Abstract
The adenosine A2A receptor (A2AR) is increasingly recognized as a novel therapeutic target in Parkinson disease. In striatopallidal neurons, the G-protein αolf subtype is required to couple this receptor to adenylyl cyclase activation. It is now well established that the βγ dimer also performs an active role in this signal transduction process. In principal, sixty distinct βγ dimers could arise from combinatorial association of the five known β and 12 γ subunit genes. However, key questions regarding which βγ subunit combinations exist and whether they perform specific signaling roles in the context of the organism remain to be answered. To explore these questions, we used a gene targeting approach to specifically ablate the G-protein γ7 subtype. Revealing a potentially new signaling paradigm, we show that the level of the γ7 protein controls the hierarchial assembly of a specific G-protein αolfβ2γ7 heterotrimer in the striatum. Providing a probable basis for the selectivity of receptor signaling, we further demonstrate that loss of this specific G-protein heterotrimer leads to reduced A2AR activation of adenylyl cyclase. Finally, substantiating an important role for this signaling pathway in pyschostimulant responsiveness, we show that mice lacking the G-protein γ7 subtype exhibit an attenuated behavioral response to caffeine. Collectively, these results further support the A2AR G-protein αolfβ2γ7 interface as a possible therapeutic target for Parkinson disease.
Keywords: Adenylate Cyclase (Adenylyl Cyclase), Cell Surface Receptor, Cyclic AMP (cAMP), Gene Knockout, Heterotrimeric G Proteins, Neurotransmitter Receptors, Parkinson Disease, Signal Transduction
Introduction
G-protein-coupled receptors represent the single largest family of target proteins for drug development. Their actions require the participation of heterotrimeric guanine nucleotide binding proteins (G-proteins) whose roles in these diverse signaling pathways may be determined by their specific αβγ subunit combinations. The existence of 16 α, 5 β, and 12 γ subtypes creates the potential to generate a large number of distinct G-protein αβγ heterotrimers (1, 2). Although their biochemical properties have been well studied (3, 4), key questions regarding which G-protein αβγ heterotrimers actually exist in vivo and determining whether they perform specific signaling roles and biological functions remain to be answered. To address these questions, a gene-targeting approach has been used to delete the various α subunit genes in mice, leading to the identification of physiological functions for most of them (5). By contrast, little attention has focused on the β and γ subunit genes. In particular, several features of the γ subunit genes suggest they may perform heterogeneous functions in vivo. Analogous to their α partners, the various γ subtypes show substantial structural diversity and exhibit pleiotropic patterns of expression (2). Accordingly, we have undertaken a gene targeting approach to systematically ablate the individual γ subtypes in mice (6, 7), with the ultimate goal of elucidating their biological functions.
Our recent work has demonstrated that knock-out of Gng7, encoding the γ7 subtype, produces a behavioral phenotype resulting in part from a localized defect in dopamine D1 receptor (D1R)2 signaling in the brain (6). Within the brain, the striatum collects and processes information from the cerebral cortex and thalamus affecting the control of voluntary movements (8). Accounting for >90% of neurons within the striatum, the medium spiny neurons are comprised of two distinct subpopulations that are classified on the basis of their distinct circuitries (9). The striato-nigral (SN) neurons projecting to the substania nigra pars reticulata and entopeduncular nucleus constitute the direct tract, whereas the striato-pallidal (SP) neurons projecting to the lateral part of the globus pallidus comprise the indirect tract (8). Typically, a coordinated balance between these two tracts produces normal movements, whereas a preponderance of one tract over the other is implicated in producing motor abnormalities associated with basal ganglia disorders (10, 11).
In the SN neurons, the D1R acts through the G-protein αolf subunit to stimulate cAMP production (12). Based on recent analyses of Gng7−/− mice, this action is also dependent on the γ7 subtype (6). Intriguingly, in the SP neurons, the adenosine A2A receptor (A2AR) also couples through the G-protein αolf subunit to enhance cAMP production even though this pathway produces the opposite behavioral effect (12). In the present study, we explored whether a specific G-protein αolfβ2γ7 subunit combination is required for this pathway in SP neurons. Using mice with targeted deletions of Gng7 or Gnal, lacking the G-protein γ7 (6) or αolf (13) subunits, respectively, we showed that levels of the αolf and β2 proteins were selectively and coordinately reduced in Gng7−/− mice, whereas levels of γ7 were largely unaffected in the Gnal−/− mice. Notably, these results indicate that assembly of the αolfβ2γ7 heterotrimer is an ordered process that is controlled by the amount of the γ7 subtype. Moreover, loss of the G-protein γ7 subunit led to defects in both D1R (6) and A2A receptor activation of adenylyl cyclase without producing any gross alterations in locomotor behavior typical of Parkinson disease. Importantly, these findings contribute to a growing literature that suggests that blockade of A2AR signaling in the striatum may be an effective strategy for treating various neurological and addictive disorders.
EXPERIMENTAL PROCEDURES
Production of Mice
Disruption of Gng7, the gene encoding the G-protein γ7 subunit in mice, was described previously (6). Gng7+/− mice were backcrossed to C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) for 5 generations, or separately to BALB/c mice (Jackson Laboratories) for 5 generations. Gng7+/− mice were intercrossed to produce the Gng7−/− mice and wild-type littermates used in these experiments. In this work, we will describe these mice as “on a C57BL/6 background,” or “on a BALB/c background.” However, after only 5 generations of backcrosses, there is still some contribution to their genetic makeup from the original ES cells (129SvEvBrd, Lexicon Genetics, Inc., The Woodlands, TX), from the dam that was bred with the chimera (C57BL/6 albino, Lexicon), and from the Cre recombinase expressing strain that we utilized (BALB/c-TgN(CMV-Cre)#Cgn, Jackson Laboratories). Hence, it was essential to use littermates to control for the possible influence of genetic background to observed responses. On the C57BL/6 background, 16 Gng7−/− mice (8 males and 8 females) and 16 wild-type littermates (8 males and 8 females) were studied. On the BALB/c background, 12 Gng7−/− mice (6 males and 6 females), and 14 wild-type littermates (4 males and 10 females) were studied. Genotypes were determined by PCR analysis of tail biopsy DNA as described previously (6).
Mice with a disrupted Gnal gene, that lack the G-protein αolf, were described previously (13). These were backcrossed for up to 9 generations with C57BL/6 mice to obtain homozygous (Gnal−/−) and their control littermates (Gnal+/+). For comparing homozygous mutant and wild-type mice, 6-week-old male and female mice were used for experiments.
Animal Care and Approval
Mice were segregated by sex and group housed in plastic microisolator cages in ventilated racks (Thoren Caging Systems, Inc., Hazelton, PA). Mice were given ad lib access to water and Mouse Diet 9F (Purina Mills, LLC, St. Louis, MO). Environmental factors included temperature and humidity control and a 12-hour light/dark cycle. The animal facility is maintained as virus antibody-free and parasite-free. The Geisinger Clinic Institutional Animal Care and Use Committee approved animal research protocols.
Adenylyl Cyclase Assay
Striatal tissues were homogenized in Buffer A (10 mm Tris, pH 7.4, 1 mm EDTA, 1 mm DTT, 0.3 mm AEBSF, 30 μm leupeptin, 1 μm pepstatin A) with 10% sucrose using a Brinkmann Homogenizer (Brinkmann Instruments Co, Westbury, NY). Membranes were then isolated by centrifugation (65 min at 100,000 × g) onto a cushion of Buffer A with 44.5% (w/v) sucrose. The membranes at the interface were transferred to a new tube and twice washed with Buffer A and collected by centrifugation (30 min at 100,000 × g). Protein concentrations were determined with Coomassie Plus (Thermo Fisher Scientific, Inc., Rockford, IL). Adenylyl cyclase activity was determined by incubating membrane protein (20 μg) at 30 °C for 15 min in 0.1 ml of buffer containing 50 mm HEPES (pH 7.4), 1 mm EGTA, 5 mm MgCl2, 0.1 mm ATP, 1 × 106 cpm of [α-32P]ATP, 10 μm rolipram, 1 unit/ml adenosine deaminase, 5 mm creatine phosphate, 50 units/ml creatine phosphokinase, and various agonists as indicated in the text. Reactions were terminated by addition of 0.1 ml of 2% SDS, 40 mm ATP, 1.4 mm cAMP, 10,000 cpm of [3H]cAMP, and heating to 100 °C for 3 min. [32P]cAMP was isolated by chromatography on Dowex and Alumina columns, using [3H]cAMP as a recovery marker, and quantified by liquid scintillation counting.
Radioligand Binding Assays
Radioligand binding to A2AR in striatal membranes prepared from Gng7−/− mice and wild-type littermates was performed using either the radiolabeled agonist [125I]2-[2-(4-amino-3-iodo-phenyl)ethylamino]adenosine (125I-APE) or the radiolabeled antagonist 125I-ZM241385 as described previously (14, 15). Binding was performed using striatal membranes prepared from wild type or Gng7−/− mice in buffer containing 10 mm HEPES pH 7.4, 1 mm EDTA, 5 mm MgCl2, and 1 unit/ml adenosine deaminase. The agonist, 125I-APE binds to two affinity states of the A2AR, a high affinity state corresponding to receptor-G-protein complexes, and a low affinity state corresponding to receptors uncoupled from G-proteins (15). Because GTPγS added to membranes uncouples receptors from G-proteins, 50 μm GPTγS was added to some membranes to measure agonist binding to largely uncoupled receptors. In the absence of added GTPγS, 125I-APE binds preferentially to G-protein coupled receptors. 125I-APE also binds to uncoupled receptors, but may under measure total receptor number because a fraction of the radioligand may dissociate for the low affinity site during washing of filters. Hence, the total number of receptors (Bmax) was more accurately detected as the number of specific binding sites for the high affinity antagonist, 125I-ZM241385. Nonspecific radioligand binding was measured in the presence of 50 μm N-ethylcarboxamidoadenosine (NECA).
Immunoblot Analysis
To examine the expression of G-protein subunits in mouse striatum, Western blot analysis was performed on cholate-solublized membranes that were prepared as described previously (6). Antisera for Gαs was used at a 1:500 dilution, for Gαolf (16) at 1:2000, and for Ras (BD Biosciences, Palo Alto, CA) at a 1:2000 dilution. Antisera for β1 (1:500), β2 (1:500), γ2, γ3, γ5 (1:100), and γ7 were described previously (17, 18, 19) and were used at a 1:200 dilution, except as indicated. His-tagged G-protein β and γ subunits (CytoSignal Research Products, Irvine, CA) and His-tagged αolf (12) were used as standards for quantitative immunoblotting.
Real-time RT-PCR
To examine levels of mRNA for αolf, β2, γ7, and DARPP-32, RNA was prepared from striatum of six Gng7−/− mice and six wild-type littermates at the N24 backcross to C57BL/6 and real-time RT-PCR was conducted as described previously (20). Primers were as follows: Gnal (CCT TCC TAC TTG CCT GAC CGC; TGA CGA TAG TGC TTT TCC CGG), Gng7 (GCT GGG ATC GAA CGC ATC AAG; CAG GAA GAT CCC GGC ATT CAC), Gnb2 (TCA TAG GTC ACG AGT CGG ACA TCA; ATG GCA TCC CAG ATG TTG CAG TTG), Ppp1r1b (CAC CAC CCA AAG TCG AAG AGA; CGA AGC TCC CCT AAC TCA TCCT), and to correct for variation in cDNA yield Eef1a1 (GGA ATG GTG ACA ACA TGC TG; CGT TGA AGC CTA CAT TGT CC).
Locomotor Activity
Locomotor activity was quantified in CLAMS cages (Columbus Instruments, Columbus, OH). The cages consist of clear plastic boxes (20 cm × 10 cm × 12.5 cm) fitted with three rows of 8 photoelectric sensors (x, y, and z directions). The mice were placed in the CLAMS cages at 11 am and remained in the cages for 4 h. During this time the mice had ad lib access to water. Every minute the numbers of individual (total) and consecutive (ambulatory) photobeam breaks for each of 3 sensor arrays, and the number of contacts with the sipper tube were recorded. For caffeine trials, mice were removed from the cages after 1 h and given an intraperitoneal injection of saline or caffeine (5 ml/kg). Mice that had been habituated to the CLAMS cages and to the injection procedure with saline were used in drug trials. The locomotor response to drug is expressed as an increment over the response to saline. Locomotor activities were studied at age 10.0 ± 0.8 weeks for C57BL/6 background mice and age 8.5 ± 0.3 weeks for BALB/c background mice. The response to caffeine was studied at age 23.6 ± 3.0 weeks for C57BL/6 background mice and age 14.6 ± 0.3 weeks for BALB/c background mice.
Statistical Analysis
Sample statistics and Student's t-tests were computed using Excel (Microsoft). Data are presented as means ± S.E. of the mean. Locomotor activity was compared by repeated measures multivariate analysis of variance (MANOVA), using JMP (SAS Institute Inc., Carey, NC).
RESULTS
The A2AR is primarily responsible for the psychostimulant action of caffeine (21, 22). Because blockade of this receptor has been shown to reverse the hypolocomotor phenotype resulting from dopamine deficiency (23) and dysfunctional dopamine signaling (24), the A2AR signaling components are increasingly recognized as valid therapeutic targets for treating Parkinson disease and for reducing the side effects of levodopa therapy (25, 26). In SP neurons, the G-protein αolf subunit has been shown to positively couple this receptor to stimulation of cAMP production (27, 12). However, little information is available on the obligatory β and γ components involved in this context. By applying biochemical and behavioral approaches to a novel mouse model, we found that the G-protein γ7 subunit is specifically required for both A2AR signaling and psychostimulant response to caffeine. Identifying a mechanistic basis for this requirement, we show that the γ7 protein drives the preferential assembly of a G-protein αolfβ2γ7 heterotrimer in the striatum that is involved in a key signaling pathway controlling locomotion and reward.
Defective A2AR Signaling in Mice Lacking the γ7 Protein
Regulation of cAMP production in medium spiny neurons represents a primary target of many neurotransmitters and psychoactive drugs that affect short- and long-term locomotor responses (28). Virtually all medium spiny neurons, including SN and SP neurons, express substantial levels of γ7 mRNA (29, 30, 31). Because adenylyl cyclase signaling by the D1R was shown to be dependent on γ7 expression in SN neurons (6), we explored whether adenylyl cyclase signaling by the A2AR is similarly dependent on expression of γ7 in SP neurons. As a biochemical assay, we used the selective A2AR agonist, CGS-21680, to stimulate adenylyl cyclase activity in striatal membranes prepared from Gng7−/− mice. By comparison to their wild type littermates, adenylyl cyclase activity in response to 10 μm or 25 μm CGS-21680 was reduced by 30–40% in striatal membranes from Gng7−/− mice (Fig. 1A). Furthermore, cAMP production in response to 100 μm forskolin was also reduced by ∼35% in striatal membranes from Gng7−/− mice (Fig. 1B).
FIGURE 1.
Adenylyl cyclase activity of membranes prepared from striata of Gng7−/− mice (KO) and wild-type littermates (WT), expressed as pmol of cAMP per mg of membrane protein per min. A, adenylyl cyclase activity in response to no added agonist (None), 1 μm GTP alone (GTP), or with 10 μm CGS-21680 (CGS) or 25 μm CGS-21680. B, in response to 100 μm forskolin (Forsk), adenylyl cyclase activity is significantly reduced in Gng7−/− striatal membranes (n = 9 mice in each group, *, p < 0.01 by Student's t-test).
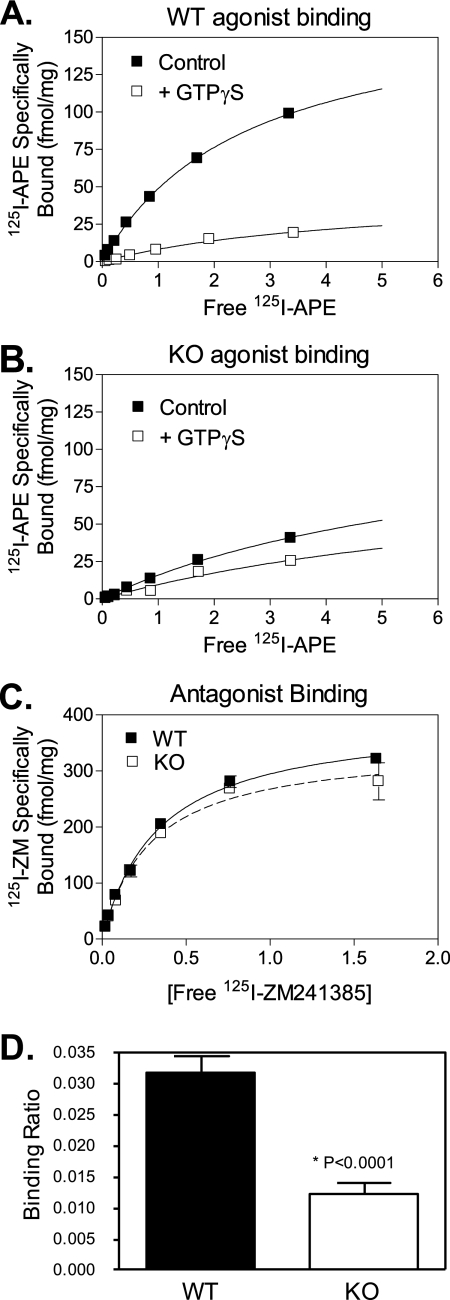
Because forskolin-stimulated adenylyl cyclase activity was reduced, we could not assess whether the impaired response to the A2AR agonist was due to a defect in G-protein coupling and/or adenylyl cyclase activation. Therefore, as a second biochemical assay, we used high affinity agonist binding to directly measure the actual interaction between the A2AR and the G-protein. Initial saturation binding experiments were performed with the A2AR agonist, 125I-APE, on pooled samples of striatal membranes from either Gng7−/− mice or their wild-type littermates (12 mice in each group). In the wild-type sample, the addition of GTPγS dramatically reduced agonist binding by greater than 75%, indicating a significant portion of the A2AR was associated with G-protein (Fig. 2A). In contrast, in the knock-out sample, addition of GTPγS produced little reduction in A2AR agonist binding (Fig. 2B), suggesting most A2AR was no longer coupled to G-protein. Finally, there was no significant difference in the binding of the A2AR antagonist, 125I-ZM241385, between the samples (Fig. 2C), indicating the total number of A2AR was comparable between the two genotypes. Taken together, these results indicate a striking reduction in the fraction of the A2AR that was coupled to G-protein in striatal membranes from Gng7−/− mice. Subsequent binding studies were performed on striatal membranes from individual mice representing each genotype. To calculate the fraction of the A2AR pool that was coupled to G-protein, we determined the ratio of specific GTPγS-sensitive 125I-APE agonist binding sites relative to 125I-ZM421385 antagonist binding sites in striatal membranes from both genotypes. By comparison to their wild-type littermates, the fraction of the A2AR pool that was coupled to G-protein was markedly reduced in striatal membranes from Gng7−/− mice (p < 0.001) (Fig. 2D). Collectively, these results confirm that A2AR signaling shows a specific requirement for G-protein γ7 expression and that its loss is associated with an impaired ability of this receptor to couple to G-protein.
FIGURE 2.
Radioligand binding to striatal membranes. Pooled membranes from striata of 12 wild-type littermates (A) or 12 Gng7−/− mice (B), showing 125I-APE specifically bound at various concentrations in the absence (filled boxes) or presence (open boxes) of GTPγS. The difference ± GTPγS is defined as GTPγ-S sensitive binding. Note reduced GTPγS-sensitive binding to Gng7−/− membranes compared with controls. The total number of receptors was determined from binding of the antagonist, 125I-ZM241385 (C). The ratio of specific binding of 125I-APE ± GTPγS/125I-ZM241385 was measured in 12 individual membrane preparations from wild type or Gng7−/− mice, as an index of receptor coupling to G proteins (D).
Impaired Assembly of Golf Heterotrimer in Mice Lacking the γ7 Protein
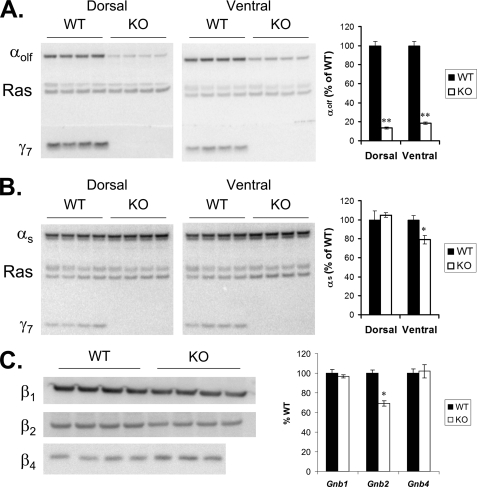
One mechanism that could account for the observed defects in both G-protein coupling and adenylyl cyclase activation is a coordinate reduction in the cellular amount of the G-protein αs or αolf subunit. These two structurally related isoforms are both able to stimulate cAMP production (12) and are both expressed in the striatum (32). Previously, we showed that loss of the γ7 subunit coordinately reduced levels of the αolf protein in the striatum (6). To confirm and extend this finding, we performed immunoblot analysis on micropunch samples from dorsal striatum (caudate) and ventral striatum (nucleus accumbens) of Gng7−/− mice on two different genetic backgrounds (i.e. C57BL/6 and BALB/c). By comparison to their wild-type littermates, αolf protein levels were strikingly reduced by >85% in both dorsal and ventral striatal membranes from knock-out animals on a C57BL/6 genetic background (Fig. 3A). The effect was remarkably specific in that αs protein levels (i.e. 45- and 52-kDA forms) were not affected in the dorsal striatum and were reduced by only 20% in the ventral striatum of knock-out mice (Fig. 3B). Attesting to the generality of this finding, immunoblot analysis of the corresponding regions of knock-out mice on a BALB/c genetic background yielded similar results (supplemental Fig. S1). Taken together, these results demonstrate that the cellular level of the αolf but not the αs subunit is dependent on expression of the γ7 subunit.
FIGURE 3.
A, immunoblot of αolf, γ7, and Ras on membranes prepared from micropunch samples of dorsal striatum (15 μg/lane) and ventral striatum (10 μg/lane) of four Gng7−/− mice (KO) and four wild-type littermates (WT) on a C57BL/6 genetic background. B, immunoblot of αs, γ7, and Ras in membranes described above. C, immunoblot of β1, β2, and β4 on membranes prepared from whole striatum of Gng7−/− mice and wild-type littermates (20 μg/lane) on a C57BL/6 genetic background. Graphs depict quantitation of 1 or 2 immunoblots for each subunit, values are normalized to Ras for each lane, then expressed as percent of wild-type (% WT) (n = 4 to 8 mice in each group; *, p < 0.01; **, p < 1 × 10−6).
Next, we investigated how loss of the γ7 protein impacts the levels of particular β protein(s) in the striatum. Because targeting of the βγ dimer to the plasma membrane is dependent upon post-translational lipid modifications of the γ subunit (33, 34, 35), we reasoned that loss of the γ7 protein could affect the level of a specific β subtype in striatal membranes from Gng7−/− mice. By comparison to their wild-type littermates, β2 protein levels were selectively reduced by 31% with no significant changes in the amounts of β1 and β4 proteins (Fig. 3C). Taken together, these findings show both coordinate and selective suppression of the αolf, β2, and γ7 subunits at the protein level.
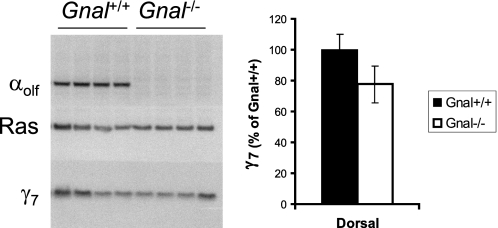
To assess whether the αolf subunit plays a reciprocal role in this process, we used the Gnal−/− mouse model (13) to determine whether loss of the αolf protein causes a corresponding suppression of the γ7 protein. By comparison to their wild type littermates, γ7 protein levels were not significantly different in striatal membranes from Gnal−/− mice (Fig. 4), indicating that the expression of the γ7 but not the αolf protein drives the assembly of a specific G-protein heterotrimer in the striatum.
FIGURE 4.
Immunoblot of membranes (15 μg/lane) prepared from micropunch samples of dorsal striatum of four Gnal+/+ mice, and four Gnal−/− mice, blotted with antisera for αolf (top), Ras (middle), or γ7 (bottom). Right panel shows quantitation of γ7 normalized to Ras for each lane, then expressed as percent of Gnal+/+.
Mechanism for Coordinate Suppression of the G-protein αolf Subunit in Mice Lacking the γ7 Subunit
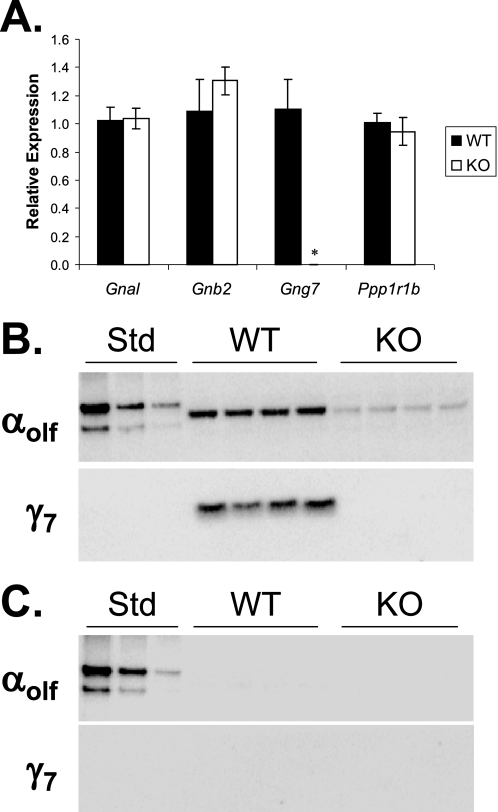
One mechanism that could account for suppression of the αolf protein is a reduced level of the corresponding mRNA transcript. To test this possibility, we performed real time RT-PCR analysis on striatal tissue from Gng7−/− mice and their wild-type littermates on the C57BL/6 background. Despite the loss of αolf protein (Fig. 4), the level of αolf mRNA was not significantly reduced in the striatum of Gng7−/− mice (Fig. 5A). Moreover, the level of β2 mRNA was not significantly different in wild type and Gng7−/− striatum. Finally, using a well validated marker of medium spiny neurons, the level of DARPP-32 (Ppp1r1b) mRNA was not significantly reduced in the striatum of Gng7−/− mice (Fig. 5A), indicating that there was no overt loss of medium spiny neurons from the brains of knock-out mice. These findings point to a post-transcriptional mechanism responsible for the coordinate suppression of the αolf and β2 proteins in mice lacking the γ7 protein. Because plasma membrane binding of the α subunit is facilitated by βγ association (36, 37), we examined whether the decreased amount of αolf protein in the striatal membrane fractions was associated with increased accumulation of this protein in the corresponding cytosolic fractions. Despite loss from the membrane fraction (Fig. 5B), no αolf protein was detectable in the cytosolic fraction of Gng7−/− mice (Fig. 5C).
FIGURE 5.
Real time RT-PCR amplification of αolf (Gnal), β2 (Gnb2), γ7 (Gng7), and DARPP-32 (Ppp1r1b) from RNA prepared from dorsal striatum of Gng7−/− mice (KO) and wild-type littermates (WT), on the C57BL/6 genetic background. Relative expression was calculated as 2−(ΔCt-ΔCtavg), where ΔCt is the threshold cycle for the gene of interest minus the threshold for Eef1a1, and ΔCtavg is the average ΔCt of the wild-type samples (n = 6 in each group, *, p < 0.001 by Student's t-test). B, immunoblot of membrane fraction (15 μg/lane) prepared from micropunch samples of dorsal striatum of Gng7−/− mice (KO) and wild-type littermates (WT), blotted with antisera for αolf (top) or γ7 (bottom). The first three lanes contain 100 ng, 50 ng, or 25 ng of recombinant αolf protein (Std). C, immunoblot of cytosol fraction (15 μg/lane) from samples in B.
Basis for γ7 Selectivity
To determine why γ7 has a special place in the assembly of the Golf heterotrimer in the native context, we performed immunoblot analysis to provide a quantitative accounting of the G-protein subunits that are expressed in the striatum of wild-type and Gng7−/− mice (Table 1). The wild-type striatum contains roughly equimolar levels of αolf and γ7 proteins (supplemental Fig. S2A and Table 1). Therefore, in the Gng7−/− striatum, the 85% reduction in αolf could be almost completely accounted for by loss of γ7. Likewise, the wild-type striatum contains a >2-fold molar excess of β2 relative to γ7 (supplemental Fig. S2A and Table 1). Therefore, in the knock-out striatum, the 30% reduction in β2 could also be attributed to loss of γ7. While our accounting is necessarily limited by the availability of G-protein subunit antibodies and standards, these results strongly suggest the existence of a specific G-protein αolfβ2γ7 complex that normally functions downstream of the A2AR in SP neurons. To explore the mechanistic role of the γ7 protein in this process, we examined the relative levels of the various γ subtypes in the striatum. Compared with the γ7 protein, there is a 4-fold molar excess of the γ2 protein and an equimolar amount of the γ3 protein in the striatum (7). Moreover, in the knock-out striatum, there is a further increase in the level of the γ3 protein (supplemental Fig. S2B). Hence, the combined levels of the γ2 and γ3 proteins appear to be sufficient to compensate for loss of the γ7 protein, suggesting other γ subtypes are not functionally interchangeable.
TABLE 1.
G-protein subunit expression in wild-type and Gng7−/− dorsal striatum as determined by quantitative immunoblotting
| Measured wild type concentration | Formula weight | Calculated wild type concentration | Measured change in Gng7−/− | Calculated change in Gng7−/− | |
|---|---|---|---|---|---|
| ng/μg membrane protein | fmol/μg membrane protein | % | fmol/μg membrane protein | ||
| γ2 | 2.84 ± 0.09 | 7850 | 362 ± 11 | −18 ± 4 | −65 ± 16 |
| γ3 | 0.71 ± 0.10 | 8305 | 85 ± 7 | +39 ± 11 | +33 ± 10 |
| γ7 | 0.65 ± 0.10 | 7611 | 86 ± 8 | −100 | −86 ± 8 |
| β2 | 8.19 ± 0.40 | 37331 | 219 ± 11 | −31 ± 4 | −68 ± 9 |
| αolf | 3.18 ± 0.12 | 44308 | 72 ± 2 | −85 ± 5 | −61 ± 4 |
Alterations in Locomotor Activity in Mice Lacking γ7
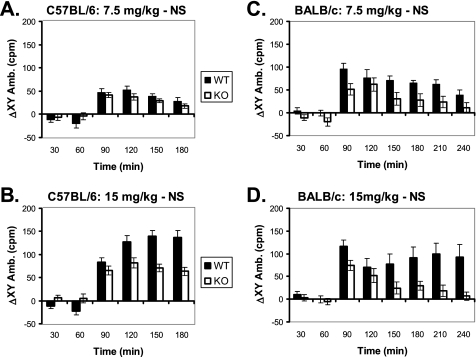
To assess the functional consequences of impaired A2AR signaling and Golf assembly, we examined the behavioral responses of Gng7−/− mice. Because the A2AR is primarily responsible for the psychostimulant actions of caffeine (21, 22), we reasoned that Gng7−/− mice on the C57BL/6 background might exhibit an attenuated response to the locomotor enhancing effects of this drug. As a quantitative measure of locomotor activity, Gng7−/− mice and their wild-type littermates were placed in CLAMS cages equipped with photobeams to record their movements. After allowing sufficient time for acclimation to their surroundings, the locomotor responses for both groups of mice were calculated as the difference between the response to caffeine versus saline injection. Although showing similar responses to low dose caffeine (Fig. 6A), Gng7−/− mice showed a significantly attenuated response to high dose caffeine that was particularly apparent at later time points (Fig. 6B; MANOVA analysis: F1,30 = 7.4, p = 0.01). Because locomotor behavior is influenced by genetic background (38), these studies were repeated on Gng7−/− mice on the BALB/c background to assess the generality of this finding. Even more strikingly, Gng7−/− mice on the BALB/c background exhibited significantly reduced responses to both low and high dose caffeine (Fig. 6, C and D; MANOVA analyses: for the low dose, F1,24 = 16.4, p = 0.0005 and for the high dose, F1,24 = 8.6, p = 0.007). Taken together, these results show a clear association between defective A2AR signaling and impaired locomotor response to caffeine in Gng7−/− mice.
FIGURE 6.
Increase in locomotor activity in CLAMS cages in response to an intraperitoneal injection, at 60 min (arrow), of caffeine 7.5 mg/kg (A and C) or 15 mg/kg (B and D) for Gng7−/− mice (KO) and wild-type littermates (WT) on either the C57BL/6 background (A and B) or the BALB/c background (C and D). Data are expressed as the average ambulatory activity in both the x- and y-dimensions, consecutive photobeam breaks per minute, over each 30-min interval for a trial with drug injection minus the average over the same interval for a trial with saline injection (ΔXY Amb.). On the C57BL/6 background, genotype was a significant factor in a repeated measures MANOVA only for the high dose (F1,30 = 7.4, p = 0.01), on the BALB/c background genotype was significant for both the low dose (F1,24 = 16.4, p = 0.0005) and the high dose (F1,24 = 8.6, p = 0.007).
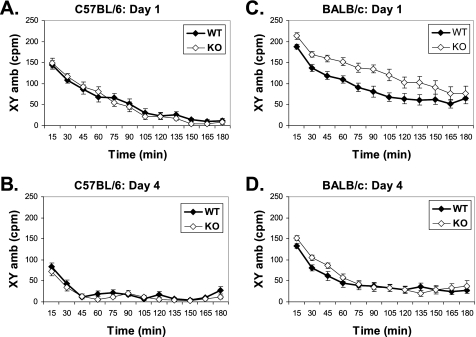
In addition to psychostimulant response, we also measured the basal locomotor activity that reflects the coordinated output from the SN and SP tracts (10, 11) in these animals. Previously, we showed that the basal locomotor activity of Gng7−/− mice was not impaired despite reduced striatal D1R signaling (6). Because increased D1R signaling is generally associated with a higher level of locomotor activity (39), this finding was somewhat surprising. To exclude the possibility that the background strain was obscuring any defect due to loss of γ7, we compared the basal locomotor activities of Gng7−/− mice and their wild-type littermates on two different genetic backgrounds. On the C57BL/6 background, both wild-type and knock-out mice displayed comparably high levels of locomotor activity when introduced into CLAMS cages (Fig. 7A) and similar abilities to acclimate to the novel environment (Fig. 7B). That is to say, the initially high levels of locomotor activity observed in both groups decreased similarly over the 3 h following initial exposure to the CLAMS cages (Fig. 7A) and over the course of 4 days upon repeated exposure to the CLAMS cages (Fig. 7B). On the BALB/c background, Gng7−/− mice exhibited a significantly higher locomotor activity than their wild-type littermates when introduced into CLAMS cages (Fig. 7C; MANOVA analysis: F1,38 = 11.3, p = 0.002) but showed no differences in their abilities to acclimate to the novel environment (Fig. 7, C and D). Collectively, these findings support the possibility that combinatorial disruption of both D1R and A2AR signaling pathways secondary to loss of the γ7 protein produces overtly normal motor activity.
FIGURE 7.
Locomotor activity in CLAMS cages during 3 h trials, expressed as the ambulatory activity per minute in both the x- and y-dimensions (XY amb) averaged over 15 min intervals. Results are for trials on the first (A and C) and the fourth (B and D) consecutive day, for Gng7−/− mice (KO) and wild-type littermates (WT) on either the C57BL/6 background (A and B) or the BALB/c background (C and D). In a repeated measures MANOVA, day (F1,136 = 108, p < 0.0001), genotype (F1,136 = 7.9, p = 0.006) and background (F1,136 = 85.3, p < 0.0001) were all significant factors, and there was a significant genotype × background interaction (F1,136 = 9.7, p = 0.002). On the BALB/c background, Gng7−/− mice were more active than wild-type littermates on Day 1 (F1,38 = 11.3, p = 0.002).
DISCUSSION
Despite their molecular cloning more than a decade ago (2), the functional significance for the large diversity of G-protein γ subunits is still not known. From analysis of Gng7−/− mice, we now show that loss of the G-protein γ7 subtype produces both biochemical and behavioral consequences. In the process of studying these effects, we also identify a fundamental role for the γ7 subtype in driving the preferential assembly of a G-protein αolfβ2γ7 heterotrimer that is required for A2AR signaling and its locomotor inhibitory effect in the striatum. These results support a growing body of data pointing to the effectiveness of A2AR blockade to better normalize motor activity in Parkinson patients (40, 41).
Golf Assembly Is Specifically Regulated by the γ7 Subtype
The functions of G-protein αβγ heterotrimers are dependent on their proper assembly and trafficking to the plasma membrane (37, 42). However, very little is known regarding which αβγ heterotrimers exist in the intact cell setting and how they are actually assembled. Previously, our analysis of Gng7−/− mice provided the first in vivo demonstration that loss of the γ7 protein disrupts the assembly of the Golf but not the Gs heterotrimer in the striatum (6). Now, we extend this finding by showing through reciprocal analysis of Gnal−/− mice that loss of the αolf protein does not substantially impact the level of the γ7 protein. Taken together, these results point to a hierarchical order of Golf formation that begins with the γ7 subunit. In hindsight, the γ7 subtype has several characteristics consistent with a primary role in this process. Spanning >66 kb in size, the mouse Gng7 gene produces multiple mRNA transcripts that encode the same protein, suggesting complex regulation of its expression. Moreover, the mouse γ7 protein encompasses only 69 amino acids that assume an α-helical structure in solution (43) that approximates that seen in the crystal structure (44). This suggests that unlike its α (45) and β (46, 47) partners, chaperone-type proteins may not be required for proper folding of the γ7 protein. Finally, indicating that a “γ subunit first” hierarchy may be generally applicable to other G-protein αβγ heterotrimers, loss of the γt1 subtype has recently been shown to disrupt assembly of a specific G-protein αt1β1γt1 heterotrimer in the retina (48).
At this time, we can only hypothesize as to the mechanism by which the γ7 protein sets the level of the Golf heterotrimer. Because the corresponding mRNAs were not altered, the simplest explanation for coordinately reduced αolf and β2 protein levels is a post-transcriptional requirement for the γ7 in the stabilization and/or trafficking of Golf to the plasma membrane. Because loss of γ7 protein was not associated with accumulation of unassembled αolf protein in the cytosol (Fig. 5), we speculate that formation of this Golf results from stabilization of the αolf and β2 subunits by the γ7 subunit, and that in the absence of this component, the unassembled αolf and β2 subunits undergo active degradation.
Likewise, at this stage, we can only speculate as to the basis for the unique requirement for γ7 subtype in this process. Refuting the long-standing dogma that most γ subunits are functionally interchangeable (reviewed in Ref. 2), the γ7 subtype must possess unique features that cannot be replaced by the other γ subtypes. To begin to identify such features, we examined the possibility that the reportedly high abundance of the γ7 subtype in the striatum (49, 50) could provide an explanation. In fact, the γ2 and γ3 proteins are present together in 4-fold molar excess to the γ7 subtype (supplemental Fig. S2 and Table 1). Therefore, abundance does not seem to be the answer. Next, we considered the possibility that the γ subtypes might be sequestered between different neuronal types making up the striatum. Two recent studies (30, 31) comparing the translational mRNA profiles of SN and SP neurons reveal that both cell types express multiple γ forms (supplemental Fig. S3). Therefore, cell type specific expression does not explain the failure of other γ subtypes to substitute for the role of γ7 in assembly process. Finally, we are contemplating the possibility that the γ7 subtype could be localized within a particular subcellular compartment in neurons, similar to that shown for the γ5 subtype in focal adhesions (51). Such compartmentation could result from a structural feature that is unique to either the γ7 mRNA or protein. In this regard, it is notable that the γ7 mRNA contains a very long 3′-UTR, a region that has been implicated in translational regulation and subcellular targeting of dendritically targeted proteins (52).
A2AR Signaling Is Dependent on a Specific G-protein αolfβ2γ7 Heterotrimer
The A2AR activates adenylyl cyclase activity in SP neurons (27, 12, 53, 54). Consistent with a requirement for the αolfβ2γ7 heterotrimer in this pathway, both A2AR-G-protein coupling and adenylyl cyclase activation are markedly reduced in knock-out membranes (Figs. 2 and 1). In looking for a functional connection, it is tempting to compare the >75% reduction in A2AR-G-protein coupling with the 85% reduction in αolf protein (Fig. 3 and supplemental Fig. S1). Because the A2AR number (Fig. 2, as determined by antagonist binding) is several orders of magnitude lower than the total Golf content (Table 1, as determined by quantitative immunoblotting), the random collision coupling model (55) would predict that the receptor represents the rate-limiting step for adenylyl cyclase activation. However, such a model is incompatible with several published reports. Notably, only about 20% of the A2AR is actually coupled to G-protein in platelets (56) and striatum (14, 57). Furthermore, in these tissues, the G-protein appears to control the rate of adenylyl cyclase activation (58). Thus, despite the apparent excess of Golf, it appears that the A2AR interacts with only a limited pool of Golf and that the latter controls the cellular response. Although not yet identified, the cellular mechanisms responsible for limiting their interaction could include post-translational modification and/or subcellular localization. In HEK293 cells, A2AR coupling to Gs and activation of adenylyl cyclase has been shown to occur within cholesterol-rich microdomains (59). In future studies, it will be interesting to investigate the functional significance for the poor coupling between the A2AR and Golf in the striatum and to elucidate the underlying mechanism. Finally, in an analogous fashion, the D1R activates adenylyl cyclase activity in SN neurons (12). Because this receptor utilizes the same G-protein αolfβ2γ7 heterotrimer (6), it will be interesting to explore whether poor coupling of the D1R is also observed in the striatum and whether the Golf represents the rate-limiting step in adenylyl cyclase activation. Supporting the latter possibility, our previous analyses of mutant Drda1a and Gnal mice have revealed that the G-protein rather than the receptor controls psychostimulant responses (60).
Taken in conjunction with our earlier studies, the G-protein γ7 subtype seems to play a special role in adenylyl cyclase stimulation in various cellular contexts. In HEK293 cells, the γ7 subtype is responsible for driving the assembly of a particular Gs heterotrimer required for β adrenergic and D1R signaling but not for PGE2 and D5R signaling (61, 62); and in the physiological relevant context of the brain, it is responsible for driving the assembly of a specific Golf heterotrimer required for D1R signaling in SN neurons (6) and for A2AR signaling in SP neurons (Figs. 1 and 2). In addition to its role in assembly, it remains to be determined whether the γ7 subunit performs additional roles in the signal transduction process. In this regard, the βγ dimer has been suggested to contribute to recognition of the upstream receptor (63, 15), as well as downstream regulation of effectors such as the striatal-enriched adenylyl cyclase type 5 isoform (64, 65). Addressing whether the γ7 subunit affects these processes will require the use of complementary approaches that bypass the requirement for the γ7 subtype in the assembly of Golf.
Altered Caffeine Responsiveness Is Associated with Impaired A2AR Signaling
Previous studies of the locomotor stimulating effect of caffeine have demonstrated the primary involvement of the A2AR (66, 21). Supporting its function acting downstream of this receptor, Gng7−/− mice lacking the γ7 subtype showed an attenuated response to caffeine that was particularly apparent on the BALB/c background (Fig. 7). Moreover, consistent with their functioning as components of the same heterotrimeric G-protein, Gnal−/− mice lacking the αolf subtype also exhibited a reduced response to caffeine (16). Finally, in agreement with a role for Golf in adenylyl cyclase stimulation and protein kinase activation, Ppp1r1b−/− mice lacking DARPP-32, which is phosphorylated by protein kinase A (67), also showed an impaired response to caffeine (68). Taken together, these results establish the importance of the A2AR-Gαolfβ2γ7-AC5-PKA-DARPP-32 pathway in mediating the psychostimulant properties of caffeine.
Clinical Significance
Despite defects in both D1R and A2AR signaling, deficiency of the G-protein γ7 subtype has little impact on basal locomotor behavior (Fig. 7). This finding is similar to reported findings for Drd1a−/− and Ador2a−/− mice, i.e. slightly increased or decreased locomotor activity (69, 21). In contrast, Drd2−/− mice display a markedly reduced locomotor activity (70–72). In this regard, the ability of A2AR signaling to modulate D2R signaling could prove beneficial for the treatment of Parkinson disease. For instance, several groups have observed attenuated stimulation of locomotor activity in response to caffeine in Drd2−/− mice (73), and a partial reversal of acute D2R antagonist-induced catalepsy in Ador2a−/− mice (66). Such results form the basis for clinical trials of adenosine antagonists in the treatment of Parkinson disease (26). In these trials, Istradefylline has been shown to reduce symptoms in patients with Parkinson disease on levodopa therapy (40, 41). Our studies further support targeting of the A2AR signaling pathway as an augmentative strategy for treating Parkinson disease and provide a better mechanistic understanding of the apparent effectiveness of this treatment.
Supplementary Material
Acknowledgments
We thank Hilary Hoffman, Cynthia Rhone, Gail Gregory, and Shannon Wescott for skilled technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants GM39867 (to J. D. R.) and HL37942 (to J. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- D1R
- dopamine D1 receptor
- A2AR
- adenosine A2A receptor
- SP
- striatopallidal
- SN
- striatonigral
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1.Hildebrandt J. D. (1997) Biochem. Pharmacol. 54, 325–339 [DOI] [PubMed] [Google Scholar]
- 2.Robishaw J. D., Berlot C. H. (2004) Curr. Opin. Cell Biol. 16, 206–209 [DOI] [PubMed] [Google Scholar]
- 3.Birnbaumer L. (2007) Biochim. Biophys. Acta 768, 772–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smrcka A. V. (2008) Cell Mol. Life Sci. 65, 2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offermanns S. (2001) Oncogene 20, 1635–1642 [DOI] [PubMed] [Google Scholar]
- 6.Schwindinger W. F., Betz K. S., Giger K. E., Sabol A., Bronson S. K., Robishaw J. D. (2003) J. Biol. Chem. 278, 6575–6579 [DOI] [PubMed] [Google Scholar]
- 7.Schwindinger W. F., Giger K. E., Betz K. S., Stauffer A. M., Sunderlin E. M., Sim-Selley L. J., Selley D. E., Bronson S. K., Robishaw J. D. (2004) Mol. Cell Biol. 24, 7758–7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matamales M., Bertran-Gonzalez J., Salomon L., Degos B., Deniau J. M., Valjent E., Hervé D., Girault J. A. (2009) PLoS One 4, e4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cauli O., Morelli M. (2005) Behav. Pharmacol. 16, 63–77 [DOI] [PubMed] [Google Scholar]
- 10.Albin R. L., Young. A. B., Penney J. B. (1989) Trends Neurosci. 12, 366–375 [DOI] [PubMed] [Google Scholar]
- 11.Popoli P., Reggio R., Pèzzola A. (2000) Neuropsychopharmacology 22, 522–529 [DOI] [PubMed] [Google Scholar]
- 12.Corvol J. C., Studler J. M., Schonn J. S., Girault J. A., Hervé D. (2001) J. Neurochem. 76, 1585–1588 [DOI] [PubMed] [Google Scholar]
- 13.Belluscio L., Gold G. H., Nemes A., Axel R. (1998) Neuron 20, 69–81 [DOI] [PubMed] [Google Scholar]
- 14.Luthin D. R., Olsson R. A., Thompson R. D., Sawmiller D. R., Linden J. (1995) Mol. Pharmacol. 47, 307–313 [PubMed] [Google Scholar]
- 15.Murphree L. J., Marshall M. A., Rieger J. M., MacDonald T. L., Linden J. (2002) Mol. Pharmacol. 61, 455–462 [DOI] [PubMed] [Google Scholar]
- 16.Hervé D., Le Moine C., Corvol J. C., Belluscio L., Ledent C., Fienberg A. A., Jaber M., Studler J. M., Girault J. A. (2001) J. Neurosci. 21, 4390–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster K. A., McDermott P. J., Robishaw J. D. (1990) Am. J. Physiol. 259, H432–H441 [DOI] [PubMed] [Google Scholar]
- 18.Cali J. J., Balcueva E. A., Rybalkin I., Robishaw J. D. (1992) J. Biol. Chem. 267, 24023–24027 [PubMed] [Google Scholar]
- 19.Wang Q., Mullah B. K., Robishaw J. D. (1999) J. Biol. Chem. 274, 17365–17371 [DOI] [PubMed] [Google Scholar]
- 20.Schwindinger W. F., Borrell B. M., Waldman L. C., Robishaw J. D. (2009) Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1494–R1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledent C., Vaugeois J. M., Schiffmann S. N., Pedrazzini T., El Yacoubi M., Vanderhaeghen J. J., Costentin J., Heath J. K., Vassart G., Parmentier M. (1997) Nature 388, 674–678 [DOI] [PubMed] [Google Scholar]
- 22.Huang Z. L., Qu W. M., Eguchi N., Chen J. F., Schwarzschild M. A., Fredholm B. B., Urade Y., Hayaishi O. (2005) Nat. Neurosci. 8, 858–859 [DOI] [PubMed] [Google Scholar]
- 23.Salamone J. D., Ishiwari K., Betz A. J., Farrar A. M., Mingote S. M., Font L., Hockemeyer J., Müller C. E., Correa M. (2008) Parkinsonism Relat. Disord. 14, S130–S134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoyama S., Kase H., Borrelli E. (2000) J. Neurosci. 20, 5848–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bara-Jimenez W., Sherzai A., Dimitrova T., Favit A., Bibbiani F., Gillespie M., Morris M. J., Mouradian M. M., Chase T. N. (2003) Neurology 61, 293–296 [DOI] [PubMed] [Google Scholar]
- 26.Schwarzschild M. A., Agnati L., Fuxe K., Chen J. F., Morelli M. (2006) Trends Neurosci. 29, 647–654 [DOI] [PubMed] [Google Scholar]
- 27.Kull B., Svenningsson P., Fredholm B. B. (2000) Mol. Pharmacol. 58, 771–777 [DOI] [PubMed] [Google Scholar]
- 28.Borgkvist A., Fisone G. (2007) Neurosci. Biobehav. Rev. 31, 79–88 [DOI] [PubMed] [Google Scholar]
- 29.Watson J. B., Coulter P. M., 2nd, Margulies J. E., de Lecea L., Danielson P. E., Erlander M. G., Sutcliffe J. G. (1994) J. Neurosci. Res. 39, 108–116 [DOI] [PubMed] [Google Scholar]
- 30.Doyle J. P., Dougherty J. D., Heiman M., Schmidt E. F., Stevens T. R., Ma G., Bupp S., Shrestha P., Shah R. D., Doughty M. L., Gong S., Greengard P., Heintz N. (2008) Cell 135, 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiman M., Schaefer A., Gong S., Peterson J. D., Day M., Ramsey K. E., Suárez-Fariñas M., Schwarz C., Stephan D. A., Surmeier D. J., Greengard P., Heintz N. (2008) Cell 135, 738–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hervé D., Lévi-Strauss M., Marey-Semper I., Verney C., Tassin J. P., Glowinski J., Girault J. A. (1993) J. Neurosci. 13, 2237–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iñiguez-Lluhi J. A., Simon M. I., Robishaw J. D., Gilman A. G. (1992) J. Biol. Chem. 267, 23409–23417 [PubMed] [Google Scholar]
- 34.Simonds W. F., Butrynski J. E., Gautam N., Unson C. G., Spiegel A. M. (1991) J. Biol. Chem. 266, 5363–5366 [PubMed] [Google Scholar]
- 35.Muntz K. H., Sternweis P. C., Gilman A. G., Mumby S. M. (1992) Mol. Biol. Cell 3, 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehm A., Ploegh H. L. (1997) J. Cell Biol. 137, 305–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrari Y., Crouthamel M., Irannejad R., Wedegaertner P. B. (2007) Biochemistry 46, 7665–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X., Orchard S. M., Sanford L. D. (2002) Behav. Brain Res. 136, 555–569 [DOI] [PubMed] [Google Scholar]
- 39.Kelly M. A., Low M. J., Rubinstein M., Phillips T. J. (2008) Genes Brain Behav. 7, 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauser R. A., Shulman L. M., Trugman J. M., Roberts J. W., Mori A., Ballerini R., Sussman N. M.for the Istradefylline 6002-US-013 Study Group (2008) Mov. Disord. 23, 2177–2185 [DOI] [PubMed] [Google Scholar]
- 41.Stacy M., Silver D., Mendis T., Sutton J., Mori A., Chaikin P., Sussman N. M. (2008) Neurology 70, 2233–2240 [DOI] [PubMed] [Google Scholar]
- 42.Dupré D. J., Robitaille M., Rebois R. V., Hébert T. E. (2009) Annu. Rev. Pharmacol. Toxicol. 49, 31–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin E. P., Neubig R. R. (1995) Biochem. J. 309, 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sondek J., Bohm A., Lambright D. G., Hamm H. E., Sigler P. B. (1996) Nature 379, 369–374 [DOI] [PubMed] [Google Scholar]
- 45.Natochin M., Campbell T. N., Barren B., Miller L. C., Hameed S., Artemyev N. O., Braun J. E. A. (2005) J. Biol. Chem. 280, 30236–30241 [DOI] [PubMed] [Google Scholar]
- 46.Wells C. A., Dingus J., Hildebrandt J. D. (2006) J. Biol. Chem. 281, 20221–20232 [DOI] [PubMed] [Google Scholar]
- 47.Lukov G. L., Baker C. M., Ludtke P. J., Hu T., Carter M. D., Hackett R. A., Thulin C. D., Willardson B. M. (2006) J. Biol. Chem. 281, 22261–22274 [DOI] [PubMed] [Google Scholar]
- 48.Lobanova E. S., Finkelstein S., Herrmann R., Chen Y. M., Kessler C., Michaud N. A., Trieu L. H., Strissel K. J., Burns M. E., Arshavsky V. Y. (2008) J. Neurosci. 28, 3510–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., Chen L., Chen L., Chen T. M., Chin M. C., Chong J., Crook B. E., Czaplinska A., Dang C. N., Datta S., Dee N. R., Desaki A. L., Desta T., Diep E., Dolbeare T. A., Donelan M. J., Dong H. W., Dougherty J. G., Duncan B. J., Ebbert A. J., Eichele G., Estin L. K., Faber C., Facer B. A., Fields R., Fischer S. R., Fliss T. P., Frensley C., Gates S. N., Glattfelder K. J., Halverson K. R., Hart M. R., Hohmann J. G., Howell M. P., Jeung D. P., Johnson R. A., Karr P. T., Kawal R., Kidney J. M., Knapik R. H., Kuan C. L., Lake J. H., Laramee A. R., Larsen K. D., Lau C., Lemon T. A., Liang A. J., Liu Y., Luong L. T., Michaels J., Morgan J. J., Morgan R. J., Mortrud M. T., Mosqueda N. F., Ng L. L., Ng R., Orta G. J., Overly C. C., Pak T. H., Parry S. E., Pathak S. D., Pearson O. C., Puchalski R. B., Riley Z. L., Rockett H. R., Rowland S. A., Royall J. J., Ruiz M. J., Sarno N. R., Schaffnit K., Shapovalova N. V., Sivisay T., Slaughterbeck C. R., Smith S. C., Smith K. A., Smith B. I., Sodt A. J., Stewart N. N., Stumpf K. R., Sunkin S. M., Sutram M., Tam A., Teemer C. D., Thaller C., Thompson C. L., Varnam L. R., Visel A., Whitlock R. M., Wohnoutka P. E., Wolkey C. K., Wong V. Y., Wood M., Yaylaoglu M. B., Young R. C., Youngstrom B. L., Yuan X. F., Zhang B., Zwingman T. A., Jones A. R. (2007) Nature 445, 168–176 [DOI] [PubMed] [Google Scholar]
- 51.Hansen C. A., Schroering A. G., Carey D. J., Robishaw J. D. (1994) J. Cell Biol. 126, 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreassi C., Riccio A. (2009) Trends Cell Biol. 19, 465–474 [DOI] [PubMed] [Google Scholar]
- 53.Fredholm B. B., IJzerman A. P., Jacobson K. A., Klotz K. N., Linden J. (2001) Pharmacol. Rev. 53, 527–552 [PMC free article] [PubMed] [Google Scholar]
- 54.Fredholm B. B., Chen J. F., Masino S. A., Vaugeois J. M. (2005) Annu. Rev. Pharmacol. Toxicol. 45, 385–412 [DOI] [PubMed] [Google Scholar]
- 55.Tolkovsky A. M., Braun S., Levitzki A. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lohse M. J., Klotz K. N., Schwabe U. (1991) Mol. Pharmacol. 39, 517–523 [PubMed] [Google Scholar]
- 57.Luthin D. R., Lee K. S., Okonkwo D., Zhang P., Linden J. (1995) J. Neurochem. 65, 2072–2079 [DOI] [PubMed] [Google Scholar]
- 58.Gross W., Lohse M. J. (1991) Mol. Pharmacol. 39, 524–530 [PubMed] [Google Scholar]
- 59.Charalambous C., Gsandtner I., Keuerleber S., Milan-Lobo L., Kudlacek O., Freissmuth M., Zezula J. (2008) J. Biol. Chem. 283, 9276–9288 [DOI] [PubMed] [Google Scholar]
- 60.Corvol J. C., Valjent E., Pascoli V., Robin A., Stipanovich A., Luedtke R. R., Belluscio L., Girault J. A., Hervé D. (2007) Neuropsychopharmacology 32, 1109–1121 [DOI] [PubMed] [Google Scholar]
- 61.Wang Q., Mullah B., Hansen C., Asundi J., Robishaw J. D. (1997) J. Biol. Chem. 272, 26040–26048 [DOI] [PubMed] [Google Scholar]
- 62.Wang Q., Jolly J. P., Surmeier J. D., Mullah B. M., Lidow M. S., Bergson C. M., Robishaw J. D. (2001) J. Biol. Chem. 276, 39386–39393 [DOI] [PubMed] [Google Scholar]
- 63.Richardson M., Robishaw J. D. (1999) J. Biol. Chem. 274, 13525–13533 [DOI] [PubMed] [Google Scholar]
- 64.Gao X., Sadana R., Dessauer C. W., Patel T. B. (2007) J. Biol. Chem. 282, 294–302 [DOI] [PubMed] [Google Scholar]
- 65.Lee K. W., Hong J. H., Choi I. Y., Che Y., Lee J. K., Yang S. D., Song C. W., Kang H. S., Lee J. H., Noh J. S., Shin H. S., Han P. L. (2002) J. Neurosci. 22, 7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J. F., Moratalla R., Impagnatiello F., Grandy D. K., Cuellar B., Rubinstein M., Beilstein M. A., Hackett E., Fink J. S., Low M. J., Ongini E., Schwarzschild M. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svenningsson P., Lindskog M., Rognoni F., Fredholm B. B., Greengard P., Fisone G. (1998) Neuroscience 84, 223–228 [DOI] [PubMed] [Google Scholar]
- 68.Lindskog M., Svenningsson P., Pozzi L., Kim Y., Fienberg A. A., Bibb J. A., Fredholm B. B., Nairn A. C., Greengard P., Fisone G. (2002) Nature 418, 774–778 [DOI] [PubMed] [Google Scholar]
- 69.Clifford J. J., Tighe O., Croke D. T., Sibley D. R., Drago J., Waddington J. L. (1998) Neuropharmacology 37, 1595–1602 [DOI] [PubMed] [Google Scholar]
- 70.Baik J. H., Picetti R., Saiardi A., Thiriet G., Dierich A., Depaulis A., Le Meur M., Borrelli E. (1995) Nature 377, 424–428 [DOI] [PubMed] [Google Scholar]
- 71.Kelly M. A., Rubinstein M., Phillips T. J., Lessov C. N., Burkhart-Kasch S., Zhang G., Bunzow J. R., Fang Y., Gerhardt G. A., Grandy D. K., Low M. J. (1998) J. Neurosci. 18, 3470–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmes A., Lachowicz J. E., Sibley D. R. (2004) Neuropharmacology 47, 1117–1134 [DOI] [PubMed] [Google Scholar]
- 73.Zahniser N. R., Simosky J. K., Mayfield R. D., Negri C. A., Hanania T., Larson G. A., Kelly M. A., Grandy D. K., Rubinstein M., Low M. J., Fredholm B. B. (2000) J. Neurosci. 20, 5949–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.