Abstract
MicroRNAs (miRNAs) are short, non-coding RNAs that target and silence protein coding genes through 3′-UTR elements. Evidence increasingly assigns an immunosuppressive role for miRNAs in immunity, but relatively few miRNAs have been studied, and an overall understanding of the importance of these regulatory transcripts in complex in vivo systems is lacking. Here we have applied multiple technologies to globally analyze miRNA expression and function in allergic lung disease, an experimental model of asthma. Deep sequencing and microarray analyses of the mouse lung short RNAome revealed numerous extant and novel miRNAs and other transcript classes. Similar to mRNAs, lung miRNA expression changed dynamically during the transition from the naive to the allergic state, suggesting numerous functional relationships. A possible role for miRNA editing in altering the lung mRNA target repertoire was also identified. Multiple members of the highly conserved let-7 miRNA family were the most abundant lung miRNAs, and we confirmed in vitro that interleukin 13 (IL-13), a cytokine essential for expression for allergic lung disease, is regulated by mmu-let-7a. However, inhibition of let-7 miRNAs in vivo using a locked nucleic acid profoundly inhibited production of allergic cytokines and the disease phenotype. Our findings thus reveal unexpected complexity in the miRNAome underlying allergic lung disease and demonstrate a proinflammatory role for let-7 miRNAs.
Keywords: Cytokine, Immunology, Inflammation, MicroRNA, RNA Modification
Introduction
MicroRNAs (miRNAs)3 are short regulatory RNAs with the potential to target and suppress multiple genes across diverse signaling pathways comprising biologically meaningful networks. Consequently, miRNAs that are inappropriately expressed in the context of specific diseases are of particular interest as therapeutic targets if they can be shown to coordinate such networks. Initially transcribed as relatively long primary transcripts, pri-miRNAs are subsequently modified by the nuclear RNAses Drosha and Pasha to yield precursor miRNAs (pre-miRNAs) that are further processed by the cytoplasmic RNase III Dicer to form short double-stranded miR-miR* duplexes, one strand of which (miR) is then integrated into the RNA-induced silencing complex that includes the enzymes Dicer and Argonaute (Ago). The mature miRNAs (∼17–24 nt) direct the RNA-induced silencing complex to specific target sites located within the 3′-UTR of target genes. Once bound to target sites, miRNAs repress translation through mRNA decay, translational inhibition, and/or sequestration into processing bodies (1–3).
Recent estimates indicate that over 60% of protein-coding genes carry 3′-UTR miRNA target sites, suggesting a role for miRNAs in the control of gene expression in diverse processes (4). Indeed, miRNAs have now been firmly linked to the regulation of early development (5), cell proliferation, and cell death (6); apoptosis and fat metabolism (7); and cell differentiation (8). In addition, studies of miRNA expression in chronic lymphocytic leukemia (9), colonic adenocarcinoma (10), Burkitt lymphoma, and cardiac disease (11) link miRNAs to cancer and numerous other diseases.
Emerging evidence further suggests an important and ancient role for miRNAs in the regulation of immunity. MicroRNAs have been implicated in antiviral defense in organisms ranging from insects to mice (12, 13). Conversely, viruses have exploited the use of host miRNAs to subvert antiviral immunity by manipulating key immune molecules (14). Surprisingly, deletion of all miRNAs does not ablate development of adaptive immune cells, such as T cells. However, mature miRNAs are generally required to preserve T cell-dependent immune homeostasis and to preclude the development of spontaneous inflammation in mice (15).
Analyses of individual miRNAs further confirm important regulatory relationships to specific immune targets. NF-κB is controlled by miR-146a while simultaneously inducing miR-9, which further contributes to the fine regulation of this transcription factor (16, 17). Numerous targets in the Toll-like receptor (TLR) signaling pathway have further been identified as targets of miR-146a, including TRAF6, IRAK1, and IRAK2 (18). Moreover, miR-147 is induced by TLR2, TLR3, and TLR4, which in turn feeds back to inhibit production of proinflammatory cytokines from macrophages (19). Moreover, miR-155, which plays a general role in host defense and dendritic cell function (20), plays a key anti-inflammatory role by suppressing molecular targets required for inflammation (21) and T regulatory cell function (22). Thus, diverse approaches to dissecting miRNA function suggest a general role for miRNAs is suppressing inflammatory reactions.
Despite these insights, global perspectives defining the potential regulatory role of miRNAs in complex inflammatory processes are lacking. One such inflammatory disease is asthma, a type of allergic lung disease. The allergic lung diseases comprise a clinically heterogeneous group of disorders that, relative to other chronic diseases, afflict a disproportionately large number of persons with transient but potentially fatal airway obstruction (23). Airway obstruction in asthma is observed in the context of local and systemic allergic inflammation that includes elevated total allergen-specific IgE levels and increased numbers of eosinophils and T helper type 2 (Th2) cells that secrete interleukin (IL)-4, IL-5, IL-6, IL-9, and IL-13 (24). Based on experimental studies of rodents, airway obstruction in the setting of allergic lung disease is largely mediated by the Th2 cytokines IL-4 and IL-13 (25, 26). However, whereas IL-4 acts as a growth factor for Th2 cells and IgE-secreting B cells, IL-13 acts on target lung tissues to induce airway hyperreactivity, a physiological hallmark of airway obstruction in asthma (27).
Given the numerous intersecting immune pathways that govern the expression of allergic disease, we hypothesized that miRNAs regulate key signaling molecules to limit the expression of allergic lung disease. Using next generation sequencing (NGS) and microarray analyses combined with detailed bioinformatics, we compared short lung transcripts from naive and asthmatic mice to gain a genome-wide perspective on the potential role of miRNAs in allergic lung disease. We further inhibited prominent miRNAs in vivo to deduce their overall function in this disease model.
EXPERIMENTAL PROCEDURES
Mice and Allergen Challenge
All experiments were performed in accordance with institutional and National Institutes of Health guidelines. Allergen challenge of C57BL/6 mice was performed with an allergenic fungal proteinase and ovalbumin as described previously (28).
Preparation of Short RNA Transcripts for Illumina Sequencing
Short RNA transcripts of <60 nucleotides in length were gel-purified after running 10 μg of total RNA extracted from whole mouse lung on 15% TBE-urea polyacrylamide gels. A synthetic 26-residue adapter RNA oligonucleotide (5′-GUU CAG AGU UCU ACA GUC CGA CGA UC-3′) was ligated to the 5′-end of the small RNAs. The ligated small RNA was gel-purified to remove unligated free adapter. A synthetic 22-residue 3′-adapter with inverted dideoxythymidine (idT) added at the 3′-end (5′-p UCG UAU GCC GUC UUC UGC UUG idT-3′) was ligated to the 5′-ligated small RNA and gel-purified. The resultant RNA library was reverse transcribed and amplified by PCR for 15 cycles using adapter-specific primers. The PCR products were sequenced using Illumina (Solexa)-based next generation sequencing. One mouse lung from each group was used for deep sequencing.
Small RNA Mapping and Classification
After filtering for the Illumina small RNA adapter sequences, the reads were mapped to the reference mouse genome (NCBI Build 37, UCSC mm9) using the Pash software package as described previously (29).
Novel miRNA Discovery
All small RNA sequences that failed to align with a known miRNA, Piwi-interacting RNA (piRNA), or short nucleolar RNA were passed through a novel miRNA discovery platform, as described in the supplemental material.
Microarray Analyses
The Illumina Sentrix Universal-12 Mouse v2 Gene Expression BeadChip Array (45,281 transcripts) was used for gene profiling, and the Illumina Mouse v2 MicroRNA Expression BeadChip Array (611 miRNAs) was used for miRNA profiling. The gene array data generated were quantile-normalized (using software kindly provided by Dr. Kerby Shedden). Significantly regulated genes and miRNAs were identified by comparing allergen-challenged with naive lungs using Student's t test (log-transformed data) and -fold change (ratio of averages of the two groups). Java TreeView (30) represented expression patterns as color maps, where gene and miRNA values were centered on the median expression of the naive group.
Isolation, Culture, and Transfection of CD4+ T Cells from Spleen
Mouse spleens were collected, and CD4 T cells were isolated by immunomagnetic selection. Th1 and Th2 cells were differentiated as described previously (26).
Nucleofection of in vitro anti-let-7a locked nucleic acids (LNAs) in to CD4 T cells was performed by using the mouse T cell Nucleofector kit (Lonza, Walkersville, MD) according to the manufacturer's protocol, and the cells were cultured for 48 h. 80 and 240 pmol of anti-let-7a LNAs and 240 pmol of scrambled LNA were used for transfection. For determining the efficiency of transfection, cells that were transfected with fluorescein-labeled LNAs were nucleofected into CD4 T cells and analyzed by flow cytometry after 48 h. For RNA extraction, cells were homogenized in TRIzol, and total RNA was isolated using the miRNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's protocol.
In Vitro Validation
HEK293T cells were used for co-transfection of plasmids expressing miRNAs, 3′-UTR of target genes, and anti-miRNA or control LNAs. Briefly, HEK293T cells that were cultured in 24-well plates were co-transfected with plasmids expressing il-13 3′-UTR (350 ng) or control 3′-UTR (350 ng) and/or mouse/human let-7a (350, 117, 39 ng) or mouse let-7a(U→G) (350 ng) or mouse miR-705 (350 ng) or scrambled miRNA (350 ng) and/or mouse/human anti-let-7a LNA (52.5, 17.5, and 5.8 pmol) or scrambled LNA (52.5 pmol) or anti-miR-705 LNA (52.5 pmol) or mouse anti-let-7e (let-7a(U→G)) LNA (52.5 pmol). Lipofectamine 2000 (Invitrogen) was used as a transfection reagent according to the manufacturer's protocol. Firefly and Renilla luciferase light units were measured after 2 days of co-transfection by using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) with the help of a FLOU star OPTIMA microplate reader (Bmg Labtech, Cary, NC). The H1 promoter was used to express pre-miRNAs, and the SV40 promoter was used to express target 3′-UTRs.
In Vivo Transfection and Allergy Induction
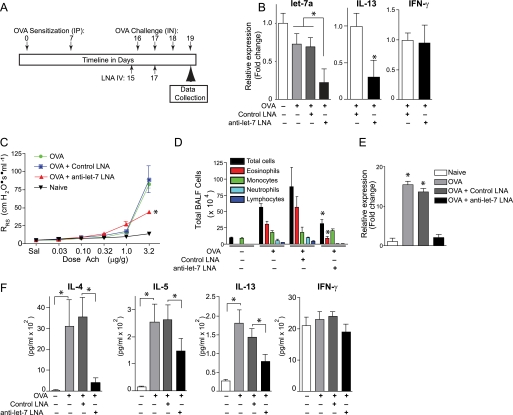
For in vivo LNA experiments, female BALB/c mice between 5 and 8 weeks were used. Mice were sensitized with 50 μl of chicken ovalbumin and alum by intraperitoneal injection twice (days 0 and 7) at 1-week intervals. On days 15 and 17, LNAs prepared in 0.9% saline were injected intravenously into mice through the tail vein. On days 16, 17, and 18 mice were intranasally challenged with chicken ovalbumin (25 mg in 50 ml of PBS) before analysis on day 19 (Fig. 5A).
FIGURE 5.
let-7 miRNAs are required for allergic lung disease. A, protocol timeline for ovalbumin (OVA) immunization intraperitoneally (IP) and challenge intranasally (IN) and anti-miRNA LNA administration intravenously (IV). B, anti-let-7 LNA suppresses T cell let-7 and il-13 in vivo. Quantitative RT-PCR analysis of let-7a, il-13, and ifn-γ transcripts in splenic CD4 T cells from mice treated under the indicated conditions. C, airway responsiveness as assessed by the change in respiratory system resistance (RRS) in response to graded intravenous acetylcholine (Ach) challenge. *, p < 0.05 relative to naive or ovalbumin or ovalbumin + control LNA groups. D, total bronchoalveolar lavage fluid (BALF) inflammatory cells (eosinophils, macrophages, neutrophils, lymphocytes, total cells). E, relative expression of Muc5AC transcripts from whole lung. *, p < 0.05 relative to naive. F, bronchoalveolar lavage fluid levels of the indicated cytokines. *, p < 0.05 for the indicated comparisons. Data are presented as means ± S.E. (error bars), n = 5 mice/group.
Quantitation of Allergic Lung Disease
24 h after the final allergen challenge, the allergic lung disease phenotype was analyzed as described previously (28).
Quantitative PCR
Quantitative PCR of miRNAs and mRNAs were performed by using Taqman miRNA expression and gene expression assays, respectively (Applied Biosystems, Foster City, CA). PCR data were analyzed by using the ΔΔCt method of relative quantification. For microRNA expression, either snoRNA202 or RNU48 were used as endogenous controls, and for gene expression, GAPDH was used as the endogenous control.
Statistical Analysis
For all statistical analyses, analysis of variance with post hoc Tukey tests or t-tests were used. Statistical significance were calculated with p value <0.05 (see also supplemental material).
RESULTS
miRNAs Dominate the Lung Short RNAome and Several Are Edited to Alter the Target Repertoire
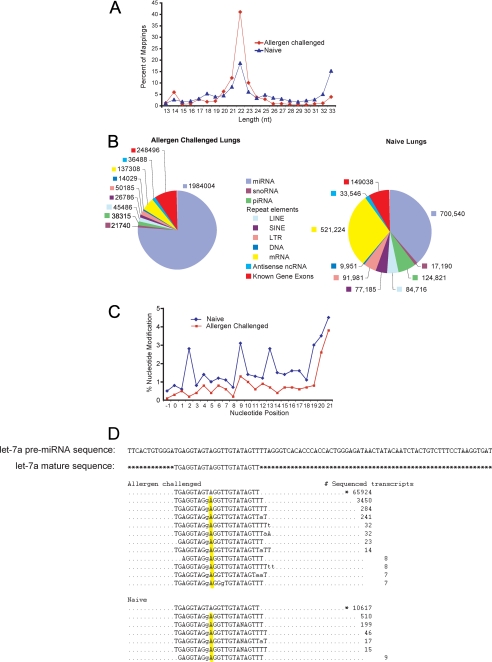
We characterized the lung short RNAomes of naive and allergen-challenged mice using the Genboree platform for mapping NGS data from the <60-nucleotide lung RNA fraction derived under each condition. This analysis revealed significant differences in the length distribution of small RNAs in naive and allergen-challenged lungs (Fig. 1A). The 21–23-nt RNA fraction was highly enriched in allergic as compared with naive lung, whereas in the latter, an increase in the 31–33-nt small RNA fraction was observed. miRNAs numerically dominated the short RNAome of both naive and allergen-challenged animals, but numerous additional transcript classes were detected (Fig. 1B). Of particular interest were the piRNAs, which previously were believed to be expressed only in haploid (gonadal) tissues of mammals (31).
FIGURE 1.
Characterization and distribution of small RNAs in mouse lung. A, frequency of NGS-derived sequences as a function of nucleotide length. The 21–23-nt peak is typical for miRNAs. B, pie charts show absolute numbers of sequenced transcripts from distinct lung RNA classes comparing allergen-challenged with naive mice. C, distribution of nucleotide modifications along the length of mature lung miRNAs comparing allergen-challenged with naive mice. D, editing of mmu-let-7a-1 as detected by NGS comparing allergen challenged with naive lungs in which the ninth nucleotide of the seed sequence, U, has been modified to G. *, canonical mature sequence. Also see supplemental Fig. 1 and Tables S1–S5.
NGS identified a total of 405 distinct miRNAs (>10 copies each) in naive and 328 miRNAs in allergen-challenged mice (supplemental Tables 1 and 2). let-7 family miRNAs were dominant, comprising 58 and 64% of total lung miRNAs from naive and allergen-challenged lungs, respectively (supplemental Tables 1 and 2). Among these, let-7f was most abundant in both naive and allergen-challenged lungs.
Upon mapping of sequences to the miRBase-14.0 pre-miRNA data base, allowing for 1–4 mismatches in the aligned reads to a given pre-miRNA, we detected several miRNAs that were post-transcriptionally modified (edited) in at least one position of the seed sequence. The distributions of nucleotide changes in relation to position for all miRNAs in naive and allergen-challenged lungs are shown in Fig. 1C and supplemental Tables 3 and 4. When the normalized numbers of nucleotide modifications were compared, the miRNA mmu-miR-101a showed a 10% increase in the number of 8th nucleotide modifications from C to U in naive lungs as compared with allergen-challenged. Using the TargetScan 5.1 algorithm, we found that the target repertoire of the modified miR-101a species had been redirected to be identical to that of mmu-miR-144. Among several predicted changes in the target repertoire, this edit potentially enhances affinity for several allergy-related genes, including gata and cd28 (32, 33). However, in keeping with our prior observations from pancreatic tissue and mouse ovary (34), post-transcriptional modifications were particularly common in the let-7 family of microRNAs. The most common such modification was a U to G change at position 9 (let-7a(9U→G)), which was detected by comparison of let-7a sequences with pre-mmu-let-7a (Fig. 1D). This post-transcriptional modification effectively converts let-7a to let-7e, which largely shares the same targets (TargetScan 5.1). Thus, post-transcriptional editing of multiple lung miRNAs occurs, potentially altering the target repertoire for some miRNAs.
Using a novel miRNA discovery bioinformatics platform (35), we further identified 25 putative novel miRNAs from naive and allergen-challenged lungs (supplemental Tables 5a and 5b). Two miRNAs, asth-miR-1 and asth-miR-2, were highly expressed in naive lung and possibly down-regulated following allergen challenge (supplemental Fig. S1).
Identification of Relevant miRNA-mRNA Functional Pairs
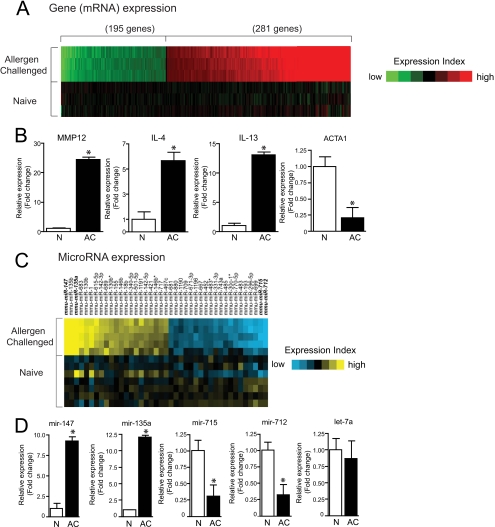
Deep sequencing is capable of identifying and enumerating both known and novel miRNAs as well as other classes of short transcripts, but the sensitivity of this technique for detecting and quantitating all known transcripts in complex samples, such as lungs, remains unknown. To circumvent this potential limitation of NGS, we also performed mRNA and miRNA microarray analyses using total RNA from naive and allergen-challenged mouse lungs and validated findings for selected genes using quantitative PCR (Fig. 2). A total of 195 genes were up-regulated, and 281 genes were down-regulated in allergen-challenged lungs relative to naive lungs (Fig. 2A). The complete listing of all mRNA changes is summarized in supplemental Table 6. In addition to numerous immunoglobulin genes, the most highly induced genes included ear11, an eosinophil-associated ribonuclease (36), gob-5 (clca3), a gene with uncertain function linked to allergic disease (37), ym2 (chi3l4), a chitinase-like molecule that is induced by IL-4 (38), and mmp12 (matrix metalloproteinase 12), an IL-13-inducible proteinase that is required for allergen-induced airway eosinophilia (39). Enhanced expression of il-4 and other Th2 cytokine transcripts was also detected in allergen-challenged lungs as expected, with the notable exception of il-13.
FIGURE 2.
Gene and miRNA expression profiling of allergen-challenged and naive mouse lungs. A, heat map of genes (mRNAs) induced or repressed (p < 0.01, >1.5-fold) in allergen-challenged versus naive lung. B, validation of gene microarray findings by quantitative RT-PCR for selected genes. C, heat map of miRNAs induced or repressed (p < 0.01, >1.5-fold) in allergen-challenged versus naive lung. D, validation of miRNA microarray findings by quantitative RT-PCR for selected miRNAs. Bar graph data are presented as means ± S.E. (error bars), n = 3; *, p < 0.05. Also see supplemental Tables S6 and S7.
Conversely, genes that were most prominently down-regulated with allergen challenge included contractile proteins (α1 actin (acta1) and troponin C (tnnc2)), chemokines (cxcl14), arntl (bmal1) (a CLOCK-associated gene linked to glucose metabolism) (40), ifitm6 (fragilis5), and lysozyme. Quantitative RT-PCR analysis of selected genes validated mRNA transcripts that were either up- or down-regulated (Fig. 2B). In contrast to microarray results, il-13 transcripts were clearly markedly enhanced by allergen challenge as assessed by quantitative RT-PCR (Fig. 2B). Moreover, the enhanced presence of both il-13 transcript and protein in allergic lungs has been repeatedly documented (25, 26, 41–43), indicating that the inability to detect this transcript by microarray was spurious. These studies thus confirm that numerous allergy-related genes are up-regulated in lungs following allergen challenge.
Microarray analyses further identified numerous miRNAs that were significantly up- and down-regulated with allergen challenge (Fig. 2C and supplemental Table 7). Expression of the most abundant miRNA transcripts, most notably let-7 miRNAs, did not change with allergen challenge. Quantitative RT-PCR again verified trends in expression of selected miRNAs that changed significantly, and we confirmed that let-7a transcripts were not altered by allergen challenge (Fig. 2D). Based on Targetscan 5.1 predictions, we identified numerous miRNAs from these analyses that putatively target genes of relevance to the asthma phenotype (Table 1). For example, a potential target of miR-135a, which was significantly up-regulated in asthmatic mice, is stat6 (signal transducer and activator of transcription 6), a transcription factor that is required for Th2 responses and experimental asthma (44).
TABLE 1.
Lung miRNAs and potential targets with relevance to allergic disease
| MicroRNA | Target gene | Gene name | Context scorea |
|---|---|---|---|
| mmu-miR-712 | gata3 | GATA-binding protein 3 | −0.12 |
| mmu-miR-699 | stat6 | Signal transducer and activator of transcription 6 | −0.26 |
| mmu-miR-743a | il13ra1 | Interleukin 13 receptor, α1 | −0.4 |
| mmu-miR-1196 | gata3 | GATA-binding protein 3 | −0.33 |
| mmu-miR-709 | cd4 | CD4 | −0.23 |
| mmu-miR-717 | adrb2 | Adrenergic, β2, receptor | −0.33 |
| mmu-miR-142–5p | jak1 | Janus kinase 1 | −0.16 |
| mmu-miR-340–5p | il-4 | Interleukin 4 | −0.25 |
| mmu-miR-340–5p | jak1 | Janus kinase 1 | −0.26 |
| mmu-miR-146b | irak1 | Interleukin-1 receptor-associated kinase 1 | −0.91 |
| mmu-miR-135a | stat6 | Signal transducer and activator of transcription 6 | −0.45 |
| mmu-let-7 | il-13 | Interleukin 13 |
a Derived from TargetScan 5.1.
il-13 Is a Target Gene of let-7a
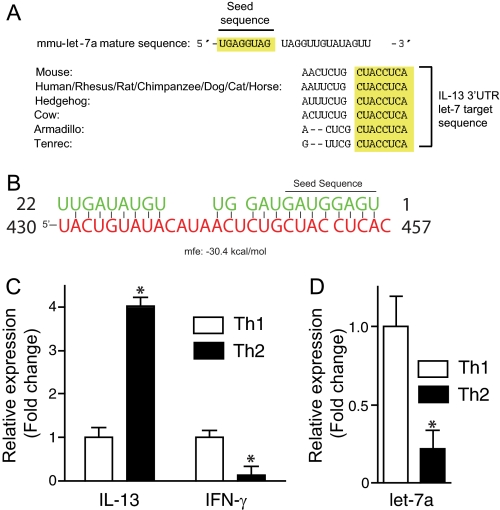
We focused subsequent efforts on the abundant and extremely conserved let-7 miRNA family, the function of which in mammals remains largely undefined. The let-7 family target recognition sequence in the il-13 3′-UTR is highly conserved across mammalian species (Fig. 3A). Moreover, all mouse let-7 miRNAs (mmu-let-7a-i; mmu-miR-98) are predicted to target il-13 (TargetScan 5.1). To verify this, we first folded the mature let-7a-1 miRNA sequence against the mouse il-13 3′-UTR target sequence. This comparison revealed a high degree of complementarity characterized by a very low mean free energy value of −30.4 kcal/mol (Fig. 3B).
FIGURE 3.
Inverse expression of il-13 and let-7a suggests a functional association. A, the let-7a target sequence in the il-13 3′-UTR is conserved across mammalia (Targetscan 5.1). B, mature let-7a sequence (green) aligned with the mouse il-13 3′-UTR target site (red) and predicted minimum free energy (mfe) value. The let-7a seed sequence is shown. C and D, quantitative RT-PCR analysis of il-13 and ifn-γ (C) and mmu-let-7a (D) transcripts from in vitro cultured Th1 and Th2 cells. Data are presented as means ± S.E. (error bars), n = 3; *, p < 0.05.
Lung il-13 transcripts were markedly enhanced with allergen challenge (Fig. 2B), whereas total lung let-7a transcripts did not change (Fig. 2D), which failed to support a functional relationship between il-13 and let-7a. However, Th2 cells are the predominant source of lung IL-13 following allergen challenge and represent a small (0.01–0.1%) fraction of total lung cells following allergen challenge in this model. We therefore quantitated both il-13 and mmu-let-7a transcripts in Th2 cells derived from naive mouse CD4 T cells. Similar to lung, let-7 miRNAs were the most abundant miRNA transcripts in T helper cells.4 As expected, il-13 transcripts were markedly enhanced, whereas interferon γ (ifn-γ) transcripts were suppressed in Th2 relative to Th1 cells (Fig. 3C), but in contrast to lung, mmu-let-7a transcripts were markedly suppressed in Th2 cells, an inverse association with il-13 that did suggest a functional interaction (Fig. 3D).
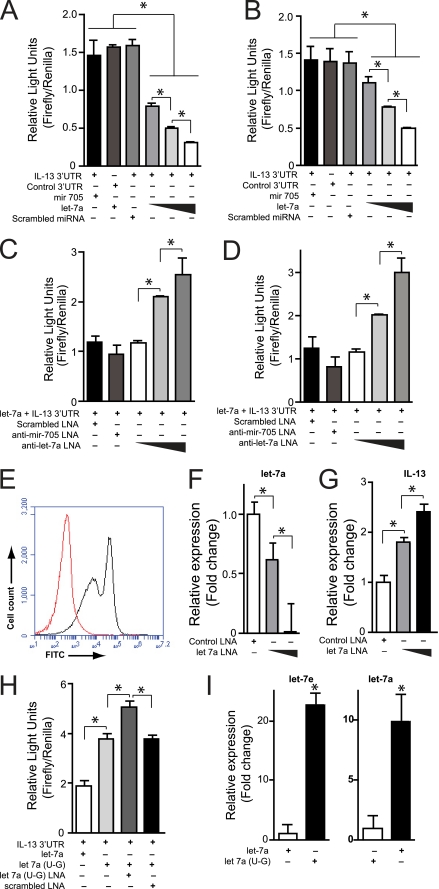
To determine if il-13 is a genuine target of mmu-let-7a, we co-transfected into HEK293T cells plasmids expressing the pre-miRNA for mmu-let-7a and a luciferase gene containing the il-13 3′-UTR. In a dose-dependent manner, mmu-let-7a suppressed luciferase production, whereas neither a scrambled miRNA nor an irrelevant miRNA (miR-705) had any effect (Fig. 4A). We further transfected into these cells scrambled or anti-let-7a LNAs (45) representing the entire reverse complement of mmu-let-7a. Again in a dose-dependent manner, anti-let-7a LNAs progressively reversed the suppressive effect of mmu-let-7a on luciferase production (Fig. 4B). Identical experiments were performed using the human IL-13 3′-UTR, human LET-7a (HSA-LET-7a, which is identical to mmu-let-7a) and the same LNAs and produced identical results (Figs. 4, C and D). Together, these studies indicated that both human and mouse il-13 are targets of let-7a and that this miRNA can be specifically inhibited by an LNA.
FIGURE 4.
IL-13 expression is suppressed by let-7a. A and B, let-7a suppresses mouse and human IL-13 in HEK293T cells. HEK293T cells were transfected with plasmids containing firefly luciferase under the control of the mouse (A) or human (B) il-13 3′-UTR or control 3′-UTR and simultaneously with plasmids expressing pre-mmu-miR-705, scrambled pre-miR, or pre-let-7a, as indicated. After 2 days, gene expression was quantitated as firefly relative light units after normalizing for transfection efficiency based on Renilla luciferase activity (Firefly/Renilla). C and D, anti-let-7a rescues mouse IL-13 expression. HEK293T cells were transfected simultaneously with mouse (C) or human (D) IL-13 3′-UTR and pre-mmu-let-7a plasmids as in A and additionally scrambled, irrelevant (anti-miR-705) or anti-let-7a locked nucleic acids. After 2 days, IL-13 expression was assessed as firefly/Renilla relative light units. E–G, let-7a suppresses IL-13 gene expression in primary T cells. E, mouse splenic CD4+ T cells were electroporated with FITC-labeled anti-let-7a LNAs (black curve) or sham (red curve), and the efficiency of transfection was assessed by flow cytometry. Additional T cells were transfected with control or anti-let-7a LNA, and relative expression of let-7a (F) and IL-13 (G) transcripts were determined by quantitative RT-PCR 24 h later. H, editing of let-7a to let-7e reduces efficiency of targeting of il-13. HEK293T cells were transfected with mouse IL-13 3′-UTR-containing luciferase plasmid, as in A, and either plasmids for expression of let-7a or edited let-7a(U→G) and either scrambled or anti-let-7a(U→G) LNA, as indicated, and the effect on il-13 gene expression was assessed as relative light units. I, pre-let-7a(U→G) is fully processed to let-7e. Quantitative RT-PCR quantitation of let-7e or let-7a in HEK293T cells transfected with either pre-let-7a or pre-let-7a(U→G) expression plasmids. Data are presented as means ± S.E. (error bars), n = 3 or 4 replicates/condition; *, p < 0.05 for the indicated comparisons.
We next confirmed these findings in primary murine CD4+ T cells. The majority (>80%) of T helper cells could be transfected with anti-let-7a LNA (Fig. 4E), which by quantitative RT-PCR reduced let-7a transcripts >90% at the highest LNA dose given (Fig. 4F). This was accompanied by a 2.5-fold greater increase in CD4 T cell il-13 transcripts following activation (Fig. 4G). Together, these findings confirm that il-13 is regulated by let-7a and demonstrate the utility of LNAs for the specific inhibition of miRNAs in primary T cells.
Finally, we used this in vitro system to compare native let-7a and let-7a(9U→G) for their ability to silence il-13 expression. Despite having identical affinities for the il-13 3′-UTR recognition site (Targetscan 5.1), let-7a(9U→G) (let-7e) was less efficient in suppressing il-13 expression relative to let-7a (Fig. 4H). The let-7a(9U→G) pre-miRNA as used in these studies is not identical to the let-7e pre-miRNA, raising the possibility that the let-7a(9U→G) pre-miRNA was not properly processed into mature let-7e. However in separate transfection experiments, we confirmed by quantitative RT-PCR that mature let-7e was fully processed from the let-7a(9U→G) pre-miRNA, as was mature let-7a from let-7a pre-miRNA (Fig. 4I). Thus, editing of let-7a to let-7a(9U→G) creates let-7e, which is less efficient at suppressing il-13 expression.
Proinflammatory Role of let-7 miRNAs in Vivo
In addition to il-13, let-7 miRNAs are predicted to inhibit other genes of interest in asthma, including the β2-adrenergic receptor (adrb2), a catecholamine receptor that is required for expression of experimental allergic lung disease (46, 47). However, the entire let-7 miRNA family is predicted to regulate over 800 conserved targets (TargetScan 5.1). We therefore reasoned that the overall in vivo function of mmu-let-7a (or indeed any miRNA) cannot alone be predicted from in silico analysis of the target repertoire even combined with knowledge of individually validated targets. Thus, to begin to assess overall function of let-7 miRNAs in vivo, we systemically administered to allergen immunized mice either a scrambled or an anti-let-7 LNA that is the reverse complement of the first 14 nucleotides (5′) of let-7a, -b, -c, and -d. LNAs were administered before intranasal allergen challenge but after allergen sensitization to determine their effect on the effector phase of the disease (Fig. 5A). We first determined the specificity of this in vivo protocol and confirmed that anti-let-7 LNA but not a scrambled LNA reduced let-7a transcripts in splenic CD4 T cells (Fig. 5B). However, unlike the immediate effect of anti-let-7a on T cells transfected in vitro (Fig. 4, F and G), after 3 days of allergen challenge in vivo, splenic CD4 T cell il-13 transcripts were reduced, whereas transcripts of an unrelated gene, ifn-γ, were unaffected (Fig. 5B).
The discrepancy in expression of the same target gene observed with immediate (Fig. 4) and delayed (Fig. 5) administration of an anti-let-7 LNA was unexpected and suggested that secondary or even tertiary effects of let-7 inhibition arise over time in vivo to suppress inflammatory gene expression. To determine if this anti-inflammatory effect is physiologically significant, we next determined the effect of anti-let-7 miRNAs on the allergic lung disease phenotype. Three canonical features of this phenotype are airway hyperreactivity, which was determined in anesthetized, mechanically ventilated animals as the change in respiratory system resistance (RRS) induced by graded injections of acetylcholine; recruitment to the airways of inflammatory cells; and enhanced secretion of mucin gene products, especially Muc5AC (48, 49). Transcripts of the latter were quantitated by quantitative RT-PCR from mRNA extracted from whole lung. As expected, scrambled LNA had no effect on these asthma-related parameters (Fig. 5, C–E). In contrast, anti-let-7 LNA markedly suppressed airway hyperresponsiveness at the highest dose of acetylcholine used (3.2 μg/g) and lung inflammation, especially eosinophil recruitment to the airways, and normalized Muc5AC transcripts. Analysis of airway cytokines confirmed that anti-let-7, but not control LNA, significantly inhibited secretion of canonical Th2 cell cytokines, including IL-4, IL-5, and IL-13 (Fig. 5F). In contrast, neither LNA influenced secretion of IFN-γ, ruling out a possible anti-viral response triggered by the exogenous LNAs. Thus, in contrast to expectations from analysis of individual gene targets in vitro, in vivo suppression of let-7 miRNAs revealed the proinflammatory role of select members of this miRNA family in allergic lung disease.
DISCUSSION
Using a combination of high resolution miRNA microarrays and NGS together with detailed bioinformatic analyses, we provide the first whole genome view of major families of short transcripts and the RNAome of the lung in its naive state and the changes it undergoes in response to challenge with a potent respiratory allergen. Lung miRNAs demonstrated profound changes in overall abundance, sequence, and composition of individual species. Many new miRNAs have been discovered through this effort, and we have determined that let-7 microRNAs are the most abundant of all miRNAs in mouse lung. Although the majority of prior studies suggested a dominant anti-inflammatory role for miRNAs in immunity, our in vivo analyses revealed a potent proinflammatory role for let-7 miRNAs in allergic lung disease. Together, our results constitute an important miRNA database and provide unique insight into the control of allergic inflammation.
Emerging evidence suggests that miRNA function is highly nuanced and can range from straightforward silencing to fine tuning of gene expression (34). A striking finding of this study is that miRNA editing potentially represents a new dimension of this essential function. Previously, we reported that miRNAs of the let-7 family were extensively edited in cells derived from human and mouse pancreas and ovary, and our current study extends this finding to the lung (34). We have shown here that relatively underrepresented, non-let-7 miRNAs show similar editing. The C-to-U modification of mmu-miR-101a effectively converts the seed sequence to that of mmu-miR-144, with significant potential alterations in the target repertoire. Moreover, we have demonstrated that conversion of let-7a to let-7e (let-7a(9U→G)) reduces the ability of mmu-let-7a to regulate established targets, such as il-13. All let-7 miRNAs are predicted to target the same genes, and let-7a and let-7e appear to target il-13 with identical affinity (TargetScan 5.1). Our studies therefore indicate that subtleties exist with respect to the efficiency of target suppression relevant to position 9 nucleotides that are not accounted for by current prediction algorithms. Further analysis of the effect of miRNA edits, both naturally occurring and induced, on target regulation will be useful in refining the accuracy of target predictions.
Many of our novel miRNAs are homologous to transcripts previously identified from humans, zebra fish, and mice as piRNAs. Some of our novel transcripts exceed the typical length of miRNAs (∼22 nt) (e.g. asth-miR-1 consists of 26 nt). However, the genomic context of all novel putative miRNAs permits the formation of a stable pre-miRNA duplex that may serve as a substrate for the nuclear Drosha/Pasha microprocessor required for miRNA biogenesis. Because piRNA precursors do not form such duplexes, and indeed the biogenesis of piRNAs remains uncertain (50), we believe that our novel sequences are most appropriately classified as miRNAs.
We have identified numerous miRNAs from mouse lung with potential relevance to the control of allergic inflammation, as suggested by a limited analysis of the target repertoire. The importance of these putative associations requires confirmation through additional studies, but such predictions nonetheless emphasize the potentially highly complex role played by miRNAs in this disease model. For the current study, we focused additional effort on understanding the global significance of let-7 miRNAs to the control of allergic lung inflammation. We chose this large miRNA family because of the high degree of conservation of family members across metazoans and unexpectedly robust expression in both T cells and lung that suggested a conserved and probably critical function (51). let-7 miRNAs and the let-7 processing regulator Lin28 (52) have previously been identified as regulators of developmental timing, morphogenesis, and cancer (53–55). However, the miRNA-controlled cellular circuitry involved in development and oncongenesis overlaps with programs governing inflammation (55, 56), suggesting that a regulatory role for let-7 miRNAs in lung inflammation was possible.
Although as predicted il-13 is regulated by let-7a, given the more than 800 predicted targets of let-7 miRNAs, we reasoned that the effects of let-7 inhibition in a complex in vivo model of inflammation could not be predicted based on target validation alone. Indeed, neither the failure of lung il-13 and let-7a transcript expression to correlate inversely nor the suppressive effect of let-7a on T cell il-13 transcripts predicted the requisite role of let-7 miRNAs in allergic lung disease. These findings emphasize the difficulty in predicting miRNA function in complex in vivo systems and indicate that the primary effects of let-7 inhibition on target gene expression translate over time into dominant secondary effects that ultimately suppress inflammation. The large size of the let-7 target repertoire and such secondary effects precluded precise identification of the proinflammatory mechanism coordinated by let-7 miRNAs, an effort made more complex by the recent discoveries that let-7 miRNAs can either promote or suppress target gene expression by binding either canonical or non-canonical mRNA elements (57, 58). Our findings therefore emphasize the need for empiric analyses of miRNA function in inflammatory diseases and reveal the critical need for more sophisticated algorithms for the prediction of miRNA function in vivo.
Assessing the biological function of let-7 miRNAs in vivo is challenging. In addition to targeting essentially the same ∼820 mRNAs, the nine known let-7 miRNAs derive from 12 genetic loci (three exist as duplicate miRNA genes), effectively precluding a direct family-wide gene silencing approach through homologous recombination. For this study, we chose to use LNAs, the safety, efficacy, and specificity of which have been demonstrated both in vitro and in vivo (45, 59, 60). LNAs have the additional advantage over alternate gene silencing approaches that potentially toxic transfection vehicles (viruses, polyethyleneimine, etc.) are not required for in vivo use (61). Our studies confirm the specificity of LNAs used in vitro and in vivo, and we further did not observe toxicity in mice receiving either control or anti-let-7 LNAs (data not shown). These studies therefore support the therapeutic application of anti-let-7 LNAs in asthma and possibly other allergic conditions specifically to target let-7 and potentially numerous other miRNAs.
In summary, we have determined using a variety of genomic approaches that numerous miRNAs and other short transcripts are expressed in mouse lung and undergo marked changes in abundance during the transition from the naive state to allergic lung disease. Selected miRNAs undergo editing, creating potentially novel means for regulating the target repertoire, and we have identified numerous novel miRNAs. We have tentatively identified miRNAs of particular interest to allergic disease and specifically demonstrated that the most abundant lung miRNAs, from the let-7 family, are required to support allergic lung disease. These findings and the resulting miRNA data base may be useful in further delineating the role of miRNAs in lung homeostasis and in numerous immune disorders.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL095382 (to F. K. and D. B. C.) and AI070973 (to F. K. and D. B. C.). This work was also supported by a grant from the Clayton Foundation for Research (to D. B. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Tables S1–S7.
L. Batts and D. Corry, manuscript in preparation.
- miRNA and miR
- microRNA
- pre-miRNA
- precursor miRNA
- TLR
- T cell receptor
- Th2
- T helper type 2
- NGS
- next generation sequencing
- LNA
- locked nucleic acid
- piRNA
- Piwi-interacting RNA
- nt
- nucleotide(s).
REFERENCES
- 1.Eulalio A., Huntzinger E., Izaurralde E. (2008) Cell 132, 9–14 [DOI] [PubMed] [Google Scholar]
- 2.Behm-Ansmant I., Rehwinkel J., Izaurralde E. (2006) Cold Spring Harbor Symp. Quant. Biol. 71, 523–530 [DOI] [PubMed] [Google Scholar]
- 3.Chu C. Y., Rana T. M. (2006) PLoS Biol. 4, e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. (2009) Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhart B. J., Slack F. J., Basson M., Pasquinelli A. E., Bettinger J. C., Rougvie A. E., Horvitz H. R., Ruvkun G. (2000) Nature 403, 901–906 [DOI] [PubMed] [Google Scholar]
- 6.Brennecke J., Hipfner D. R., Stark A., Russell R. B., Cohen S. M. (2003) Cell 113, 25–36 [DOI] [PubMed] [Google Scholar]
- 7.Xu P., Vernooy S. Y., Guo M., Hay B. A. (2003) Curr. Biol. 13, 790–795 [DOI] [PubMed] [Google Scholar]
- 8.Chen X. (2004) Science 303, 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calin G. A., Cimmino A., Fabbri M., Ferracin M., Wojcik S. E., Shimizu M., Taccioli C., Zanesi N., Garzon R., Aqeilan R. I., Alder H., Volinia S., Rassenti L., Liu X., Liu C. G., Kipps T. J., Negrini M., Croce C. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5166–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael M. Z., O'Connor S. M., van Holst Pellekaan N. G., Young G. P., James R. J. (2003) Mol. Cancer Res. 1, 882–891 [PubMed] [Google Scholar]
- 11.Zhao Y., Ransom J. F., Li A., Vedantham V., von Drehle M., Muth A. N., Tsuchihashi T., McManus M. T., Schwartz R. J., Srivastava D. (2007) Cell 129, 303–317 [DOI] [PubMed] [Google Scholar]
- 12.van Rij R. P., Saleh M. C., Berry B., Foo C., Houk A., Antoniewski C., Andino R. (2006) Genes Dev. 20, 2985–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen I. M., Cheng G., Wieland S., Volinia S., Croce C. M., Chisari F. V., David M. (2007) Nature 449, 919–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Z., Kearney P., Plaisance K., Parsons C. H. (2010) J. Leukoc. Biol. 87, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong M. M., Rasmussen J. P., Rudensky A. Y., Littman D. R. (2008) J. Exp. Med. 205, 2005–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukiw W. J., Zhao Y., Cui J. G. (2008) J. Biol. Chem. 283, 31315–31322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazzoni F., Rossato M., Fabbri M., Gaudiosi D., Mirolo M., Mori L., Tamassia N., Mantovani A., Cassatella M. A., Locati M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 5282–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachmani D., Stern-Ginossar N., Sarid R., Mandelboim O. (2009) Cell Host Microbe 5, 376–385 [DOI] [PubMed] [Google Scholar]
- 19.Liu G., Friggeri A., Yang Y., Park Y. J., Tsuruta Y., Abraham E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 15819–15824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez A., Vigorito E., Clare S., Warren M. V., Couttet P., Soond D. R., van Dongen S., Grocock R. J., Das P. P., Miska E. A., Vetrie D., Okkenhaug K., Enright A. J., Dougan G., Turner M., Bradley A. (2007) Science 316, 608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He M., Xu Z., Ding T., Kuang D. M., Zheng L. (2009) Cell Mol. Immunol. 6, 343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohlhaas S., Garden O. A., Scudamore C., Turner M., Okkenhaug K., Vigorito E. (2009) J. Immunol. 182, 2578–2582 [DOI] [PubMed] [Google Scholar]
- 23.Wills-Karp M. (1999) Annu. Rev. Immunol. 17, 255–281 [DOI] [PubMed] [Google Scholar]
- 24.Fahy J. V., Corry D. B., Boushey H. A. (2000) Curr. Opin. Pulm. Med. 6, 15–20 [DOI] [PubMed] [Google Scholar]
- 25.Corry D. B., Folkesson H. G., Warnock M. L., Erle D. J., Matthay M. A., Wiener-Kronish J. P., Locksley R. M. (1996) J. Exp. Med. 183, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grünig G., Warnock M., Wakil A. E., Venkayya R., Brombacher F., Rennick D. M., Sheppard D., Mohrs M., Donaldson D. D., Locksley R. M., Corry D. B. (1998) Science 282, 2261–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corry D. B. (1999) Curr. Opin. Immunol. 11, 610–614 [DOI] [PubMed] [Google Scholar]
- 28.Kheradmand F., Kiss A., Xu J., Lee S. H., Kolattukudy P. E., Corry D. B. (2002) J. Immunol. 169, 5904–5911 [DOI] [PubMed] [Google Scholar]
- 29.Kalafus K. J., Jackson A. R., Milosavljevic A. (2004) Genome Res. 14, 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saldanha A. J. (2004) Bioinformatics 20, 3246–3248 [DOI] [PubMed] [Google Scholar]
- 31.Xu M., You Y., Hunsicker P., Hori T., Small C., Griswold M. D., Hecht N. B. (2008) Biol. Reprod. 79, 51–57 [DOI] [PubMed] [Google Scholar]
- 32.Keane-Myers A., Gause W. C., Linsley P. S., Chen S. J., Wills-Karp M. (1997) J. Immunol. 158, 2042–2049 [PubMed] [Google Scholar]
- 33.Das J., Chen C. H., Yang L., Cohn L., Ray P., Ray A. (2001) Nat. Immunol. 2, 45–50 [DOI] [PubMed] [Google Scholar]
- 34.Reid J. G., Nagaraja A. K., Lynn F. C., Drabek R. B., Muzny D. M., Shaw C. A., Weiss M. K., Naghavi A. O., Khan M., Zhu H., Tennakoon J., Gunaratne G. H., Corry D. B., Miller J., McManus M. T., German M. S., Gibbs R. A., Matzuk M. M., Gunaratne P. H. (2008) Genome Res. 18, 1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu P., Reid J. G., Gao X., Shaw C. A., Creighton C., Tran P. L., Zhou X., Drabek R. B., Steffen D. L., Hoang D. M., Weiss M. K., Naghavi A. O., El-daye J., Khan M. F., Legge G. B., Wheeler D. A., Gibbs R. A., Miller J. N., Cooney A. J., Gunaratne P. H. (2008) PLoS ONE 3, e2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cormier S. A., Larson K. A., Yuan S., Mitchell T. L., Lindenberger K., Carrigan P., Lee N. A., Lee J. J. (2001) Mamm. Genome 12, 352–361 [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi A., Morita S., Iwashita H., Sagiya Y., Ashida Y., Shirafuji H., Fujisawa Y., Nishimura O., Fujino M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5175–5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb D. C., McKenzie A. N., Foster P. S. (2001) J. Biol. Chem. 276, 41969–41976 [DOI] [PubMed] [Google Scholar]
- 39.Pouladi M. A., Robbins C. S., Swirski F. K., Cundall M., McKenzie A. N., Jordana M., Shapiro S. D., Stämpfli M. R. (2004) Am. J. Respir. Cell Mol. Biol. 30, 84–90 [DOI] [PubMed] [Google Scholar]
- 40.Rudic R. D., McNamara P., Curtis A. M., Boston R. C., Panda S., Hogenesch J. B., Fitzgerald G. A. (2004) PLoS Biol. 2, e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasaian M. T., Donaldson D. D., Tchistiakova L., Marquette K., Tan X. Y., Ahmed A., Jacobson B. A., Widom A., Cook T. A., Xu X., Barry A. B., Goldman S. J., Abraham W. M. (2007) Am. J. Respir. Cell Mol. Biol. 36, 368–376 [DOI] [PubMed] [Google Scholar]
- 42.Arima K., Umeshita-Suyama R., Sakata Y., Akaiwa M., Mao X. Q., Enomoto T., Dake Y., Shimazu S., Yamashita T., Sugawara N., Brodeur S., Geha R., Puri R. K., Sayegh M. H., Adra C. N., Hamasaki N., Hopkin J. M., Shirakawa T., Izuhara K. (2002) J. Allergy Clin. Immunol. 109, 980–987 [DOI] [PubMed] [Google Scholar]
- 43.Huang S. K., Xiao H. Q., Kleine-Tebbe J., Paciotti G., Marsh D. G., Lichtenstein L. M., Liu M. C. (1995) J. Immunol. 155, 2688–2694 [PubMed] [Google Scholar]
- 44.Kuperman D., Schofield B., Wills-Karp M., Grusby M. J. (1998) J. Exp. Med. 187, 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahlestedt C., Salmi P., Good L., Kela J., Johnsson T., Hökfelt T., Broberger C., Porreca F., Lai J., Ren K., Ossipov M., Koshkin A., Jakobsen N., Skouv J., Oerum H., Jacobsen M. H., Wengel J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5633–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callaerts-Vegh Z., Evans K. L., Dudekula N., Cuba D., Knoll B. J., Callaerts P. F., Giles H., Shardonofsky F. R., Bond R. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4948–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L. P., Lin R., Parra S., Omoluabi O., Hanania N. A., Tuvim M. J., Knoll B. J., Dickey B. F., Bond R. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2435–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornton D. J., Carlstedt I., Howard M., Devine P. L., Price M. R., Sheehan J. K. (1996) Biochem. J. 316, 967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuhdi Alimam M., Piazza F. M., Selby D. M., Letwin N., Huang L., Rose M. C. (2000) Am. J. Respir. Cell Mol. Biol. 22, 253–260 [DOI] [PubMed] [Google Scholar]
- 50.Kim V. N., Han J., Siomi M. C. (2009) Nat. Rev. Mol. Cell Biol. 10, 126–139 [DOI] [PubMed] [Google Scholar]
- 51.Lee R. C., Ambros V. (2001) Science 294, 862–864 [DOI] [PubMed] [Google Scholar]
- 52.Viswanathan S. R., Daley G. Q., Gregory R. I. (2008) Science 320, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viswanathan S. R., Powers J. T., Einhorn W., Hoshida Y., Ng T. L., Toffanin S., O'Sullivan M., Lu J., Phillips L. A., Lockhart V. L., Shah S. P., Tanwar P. S., Mermel C. H., Beroukhim R., Azam M., Teixeira J., Meyerson M., Hughes T. P., Llovet J. M., Radich J., Mullighan C. G., Golub T. R., Sorensen P. H., Daley G. Q. (2009) Nat. Genet. 41, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammell C. M., Karp X., Ambros V. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18668–18673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iliopoulos D., Hirsch H. A., Struhl K. (2009) Cell 139, 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidson-Moncada J., Papavasiliou F. N., Tam W. (2010) Ann. N.Y. Acad. Sci. 1183, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasudevan S., Tong Y., Steitz J. A. (2007) Science 318, 1931–1934 [DOI] [PubMed] [Google Scholar]
- 58.Lytle J. R., Yario T. A., Steitz J. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9667–9672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanford R. E., Hildebrandt-Eriksen E. S., Petri A., Persson R., Lindow M., Munk M. E., Kauppinen S., Ørum H. (2010) Science 327, 198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elmén J., Lindow M., Schütz S., Lawrence M., Petri A., Obad S., Lindholm M., Hedtjärn M., Hansen H. F., Berger U., Gullans S., Kearney P., Sarnow P., Straarup E. M., Kauppinen S. (2008) Nature 452, 896–899 [DOI] [PubMed] [Google Scholar]
- 61.Stein C. A., Hansen J. B., Lai J., Wu S., Voskresenskiy A., Høg A., Worm J., Hedtjärn M., Souleimanian N., Miller P., Soifer H. S., Castanotto D., Benimetskaya L., Ørum H., Koch T. (2010) Nucleic Acids Res. 38, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.