that LV performance is influenced by the arterial load and that the arterial properties are, in turn, influenced by LV performance. Thus, studying the interaction between the LV and the arterial system may provide a useful framework to gain insights into cardiovascular performance.
Compared with normotensive subjects, women who have systolic hypertension have a lower resting Ea/ELV; a higher energetic requirement at rest, at peak exercise, and during recovery; and a markedly attenuated Ea/ELV reserve.75 No differences were noted between normotensive and hypertensive men. The diminished Ea/ELV reserve in women who have systolic hypertension deserves further study as a possible cause of future functional limitations that could putatively explain the increased incidence of heart failure with preserved ejection fraction in older women who have systolic hypertension.
In summary, the metabolic, enzymatic, cellular, and molecular alterations and lifestyle factors (eg, high dietary sodium) that have been implicated in age-associated structural remodeling (see Table 1) are linked to Ang II signaling (see Fig. 7) and increasingly are recognized as playing a critical role in the genesis or promotion of inflammatory arterial diseases, including hypertension. In other words, many of the same factors that underlie the age-associated structural and functional alterations of the arterial wall also are implicated in the pathogenesis of hypertension. These and other previously well-defined factors are the culprits that accumulate and underlie the “risky” component of arterial aging and are linked to the increased incidence of the quintessential cardiovascular diseases, such as hypertension, in the elderly.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
The authors thank Robert E. Monticone for his assistance in preparing this document.
REFERENCES
- 1.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46(3):454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set-up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Najjar SS, Lakatta EG. Vascular aging: from molecular to clinical cardiology. In: Patterson WC, Runge M, editors. Principles of molecular cardiology. Totowa (NJ): Humana Press; 2005. pp. 517–547. [Google Scholar]
- 4.Wang M, Lakatta EG. Central arterial aging: humans to molecules. In: Safar M, editor. Handbook of hypertension: arterial stiffness in hypertension. Amsterdam: Elsevier; 2006. pp. 137–160. [Google Scholar]
- 5.Lakatta EG. Central arterial aging and the epidemic of systolic hypertension and atherosclerosis. J Am Soc Hypertens. 2007;1(5):302–340. doi: 10.1016/j.jash.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139(5):1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 7.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Lakatta EG. The salted artery and angiotensin ii signaling: a deadly duo in arterial disease. J Hypertens. 2009;27(1):19–21. doi: 10.1097/HJH.0b013e32831d1fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Zhang J, Spinetti G, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167(5):1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20(6):1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41(6):1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50(1):219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39(4):865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Zhao D, Spinetti G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26(7):1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 15.Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Wang M, Zhang J, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE. 2008;3:1–12. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiguchi K, Kurabayashi M, Oyama Y, et al. Homeobox protein Hex induces SMemb/nonmuscle myosin heavy chain-B gene expression through the cAMPresponsive element. Circ Res. 2001;88(1):52–58. doi: 10.1161/01.res.88.1.52. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki M, Takai S, Jin D, et al. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112(3):668–676. doi: 10.1016/j.pharmthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Pauly RR, Passaniti A, Crow M, et al. Experimental models that mimic the differentiation and dedifferentiation of vascular cells. Circulation. 1992;86 Suppl:III68–III73. [PubMed] [Google Scholar]
- 20.Li Z, Cheng H, Lederer WJ, et al. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol. 1997;64(1):1–11. doi: 10.1006/exmp.1997.2204. [DOI] [PubMed] [Google Scholar]
- 21.Hariri RJ, Hajjar DP, Coletti D, et al. Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol. 1988;131(1):132–136. [PMC free article] [PubMed] [Google Scholar]
- 22.Celermajer DS, Sorensen KE, Spiegelhalter DJ, et al. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 23.Csiszar A, Wang M, Lakatta EG, et al. Inflammation and endothelial dysfunction during aging: role of NF-{kappa} B. J Appl Phys. 2008;105(4):1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Q, Aida K, Vandeberg JL, et al. Passage-dependent changes in baboon endothelial cells–relevance to in vitro aging. DNA Cell Biol. 2004;23(8):502–509. doi: 10.1089/1044549041562294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner M, Hampel B, Bernhard D, et al. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol. 2001;36(8):1327–1347. doi: 10.1016/s0531-5565(01)00105-x. [DOI] [PubMed] [Google Scholar]
- 26.Yeh HI, Chang HM, Lu WW, et al. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J Histochem Cytochem. 2000;48(0):1377–1389. doi: 10.1177/002215540004801008. [DOI] [PubMed] [Google Scholar]
- 27.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92(24):11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwama H, Ohyashiki K, Ohyashiki JH, et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet. 1998;102(4):397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- 29.Van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 31.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 32.Berkowitz DE, White R, Li D, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108(16):2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 33.Bode-Boger SM, Muke J, Surdacki A, et al. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8(2):77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 34.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83(3):279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton CA, Brosnan MJ, McIntyre M, et al. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37(2 Part 2):529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 36.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 Part 1):1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 37.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 38.Laurent S, Lacolley P, Girerd X, et al. Arterial stiffening: opposing effects of ageand hypertension-associated structural changes. Can J Physiol Pharmacol. 1996;74(7):842–849. [PubMed] [Google Scholar]
- 39.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Phys. 2008;105(5):1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldstein SR, Rice SC, Thayer JF, et al. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 41.Kelly R, Hayward C, Avolio A, et al. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80(6):1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 43.McVeigh GE, Allen PB, Morgan DR, et al. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci (Lond) 2001;100(4):387–393. [PubMed] [Google Scholar]
- 44.Foster JA, Rich CB, Miller M, et al. Effect of age and IGF-I administration on elastin gene expression in rat aorta. J Gerontol. 1990;45(4):B113–B118. doi: 10.1093/geronj/45.4.b113. [DOI] [PubMed] [Google Scholar]
- 45.Quaglino D, Fornieri C, Nanney LB, et al. Extracellular matrix modifications in rat tissues of different ages. Correlations between elastin and collagen type I mRNA expression and lysyl-oxidase activity. Matrix. 1993;13(6):481–490. [PubMed] [Google Scholar]
- 46.Behmoaras J, Slove S, Seve S, et al. Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation Res. 2008;11(5):883–889. doi: 10.1089/rej.2008.0760. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan D, Meyer K. Mucopolysaccharides of aorta at various ages. Proc Soc Exp Biol Med. 1960;105:78–81. doi: 10.3181/00379727-105-26015. [DOI] [PubMed] [Google Scholar]
- 48.Harding SE, Jones SM, O’Gara P, et al. Isolated ventricular myocytes from failing and nonfailing human heart: the relation of age and clinical status of patients to isoproterenol response. J Mol Cell Cardiol. 1992;24(5):549–564. doi: 10.1016/0022-2828(92)91843-t. [DOI] [PubMed] [Google Scholar]
- 49.King AL. Pressure-volume relation for cylindrical tubes with elastomeric walls: the human aorta. J Appl Phys. 1946;17:501–505. [Google Scholar]
- 50.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 51.Roach MR, Burton AC. The effect of age on the elasticity of human iliac arteries. Can J Biochem Physiol. 1959;37(4):557–570. [PubMed] [Google Scholar]
- 52.Navar LG, Harrison-Bernard LM, Wang CT, et al. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10 Suppl 11:S189–S195. [PubMed] [Google Scholar]
- 53.Diz D, Lewis K. Dahl memorial lecture: the renin-angiotensin system and aging. Hypertension. 2008;52(1):37–43. doi: 10.1161/HYPERTENSIONAHA.107.108985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 55.Nichols WW, O’Rourke MF. McDonald’s blood flow in arteries: theoretical, experimental and clinical principles. 4th edition. London: Edward Arnold; 1998. [Google Scholar]
- 56.Wang KL, Cheng HM, Chuang SY, et al. Central or peripheral systolic or pulse pressure: which best predicts target-organ damage and mortality? J Hypertens. doi: 10.1097/hjh.0b013e3283220ea4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressurelowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 58.Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98(15):1504–1509. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- 59.O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 60.London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38(3):434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 61.Arnett DK, Tyroler HA, Burke G, et al. Hypertension and subclinical carotid artery atherosclerosis in blacks and whites: the Atherosclerosis Risk in Communities Study. ARIC Investigators. Arch Intern Med. 1996;156(17):1983–1989. [PubMed] [Google Scholar]
- 62.Nichols WW, Nicolini FA, Pepine CJ. Determinants of isolated systolic hypertension in the elderly. J Hypertens Suppl. 1992;10(6):S73–S77. [PubMed] [Google Scholar]
- 63.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45(4):652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell GF, Lacourciere Y, Ouellet JP, et al. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108(13):1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 65.Farasat SM, Morrell CH, Scuteri A, et al. Pulse pressure is inversely related to aortic root diameter implications for the pathogenesis of systolic hypertension. Hypertension. 2008;51(2):196–202. doi: 10.1161/HYPERTENSIONAHA.107.099515. [DOI] [PubMed] [Google Scholar]
- 66.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giles TD, Berk BC, Black HR, et al. Expanding the definition and classification of hypertension (Greenwich) J Clin Hypertens. 2005;7(9):505–512. doi: 10.1111/j.1524-6175.2005.04769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endolethial dysfunction in hypertensive patients. Circulation. 2001;104(2):191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 69.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 70.Taddei S, Virdis A, Mattei P, et al. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29(3):736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- 71.Taddei S, Virdis A, Mattei P, et al. Defective Larginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94(6):1298–1303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 72.Guerin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 73.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Phys. 2008;105(4):1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Najjar SS, Schulman SP, Gerstenblith G, et al. Age and gender affect ventricularvascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44(3):611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 75.Chantler PD, Melenovsky V, Schulman SP, et al. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol. 2008;295(1):H145–H153. doi: 10.1152/ajpheart.01179.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]