Abstract
The Arabidopsis ABC transporter Comatose (CTS; AtABCD1) is required for uptake into the peroxisome of a wide range of substrates for β-oxidation, but it is uncertain whether CTS itself is the transporter or if the transported substrates are free acids or CoA esters. To establish a system for its biochemical analysis, CTS was expressed in Saccharomyces cerevisiae. The plant protein was correctly targeted to yeast peroxisomes, was assembled into the membrane with its nucleotide binding domains in the cytosol, and exhibited basal ATPase activity that was sensitive to aluminum fluoride and abrogated by mutation of a conserved Walker A motif lysine residue. The yeast pxa1 pxa2Δ mutant lacks the homologous peroxisomal ABC transporter and is unable to grow on oleic acid. Consistent with its exhibiting a function in yeast akin to that in the plant, CTS rescued the oleate growth phenotype of the pxa1 pxa2Δ mutant, and restored β-oxidation of fatty acids with a range of chain lengths and varying degrees of desaturation. When expressed in yeast peroxisomal membranes, the basal ATPase activity of CTS could be stimulated by fatty acyl-CoAs but not by fatty acids. The implications of these findings for the function and substrate specificity of CTS are discussed.
Keywords: ABC Transporter, Fatty Acid Oxidation, Fatty Acid Transport, Peroxisomes, Plant, Protein Targeting, Yeast
Introduction
Peroxisomes perform a range of different functions, including β-oxidation of fatty acids (FA)2 and synthesis and degradation of bioactive, lipid-derived molecules. Import of substrates for peroxisomal metabolism is mediated by ATP binding cassette (ABC) transporters belonging to subclass D (1, 2). ABC transporters are composed of a minimum of four functional domains: two transmembrane domains, involved in substrate binding and translocation, and two nucleotide binding domains (NBDs) that bind and hydrolyze ATP, providing energy for transport (3, 4). The domains may be fused into a single polypeptide, but are frequently expressed as half-size transporters composed of a transmembrane domain fused to an NBD, which hetero- or homodimerize to form a functional transporter. Bakers' yeast (Saccharomyces cerevisiae) contains two ABCD genes that encode half-size ABC proteins: Pxa1p (peroxisomal ABC-transporter 1), and Pxa2p (5–7). The single pxa1Δ and pxa2Δ deletion mutants are unable to grow on oleate (C18:1) as the sole carbon source and exhibit reduced β-oxidation of this long-chain FA. It has been proposed that Pxa1p and Pxa2p operate as a heterodimer to form a functional transporter (6, 8, 9), which has been shown by indirect evidence to be required for the peroxisomal transport of the C18:1-CoA, a long-chain acyl-CoA ester, but not for import of C8:0-CoA (10). In contrast, medium-chain FAs enter yeast peroxisomes as free acids independently of Pxa1p/Pxa2p, and are activated by peroxisomal acyl-CoA synthetase, Faa2p, prior to β-oxidation (6).
The human ABCD transporter subfamily comprises four half-size members: adrenoleukodystrophy protein (ALDP), ALD-related protein, the 70-kDa peroxisomal membrane protein (PMP70), and PMP70-related protein (PMP70R/PMP69) (1, 2). Although ALDP, ALD-related protein, and PMP70 have been implicated in lipid transport across the peroxisomal membrane (2, 11–13), the precise biochemical functions of these transporters are not well understood. However, ALDP partially complements the yeast pxa1 pxa2Δ mutant for growth on oleate and β-oxidation of long-chain FAs (9), suggesting that ALDP acts as a homodimeric transporter that accepts acyl-CoA esters (9). In addition, in a study using protease sensitivity as a probe of conformational changes in ALDP, increased sensitivity was observed upon addition of long and very long-chain FA-CoAs but not free FAs (14). Taken together, these studies suggest a role of ALDP in the transport of very long-chain FA-CoAs across the peroxisome membrane, although this hypothesis awaits confirmation by carrying out transport studies in a reconstituted system.
The reference plant Arabidopsis thaliana contains a single peroxisomal ABC transporter that has been identified in at least four independent forward genetic screens and is known as AtABCD1 (15), CTS (Comatose (16)), AtPXA1 (A. thaliana peroxisomal ABC transporter 1 (17)), PED3 (peroxisomal defective 3 (18)), and ACN2 (acetate non-utilizing 2 (19)), referred to hereafter as CTS. Unlike the yeast and mammalian peroxisomal ABC proteins, CTS encodes a full-size ABC protein with two dissimilar halves. Analysis of cts null mutants has demonstrated that CTS plays key roles in a number of developmental and physiological processes, including germination, seedling establishment, fertility, root growth, and dark-induced senescence (16–18, 20–27). The different roles of CTS in planta are separable by mutagenesis (28) and can be related to different biochemical roles, specifically the ability to metabolize distinct substrates such as fatty acids and hormone precursors via β-oxidation. Taken together, these findings strongly suggest that CTS is a transporter with broad acyl chain specificity, which mediates uptake of substrates for β-oxidation into the peroxisome. However, this conclusion is based on inferences from amino acid sequence identity with better characterized ABC transporters and in vivo mutant phenotypes that can be pleiotropic. There has been no direct biochemical evidence to support this interpretation. Additionally, there are arguments in favor of both acyl-CoAs (16) and free fatty acids as substrates (29).
Because plant peroxisomes are fragile and difficult to purify from Arabidopsis seedlings free from mitochondrial contamination, in this paper we have carried out biochemical characterization of heterologously expressed CTS, and provide evidence for its role as a transporter of fatty acyl-CoAs.
EXPERIMENTAL PROCEDURES
Yeast Strains and Culture Conditions
The yeast strains used in this study are shown in Table 1. Yeast transformants were selected and grown on minimal medium with appropriate supplements. A single colony of yeast was inoculated into 10 ml of WOYD medium (0.67% (w/v) yeast nitrogen base (BD Biosciences, Oxford, UK), 0.3% (w/v) glucose, 0.1% (w/v) yeast extract) and incubated at 28 °C for 18 h. For peroxisome proliferation, 5 ml of the resultant yeast suspension was used to inoculate a liter of oleate medium (0.5% (w/v) Bacto-peptone, 0.3% (w/v) yeast extract, 0.12% (v/v) oleic acid, 0.2% (v/v) Tween 20, 0.5% (w/v) KH2PO4, pH 6.0) followed by incubation at 28 °C for 18 h (A600 of 1.0 to 1.5) (30).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BSL1- 11B (WT) | MATa, lys2Δ0, his4Δ519, Ura3Δ0, leu2Δ2 | Ref. 79 |
| BSL1–11B (pxa1Δ) | MATa, lys2Δ0, his4Δ519, Ura3Δ0, leu2Δ2, YPL147w::kanMX4 | This study |
| BJ1991 (WT) | MATα, leu2, trp1, ura3–251, prb1–1122, pep4–3 | Ref. 9 |
| BJ1991 (pxa1Δ) | MATα, leu2, trp1, ura3–251, prb1–1122, pep4–3 YPL147w::LEU2 | Ref. 9 |
| BJ1991(pxa1 pxa2Δ) | MATα, leu2, trp1, ura3–251, prb1–1122, pep4–3 YKL188c::kanMX4; YPL147w::LEU2 | Ref. 9 |
| BJ1991 (fox1Δ) | MATα, leu2, trp1, ura3–251, prb1–1122, pep4–3 (YGL205W::kanMX4) | Ref. 9 |
Construction of a pxa1Δ Mutant Strain
Gene replacement was used to generate a BSL1-11 B strain lacking the PXA1 gene. The KanMX4 disruption cassette was amplified using primers PXA1KOF (CGTTTCAGACAATCTGGAAGTCTTAGAACGCATAACACAGAAATGGCTTCGTACGCTGCAGGTCG) and PXA1KOR (ATTATATTCGCTAAATAAAATCTCTCCCTTTCTAGGGTGTTTTCACACTATAGGGAGACCGGCAGATCC) with plasmid pFA6kanMX4 (31) as template. The following program was used: 95 °C, 2 min; 30 cycles of 94 °C, 30 s; 60 °C, 30 s; 72 °C, 1 min, followed by a final extension at 72 °C for 5 min. The PCR product was transformed into BSL1-11B cells and putative pxa1Δ knockouts were selected on SD agar supplemented with 500 μg/ml of geneticin (G418; Melford, Ispwich, Suffolk, UK). Following re-streaking on SD-agar plates containing geneticin, colonies were inoculated in WOYD medium for genomic DNA extraction (32). Integration of the cassette in the correct locus was confirmed by PCR.
Construction of CTS Expression Plasmids
To generate a construct for expression of CTS in S. cerevisiae, a VspI (blunt)/EcoRI fragment containing the CTS ORF was ligated into the SmaI/EcoRI sites of pRS416-GPD (33, 34), to give CTS/pRS416-GPD. The K487A mutant was generated by site-directed mutagenesis, as described in Dietrich et al. (28). To create an in-frame CFP fusion with a 10-amino acid spacer between the C terminus of CTS and CFP, the native stop codon of CTS was removed by site-directed mutagenesis using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA), with primer pair CTSstopF (GAACAGACAACAGAGTCAGAATTCGATATCAAGC) and CTSstopR (CTTGTCTGTTGTCTCAGTCTTAAGCTATAGTTCG), according to the manufacturer's instructions. The removal of the stop codon and absence of second site mutations were confirmed by sequencing of the entire CTS coding sequence. CFP was then amplified using primers CFPsF (AATTGGGGAATTCGGTTCAGCAGCTGGATCAGGTGCTAGCGCAATGGTGAGCAAGGGCGAGG) and CFPR (CCCAATTGAATTCTTACTTGTACAGCACGTCCATGCC), with plasmid pVKh18En6 CFP-SKL (35) as template. The following PCR program was used: 94 °C, 2 min; 94 °C, 30 s; 65 °C, 30 s; 72 °C, 1 min (30 cycles) plus a final extension for 5 min at 72 °C. The primers introduced EcoRI restriction sites for subsequent cloning into CTS/pRS416-GPD linearized with EcoRI. The orientation of CFP was confirmed by sequencing. To generate a construct for complementation studies, a BamHI/SalI fragment containing the CTS ORF was excised from the CTS/pRS416-GPD plasmid and ligated into the BamHI/SalI sites of pEL30 (36), to give CTS/pEL30. The construction of ALDP/pEL30 is described in Ref. 9.
Subcellular Fractionation
A light mitochondrial fraction was prepared essentially according to Ref. 30. Briefly, yeast cells grown overnight on oleate medium were converted to spheroplasts with Zymolase 20T (ICN Biomedicals, Illkirch, France) at a concentration of 1 mg/g of cells. Spheroplasts were washed twice in wash buffer (50 mm potassium phosphate, pH 7.5, 1 mm EDTA, 1 mm KCl, 1.2 m sorbitol) before osmotic lysis in breaking buffer (5 mm MES, pH 5.5, 1 mm EDTA, 1 mm KCl, 0.65 m sorbitol) followed by Dounce homogenization. This and all subsequent steps were carried out at 4 °C. Unbroken cells and nuclei were removed by centrifuging the homogenate twice at 2,000 × g for 10 min. The post-nuclear supernatants were further centrifuged at 20,000 × g for 30 min to obtain a light mitochondrial fraction (pellet), containing mitochondria and peroxisomes. Organelles were purified further by centrifugation through an iodixanol density gradient. The organelle pellet was resuspended in 5 ml of homogenization buffer using 2 gentle strokes with a loose-fitting (Wheaton type B) Dounce homogenizer pestle and an equal volume of 47% (w/v) Optiprep (Axis Shield POC Oslo, Norway) was added. The mixture was carefully overlaid on a 2-ml cushion of 35% (w/v) Optiprep and centrifuged at 110,000 × g for 2 h in a swing-out rotor. Homogenization buffer and gradient medium contained protease inhibitors: 1 mm PMSF (Sigma), 1 μg/ml of Pefabloc® SC (Sigma) and 1:50 dilution of complete protease inhibitor mixture tablets (Roche Applied Science).
Immunoblotting
Immunoblotting was carried out as described in Ref. 16. Dilutions of primary antibodies were as follows: affinity purified anti-CTS, 1:1,000 (16); anti-thiolase, 1:100 (37); anti-AAC, 1:2,000 (38); anti-SRP72, 1:4,000 (39).
Carbonate and Salt Extractions
A light mitochondrial fraction was prepared as above. The pellet was resuspended in ×10 volume of hypotonic lysis buffer at 4 °C (10 mm Tris-HCl, pH 8.5, containing 1 mm PMSF, 1 μg/ml of Pefabloc SC (Sigma), and 1:50 dilution of complete protease inhibitor mixture tablets (Roche)). The pellet was disrupted in a Dounce homogenizer with a tight fitting pestle, using 10 stokes at 4 °C. The mixture was incubated at 4 °C for 20 min followed by homogenization as above. The mixture was centrifuged at 100,000 g for 1 h at 4 °C to pellet membranes and the supernatant was collected as the soluble fraction. The pellet (membrane fraction) was resuspended either in salt extraction buffer (1 m NaCl, 10 mm Tris-HCl, pH 8.5, or 100 mm Na2CO3, pH 11.5, supplemented with 10 mm DTT), to a concentration of 500 μg/ml; each solution contained protease inhibitors. The mixture was homogenized and incubated at 4 °C for 30 min followed by repeat homogenization and centrifugation to pellet membrane proteins. The pellet and supernatant fractions were assayed by SDS-PAGE and immunoblotting.
Determination of the Orientation of NBDs
A light mitochondrial fraction was homogenized in trypsin digestion buffer (0.65 m sorbitol, 50 mm Tris-HCl buffer, pH 7.5). For protease protection experiments, reactions contained 100 μl of homogenate (1 mg/ml of protein) and 0–50 μg of trypsin (Sigma), in the presence or absence of 0.1% (v/v) Triton X-100. Reactions were incubated on ice for 30 min and the reaction was stopped by the addition of 200 μl of 10 mg/ml of soybean trypsin inhibitor (Sigma). The samples were homogenized in ×10 volume of 0.1 m Na2CO3, pH 11.5, supplemented with 10 mm DTT and carbonate extraction carried out as above. The pellets and supernatants were analyzed by immunoblotting with anti-CTS and anti-thiolase antibodies.
Confocal Microscopy of Yeast Cells
Yeast transformed with plasmids to co-express CTS-CFP (CTS-CFP/pRS416-GPD) and YFP-PTS1 (pEW205) was grown in 10 ml of WOYD medium at 28 °C for 18 h. Cells were harvested by centrifugation at 5,000 × g, and resuspended in 1 ml of water. The suspension was used to inoculate oleate medium at an A600 of 0.15. Following growth at 28 °C for 12 h, cells were harvested by centrifugation at 5,000 × g for 5 min, washed in deionized water, and resuspended in WOYD medium supplemented with Mitotracker Orange® (Invitrogen) at a final concentration of 12.5 nm. The cells were incubated at 30 °C for 30 min, harvested, and washed twice in WOYD medium pre-warmed to 37 °C. The cells were mounted in solution containing 90% (v/v) glycerol and 100 mm Tris-HCl, pH 8.5, and visualized by confocal laser scanning microscopy. An inverted laser scanning microscope (LSM 510; Zeiss, Jena, Germany) with argon, HeNe1, diode lasers, and a ×63 oil immersion objective was used for confocal imaging. The pinhole was set to give confocal sections of 1.2 μm. Cyan fluorescent protein was excited with a 405-nm laser and fluorescence was collected through a band pass of 475–525 nm, yellow fluorescent protein was excited at 514 nm, and fluorescence was collected through a band pass of 530–600 nm, and Mitotracker® was excited with a 543 nm laser and fluorescence was collected through a band pass filter of 560–615 nm. All images were scanned using identical conditions (laser power, gain of photomultiplier tube, pinhole size, and zoom). Post-acquisition image processing was done using the LSM 5 browser (Zeiss) and Adobe Photoshop 9.0 software (Adobe Systems, Mountain View, CA).
ATPase Assays
The basal Mg2+-dependent ATPase activity of peroxisomes isolated from control yeast and yeast expressing CTS was determined by measuring the release of inorganic phosphate from ATP using a colorimetric method (40). The assay was optimized to be linear with respect to time and enzyme concentration (peroxisome protein) (supplemental Fig. S2). Reactions contained 40 mm Tris-HCl, pH 7.4, 0.1 mm EGTA, 1 mm DTT, 15 mm MgSO4, 10 mm sodium azide, 2 mm ouabain, 55 μm molybdate, and 10 μg of peroxisome proteins in a volume of 95 μl. Following equilibration at 37 °C for 10 min, the reaction was started by the addition of 5 μl of Mg-ATP solution (7 mm MgSO4, 5 mm ATP) and incubated at 37 °C for 20 min. Reactions were stopped by the addition of 100 μl of 12% (w/v) SDS, before color development as described (40). Where indicated, inhibitors (10 mm AlFx, (added by mixing an equimolar concentration of aluminum chloride and sodium fluoride), 100 μm sodium orthovanadate, or 10 μm concanamycin A) were added to the reaction mixture before allowing the reactants to equilibrate. ATPase activity was also assayed in the presence of fatty acids (Nu-Chek Prep, Inc., Elysian, MN) or fatty acyl-CoAs (Avanti Polar Lipids Inc., Alabaster, AL) in the range of 0 to 1000 μm (final concentration). In these assays, MgSO4 was reduced to 2.5 mm to avoid precipitation of acyl-CoAs (41, 42). Substrates were solubilized in 5% (w/v) 2-hydroxypropylated β-cyclodextrin (43) and incubated in the ATPase reaction mixture at 37 °C for 10 min to equilibrate the reactants and allow substrate recognition and binding before starting the reaction with MgATP as above. Data were analyzed by one-way or two-way analysis of variance and expressed as means (nanamole of Pi/mg/min) ± 95% confidence limit (CL). The stimulation indices (SI) for different compounds were calculated as (CTS expressing (stimulated) − control (stimulated))/(CTS (unstimulated) − control (unstimulated)). Data were expressed as mean SI ± 95% CL, where 95% CL was determined according to the formula: 95% CL = mean ± (t × S.E.), where n = number of samples, t = value of the Student's t distribution at (n-1) degrees of freedom and S.E. is the mean ± S.E. All statistical analysis and calculations were done using the Origin® 8.0 Data Analysis and Graphing Software statistical package and Windows® Microsoft Excel.
Complementation of pxa1 pxa2Δ
Transformed yeast cells were grown in minimal medium containing 0.67% (w/v) yeast nitrogen base without amino acids, supplemented with 0.3% (w/v) glucose and amino acids (20 mg/liter), if required, at 28 °C for 24 h. For the induction of peroxisome proliferation, cells were shifted to YPO medium containing 0.5% potassium phosphate buffer, pH 6.0, 0.3% (w/v) yeast extract, 0.5% (w/v) peptone supplemented with 0.12% (w/v) oleate and 0.2% (w/v) Tween 80. Cultures were incubated for 150 h in an orbital shaker at 200 rpm. The A600 was measured every 12 h by mixing 100 μl of culture, 50 μl of 10% (v/v) Triton X-100, and 850 μl of water. Oleic acid plates contained 0.125% (w/v) oleic acid, 0.4% (w/v) Tween 80, 2% (w/v) agar, 0.67% (w/v) yeast nitrogen base without amino acids, 0.1% (w/v) yeast extract (Difco) and amino acids (20 μg/ml) as needed. β-Oxidation assays in intact cells were performed as described previously (9) with some modifications. Cells were grown overnight in medium containing oleate to induce fatty acid β-oxidation. The β-oxidation capacity was measured in 50 mm MES, pH 6.0, supplemented with 10 μm 1-14C-labeled fatty acids. Subsequently, [14C]CO2 was trapped in 2 m NaOH and used to quantify the rate of fatty acid oxidation.
RESULTS
CTS Is Targeted to the Peroxisome in S. cerevisiae
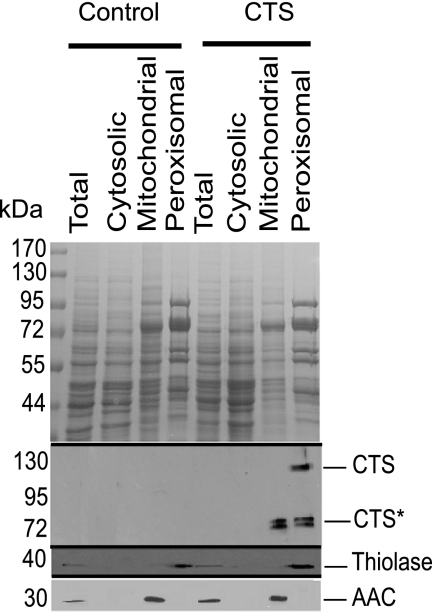
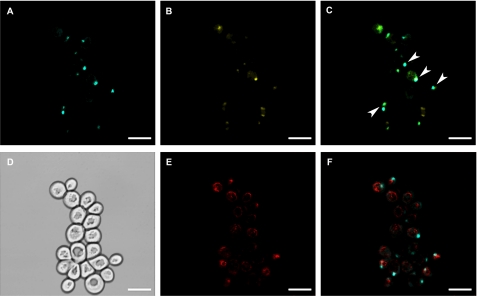
To determine the suitability of S. cerevisiae (BSL1-11B) as a heterologous system for the biochemical characterization of CTS, the CTS ORF was expressed from a low copy plasmid (pRS416-GPD) under the control of the strong, constitutive glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter (34). To investigate whether CTS was appropriately targeted in the heterologous host, peroxisomes were purified on Iodixanol gradients from a “light mitochondrial fraction” of oleate-grown cells transformed with CTS/pRS416-GPD or the non-recombinant vector, as described (30). Total, cytosolic, and the mitochondrial and peroxisomal fractions from the gradient were analyzed by SDS-PAGE, followed by immunoblotting with specific antibodies for the peroxisomal marker, thiolase (37), and the mitochondrial marker, adenine nucleotide carrier (AAC; Ref. 38). Clean separation of mitochondria and peroxisomes was demonstrated by the absence of the peroxisomal marker from the mitochondrial fraction, and vice versa (Fig. 1, bottom two panels). When the fractions were probed with an affinity-purified antiserum raised to the C-terminal NBD of CTS (16), a band of the expected size (about 130 kDa) was observed in the peroxisomal fraction isolated from cells transformed with CTS/pRS416-GPD (Fig. 1, middle panel). A doublet of cross-reacting bands (CTS*) of about 70 kDa was detected in the peroxisomal and mitochondrial fractions but was absent from the control (non-recombinant vector) samples. Further experiments suggested that these bands arose from cleavage between the two halves of CTS by an endogenous yeast protease (supplemental Fig. S1). Cleavage was not prevented in a Δpep4 knock-out strain of yeast (data not shown). To provide independent corroboration of peroxisomal localization, CTS was tagged with CFP at the C terminus and co-expressed in yeast with YFP-PTS1, a peroxisomal marker. Peroxisomes were observed as discrete spots in the YFP channel (Fig. 2B) and many of these coincided with the CFP signal (A), as indicated in the merge panel (C). Cells were also stained with Mitotracker to visualize mitochondria (E). The Mitotracker and CFP signals did not co-localize (F), suggesting that there was no significant amount of CTS present in the mitochondria and that the degradation products seen in immunoblots of the mitochondrial fraction could represent association with membranous fragments that co-fractionate with mitochondrial markers in the gradients.
FIGURE 1.
CTS targets to peroxisomes in yeast. A light mitochondrial fraction was prepared from oleate-grown BSL1-11B cells transformed with a CTS expression plasmid (CTS/pRS416-GPD) or with non-recombinant vector (control) and separated on an Iodixanol density gradient. Mitochondrial and peroxisomal fractions from the gradient along with total and cytosolic samples were separated by SDS-PAGE (30 μg of protein/lane), stained with Coomassie Blue (upper panel) or immunoblotted with antibodies specific for CTS, thiolase (peroxisomal marker), and mitochondrial AAC (mitochondrial marker). The mobilities of marker proteins of known mass (kDa) are indicated on the left-hand side of the figure.
FIGURE 2.
CTS-CFP targets to peroxisomes in yeast. Yeast strain BLS1-11B cells co-transformed with the CTS-CFP and YFP-PTS1 expression plasmids were grown on WOYD and transferred to oleate medium. Mitochondria were labeled with 12.5 nm Mitotracker and visualized by confocal microscopy, as described under “Experimental Procedures.” A, CTS-CFP; B, YFP-PTS1; C, merge of A and B; D, differential interference contrast image; E, Mitotracker; F, Mitotracker and CTS-CFP. E and F are the same group of cells as A and B but a different optical section. Examples of co-localizations are indicated by arrowheads. Scale bar, 5 μm.
Membrane Integration and Topology of CTS
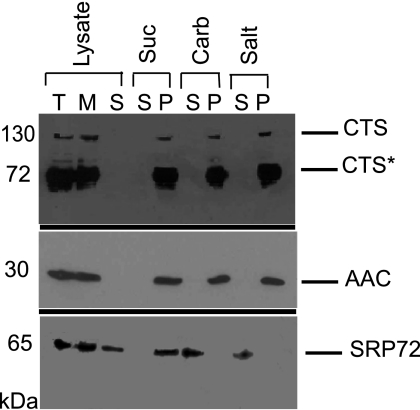
As a prerequisite to functional testing, the integration and topology of CTS in the yeast peroxisomal membrane were next evaluated. A light mitochondrial fraction (which contains mitochondria and peroxisomes, as well as some contaminating endoplasmic reticulum) was subjected to hypotonic lysis and centrifugation, to provide a membrane and soluble fraction. CTS, its associated degradation products, and the integral membrane protein marker, AAC, were detected exclusively in the membrane fraction, whereas the peripheral ER membrane protein, SRP72 (39), was partially soluble and partially membrane-associated (Fig. 3). Membranes were resuspended in sucrose buffer, alkaline sodium carbonate, or high salt and re-centrifuged. CTS, AAC, and SRP72 all remained membrane-associated on resuspension and re-pelleting in sucrose buffer, but SRP72 was extracted by both high salt and sodium carbonate showing that, as expected, these treatments were effective at removing peripheral membrane proteins. Both CTS and AAC were present in the pellets from the carbonate and salt treatments, indicating that CTS behaves as an integral membrane protein. Notably, the cleavage products were also found in the pellets, indicating that they are also firmly integrated in the membrane.
FIGURE 3.
CTS is an integral membrane protein in S. cerevisiae. Organelles (light mitochondrial fraction) were lysed in a hypotonic buffer in the presence of protease inhibitors and membranes were separated from soluble proteins by centrifugation at 100,000 × g. Membranes were then extracted with alkaline sodium carbonate buffer, pH 11.5 (carb), 1 m NaCl (salt), or sucrose buffer (suc), followed by centrifugation at 100,000 × g to pellet membranes. Lysate fractions (total, T; membrane, M; soluble, S) plus pellet (P) and supernatant fractions (S) were separated by SDS-PAGE (30 μg/lane) followed by immunoblotting with antibodies raised against CTS, AAC (integral membrane protein marker), and SRP72 (peripheral membrane protein marker).
Partial proteolysis was used to investigate the topology of CTS within the peroxisomal membrane. A light mitochondrial fraction (100 μg) was incubated with increasing amounts of trypsin, then separated into membrane-bound and soluble fractions and analyzed by immunoblotting with the anti-CTS C-terminal antiserum, which recognizes both NBDs (44). The membrane fractions (Fig. 4A) showed that full-length CTS was very sensitive to proteolysis and was almost completely degraded following incubation for 30 min at 0 °C with 1% (w/w) trypsin. The endogenous cleavage products were also degraded at higher trypsin concentrations. Analysis of the soluble fractions (Fig. 4B) revealed the presence of a fragment of the expected size of the NBDs (about 25 kDa), which was produced by treatment with 0.5% (w/w) trypsin but was completely degraded at concentrations greater than 10% (w/w). Under these conditions, thiolase, which is a peroxisomal matrix protein, remained completely protected from digestion, unless Triton X-100 was added. This is consistent with the cleavage of the NBDs from the membrane at low concentrations of trypsin and their degradation at higher concentrations. Thus we conclude that the NBDs face the cytosol. The addition of 0.1% (v/v) Triton X-100 did not result in a qualitative change in the cleavage pattern of the membrane-associated species but rather accelerated the rate of cleavage, due to the solubilizing effect of the detergent (not shown).
FIGURE 4.
Determination of orientation of NBDs by controlled proteolysis. Aliquots (100 μg of protein) of a light mitochondrial fraction were digested with the quantities of trypsin indicated for 30 min at 0 °C, in the presence or absence of detergent (Triton X-100). The reactions were stopped by the addition of soybean trypsin inhibitor and subjected to carbonate extraction followed by centrifugation at 100,000 × g to pellet the membranes. Pellet and supernatant fractions were separated by SDS-PAGE, transferred to nitrocellulose, and probed with CTS- or thiolase-specific antibodies. Mock indicates a reaction in which soybean trypsin inhibitor was added before trypsin. A, membrane fraction following tryptic digestion in the absence of detergent. B, supernatant fraction following tryptic digestion in the absence (upper two panels) and presence (lower panel) of Triton X-100.
CTS Exhibits ATPase Activity Which Is Inhibited by AlFx
To assess the functionality of expressed CTS, the ATPase activity was measured in peroxisomes purified from CTS-expressing and control yeast. Initial experiments showed that activity was linear with respect to the amount of peroxisomal protein added, in the range of 0–20 μg, and linear with respect to time up to 60 min (supplemental Fig. S2, A and B). In wild-type (BSL1-11B) and pxa1Δ backgrounds, control peroxisomes had a modest ATPase activity (black bars) that was not inhibited by ouabain, azide, concanamycin A, or molybdate (data not shown), but which was strongly inhibited by AlFx (Fig. 5A, white bars). The pxa1Δ knock-out mutant had a similar level of activity to wild-type yeast, indicating that the background activity was not substantially contributed by Pxa1/Pxa2p. Expression of CTS in both wild-type and pxa1Δ cells gave a 2.5-fold increase in activity, which was also sensitive to AlFx. The CTS-dependent ATPase activity was insensitive to sodium orthovanadate (not shown). Introduction of the NBD1 Walker A motif mutation, K487A, which abrogates function in planta (28), reduced ATPase activity to a level similar to that of the control. As a control, we determined that the mutant was correctly targeted to the peroxisome and expressed at a similar level to the wild-type protein (Fig. 5B).
FIGURE 5.
Basal ATPase activity of wild-type CTS and K478A mutant. Peroxisomes were isolated from oleate-grown wild-type BSL1-11B or pxa1Δ mutant yeast (PXA1KO), transformed with vectors encoding CTS, CTSK487A, or lacking an insert (control). A, ATPase activity was measured in the absence (black bars) or presence (open bars) of 10 mm AlFx. All samples were analyzed in triplicate, and the experiment was repeated three times. The data were used to compute the means and the mean ± S.E. The plot shows mean ± 95% confidence limit (computed from S.E. for n = 9) as indicated by the error bars. Data were analyzed by two-way analysis of variance, which indicated significantly higher ATPase activity of wild-type CTS compared with the K487A mutant (p < 0.05). The difference in the ATPase activity between the control BSL1–11B and the control pxa1Δ mutant was insignificant. B, total, cytosol, peroxisome, and mitochondria fractions were isolated from oleate-grown BSL1-11B cells (upper panel) or pxa1Δ cells (lower panel), transformed with the indicated constructs. Proteins (30 μg/lane) were separated by SDS-PAGE and immunoblotted with a CTS-specific antibody.
To investigate whether the ATPase activity ascribed to CTS demonstrated structure-linked latency, the ATPase assays were carried out both on intact and on osmotically ruptured peroxisomes (supplemental Fig. S3). Whereas peroxisomes from cells expressing CTS showed more than double the activity of the control, as seen in Fig. 5A, there was no difference in activity between ruptured and intact peroxisomes. The absence of latency is consistent with a cytosolic location of the NBDs as deduced from partial proteolysis experiments (Fig. 4).
As an independent means of testing CTS functionality, its ability to bind to ATP-agarose was also tested. Both full-length CTS and the cleavage products bound to ATP-agarose (supplemental Fig. S4A) and were eluted with 10 or 20 mm ATP (supplemental Fig. S4B), providing confirmation of proper folding and suggesting that the cleavage products most likely represent the two CTS halves that remain associated to form composite ATP binding sites, as would occur in the intact transporter (3, 28).
CTS Complements the Yeast pxa1 pxa2Δ Mutant
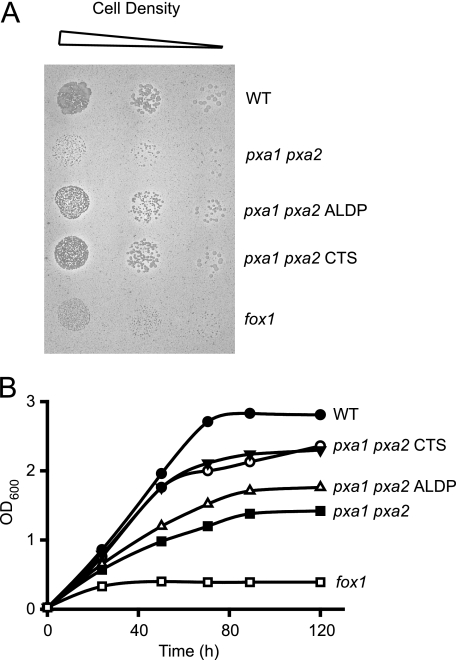
Because the biochemical data suggested that CTS was functional when expressed in yeast, we investigated the ability of CTS to complement an S. cerevisiae pxa1 pxa2Δ mutant, for β-oxidation of different fatty acids. The CTS ORF was subcloned into pEL30, a yeast expression vector that contains the oleate-inducible catalase promoter and was used successfully for expression of ALDP in S. cerevisiae (9). Peroxisomal targeting of CTS was confirmed by fractionation of organelles and immunoblotting (supplemental Fig. S5). Mutant cells transformed with CTS/pEL30 grew almost as well as the wild-type on oleate plates (Fig. 6A), and somewhat better than cells transformed with the human peroxisomal ABC transporter, ALDP, in liquid medium (Fig. 6B).
FIGURE 6.
CTS complements the S. cerevisiae pxa1 pxaΔ2 mutant for growth on oleate. Growth of wild-type and mutant cells on solid (A) and liquid (B) oleate medium. Strains shown are as follows: wild-type (WT; BJ1991), fox1Δ (acyl-CoA oxidase deletion mutant; negative control), pxa1 pxa2Δ mutant cells transformed with CTS/pEL30, ALDP/pEL30, or vector lacking insert (two independent transformants of CTS/pEL30 are shown in B). Data are representative of at least two independent experiments.
CTS Expressing pxa1 pxa2Δ Mutant Yeast Strains Can Oxidize a Wide Range of Fatty Acids
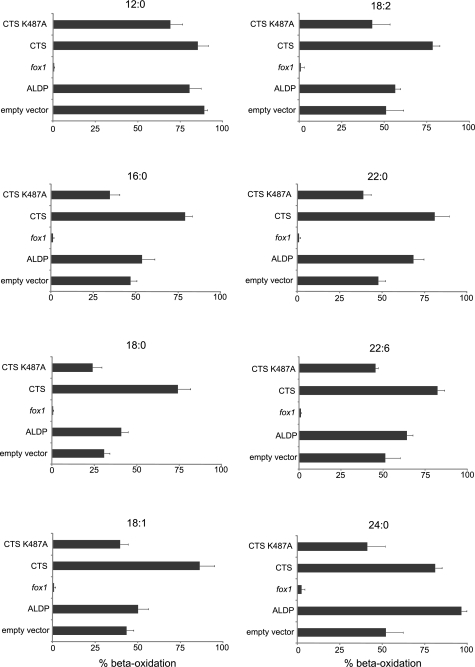
The ability of pxa1 pxa2Δ− mutant cells expressing CTS to metabolize fatty acids of different chain length and saturation was tested and compared with that of cells expressing ALDP (Fig. 7). For each substrate, data were normalized to the rate of β-oxidation for wild-type (WT = BJ1991) cells. WT rates of β-oxidation for lauric (C12:0), palmitic (C16:0), stearic (C18:0), oleic (C18:1), linoleic (C18:2), behenic (C22:0), and docosahexanoic (C22:6) acids were 7.24 ± 0.8, 1.06 ± 0.1, 0.95 ± 0.2, 2.6 ± 0.5, 2.73 ± 0.51, 0.15 ± 0.008, and 0.62 ± 0.08 nmol/min/OD, respectively, and the rate for lignoceric (C24:0) was 4.46 ± 0.15 pmol/h/OD. As shown previously, Pxa1/Pxa2p contribute to β-oxidation of fatty acids of chain length C16 or greater, but not to the β-oxidation of C12:0, as evidenced by the relative level of β-oxidation in the pxa1 pxa2Δ mutant transformed with vector lacking insert (6). For C16:0 and longer acyl chains, expression of CTS increased β-oxidation above the level seen for the non-recombinant control vector, restoring β-oxidation to 75–80% of the WT level. The Walker A motif mutant, CTSK487A, did not restore β-oxidation, compared with the vector-transformed control. ALDP was only effective with longer chain substrates of length C22:0 and longer, in agreement with Ref. 9. The β-oxidation mutant, fox1Δ, which lacks acyl-CoA oxidase, did not metabolize any of the fatty acids tested (Fig. 7). These data support the hypothesis that CTS has broad substrate specificity with regard to chain length and degree of desaturation. However, these in vivo measurements are constrained by the ability of yeast to metabolize different fatty acids and only provide indirect evidence for substrate specificity. Therefore we sought alternative, biochemical evidence for the ability of CTS to accept a range of substrates.
FIGURE 7.
β-Oxidation of different fatty acids in wild-type and transformed pxa1 pxa2Δ mutant cells. Cells grown on oleate medium were incubated with 1-14C-labeled fatty acids, followed by β-oxidation activity measurements. Results are presented as % relative to the rate of oxidation by wild-type cells for each fatty acid. Bars represent mean ± S.E. (n = 3).
Effects of Potential Substrates on the ATPase Activity of CTS
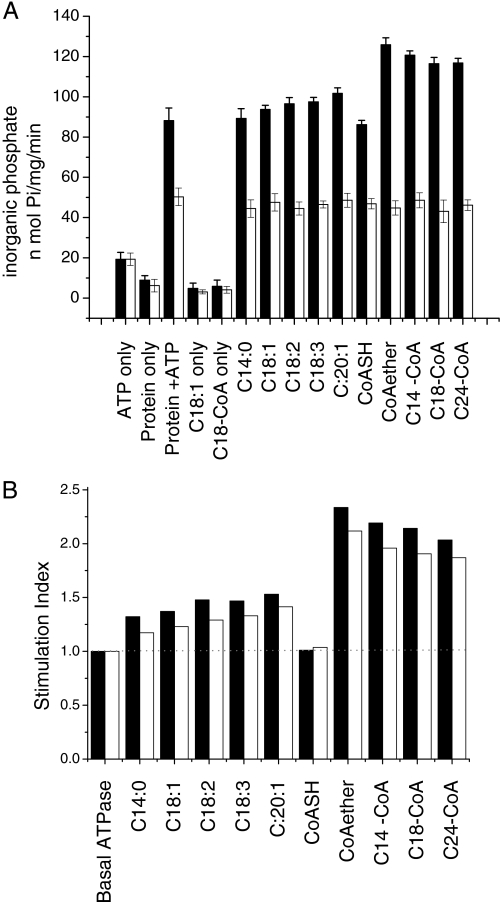
The ATPase activity of many ABC transporters is increased above basal rates upon substrate binding (45–50). Exploiting this behavior, it is possible to screen a range of compounds to identify putative substrates for a given ATP transporter. Therefore we tested the effects of fatty acids and fatty acyl-CoAs on the ATPase activity of CTS when expressed from the vector pEL30 in the BJ1991 pxa1Δ strain. Substrates (10–1000 μm) were solubilized in 5% (w/v) hydroxyl-propylated β-cyclodextrin. The effects of 10 μm substrates on activity are presented in Fig. 8A, black bars indicate activity with CTS; white bars are non-recombinant vector controls. Control reactions in the presence of individual assay components (ATP (“ATP only”), peroxisome extract (“protein only”), FA-CoA (“C-18-CoA only”), or FA (“C18:1 only”)) showed only a very low rate of inorganic phosphate production (20 nmol of Pi/min or less). In the CTS/pEL30-transformed cells, basal ATPase activity was 86.64 ± 2.49 nmol of Pi/min/mg of protein. This was increased in the presence of all fatty acyl-CoAs tested, although the effects of fatty acids were marginal (Fig. 8A). CoASH had no effect on activity, but the non-hydrolyzable C14-etheno-CoA increased CTS-dependent basal ATPase activity, demonstrating that simulation of activity by FA-CoA was not due to release of FA by hydrolysis. Indole butyric acid and 2,4-dichlorophenoxybutyric acid have both been proposed as substrates of CTS (17, 18), but these compounds did not stimulate ATPase activity (data not shown). Unfortunately the corresponding CoA esters could not be tested as they are not commercially available. No effect of any of the potential substrates was seen on the ATPase activity of non-recombinant vector-transformed cells. To evaluate more precisely the effects of the different compounds, a stimulation index was calculated for each potential substrate, as defined under “Experimental Procedures.” From this, it is clear that FA-CoA give a higher stimulation of activity: about 2-fold, compared with 1.2–1.4-fold for FA (Fig. 8B).
FIGURE 8.
Effects of fatty acids and fatty acyl-CoAs on the ATPase activity of CTS. ATPase activity was measured in the presence of 10 or 100 μm fatty acids or fatty acyl-CoAs using peroxisomes (10 μg of protein) isolated from pxa1Δ cells transformed with CTS/pEL30 (black bars) or vector lacking insert (open bars). A, ATPase activities measured in the presence or absence of 10 μm fatty acids/fatty acyl-CoAs; bars represent mean ± S.E. (n = 3). B, the ATPase activities obtained in A, or in a similar experiment performed using 100 μm fatty acids/fatty acyl-CoAs, were used to calculate the CTS ATPase activity relative to that seen in the basal state in the absence of substrate (“stimulation index”), according to the formula: (CTS expressing (stimulated) − control (stimulated))/(CTS (unstimulated) − control (unstimulated)). The basal ATPase activity is defined as having a stimulation index of 1 (represented by a broken line across the graph) and values above 1 indicate the degree of stimulation relative to the basal ATPase activity of CTS. Black bars, 100 μm substrate; open bars, 10 μm substrate.
DISCUSSION
Genetic and physiological evidence has indicated that CTS is involved in the transport of substrates into the peroxisome for β-oxidation but, to date, no direct biochemical data have been available to support this hypothesis. To address this, we have expressed CTS in S. cerevisiae, demonstrated that it is correctly targeted to peroxisomes in the heterologous host, and is integrated into the peroxisomal membrane in a functional form, allowing complementation of a pxa1 pxa2Δ mutant.
Through complementary cell fractionation and confocal microscopy approaches, it was shown that full-length CTS is targeted to the peroxisome in yeast, which, taken together with other findings, suggests conservation of targeting of peroxisomal membrane proteins between plants, yeast, mammals, and protozoa (51–54). Attempts to complement a pxa1Δ mutant with the CTS/pRS416-GPD construct gave inconsistent results (data not shown), suggesting that for complementation to work effectively either the expression of CTS has to be coordinated with the proliferation of peroxisomes (as would be expected to be the case when CTS expression is driven from the CAT promoter of pEL30) or that the presence of Pxa2p somehow interferes with the function of CTS. When ALDP (HsABCD1) was co-expressed with Pxa1p in yeast it was inactive but like CTS could complement a pxa1 pxa2Δ mutant (9).
Carbonate and salt extraction demonstrated that this protein is integrated into the yeast peroxisomal membrane, consistent with its behavior as an integral membrane protein in Arabidopsis (18), and partial proteolysis experiments indicated that the NBDs face the cytosol, as has been shown for the human peroxisomal ABC transporters, ALDP and PMP70 (55, 56). This topology is consistent with the canonical model of ATP-dependent transport of substrates from the cytosol to the peroxisome lumen and rules out a model in which CTS utilizes the peroxisomal pool of ATP supplied by the adenine nucleotide translocators, PNC1 and -2 (57, 58).
We were able to demonstrate ATPase activity in peroxisomes purified from cells expressing CTS, which was consistently 2–3-fold higher than that of peroxisomes isolated from control cells transformed with vector lacking insert. Interestingly, the level of control ATPase activity in the pxa1Δ knock-out was not greatly different to that of wild-type cells, suggesting that the basal activity of endogenous Pxa1/Pxa2p is low relative to other ATPases in the peroxisome fraction. Independent confirmation of functionality was obtained by ATP binding experiments in which full-length CTS bound to ATP-agarose and could be eluted with ATP. Expression of CTS bearing a K487A mutation in the Walker A motif, which abolishes function in planta, reduced peroxisomal ATPase activity to close to control levels, providing evidence of specificity. Both control and CTS-dependent ATPase activities were insensitive to vanadate (Vi), but were inhibited by AlFx. Vanadate and metal fluorides act as phosphate analogues, arresting ATP-utilizing enzymes in the catalytic cycle by forming a stable Mg·ADP·inhibitor complex, but ABC transporters differ in their sensitivity to these inhibitors (46, 47, 59, 60). In common with CTS, ALDP, PMP70, and TAP (transporter associated with antigen processing) are insensitive to vanadate, but susceptible to inhibition by metal fluorides (61–63).
The ability of CTS to complement a pxa1 pxa2Δ mutant for growth on oleic acid and to support the β-oxidation of a range of fatty acids (C16:0-C24:0) provides further support for the ability of CTS to handle a broad range of substrates, in contrast to ALDP, which shows a marked preference for very long-chain substrates (9). Evidence from yeast suggests that Pxa1/Pxa2p transports FA-CoAs and not FA (10), suggesting, by extension, that the same is also true for ALDP and CTS, because they complement the pxa1 pxa2Δ mutant (Ref. 9 and this study) and the peroxisomal synthetase Faa2p is not required for metabolism of fatty acids with chain lengths longer than C14 (2). Both cytosolic and peroxisomal acyl-CoA synthetases exist in Arabidopsis (29, 64, 65) but genetic data imply that CTS might transport free acids and not CoAs. Arabidopsis mutants and transgenics in which peroxisomal acyl-CoA synthetases and peroxisomal adenine nucleotide translocators are down-regulated share the lipid mobilization defect of cts mutants (29, 57, 58) and the peroxisomal acetyl-CoA synthetase mutant, acn1, and the cts allele, acn2 are both impaired in acetate metabolism (19, 66), suggesting that free acids must be activated in the peroxisome prior to β-oxidation. Therefore, the precise nature of the CTS substrates is of particular interest. As a number of ABC transporters exhibit substrate-stimulated ATPase activity (46–49, 67), we tested both FA and FA-CoA for effects on basal ATPase activity. Comparison of the stimulation indices for free acids and acyl-CoA esters indicated that CoAs gave a markedly greater stimulation than the corresponding free FA. The non-hydrolysable etheno-CoA species also stimulated basal ATPase, providing evidence that the CoA ester, rather than the free FA hydrolysis product is responsible for this effect.
Stimulation of CTS-dependent ATPase activity was observed in the concentration range of 10–100 μm FA-CoA. It is noteworthy that higher concentrations (1000 μm) of FA and FA-CoA had an inhibitory effect on the basal ATPase activity of CTS (not shown), possibly due to a detergent effect. However, the observation that 100 μm of various non-ionic and anionic detergents did not affect the basal ATPase activity of CTS (data not shown) suggests that the effects observed at 100 μm reflect a specific interaction of the substrates with CTS. The critical micelle concentrations of medium to long-chain acyl-CoAs are reported to be in the range of 5–200 μm depending on chain length, degree of saturation, and the ionic strength of the buffer (68). Below the critical micelle concentration, acyl-CoAs exist in molecular solution, but above the critical micelle concentration, the concentration of free acyl-CoA is independent of the total concentration. In mammalian cells, the total cellular acyl-CoA concentration was reported to be in the range of 5–160 μm, depending on the metabolic state of the cell, although the majority of the acyl-CoA pool is likely to be protein-bound (68, 69). Acyl-CoA concentrations in plants are lower: in the range of 3–6 μm in developing Brassica napus embryos and in Arabidopsis seedlings (70). The potential disparity between the stimulatory concentration determined in this study and the reported concentration of acyl-CoAs in plant cells possibly reflects the differences between in vivo and in vitro conditions. For FA and FA-CoA to interact with CTS, it is probable that they partition into the peroxisomal membrane, where they reach a much higher local concentration (71–74). Nevertheless, our data are consistent with those of Guimarães et al. (14), in which FA-CoA (but not FA) provoked substrate-induced conformational changes in ALDP when supplied at 200 μm.
Although the stimulation of basal ATPase activity by the FA-CoA species is consistent with CoA esters, rather than free FA, being bona fide CTS substrates, other interpretations are possible: not all ABC transport substrates stimulate ATP hydrolysis and conversely, not all compounds that stimulate ATPase activity are substrates (46, 47, 49, 75–77), therefore FA as potential substrates cannot be excluded. Nevertheless, collectively, our data support the notion that CTS is a transporter of acyl-CoAs. It has been proposed that CTS might cleave the CoA moiety during transport, thus requiring its re-esterification inside the peroxisome (29); alternatively, cleavage may be carried out by peroxisomal thioesterases (78), which would agree with both the biochemical and genetic data. Ultimately, transport assays using purified, reconstituted CTS protein will be essential to determine unequivocally the biochemical function of this intriguing membrane protein. However, this study has advanced our understanding of the function of CTS by identifying potential substrates and developing a yeast expression system that may prove useful for the expression, purification, and reconstitution of CTS to enable testing of these substrates via transport assays.
Supplementary Material
Acknowledgments
We gratefully acknowledge the advice and assistance of Dr. Gareth Howell with confocal microscopy experiments. We thank Dr. Steve Slocombe for CTS/pRS416-GPD, Dr. Ewald Hettema for pEW205, and Dr. Martin Pool for SRP72 antibody.
This work was supported by Biotechnology and Biosciences Research Council Grants BB/F007299/1 (to A. B. and S. A. B.) and BB/F007108/1 (to F. L. T.) and a BBSRC doctoral training studentship (to Y. N.). Rothamsted Research receives support from the BBSRC of the United Komgdom.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- FA
- fatty acid
- ABC
- ATP binding cassette
- CTS
- Comatose
- FA-CoA
- fatty acid acyl-CoA ester
- NBD
- nucleotide binding domain
- GPD
- glyceraldehyde-3-phosphate dehydrogenase
- AAC
- adenine nucleotide carrier
- PMP
- peroxisomal membrane protein
- ALDP
- adrenoleukodystrophy protein.
REFERENCES
- 1.Theodoulou F. L., Holdsworth M., Baker A. (2006) FEBS Lett. 580, 1139–1155 [DOI] [PubMed] [Google Scholar]
- 2.Wanders R. J., Visser W. F., van Roermund C. W., Kemp S., Waterham H. R. (2007) Pflugers Arch. 453, 719–734 [DOI] [PubMed] [Google Scholar]
- 3.Linton K. J., Higgins C. F. (2007) Pflugers Arch. 453, 555–567 [DOI] [PubMed] [Google Scholar]
- 4.Procko E., O'Mara M. L., Bennett W. F., Tieleman D. P., Gaudet R. (2009) FASEB J. 23, 1287–1302 [DOI] [PubMed] [Google Scholar]
- 5.Shani N., Watkins P. A., Valle D. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 6012–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hettema E. H., van Roermund C. W., Distel B., van den Berg M., Vilela C., Rodrigues-Pousada C., Wanders R. J., Tabak H. F. (1996) EMBO J. 15, 3813–3822 [PMC free article] [PubMed] [Google Scholar]
- 7.Swartzman E. E., Viswanathan M. N., Thorner J. (1996) J. Cell Biol. 132, 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shani N., Valle D. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 11901–11906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Roermund C. W., Visser W. F., Ijlst L., van Cruchten A., Boek M., Kulik W., Waterham H. R., Wanders R. J. (2008) FASEB J. 22, 4201–4208 [DOI] [PubMed] [Google Scholar]
- 10.Verleur N., Hettema E. H., van Roermund C. W., Tabak H. F., Wanders R. J. (1997) Eur. J. Biochem. 249, 657–661 [DOI] [PubMed] [Google Scholar]
- 11.Imanaka T., Aihara K., Takano T., Yamashita A., Sato R., Suzuki Y., Yokota S., Osumi T. (1999) J. Biol. Chem. 274, 11968–11976 [DOI] [PubMed] [Google Scholar]
- 12.Fourcade S., Ruiz M., Camps C., Schlüter A., Houten S. M., Mooyer P. A., Pàmpols T., Dacremont G., Wanders R. J., Giròs M., Pujol A. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E211–221 [DOI] [PubMed] [Google Scholar]
- 13.Rottensteiner H., Theodoulou F. L. (2006) Biochim. Biophys. Acta 1763, 1527–1540 [DOI] [PubMed] [Google Scholar]
- 14.Guimarães C. P., Sá-Miranda C., Azevedo J. E. (2005) J. Hum. Genet. 50, 99–105 [DOI] [PubMed] [Google Scholar]
- 15.Verrier P. J., Bird D., Burla B., Dassa E., Forestier C., Geisler M., Klein M., Kolukisaoglu U., Lee Y., Martinoia E., Murphy A., Rea P. A., Samuels L., Schulz B., Spalding E. J., Yazaki K., Theodoulou F. L. (2008) Trends Plant Sci. 13, 151–159 [DOI] [PubMed] [Google Scholar]
- 16.Footitt S., Slocombe S. P., Larner V., Kurup S., Wu Y., Larson T., Graham I., Baker A., Holdsworth M. (2002) EMBO J. 21, 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zolman B. K., Silva I. D., Bartel B. (2001) Plant Physiol. 127, 1266–1278 [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi M., Nito K., Takei-Hoshi R., Yagi M., Kondo M., Suenaga A., Yamaya T., Nishimura M. (2002) Plant Cell Physiol. 43, 1–11 [DOI] [PubMed] [Google Scholar]
- 19.Hooks M. A., Turner J. E., Murphy E. C., Johnston K. A., Burr S., Jarosławski S. (2007) Biochem. J. 406, 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theodoulou F. L., Job K., Slocombe S. P., Footitt S., Holdsworth M., Baker A., Larson T. R., Graham I. A. (2005) Plant Physiol. 137, 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinfield-Wells H., Rylott E. L., Gilday A. D., Graham S., Job K., Larson T. R., Graham I. A. (2005) Plant J. 43, 861–872 [DOI] [PubMed] [Google Scholar]
- 22.Footitt S., Marquez J., Schmuths H., Baker A., Theodoulou F. L., Holdsworth M. (2006) J. Exp. Bot. 57, 2805–2814 [DOI] [PubMed] [Google Scholar]
- 23.Footitt S., Dietrich D., Fait A., Fernie A. R., Holdsworth M. J., Baker A., Theodoulou F. L. (2007) Plant Physiol. 144, 1467–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrera E., Holman T., Medhurst A., Peer W., Schmuths H., Footitt S., Theodoulou F. L., Holdsworth M. J. (2007) Plant Physiol. 143, 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunz H. H., Scharnewski M., Feussner K., Feussner I., Flügge U. I., Fulda M., Gierth M. (2009) Plant Cell 21, 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slocombe S. P., Cornah J., Pinfield-Wells H., Soady K., Zhang Q., Gilday A., Dyer J. M., Graham I. A. (2009) Plant Biotechnol. J. 7, 694–703 [DOI] [PubMed] [Google Scholar]
- 27.Kanai M., Nishimura M., Hayashi M. (2010) Plant J. 62, 936–947 [DOI] [PubMed] [Google Scholar]
- 28.Dietrich D., Schmuths H., De Marcos Lousa C., Baldwin J. M., Baldwin S. A., Baker A., Theodoulou F. L., Holdsworth M. J. (2009) Mol. Biol. Cell 20, 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulda M., Schnurr J., Abbadi A., Heinz E., Browse J. (2004) Plant Cell 16, 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Distel B., Kragt A. (2006) Methods Mol. Biol. 313, 21–26 [DOI] [PubMed] [Google Scholar]
- 31.Wach A., Brachat A., Pöhlmann R., Philippsen P. (1994) Yeast 10, 1793–1808 [DOI] [PubMed] [Google Scholar]
- 32.Philippson P., Stotz A., Scherf C. (1991) in Methods in Enzymology (Guthrie C., Fink G. R. eds) Vol. 194, pp. 169–182 [DOI] [PubMed] [Google Scholar]
- 33.Sikorski R. S., Hieter P. (1989) Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumberg D., Müller R., Funk M. (1995) Gene 156, 119–122 [DOI] [PubMed] [Google Scholar]
- 35.Sparkes I. A., Brandizzi F., Slocombe S. P., El-Shami M., Hawes C., Baker A. (2003) Plant Physiol. 133, 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elgersma Y., van den Berg M., Tabak H. F., Distel B. (1993) Genetics 135, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erdmann R., Kunau W. H. (1994) Yeast 10, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 38.Daum G., Gasser S. M., Schatz G. (1982) J. Biol. Chem. 257, 13075–13080 [PubMed] [Google Scholar]
- 39.Halic M., Gartmann M., Schlenker O., Mielke T., Pool M. R., Sinning I., Beckmann R. (2006) Science 312, 745–747 [DOI] [PubMed] [Google Scholar]
- 40.Chifflet S., Torriglia A., Chiesa R., Tolosa S. (1988) Anal. Biochem. 168, 1–4 [DOI] [PubMed] [Google Scholar]
- 41.Constantinides P. P., Steim J. M. (1985) J. Biol. Chem. 260, 7573–7580 [PubMed] [Google Scholar]
- 42.Constantinides P. P., Steim J. M. (1986) Arch. Biochem. Biophys. 250, 267–270 [DOI] [PubMed] [Google Scholar]
- 43.Szente L., Szejtli J., Szeman J., Kato L. (1993) J. Incl. Phenom. Mol. Recos. Chem. 16, 339–354 [Google Scholar]
- 44.De Marcos Lousa C., Dietrich D., Johnson B., Baldwin S. A., Holdsworth M. J., Theodoulou F. L., Baker A. (2009) Commun. Integr. Biol. 2, 97–99 [PMC free article] [PubMed] [Google Scholar]
- 45.Romsicki Y., Sharom F. J. (1999) Biochemistry 38, 6887–6896 [DOI] [PubMed] [Google Scholar]
- 46.Kerr K. M., Sauna Z. E., Ambudkar S. V. (2001) J. Biol. Chem. 276, 8657–8664 [DOI] [PubMed] [Google Scholar]
- 47.Sauna Z. E., Nandigama K., Ambudkar S. V. (2004) J. Biol. Chem. 279, 48855–48864 [DOI] [PubMed] [Google Scholar]
- 48.Müller M., Klein I., Kopácsi S., Remaley A. T., Rajnavölgyi E., Sarkadi B., Váradi A. (2006) FEBS Lett. 580, 6139–6144 [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K., Kimura Y., Kioka N., Matsuo M., Ueda K. (2006) J. Biol. Chem. 281, 10760–10768 [DOI] [PubMed] [Google Scholar]
- 50.Kimura Y., Kioka N., Kato H., Matsuo M., Ueda K. (2007) Biochem. J. 401, 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landgraf P., Mayerhofer P. U., Polanetz R., Roscher A. A., Holzinger A. (2003) Eur. J. Cell Biol. 82, 401–410 [DOI] [PubMed] [Google Scholar]
- 52.Rottensteiner H., Kramer A., Lorenzen S., Stein K., Landgraf C., Volkmer-Engert R., Erdmann R. (2004) Mol. Biol. Cell 15, 3406–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halbach A., Lorenzen S., Landgraf C., Volkmer-Engert R., Erdmann R., Rottensteiner H. (2005) J. Biol. Chem. 280, 21176–21182 [DOI] [PubMed] [Google Scholar]
- 54.Saveria T., Halbach A., Erdmann R., Volkmer-Engert R., Landgraf C., Rottensteiner H., Parsons M. (2007) Eukaryot. Cell 6, 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. (1990) J. Biol. Chem. 265, 4534–4540 [PubMed] [Google Scholar]
- 56.Contreras M., Sengupta T. K., Sheikh F., Aubourg P., Singh I. (1996) Arch. Biochem. Biophys. 334, 369–379 [DOI] [PubMed] [Google Scholar]
- 57.Arai Y., Hayashi M., Nishimura M. (2008) Plant Cell 20, 3227–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linka N., Theodoulou F. L., Haslam R. P., Linka M., Napier J. A., Neuhaus H. E., Weber A. P. (2008) Plant Cell 20, 3241–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbatsch I. L., Sankaran B., Weber J., Senior A. E. (1995) J. Biol. Chem. 270, 19383–19390 [DOI] [PubMed] [Google Scholar]
- 60.Wittinghofer A. (1997) Curr. Biol. 7, R682–685 [DOI] [PubMed] [Google Scholar]
- 61.Chen M., Abele R., Tampé R. (2003) J. Biol. Chem. 278, 29686–29692 [DOI] [PubMed] [Google Scholar]
- 62.Morita M., Kurisu M., Kashiwayama Y., Yokota S., Imanaka T. (2006) Biol. Pharm. Bull. 29, 1836–1842 [DOI] [PubMed] [Google Scholar]
- 63.Tanaka A. R., Tanabe K., Morita M., Kurisu M., Kasiwayama Y., Matsuo M., Kioka N., Amachi T., Imanaka T., Ueda K. (2002) J. Biol. Chem. 277, 40142–40147 [DOI] [PubMed] [Google Scholar]
- 64.Shockey J. M., Fulda M. S., Browse J. A. (2002) Plant Physiol. 129, 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fulda M., Shockey J., Werber M., Wolter F. P., Heinz E. (2002) Plant J. 32, 93–103 [DOI] [PubMed] [Google Scholar]
- 66.Turner J. E., Greville K., Murphy E. C., Hooks M. A. (2005) J. Biol. Chem. 280, 2780–2787 [DOI] [PubMed] [Google Scholar]
- 67.Chloupková M., Pickert A., Lee J. Y., Souza S., Trinh Y. T., Connelly S. M., Dumont M. E., Dean M., Urbatsch I. L. (2007) Biochemistry. 46, 7992–8003 [DOI] [PubMed] [Google Scholar]
- 68.Knudsen J., Jensen M. V., Hansen J. K., Faergeman N. J., Neergaard T. B., Gaigg B. (1999) Mol. Cell. Biochem. 192, 95–103 [DOI] [PubMed] [Google Scholar]
- 69.Faergeman N. J., Knudsen J. (1997) Biochem. J. 323, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larson T. R., Graham I. A. (2001) Plant J. 25, 115–125 [DOI] [PubMed] [Google Scholar]
- 71.Cohen Simonsen A., Bernchou Jensen U., Faergeman N. J., Knudsen J., Mouritsen O. G. (2003) FEBS Lett. 552, 253–258 [DOI] [PubMed] [Google Scholar]
- 72.Eckford P. D., Sharom F. J. (2008) Biochemistry 47, 13686–13698 [DOI] [PubMed] [Google Scholar]
- 73.Eckford P. D., Sharom F. J. (2008) J. Biol. Chem. 283, 12840–12850 [DOI] [PubMed] [Google Scholar]
- 74.Belli S., Elsener P. M., Wunderli-Allenspach H., Krämer S. D. (2009) J. Pharm. Sci. 98, 1905–1918 [DOI] [PubMed] [Google Scholar]
- 75.Wang J., Sun F., Zhang D. W., Ma Y., Xu F., Belani J. D., Cohen J. C., Hobbs H. H., Xie X. S. (2006) J. Biol. Chem. 281, 27894–27904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Telbisz A., Müller M., Ozvegy-Laczka C., Homolya L., Szente L., Váradi A., Sarkadi B. (2007) Biochim. Biophys. Acta 1768, 2698–2713 [DOI] [PubMed] [Google Scholar]
- 77.Velamakanni S., Janvilisri T., Shahi S., van Veen H. W. (2008) Mol. Pharmacol. 73, 12–17 [DOI] [PubMed] [Google Scholar]
- 78.Hunt M. C., Alexson S. E. (2008) Prog. Lipid Res. 47, 405–421 [DOI] [PubMed] [Google Scholar]
- 79.Taylor K. M., Kaplan C. P., Gao X., Baker A. (1996) Biochem. J. 319, 255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.