Abstract
Oral squamous cell carcinoma has a striking tendency to migrate and metastasize. Cyclooxygenase (COX)-2, the inducible isoform of prostaglandin (PG) synthase, has been implicated in tumor metastasis. However, the effects of COX-2 on human oral cancer cells are largely unknown. We found that overexpression of COX-2 or exogenous PGE2 increased migration and intercellular adhesion molecule 1 (ICAM)-1 expression in human oral cancer cells. Using pharmacological inhibitors, activators, and genetic inhibition of EP receptors, we discovered that the EP1 receptor, but not other PGE receptors, is involved in PGE2-mediated cell migration and ICAM-1 expression. PGE2-mediated migration and ICAM-1 up-regulation were attenuated by inhibitors of protein kinase C (PKC)δ, and c-Src. Activation of the PKCδ, c-Src, and AP-1 signaling pathway occurred after PGE2 treatment. PGE2-induced expression of ICAM-1 and migration activity were inhibited by a specific inhibitor, siRNA, and mutants of PKCδ, c-Src, and AP-1. In addition, migration-prone sublines demonstrated that cells with increased migration ability had higher expression of COX-2 and ICAM-1. Taken together, these results indicate that the PGE2 and EP1 interaction enhanced migration of oral cancer cells through an increase in ICAM-1 production.
Keywords: Cell Adhesion, Cell Migration, Cyclooxygenase (COX) Pathway, Signal Transduction, Src
Introduction
Oral squamous cell carcinoma (SCC)2 represents 1–2% of all human malignancies. It is characterized by a high degree of local invasiveness and a high rate of metastasis to cervical lymph nodes. The migration of oral SCC into maxillary and mandibular bones is a common clinical problem (1). Because oral cancer is a type of highly malignant tumor with a potent capacity to invade locally and metastasize distantly (2, 3), an approach that decreases its ability to invade and metastasize may facilitate the development of effective adjuvant therapy.
Cyclooxygenases (COXs) are the rate-limiting enzymes that catalyze the conversion of arachidonic acid to prostaglandins (PGs). Two COX isoforms with distinct tissue distributions and physiological functions have been identified (4, 5). COX-1 is constitutively expressed in many tissues and plays important roles in the control of homeostasis (6). Conversely, COX-2 is an inducible enzyme and is activated by extracellular stimuli such as growth factors and proinflammatory cytokines (7). Overexpression of COX-2 is frequently found in many types of cancer, including colon, lung, breast, pancreas, head, and neck cancers (8–10) and is usually associated with poor prognosis and short survival. Identification of four subtypes of the PGE receptor (EP1–EP4) has made it possible to analyze their effects on human cancer cells (11, 12). EP1 is coupled to Ca2+ mobilization, EP2 and EP4 activate adenylate cyclase, and EP3 inhibits adenylate cyclase (13, 14). Furthermore, these studies have indicated that cancer cells express multiple PGE receptor subtypes and that each subtype may be linked to different actions of PGE2. Tumor invasion and metastasis are the critical steps in determining the aggressive phenotype of human cancers. Mortality in patients with cancer principally results from the metastatic spread of cancer cells to distant organs (15). To facilitate cell motility, invading cells need to change their cell-cell adhesion properties, rearrange the extracellular matrix environment, suppress anoikis, and reorganize their cytoskeletons (16). Cell adhesion molecules belonging to the integrin, cadherin, and immunoglobulin superfamilies have been implicated in tumor progression (17). Intercellular adhesion molecule-1 (ICAM-1, also called CD54), a member of the immunoglobulin supergene family, is an inducible surface glycoprotein that mediates adhesion-dependent cell-to-cell interactions (18, 19). The extracellular domain of ICAM-1 is essential for the transendothelial migration of leukocytes from the capillary bed into the tissue (20), and ICAM-1 may also facilitate movement (or retention) of cells through the extracellular matrix (20). ICAM-1 plays an important role in lung cancer cell invasion (21), and ICAM-1 antibody or antisense ICAM-1 cDNA has also been reported to rescues the invasiveness of breast cancer cells (22). Therefore, ICAM-1 may play a critical role in tumorigenesis, and its disruption may prevent metastasis.
The contribution of COX-2 to tumorigenesis has been intensively studied. COX-2 modulates the cell migration and invasion of several types of cancer cells (23, 24). The interaction of COX-2 with its specific EP receptors on the surface of cancer cells induces cancer invasion (25). The effect of COX-2 and EP receptors on migration activity in human oral cells is, however, mostly unknown. Here, we show that COX-2 and PGE2 increase migration and up-regulate ICAM-1 expression in human oral cancer cells. In addition, EP1 receptor, protein kinase Cδ (PKCδ), c-Src, and activator protein-1 (AP-1) signaling pathways are involved.
EXPERIMENTAL PROCEDURES
Materials
Anti-mouse and anti-rabbit IgG-conjugated horseradish peroxidase, rabbit polyclonal antibodies specific for β-actin, PKCδ, c-Src, c-Jun, p-c-Jun, lamin B, and the small interfering RNAs (siRNAs) against ICAM-1, c-Jun and control (for experiments using targeted siRNA transfection; each consists of a scrambled sequence that will not lead to the specific degradation of any known cellular mRNA) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ON-TARGET smart pool EP1 and PKCδ siRNA and ON-TARGET plus siCONTROL nontargeting pool siRNA were purchased from Dharmacon (Lafayette, CO). Rabbit polyclonal antibodies specific for PKCδ phosphorylated at Thr505 and c-Src phosphorylated at Tyr416 were purchased from Cell Signaling and Neuroscience (Danvers, MA). Mouse monoclonal antibody specific for ICAM-1 was purchased from R&D Systems (Minneapolis, MN). PGE2, 17-phenyl trinor PGE2, butaprost, sulprostone, 11-deoxy-PGE1, SC19220, and rabbit polyclonal antibody specific for COX-2 and EP1 were purchased from Cayman Chemical (Ann Arbor, MI). Valeryl salicylate, NS398, GF109203X, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2), and IPTG (isopropyl-β-d-thiogalactopyranoside) were purchased from Calbiochem. Celebrex was purchased from Pharmacia Co. Tanshinone IIA was purchased from BIOMOL (Butler Pike, PA). The COX-2 IPTG-induced expression plasmid p-NLR-COX2 was a gift from Dr. M. L. Kuo (National Taiwan University) (26). A 1.9-kbp cDNA fragment of human COX-2 (generously provided by Dr. Shuang-En Chuang, National Health Research Institute) was cloned into the pRSVNOT plasmid (27). The pRSVNOT plasmid can be relieved by addition of IPTG, allowing regulated expression of the target gene. The c-Src dominant negative mutant was a gift from Dr. S. Parsons (University of Virginia Health System, Charlottesville, VA). All other chemicals were obtained from Sigma-Aldrich.
Cell Culture
The human oral cancer cell line SCC4 (original site, tongue) was obtained from the American Type Culture Collection (Rockville, MD). The cells were maintained in DMEM supplemented with 20 mm HEPES and 10% heat-inactivated FCS, 2 mm glutamine, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C with 5% CO2. We also used a migration-prone subline, SCC4-S10, which was established from SCC4 cells (28).
Migration Assay
The migration assay was performed using Transwell (Costar; pore size, 8 μm) in 24-well dishes. Before the migration assay, cells were pretreated for 30 min with different concentrations of inhibitors, including the SC19220, GF109203X, PP2, or vehicle control (0.1% dimethyl sulfoxide). Approximately 1 × 104 cells in 100 μl of serum-free medium were placed in the upper chamber, and 300 μl of the same medium containing PGE2 was placed in the lower chamber. The plates were incubated for 24 h at 37 °C in 5% CO2, and then cells were fixed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min. Cells on the upper side of the filters were removed with cotton-tipped swabs, and the filters were washed with PBS. Cells on the underside of the filters were examined and counted under a microscope. Each clone was plated in triplicate in each experiment, and each experiment was repeated at least three times. The number of migrating cells in each experiment was adjusted with a cell viability assay to correct for proliferation effects of PGE2 (corrected migrating cell number = counted migrating cell number/percent of viable cells) (29).
Quantitative Real-time PCR (qPCR)
Total RNA was extracted from oral cancer cells using a TRIzol kit (MDBio Inc., Taipei, Taiwan). The reverse transcription reaction was performed using 2 μg of total RNA that was reverse transcribed into cDNA using oligo(dT) primer. The qPCR analysis was carried out using Taqman® one-step PCR Master Mix (Applied Biosystems). 100 ng of total cDNA was added per 25-μl reaction with sequence-specific primers and Taqman® probes. Sequences for all target gene primers and probes were purchased commercially (β-actin was used as internal control) (Applied Biosystems). qPCR assays were carried out in triplicate (one independent RNA sample for each treatment) on a StepOnePlus sequence detection system. The cycling conditions were 10-min polymerase activation at 95 °C followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s. The threshold was set above the nontemplate control background and within the linear phase of target gene amplification to calculate the cycle number at which the transcript was detected (denoted CT).
Western Blot Analysis
Cellular lysates were prepared as described (29). Proteins were resolved on SDS-PAGE and transferred to Immobilon polyvinyldifluoride membranes. The blots were blocked with 4% BSA for 1 h at room temperature and then probed with rabbit anti-human antibodies against PKCδ, p-PKCδ, c-Src, or p-c-Src (1:1,000) for 1 h at room temperature. After three washes, the blots were subsequently incubated with a donkey anti-rabbit peroxidase-conjugated secondary antibody (1:1,000) for 1 h at room temperature. The blots were visualized with enhanced chemiluminescence and Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY).
Tissue Collection
Upon approval by the local ethics committee, specimens of tumor tissues or normal tissues were obtained from patients who had been pathologically diagnosed with oral cancer and had undergone surgical resection at the China Medical University Hospital. Tissue specimens were ground and sonicated in a lysis buffer. The protein expression levels were analyzed using Western blot analysis.
Kinase Activity Assay
PKCδ and c-Src activity were assessed with a PKC Kinase Activity Assay kit (Assay Designs, Ann Arbor, MI) and a c-Src Kinase Activity Assay kit (Abnova, Taipei, Taiwan). The kinase activity kits are based on a solid phase ELISA that uses a specific synthetic peptide as a substrate for PKCδ or c-Src and a polyclonal antibody that recognizes the phosphorylated form of the substrate.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation analysis was performed as described (30). DNA immunoprecipitated with anti-c-Jun was purified and extracted with phenol-chloroform. The purified DNA pellet was subjected to PCR, and PCR products were resolved with 1.5% agarose gel electrophoresis and visualized with UV light. The primers 5′-AGACCTTAGCGCGGTGTAGA-3′ and 5′-AGTAGCAGAGGAGCTCAGCG-3′ were utilized to amplify across the ICAM-1 promoter region (−346 to −24) (30).
Statistics
For statistical evaluation, the Mann-Whitney U test was used for non-Gaussian parameters, and the Student's t test was used for Gaussian parameters (including Bonferroni correction). Differences were considered significant if the p value was <0.05.
RESULTS
COX-2 Directed Migration of Oral Cancer Cells via the EP1 Receptor
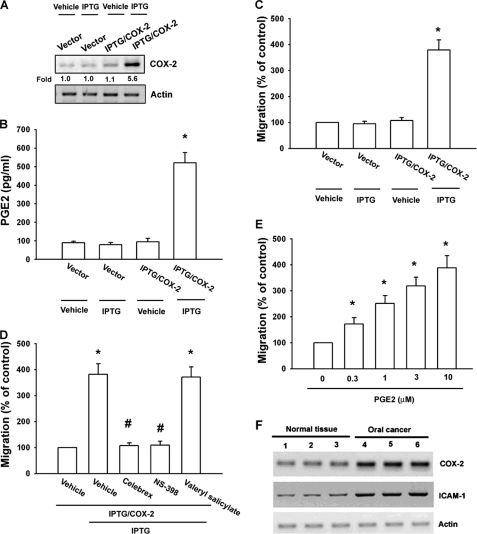
COX-2 expression stimulates directional migration and invasion of human cancer cells (23, 24). We used an IPTG-inducible COX-2 gene expression vector to examine the role of COX-2 in oral cancer cells. SCC4 cells were transfected with IPTG-inducible COX-2 gene expression vector or a control vector, and then IPTG (5 mm) was added for 24 h. Using Western blot analysis and ELISA, we found that IPTG induced COX-2 and PGE2 expression, respectively (Fig. 1, A and B). Furthermore, overexpression of COX-2 enhanced cell migration in oral cancer cells (Fig. 1C). To confirm IPTG-inducible COX-2-mediated cell migration, COX-2 specific inhibitors (Celebrex and NS-398) were used. Celebrex and NS-398, but not a COX-1-specific inhibitor (valeryl salicylate), reduced IPTG-inducible COX-2-mediated cell migration (Fig. 1D). We then directly exposed SCC4 cells to PGE2 and examined their migration activity. Stimulation of cells with PGE2 increased the migration activity in oral cancer cells in a dose-dependent manner (Fig. 1E). We also examined human oral cancer tissues for expression of COX-2 using Western blot analysis. Protein levels of COX-2 in human oral cancer tissues were significantly higher than those in normal tissues (Fig. 1F). Thus, expression of COX-2 was associated with a metastatic phenotype of oral cancer cells.
FIGURE 1.
COX-2-directed migration of human oral cancer cells. SCC4 cells were transfected with IPTG/COX-2 expression plasmid or control vector for 24 h followed by stimulation with IPTG (5 mm) for 24 h. A–C, COX-2 expression, PGE2 production, and migration activity were determined by Western blot analysis (A), ELISA (B), and migration assay (C). D, SCC4 cells were transfected with IPTG/COX-2 expression plasmid or control vector for 24 h and pretreated with valeryl salicylate (20 μm), Celebrex (10 μm), or NS-398 (20 μm) for 30 min followed by stimulation with IPTG (5 mm), and in vitro migration was measured after 24 h. E, SCC4 cells were incubated with various concentrations of PGE2, and in vitro migration activity was measured after 24 h. F, total protein were extracted from normal tissues or from human oral cancer tissues and subjected to Western blot analysis for COX-2 and ICAM-1. Results are expressed as the mean ± S.E. (error bars). *, p < 0.05 compared with control; #, p < 0.05 compared with IPTG/COX-2 plus IPTG-treated group.
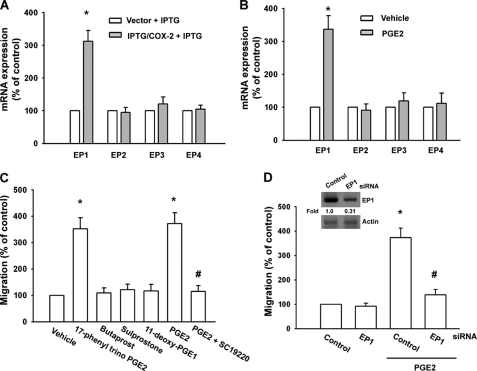
PGs exert their effects through interaction with specific EP1–EP4 subtype receptors (11, 12). To investigate the role of EP1–EP4 subtype receptors in COX-2-mediated increase of cell migration, we assessed the distribution of these EP subtype receptors in human oral cancer cells by qPCR analysis. The mRNAs of EP1, EP2, EP3, and EP4 subtype receptors could be detected in SCC4 cells (Fig. 2A). After IPTG/COX-2-transfected SCC4 cells were treated for 24 h with IPTG, the mRNA level of EP1 subtype receptor was increased, whereas EP2, EP3, and EP4 receptor mRNA remained unchanged (Fig. 2A). In addition, a similar induction of EP1 receptor mRNA, but not EP2, EP3, and EP4 receptor subtypes, was observed in SCC4 cells treated with PGE2 (Fig. 2B). To determine the role of EP1 receptor-dependent signaling in the regulation of cell migration in oral cancer cells, the cells were treated with EP1–EP4-specific agonists, and then the cell migration activity was examined. Of the agonists tested, only the EP1/EP3-selective receptor agonist, 17-phenyl trinor PGE2 (3 μm), significantly increased the migration activity (Fig. 2C). In contrast, butaprost (EP2 agonist; 10 μm), sulprostone (EP3 agonist; 10 μm) and 11-deoxy-PGE1 (EP3-selective agonist; 10 μm) did not up-regulate cell migration (Fig. 2C). In addition, treatment with the EP1 receptor antagonist SC19220 (10 μm) effectively antagonized the potentiating effect of PGE2 on cell migration activity (Fig. 2C). To confirm further this stimulation-specific mediation by EP1 receptor, we assessed the role of EP1 by using ON-TARGET smart pool EP1 siRNA, which decreases nonspecific effects by chemical modification and pooling (31). Transfection of cells with ON-TARGET smart pool EP1 siRNA reduced EP1 expression (Fig. 2D, inset). Transfection of cells with siRNA for EP1 but not with control siRNA effectively inhibited the PGE2-mediated migration of oral cancer cells (Fig. 2D inset, lower panel). These results indicate that PGE2 increased cell migration in human oral cancer cells via EP1 receptor.
FIGURE 2.
EP1 receptor is involved in PGE2-mediated migration of human oral cancer cells. A, SCC4 cells were transfected with IPTG/COX-2 expression plasmid or control vector for 24 h followed by stimulation with IPTG (5 mm) for 24 h, and the mRNA expression of EP receptors was determined by qPCR. B, SCC4 cells were incubated with PGE2 for 24 h, and the mRNA expression of EP receptors was determined by qPCR. C, SCC4 cells were treated with 17-phenyl trinor PGE2 (3 μm), butaprost (10 μm), sulprostone (10 μm), 11-deoxy-PGE1 (10 μm), PGE2 and PGE2 plus SC19220 (10 μm), and in vitro migration activity was measured after 24 h. D, inset, cells were transfected with EP1 siRNA for 24 h, and the EP1 expression was examined by Western blotting. D, cells were transfected with EP1 siRNA for 24 h followed by stimulation with PGE2, and in vitro migration was measured after 24 h. Results are expressed as the mean ± S.E. (error bars). *, p < 0.05 compared with control; #, p < 0.05 compared with PGE2-treated group.
PGE2-directed Migration of Oral Cancer Cells Involves ICAM-1 Up-regulation
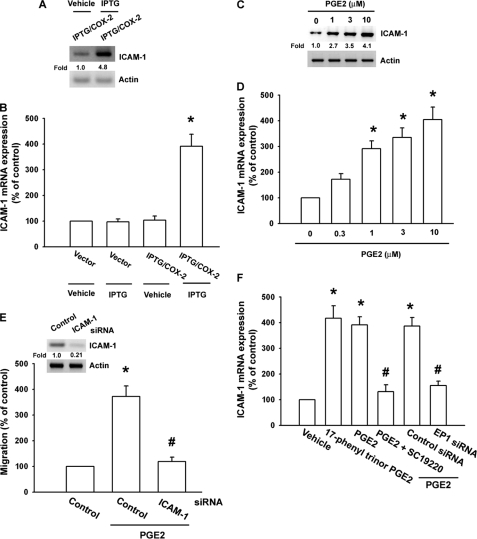
ICAM-1 is expressed at significant levels in human oral cancer cells (1). Therefore, we hypothesized that ICAM-1 may be involved in PGE2-directed migration of oral cancer cells. Western blotting and qPCR analysis showed that IPTG/COX-2-mediated COX-2 induced the protein and mRNA expression of ICAM-1 in SCC4 cells (Fig. 3, A and B). In addition, treatment of cells with PGE2 also increased protein and mRNA expression of ICAM-1 in a dose-dependent manner (Fig. 3, C and D). Transfection of cells with ICAM-1 siRNA markedly inhibited PGE2-induced cell migration (Fig. 3E). In contrast, the EP1/3 agonist enhanced mRNA expression of ICAM-1 (Fig. 3F). Pretreatment of cells with SC19220 or transfection of cells with EP1 siRNA reduced PGE2-mediated ICAM-1 expression (Fig. 3F). Furthermore, compared with normal tissues, human oral cancer tissues expressed higher levels of ICAM-1 (Fig. 1F). These data suggest that PGE2-induced cancer migration may occur via activation of the ICAM-1.
FIGURE 3.
COX-2-directed migration of human oral cancer cells involves up-regulation of ICAM-1. A and B, SCC4 cells were transfected with IPTG/COX-2 expression plasmid or control vector for 24 h followed by stimulation with IPTG (5 mm) for 24 h, and the protein and mRNA expression of ICAM-1 was determined by Western blotting (A) and qPCR (B). C and D, SCC4 cells (without transfected with control siRNA) were incubated with PGE2 for 24 h, and protein and mRNA expression of ICAM-1 was examined by Western blotting (C) and qPCR (D). E, cells were transfected with ICAM-1 siRNA for 24 h followed by stimulation with PGE2, and in vitro migration was measured after 24 h. F, SCC4 cells were treated with 17-phenyl trinor PGE2 (3 μm), 11-deoxy-PGE1 (10 μm), PGE2, and PGE2 plus SC19220 (10 μm), and mRNA expression of ICAM-1 was determined using qPCR. Results are expressed as the mean ± S.E. (error bars). *, p < 0.05 compared with control; #, p < 0.05 compared with PGE2-treated group.
Signaling Pathways of PKCδ and c-Src Are Involved in Potentiating Action of COX-2
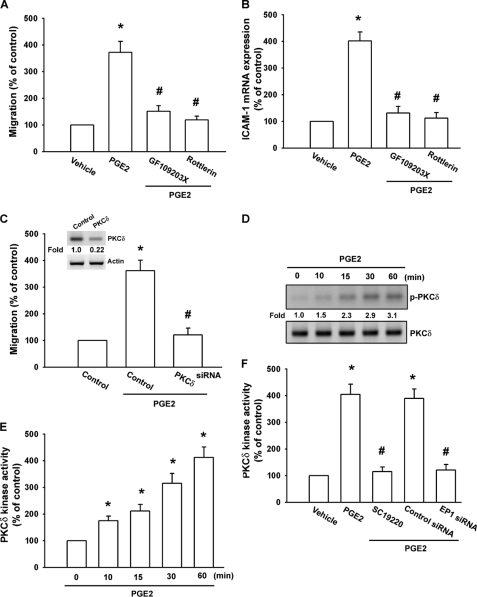
PKCδ plays a crucial role in the regulation of gene expression (32, 33). To determine whether PKC isoforms were involved in PGE2-triggered cell migration, SCC4 cells were pretreated with either GF109203X, a pan-PKC inhibitor, or rottlerin, a selective PKCδ inhibitor (34) for 30 min and then incubated with PGE2 for 24 h. As shown in Fig. 4, A and B, pretreatment with GF109203X and rottlerin reduced PGE2-induced cell migration and ICAM-1 expression, suggesting that PKCδ plays a potential role in PGE2-induced cell motility in oral cancer cells. Transfection with a PKCδ siRNA specifically blocked protein expression of PKCδ (Fig. 4C inset, upper panel). In addition, PKCδ siRNA also reduced PGE2-induced cancer cell migration (Fig. 4C inset, lower panel). We then directly measured PKCδ phosphorylation in response to PGE2. Stimulation of SCC4 cells led to a significant increase in phosphorylation of PKCδ (Fig. 4D). In addition, PKCδ activity was also increased by PGE2 treatment in SCC4 cells in a time-dependent manner (Fig. 4E). Pretreatment of cells with SC19220 or transfection of cells with EP1 siRNA also reduced PGE2-mediated PKCδ kinase activity (Fig. 4F). Based on these results, PGE2 appears to act through the EP1- and PKCδ-dependent signaling pathway to enhance ICAM-1 expression and cell migration in human oral cancer cells.
FIGURE 4.
PKCδ is involved in COX-2-induced migration and ICAM-1 production. A and B, SCC4 cells were pretreated for 30 min with GF109203X (3 μm) or rottlerin (3 μm) followed by stimulation with PGE2 (10 μm) for 24 h, and in vitro migration (A) and ICAM-1 mRNA expression (B) were measured after 24 h. C, inset, cells were transfected with PKCδ or control siRNA for 24 h, and the protein levels of PKCδ were determined by using Western blot analysis. C, cells were transfected with PKCδ siRNA or control siRNA for 24 h and then stimulated with PGE2 (10 μm) for 24 h. The in vitro migration was measured after 24 h. D, cells were incubated with PGE2 (10 μm) for the indicated time intervals, and p-PKCδ expression was examined by Western blot analysis. E and F, cells were incubated with PGE2 (10 μm) for indicated time intervals (E) or pretreated 30 min with SC19220 or transfected with EP1 siRNA for 24 h, followed by stimulation with PGE2 (10 μm) for 60 min, and PKCδ activity was determined by the PKCδ kinase kit (F). Results are expressed as the mean ± S.E. (error bars). *, p < 0.05 compared with control; #, p < 0.05 compared with PGE2-treated group.
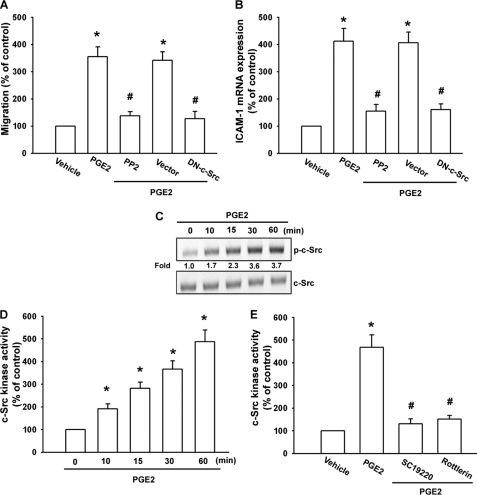
PKCδ-dependent c-Src activation is involved in the regulation of COX-2 expression (35). Therefore, we investigated the role of Src in mediating PGE2-induced ICAM-1 expression with the specific Src inhibitor PP2. As shown in Fig. 5, A and B, PGE2-induced cell migration and ICAM-1 expression was markedly attenuated by pretreatment of cells for 30 min with PP2 or transfected of cells for 24 h with c-Src mutant. The major phosphorylation site of c-Src at the Tyr416 residue results in activation from c-Src autophosphorylation (36). To confirm directly the crucial role of Src in cell motility, we measured the level of Src phosphorylation at Tyr416 in response to PGE2. As shown in Fig. 5C, treatment of SCC4 cells with PGE2 resulted in a time-dependent phosphorylation of c-Src at Tyr416. Next, we directly examined c-Src kinase activity in response to PGE2. Stimulation of cells with PGE2 also increased the kinase activity of c-Src in a time-dependent manner (Fig. 5D). To determine the relationship among EP1, PKCδ, and c-Src in the PGE2-mediated signaling pathway, we found that pretreatment of cells for 30 min with SC19220 and rottlerin markedly inhibited the PGE2-induced c-Src kinase activity (Fig. 5E). Based on these results, PGE2 appears to act through a signaling pathway involving EP1 receptors, PKCδ, and c-Src to enhance cell migration and ICAM-1 expression in oral cancer cells.
FIGURE 5.
c-Src is involved in PGE2-mediated migration and ICAM-1 expression in oral cancer cells. A and B, cells were pretreated for 30 min with PP2 (3 μm) or transfected for 24 h with c-Src mutant followed by stimulation with PGE2 for 24 h, and in vitro migration (A) and ICAM-1 mRNA expression (B) were measured after 24 h. C, cells were incubated with PGE2 for indicated the time intervals, and c-Src phosphorylation was examined by Western blotting. D and E, cells were incubated with PGE2 for the indicated time intervals (D) or pretreated 30 min with SC19220 or rottlerin for 30 min, followed by stimulation with PGE2 for 60 min, and c-Src kinase activity was determined by the c-Src kinase kit (E). Results are expressed as the mean ± S.E. (error bars). *, p < 0.05 compared with control; #, p < 0.05 compared with PGE2-treated group.
Involvement of AP-1 in COX-2-induced Cell Migration and ICAM-1 Expression
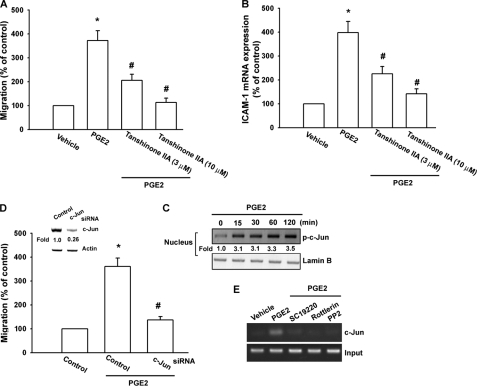
The promoter region of human ICAM-1 contains AP-1, NF-κB, CCAAT/enhancer-binding protein, and SP binding sites (37). AP-1 plays a critical role in ICAM-1 expression (38). To examine the role of the AP-1 binding site in PGE2-mediated ICAM-1 expression, an AP-1 inhibitor (tanshinone IIA) was used. Pretreatment of cells with tanshinone IIA reduced PGE2-induced cell migration and ICAM-1 expression (Fig. 6, A and B). It has been reported that the AP-1 binding site between −284 and −279 was important for the activation of the ICAM-1 gene (37). AP-1 activation was further evaluated by analyzing the accumulation of phosphorylated c-Jun in the nucleus as well as by the chromatin immunoprecipitation assay. Treatment of cells with PGE2 resulted in a marked accumulation of phosphorylated c-Jun in the nucleus (Fig. 6C). Transfection of cells with c-Jun siRNA suppressed the expression of c-Jun (Fig. 6D inset, upper panel). PGE2-induced cell migration was also inhibited by c-Jun siRNA but not by control siRNA (Fig. 6D inset, lower panel). We next investigated whether c-Jun binds to the AP-1 element on the ICAM-1 promoter after PGE2 stimulation. The in vivo recruitment of c-Jun to the ICAM-1 promoter (−346 to −24) was assessed by the chromatin immunoprecipitation assay (30). In vivo binding of c-Jun to the AP-1 element of the ICAM-1 promoter occurred after PGE2 stimulation (Fig. 6E). Binding of c-Jun to the AP-1 element by PGE2 was attenuated by SC19220, rottlerin, and PP2 (Fig. 6E). Taken together, these data suggest that activation of the EP1, PKCδ, c-Src, c-Jun, and AP-1 pathways is required for the PGE2-induced increase of cell migration and ICAM-1 expression in human oral cancer cells.
FIGURE 6.
AP-1 is involved in the potentiation of ICAM-1 expression by PGE2. A and B, cells were pretreated for 30 min with tanshinone IIA followed by stimulation with PGE2 for 24 h, and in vitro migration (A) and ICAM-1 mRNA expression (B) were measured after 24 h. C, cells were incubated with PGE2 for the indicated time intervals, and c-Jun phosphorylation in the nucleus was determined by Western blotting. D, inset, upper panel, cells were transfected with c-Jun or control siRNA for 24 h, and the protein levels of c-Jun were determined by using Western blot analysis. D, inset, lower panel, cells were transfected with c-Jun or control siRNA for 24 h and then stimulated with PGE2 for 24 h. The in vitro migration was measured after 24 h. E, cells were pretreated with SC19220, rottlerin, and PP2 and then stimulated with PGE2 for 120 min, and the chromatin immunoprecipitation assay was then performed. Chromatin was immunoprecipitated with anti-c-Jun. One percentage of the precipitated chromatin was assayed to verify equal loading (input). Results are expressed as the mean ± S.E. (error bars). *, p < 0.05 compared with control; #, p < 0.05 compared with the PGE2-treated group.
Increase of COX-2 and ICAM-1 Expression in Migration-prone Cells
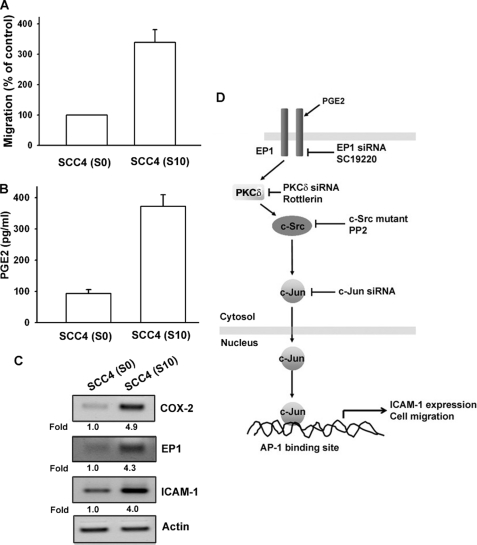
To confirm the COX-2 mediated cell migration and ICAM-1 expression in human oral cancer cells further, the higher cell mobility SCC4 sublines were used (28). In our previous report, we selected SSC4 sublines with higher cell mobility (28). We also found the a similar result with our previous report (28) that migration-prone subline SCC4-S10 had higher cell motility compared with original SCC4-S0 (Fig. 7A). Moreover, it was found that SCC4-S10 markedly increased the protein expression of PGE2 (Fig. 7B) or COX-2, EP1, and ICAM-1 (Fig. 7C). Therefore, human oral cancer cells with a higher tendency to migrate expressed more COX-2 and ICAM-1.
FIGURE 7.
Up-regulation of COX-2 and ICAM-1 expression in migration-prone cells. A, after 10 rounds of selection of SCC4 cells by cell culture insert system, the migration-prone subline (S10) exhibited more migration than original SCC4 cells (S0). B, S10 expressed more PGE2 in culture medium by ELISA than original SCC4 cells (S0). C, S10 expressed more COX-2, EP1, and ICAM-1 protein expression than original SCC4 cells (S0). Results are expressed as the mean ± S.E. (error bars). D, schematic of the signaling pathways involved in COX-2-induced migration and ICAM-1 expression of oral cancer cells is shown. COX-2 and EP1 interaction activates PKCδ and c-Src pathways, which in turn induces AP-1 activation, which leads to ICAM-1 expression and increases the migration of human oral cancer cells.
DISCUSSION
The elucidation of the molecular biology of cancer cells in recent years has identified various molecular pathways that are altered in different cancers. This information is currently being exploited to develop potential therapies that target molecules in these pathways. To achieve metastasis, cancer cells must evade multiple barriers and overcome certain rules. Several discrete steps are discernible in the biological cascade leading to metastasis: loss of cellular adhesion, increased motility and invasiveness, entry and survival into the circulation, entrance into new tissue, and eventual colonization of a distant site (15). The mechanism of metastasis is a complicated and multistage process; however, our study showed that COX-2/PGE2 promotes cell migration and the expression of ICAM-1 in human oral cancer cells. Here, we provide evidence that ICAM-1 acts as a crucial transducer of cell signaling, regulating cell migration, and COX-2 acts as a critical mediator of the metastasis activity of cancer cells in the tumor microenvironment. In addition, EP1, PKCδ, c-Src, and c-Jun inhibitor or siRNA reduced PGE2-mediated cell migration in the other oral cancer cell line HSC3 cells (supplemental Fig. S1). Furthermore, EP1, PKCδ, c-Src, and c-Jun inhibitor or siRNA also abolished PGE2-increased ICAM-1 expression in HSC3 cells (supplemental Fig. S1). Therefore, the same signaling pathways are involved in these two oral cancer cell lines. However, whether the same signaling pathways are involved in all oral cancer cells needs further examination. Using Western blot analysis, we found that the expression of COX-2 and ICAM-1 in human oral cancer tissues was significantly higher than in normal oral tissues. Therefore, these clinical results also confirm our in vitro data that the expression of COX-2 and ICAM-1 was associated with the migratory phenotype of oral cancer cells.
COX-2 is a pleiotropic enzyme that mediates many physiological functions such as inhibition of cell apoptosis, augmentation of angiogenesis, and increased cell motility. These COX-2-mediated functions are regulated in part by various proteins such as B-cell lymphoma (39), myeloid cell leukemia-1, VEGF-A (40), and metalloproteinases (41). However, the effect of COX-2 on migration activity in human oral cancer cells is mostly unknown. We found the expression of mRNA levels of COX-2 in oral cancer cells by qPCR analysis. Moreover, COX-2 and exogenous PGE2 increased migration of oral cancer cells. Our data provided the evidence that the expression of COX-2 is associated with a metastatic phenotype of oral cancer cells. We also examined the other PGE production after cells were transfected with IPTG-inducible COX-2 gene expression vector. By ELISA, we found that COX-2 also increased other PGE production ∼2-fold (PGD2, PGF2α, or PGI2; supplemental Fig. S2). However, COX-2 induced PGE2 production ∼5-fold (Fig. 1B). Therefore, PGE2 is much more important in COX-2-mediated cell migration in oral cancer cells. In this study, the 200-fold difference in PGE2 levels between that caused by COX-2 overexpression (550 pg/ml, which is ∼1.4 nm) led to significant cell migration and exogenous PGE2 (0.3 μm) required for inducing cell migration. However, we also found that COX-2 increased the other PGs (PGD2, PGF2α, and PGI2) production. Therefore, the other PGEs may also contributed COX-2-mediated cell migration. COX-2 exert it effects through interaction with specific EP1–EP4 receptors (11, 12). However, the expression of EP receptors in oral cancer cells is largely unknown. We found that the SCC4 cells expressed EP1–EP4 receptors. However, EP1 but not other EP receptors was required for PGE2-induced migration activity. Treatment with butaprost (EP2 agonist), sulprostone (EP3 agonist), and 11-deoxy-PGE1 (EP3 selective agonist) failed to up-regulate cell migration. To further rule out an effect of the EP4 receptor, EP4 siRNA was used. Compared with EP1 siRNA, EP4 siRNA did not affect PGE2-induced cell migration in SSC4 cells (supplemental Fig. S3). Therefore, an effect of the EP4 receptor can be ruled out. Our data thus suggest a critical role for EP1 receptor in PGE2-mediated cell migration in human oral cancer cells.
Several isoforms of PKC have been characterized at the molecular level and have been found to mediate several cellular molecular responses (42). We demonstrated that the PKC inhibitor GF109203X (at 3 μm dose used inhibited all PKC isoforms except ζ) antagonized the PGE2-mediated potentiation of cell migration and ICAM-1 expression, suggesting that PKC activation is an obligatory event in PGE2-induced motility in these cells. In addition, rottlerin also inhibited PGE2-induced migration and ICAM-1 expression. However, the current report indicates that rottlerin is not a specific PKCδ inhibitor but inhibits may other targets (43). Therefore, we used PKCδ siRNA to confirm PKCδ function in oral cancer cells. We found that PKCδ siRNA inhibited the enhancement of cell migration in oral cancer cells. Incubation of oral cancer cells with PGE2 also increased PKCδ phosphorylation and kinase activity. On the other hand, SC19220 and EP1 siRNA reduced PGE2-mediated PKC kinase activity. These data suggest that the EP1 and PKCδ pathways are required for PGE2-induced migration and ICAM-1 expression. On the other hand, we found that PKCδ siRNA did not affect leptin or adiponectin-induced cell migration in SCC4 cells (supplemental Fig. S4). Therefore, leptin or adiponectin induces cell migration in oral cancer cell through a PKCδ-independent pathway. Src, a tyrosine kinase, plays a critical role in the induction of chemokine transcription (44). Because c-Src is a downstream effector of PKCδ (35), we examined the potential role of c-Src in the signaling pathway PGE2-induced ICAM-1 expression. Treatment of cells with c-Src inhibitor PP2 or transfection of cells with c-Src mutant reduced PGE2-mediated cell migration and ICAM-1 expression. In addition, we also found that treatment of oral cancer cells with PGE2 induced increases in c-Src phosphorylation at Tyr416 and in c-Src kinase activity. These effects were inhibited by SC19220 and rottlerin, indicating the involvement of EP1, PKCδ-dependent c-Src activation in PGE2-mediated migration and ICAM-1 induction. Taken together, our results provide evidence that PGE2 up-regulates cell motility and ICAM-1 expression in human oral cancer cells via the EP1/PKCδ/c-Src signaling pathway.
There are several binding sites on the human ICAM-1 promoter for a number of transcription factors, including sites for binding AP-1, NF-κB, CCAAT/enhancer-binding protein, and SP (37). The results of this study show that AP-1 activation contributes to PGE2-induced migration and ICAM-1 production in oral cancer cells. The AP-1 sequence binds to members of the Jun and Fos families of transcription factors. These nuclear proteins interact with the AP-1 site as Jun homodimers or Jun-Fos heterodimers formed by protein dimerization through their leucine zipper motifs. The results of this study show that PGE2 induced c-Jun nuclear accumulation. In addition, c-Jun siRNA abolished the PGE2-induced cell migration in oral cancer cells. Furthermore, PGE2 also increased the binding of c-Jun to the AP-1 element on the ICAM-1 promoter, as shown by chromatin immunoprecipitation assay. Binding of c-Jun to the AP-1 element was attenuated by SC19220, rottlerin, and PP2. These results indicate that PGE2 and EP1 interaction might act through the PKCδ, c-Src, c-Jun, and AP-1 pathway to induce ICAM-1 activation in human oral cancer cells.
To conclude, we present a novel mechanism of COX-2-directed migration of oral cancer cells via up-regulation of ICAM-1 production. PGE2 increases cell migration and ICAM-1 expression by activation of EP1, PKCδ, c-Src, c-Jun, and AP-1-dependent pathway (Fig. 7D).
Supplementary Material
Acknowledgments
We thank Dr. S. Parsons for the c-Src mutant and Dr. M. L. Kuo for COX-2 IPTG-induced expression plasmid.
This work was supported by National Science Council of Taiwan Grant NSC-97-2314-B-040-025-MY3, China Medical University Grant CMU98-OC-02, China Medical University Hospital Cancer Research of Excellence Grant DOH99-TD-C-111-005, and by the Taiwan Department of Health.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- SCC
- squamous cell carcinoma
- ICAM-1
- intercellular adhesion molecule 1
- IPTG
- isopropyl-β-d-thiogalactopyranoside
- PG
- prostaglandin
- qPCR
- quantitative real-time PCR.
REFERENCES
- 1.Lyons A. J., Jones J. (2007) Int. J. Oral Maxillofac. Surg. 36, 671–679 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg J. S., El Naggar A. K., Mo V., Roberts D., Myers J. N. (2003) Cancer 98, 508–515 [DOI] [PubMed] [Google Scholar]
- 3.Thomas G. J., Speight P. M. (2001) Crit. Rev. Oral Biol. Med. 12, 479–498 [DOI] [PubMed] [Google Scholar]
- 4.Smith W. L., DeWitt D. L., Garavito R. M. (2000) Annu. Rev. Biochem. 69, 145–182 [DOI] [PubMed] [Google Scholar]
- 5.Warner T. D., Mitchell J. A. (2004) FASEB J. 18, 790–804 [DOI] [PubMed] [Google Scholar]
- 6.Morita I. (2002) Prostaglandins Other Lipid Mediat. 68–69, 165–175 [DOI] [PubMed] [Google Scholar]
- 7.Turini M. E., DuBois R. N. (2002) Annu. Rev. Med. 53, 35–57 [DOI] [PubMed] [Google Scholar]
- 8.Sano H., Kawahito Y., Wilder R. L., Hashiramoto A., Mukai S., Asai K., Kimura S., Kato H., Kondo M., Hla T. (1995) Cancer Res. 55, 3785–3789 [PubMed] [Google Scholar]
- 9.Hida T., Yatabe Y., Achiwa H., Muramatsu H., Kozaki K., Nakamura S., Ogawa M., Mitsudomi T., Sugiura T., Takahashi T. (1998) Cancer Res. 58, 3761–3764 [PubMed] [Google Scholar]
- 10.Hwang D., Scollard D., Byrne J., Levine E. (1998) J. Natl. Cancer Inst. 90, 455–460 [DOI] [PubMed] [Google Scholar]
- 11.Suzawa T., Miyaura C., Inada M., Maruyama T., Sugimoto Y., Ushikubi F., Ichikawa A., Narumiya S., Suda T. (2000) Endocrinology 141, 1554–1559 [DOI] [PubMed] [Google Scholar]
- 12.Weinreb M., Machwate M., Shir N., Abramovitz M., Rodan G. A., Harada S. (2001) Bone 28, 275–281 [DOI] [PubMed] [Google Scholar]
- 13.Watabe A., Sugimoto Y., Honda A., Irie A., Namba T., Negishi M., Ito S., Narumiya S., Ichikawa A. (1993) J. Biol. Chem. 268, 20175–20178 [PubMed] [Google Scholar]
- 14.Sugimoto Y., Namba T., Honda A., Hayashi Y., Negishi M., Ichikawa A., Narumiya S. (1992) J. Biol. Chem. 267, 6463–6466 [PubMed] [Google Scholar]
- 15.Gupta G. P., Massagué J. (2006) Cell 127, 679–695 [DOI] [PubMed] [Google Scholar]
- 16.Desgrosellier J. S., Cheresh D. A. (2010) Nat. Rev. Cancer 10, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makrilia N., Kollias A., Manolopoulos L., Syrigos K. (2009) Cancer Invest. 27, 1023–1037 [DOI] [PubMed] [Google Scholar]
- 18.Lawson C., Wolf S. (2009) Pharmacol. Rep. 61, 22–32 [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman T., Blanco F. J. (2008) Curr. Pharm. Des. 14, 2128–2139 [DOI] [PubMed] [Google Scholar]
- 20.Duperray A., Languino L. R., Plescia J., McDowall A., Hogg N., Craig A. G., Berendt A. R., Altieri D. C. (1997) J. Biol. Chem. 272, 435–441 [DOI] [PubMed] [Google Scholar]
- 21.Huang W. C., Chan S. T., Yang T. L., Tzeng C. C., Chen C. C. (2004) Carcinogenesis 25, 1925–1934 [DOI] [PubMed] [Google Scholar]
- 22.Rosette C., Roth R. B., Oeth P., Braun A., Kammerer S., Ekblom J., Denissenko M. F. (2005) Carcinogenesis 26, 943–950 [DOI] [PubMed] [Google Scholar]
- 23.Rozic J. G., Chakraborty C., Lala P. K. (2001) Int. J. Cancer 93, 497–506 [DOI] [PubMed] [Google Scholar]
- 24.Kozaki K., Koshikawa K., Tatematsu Y., Miyaishi O., Saito H., Hida T., Osada H., Takahashi T. (2001) Oncogene 20, 4228–4234 [DOI] [PubMed] [Google Scholar]
- 25.Fulton A. M., Ma X., Kundu N. (2006) Cancer Res. 66, 9794–9797 [DOI] [PubMed] [Google Scholar]
- 26.Su J. L., Shih J. Y., Yen M. L., Jeng Y. M., Chang C. C., Hsieh C. Y., Wei L. H., Yang P. C., Kuo M. L. (2004) Cancer Res. 64, 554–564 [DOI] [PubMed] [Google Scholar]
- 27.Fieck A., Wyborski D. L., Short J. M. (1992) Nucleic Acids Res. 20, 1785–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang J. Y., Yang W. H., Chen H. T., Huang C. Y., Tan T. W., Lin Y. T., Hsu C. J., Fong Y. C., Tang C. H. (2009) J. Cell. Physiol. 220, 418–426 [DOI] [PubMed] [Google Scholar]
- 29.Tang C. H., Tan T. W., Fu W. M., Yang R. S. (2008) Carcinogenesis 29, 35–43 [DOI] [PubMed] [Google Scholar]
- 30.Huang W. C., Chen C. C. (2005) Mol. Cell. Biol. 25, 6592–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedorov Y., Anderson E. M., Birmingham A., Reynolds A., Karpilow J., Robinson K., Leake D., Marshall W. S., Khvorova A. (2006) RNA 12, 1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokuda H., Hosoi T., Hayasaka K., Okamura K., Yoshimi N., Kozawa O. (2009) Horm. Metab. Res. 41, 333–338 [DOI] [PubMed] [Google Scholar]
- 33.Page K., Li J., Zhou L., Iasvovskaia S., Corbit K. C., Soh J. W., Weinstein I. B., Brasier A. R., Lin A., Hershenson M. B., Iasvoyskaia S. (2003) J. Immunol. 170, 5681–5689 [DOI] [PubMed] [Google Scholar]
- 34.Basu A., Adkins B., Basu C. (2008) Cancer Res. 68, 2795–2802 [DOI] [PubMed] [Google Scholar]
- 35.Hsieh H. L., Sun C. C., Wang T. S., Yang C. M. (2008) Biochim. Biophys. Acta 1783, 1563–1575 [DOI] [PubMed] [Google Scholar]
- 36.Roskoski R., Jr. (2005) Biochem. Biophys. Res. Commun. 331, 1–14 [DOI] [PubMed] [Google Scholar]
- 37.van de Stolpe A., van der Saag P. T. (1996) J. Mol. Med. 74, 13–33 [DOI] [PubMed] [Google Scholar]
- 38.Roebuck K. A. (1999) Int. J. Mol. Med. 4, 223–230 [DOI] [PubMed] [Google Scholar]
- 39.Sun Y., Tang X. M., Half E., Kuo M. T., Sinicrope F. A. (2002) Cancer Res. 62, 6323–6328 [PubMed] [Google Scholar]
- 40.Ma L., del Soldato P., Wallace J. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13243–13247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dohadwala M., Batra R. K., Luo J., Lin Y., Krysan K., Pold M., Sharma S., Dubinett S. M. (2002) J. Biol. Chem. 277, 50828–50833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berk B. C., Taubman M. B., Cragoe E. J., Jr., Fenton J. W., 2nd, Griendling K. K. (1990) J. Biol. Chem. 265, 17334–17340 [PubMed] [Google Scholar]
- 43.Leitges M., Elis W., Gimborn K., Huber M. (2001) Lab. Invest. 81, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 44.Yeh M., Gharavi N. M., Choi J., Hsieh X., Reed E., Mouillesseaux K. P., Cole A. L., Reddy S. T., Berliner J. A. (2004) J. Biol. Chem. 279, 30175–30181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.