Abstract
β-Arrestins, originally discovered to desensitize activated G protein-coupled receptors, (aka seven-transmembrane receptors, 7TMRs) also mediate 7TMR internalization and G protein-independent signaling via these receptors. More recently, several regulatory roles of β-arrestins for atypical 7TMRs and non-7TM receptors have emerged. Here, we uncover an entirely novel regulatory role of β-arrestins in cross-talk between the angiotensin receptor (AT1aR) and a member of the transient receptor potential (TRP) ion channel family, TRPV4. AT1aR and TRPV4 form a constitutive complex in the plasma membrane, and angiotensin stimulation leads to recruitment of β-arrestin 1 to this complex. Surprisingly, angiotensin stimulation results in ubiquitination of TRPV4, a process that requires β-arrestin 1, and subsequently to internalization and functional down-regulation of TRPV4. β-Arrestin 1 interacts with, and acts as an adaptor for AIP4, an E3 ubiquitin ligase responsible for TRPV4 ubiquitination. Thus, our data provide the first evidence of a functional link between β-arrestins and TRPV4 and uncovers an entirely novel mechanism to maintain appropriate intracellular Ca2+ concentration to avoid excessive Ca2+ signaling.
Keywords: Calcium, Endocytosis, G Protein-coupled Receptors (GPCR), TRP Channels, Ubiquitination
Introduction
G protein-coupled receptors, also known as seven-transmembrane receptors (7TMRs),4 constitute the largest family of cell surface receptors in the human genome (1). They mediate a wide range of cellular and physiological responses upon activation by different stimuli (2). To avoid harmful effects of sustained signaling and to exert tight regulation, cells have evolved several different mechanisms for down-regulating these receptors (3). For example, upon activation by an agonist, 7TMRs are phosphorylated by G protein-coupled receptor kinases followed by recruitment of β-arrestins. This receptor-β-arrestin interaction sterically hinders further G protein coupling and thus terminates the G protein-dependent cellular responses, leading to receptor desensitization (4). Subsequently, the activated receptors are internalized and targeted either for degradation or for recycling back to the cell surface (5). β-Arrestins, originally discovered in the context of receptor desensitization, have more recently emerged as key players in almost every aspect of 7TMR function and regulation. For example, they not only mediate 7TMR internalization by acting as adaptors for several key proteins involved in clathrin-mediated endocytosis but also mediate G protein-independent signaling via these receptors (6). Moreover, β-arrestins also act as adaptors for E3 ubiquitin ligases to mediate 7TMR ubiquitination and degradation (6). Somewhat surprisingly, β-arrestins were also found to interact with and regulate atypical 7TMRs such as Smoothened (7) and Frizzled (8) and non-7TM receptors such as the insulin like growth factor 1 receptor (IGF-1R) (9) and type III transforming growth factor-β receptor (TGF-β RIII) (10). These previously unanticipated functional capabilities of β-arrestins hint at much broader roles of β-arrestins in cellular signaling and clearly demand more in depth investigation. To better understand their functional and regulatory roles in cellular processes, we recently carried out a global interactomics screen using a shotgun proteomics approach to generate an extensive list of putative interaction partners of β-arrestins (11). Among a small number of non-receptor membrane proteins such as ion channels and transporters present in this screen, we surprisingly identified a transient receptor potential (TRP) channel TRPV4 as a specific interacting partner of β-arrestins in response to angiotensin stimulation.
TRP channels are a family of non-selective cation channels permeable to Na+, Ca2+, and Mg2+, and they mediate numerous cellular and physiological processes (12). TRP channels are ubiquitously expressed, and they are activated and regulated by a wide array of external stimuli such as chemicals, temperature, mechanical and osmotic stress, and intracellular signals such as phosphorylation and ubiquitination (12, 13). TRPV4 is a member of the vanilloid subfamily of the TRP channel family and is widely expressed in the central nervous system as well as the peripheral nervous system, cardiovascular tissues, epithelial cells such as keratinocytes, bronchial, and renal epithelia, mesenchymally derived cells such as skeletal (chondrocytes, osteoclasts, and osteoblasts), and endothelial cells (14, 15). TRPV4 is activated in response to moderate heating, hypotonic stress, and phorbol ester. Mice lacking TRPV4 exhibit reduced defense to systemic osmotic stress, reduced sensation of noxious mechanical stimuli, reduced inflammatory and neuropathic pain, and deficits in thermal preference and vascular, skin, gall, and urinary bladder regulation (16–24). Moreover, TRPV4-mediated entry of Ca2+ in endothelial cells appears to be critical for steady state production of nitric oxide and vasoconstriction and vasodilatation of peripheral blood vessels (25–28). Additionally, activators of TRPV4 also appear to influence vascular tone (29, 30). Interestingly, signaling via AT1aR has been shown to be involved in regulation of vasoconstriction, cardiac contractility, central osmocontrol and extracellular matrix formation, cardiac hypertrophy, and vascular smooth muscle cell proliferation (31). β-Arrestin-dependent signaling via AT1aR and β-arrestin-mediated transactivation of EGF receptor are also known to be critically involved in cardiovascular regulation (32, 33). This intriguing physiological convergence of TRPV4, AT1aR, and β-arrestin functions prompted us to explore the consequences of the interaction between TRPV4 and β-arrestin. In this study, we set out to investigate the interaction of β-arrestins with TRPV4 upon stimulation of AT1aR and the functional implications of this novel interaction.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
HA-AT1aR and Myc-AIP4 constructs have been described previously (34, 35). FLAG-TRPV4 construct was generated by subcloning the TRPV4 coding sequence in the pcDNA 3.0 vector using standard subcloning procedure. Chemically synthesized, double-stranded siRNAs with 19-nucleotide duplex RNA and 2-nucleotide 3′-dTdT overhangs (or 17-nucleotide duplex RNA without 2-nucleotide 3′-dTdT overhangs) were purchased from Dharmacon (Lafayette, CO) in deprotected and desalted forms. The sequences of siRNAs used in this study are presented here: control, 5′-UUCUCCGAACGUGUCACGU-3′; human β-arrestin 1, 5′-AAAGCCUUCUGCGCGGAGAAU-3′ (start position 439) (36); human β-arrestin 2, 5′-AAGGACCGCAAAGUGUUUGUG-3′ (start position 201) (36); rat β-arrestin 1, 5′-AGCCUUCUGUGCUGAGAAC-3′ (start position 431) (37); rat β-arrestin 2, 5′-GGACCGCAAAGUGUUUGUG-3′ (start position 150) (37); AIP4-1, 5′-GGUGACAAAGAGCCAACAGAG-3′ (38); AIP4-2, 5′-CAGCAAAACUUAAGGAAAA-3′ (start position 2610); AIP4-3, 5′-GAGCAAUGGACCACAGAAA-3′ (start position 649).
The following antibodies were used: rabbit polyclonal anti-β-arrestins A1CT and A2CT (1:1000 for IP, 1:3000 for IB), polyclonal goat anti-TRPV4 (1:500–1:1000 dilution for IB, 1:200 for immunostaining) (Santa Cruz Biotechnology), polyclonal rabbit anti-TRPV4 (1:500–1:1000 dilution for IB, 1:500 for IP) (Alomone Labs), mouse monoclonal anti-AIP4 (1:200 for IB) (BD Biosciences), mouse monoclonal anti-β-arrestins (1:500 for IP and IB, 1:200 for immunostaining) (BD Biosciences), mouse monoclonal P4D1 anti-ubiquitin (1:200 for IB) (Santa Cruz Biotechnology), mouse monoclonal anti-FLAG (1:3000 for IB) (Sigma), and mouse monoclonal and rabbit polyclonal anti-HA antibodies (1:1000 for IP and IB) (Santa Cruz Biotechnology). HRP-conjugated secondary antibodies were purchased from GE healthcare. FITC-conjugated and Alexa Fluor 633-conjugated secondary antibodies were obtained from Invitrogen. 4-α-Phorbol 12,13-didecanoate (4-α-PDD), MG132, and angiotensin II were purchased from Sigma. Sulfo-NHS-biotin and NeutrAvidin beads were from Pierce. The Fluo-4 NW calcium assay kit was purchased from Invitrogen.
Cell Culture and Transfection
HEK-293 cells were grown in regular minimum Eagle's medium supplemented with 10% FBS. Plasmid transfections and siRNA transfections were carried out essentially as described before (34–38). Isolation and culture of rat vascular smooth muscle cells (rVSMCs) has been described in detail previously (39). Cells were incubated at 25 °C for 8–12 h before immunoprecipitation or confocal experiments.
Immunoprecipitation Experiments
Cells were stimulated with angiotensin or SII-angiotensin for an appropriate time interval and lysed using glycerol lysis buffer (37). Subsequently, 1–3 mg of total cell lysate was used to immunoprecipitate the protein of interest with tumbling overnight in the cold room. Subsequently, the beads were washed three times (with 1 ml of lysis buffer each) and eluted with SDS-PAGE loading buffer. The proteins were separated on 4–20% SDS-PAGE and used for Western blotting using appropriate antibodies.
Confocal Microscopy
Cells were fixed on conformal chambers using 6% formaldehyde for 15 min followed by permeabilization with 0.1–0.2% Triton X-100 for 20 min. After washing, the cells were incubated with an appropriate antibody overnight followed by incubation with secondary antibody. Confocal images were obtained on a Zeiss LSM510 laser scanning microscope using single line (488 nm) or multitrack sequential excitation (488 and 633 nm) and emission (515–540 nm, boron-dipyrromethene (BODIPY) fluorescein; 650 nm, Alexa Fluor 633) filter sets. The color for 488 nm excitation line was set as pseudo green, and the color for 633 nm excitation line was set as pseudo red. The merge of these two colors (pseudo green and pseudo red) yielded yellow when colocalization of two proteins occurred (see Fig. 1E).
FIGURE 1.
Angiotensin stimulation leads to formation of a multiprotein complex involving β-arrestin, TRPV4, and AT1aR. A–D, interaction of β-arrestins (β-arr) and TRPV4 upon angiotensin (Ang) stimulation. A, rVSMCs were stimulated with angiotensin (1 μm) for the indicated time points and coimmunoprecipitated using a goat polyclonal TRPV4 antibody. β-Arrestins were detected in the immunoprecipitate using rabbit polyclonal A1CT antibody. Blots were reprobed with rabbit anti-TRPV4 polyclonal antibody. B, quantification of coimmunoprecipitated β-arrestin (shown in panel A) by densitometry. Data are expressed as -fold over basal interaction detected between β-arrestin and TRPV4. C, rVSMCs were stimulated with angiotensin for the indicated time points and coimmunoprecipitated using rabbit polyclonal A1CT antibody. TRPV4 was detected in the immunoprecipitate using goat polyclonal anti-TRPV4 antibody. Blots were reprobed with goat anti-TRPV4 polyclonal antibody. D, quantification of coimmunoprecipitated TRPV4 (shown in panel C) by densitometry. Data are expressed as -fold over basal interaction detected between β-arrestin and TRPV4. Data presented in panels B and D show mean ± S.E. from at least 3 independent experiments. E, colocalization analysis of AT1aR and TRPV4 by confocal microscopy. rVSMCs were transfected with HA-AT1aR, fixed, permeabilized, and immunostained using goat polyclonal anti-TRPV4 antibody (green) and a polyclonal rabbit anti-HA antibody (blue). F and G, interaction of AT1aR and TRPV4 in rVSMCs. F, rVSMCs were stimulated with angiotensin (1 μm) for the indicated time points and subsequently coimmunoprecipitated using a goat polyclonal anti-TRPV4 antibody. AT1aR was detected in the immunoprecipitate using a rabbit polyclonal anti-HA antibody. Blots were reprobed with rabbit anti-TRPV4 polyclonal antibody. G, rVSMCs were stimulated with angiotensin for the indicated time points and coimmunoprecipitated using a mouse monoclonal anti-HA antibody, and TRPV4 was detected in the immunoprecipitate using a goat polyclonal anti-TRPV4 antibody. Representative blots and confocal images are shown from at least 3 independent experiments.
Surface Biotinylation Experiment
Cells were serum-starved for 2–4 h and subsequently stimulated with 1 μm angiotensin for time point as mentioned in the legends for Figs. 1–5. Cells were washed three times with ice-cold PBS and incubated on ice for 30 min. 1 mm Sulfo-NHS-biotin was added to the cells and incubated for 1 h with mild shaking. The biotinylation reaction was stopped by using 1 mm glycine containing PBS for 15 min on ice. Cells were washed 3–5 times with ice-cold PBS and lysed using the lysis buffer. Immunoprecipitation of biotinylated proteins was performed as described earlier.
FIGURE 2.
β-Arrestin 1 is required for angiotensin-induced internalization and functional down-regulation of TRPV4. A–C, angiotensin-induced internalization of TRPV4. A, rVSMCs were stimulated with angiotensin (Ang) (1 μm) for the indicated time period, fixed, permeabilized, and stained using goat polyclonal anti-TRPV4 antibody followed by Alexa Fluor 488-conjugated secondary antibody (green: TRPV4). B, HEK-293 cells expressing HA-AT1aR and FLAG-TRPV4 were stimulated with angiotensin for the indicated time points, and surface TRPV4 was labeled with NHS-S-S-biotin. The cells were lysed, immunoprecipitated using NeutrAvidin beads, and immunoblotted with a mouse monoclonal anti-FLAG antibody. C, densitometry analysis of angiotensin-induced TRPV4 internalization as measured by surface biotinylation (shown in panel B). Data are presented as the percentage of initial levels of TRPV4 at the cell surface and show mean ± S.E. from at 3 independent experiments. D–F, involvement of β-arrestin 1 in TRPV4 internalization. D, 4-α-PDD-induced Ca2+ influx measured in HEK-293 cells. The cells were prestimulated with either angiotensin or SII-angiotensin (SII-Ang), and subsequently 4-α-PDD-induced Ca2+ influx was measured using the Fluo-4 NW calcium assay kit. The data are presented as the percentage of control cells, i.e. cells prestimulated with saline. NS, non-stimulated. E, 4-α-PDD-induced Ca2+ influx measured in HEK-293 cells after depletion of β-arrestin 1 (β-arr 1) or β-arrestin 2 (β-arr 2) using siRNA. CTL is control scrambled siRNA. F, angiotensin-induced internalization was measured in HEK-293 cells by surface biotinylation after depletion of β-arrestin 1 or 2 using siRNA using mouse monoclonal anti-FLAG antibody. Representative blots are shown from at least 3 independent experiments. G, densitometry analysis of angiotensin-induced internalization of TRPV4 (shown in panel F). Data are normalized with respect to the non-treated control siRNA sample (100%). The data presented in panels D–F show the mean ± S.E. from 3–4 independent experiments, and the statistical analysis was carried out using one-way ANOVA with Bonferroni post test.
FIGURE 3.
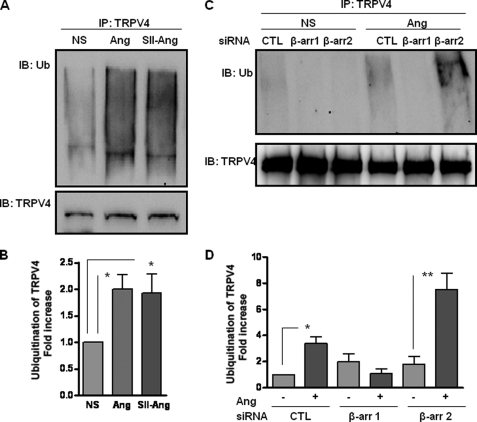
β-Arrestin mediates angiotensin-induced ubiquitination of TRPV4. A, rVSMCs were preincubated with MG132 (10 μm) for 2 h and then stimulated with angiotensin (Ang) (0.1 μm) or SII-angiotensin (SII-Ang) (10 μm) for 1 h. Subsequently, the cells were lysed and immunoprecipitated with goat polyclonal anti-TRPV4 antibody followed by Western blot using mouse monoclonal anti-ubiquitin antibody. The levels of TRPV4 in the lysate were detected by goat polyclonal anti-TRPV4 antibody. NS, non-stimulated; Ub, ubiquitin. B, densitometry analysis of angiotensin-induced TRPV4 ubiquitination (shown in panel A). Data are presented as -fold increase over the basal ubiquitination of TRPV4. C, rVSMCs transfected with either control or siRNA against β-arrestin 1 (β-arr 1) or β-arrestin 2 (β-arr 2) were preincubated with MG132 (10 μm) for 2 h and then stimulated with angiotensin (0.1 μm) for 1 h. Subsequently, the cells were lysed and immunoprecipitated with TRPV4 antibody followed by Western blot using anti-ubiquitin antibody. The levels of TRPV4 in the lysate were detected by rabbit polyclonal anti-TRPV4 antibody. CTL, control siRNA. D, densitometry analysis of angiotensin-induced TRPV4 ubiquitination (shown in panel C). Data are presented as -fold increase over the basal ubiquitination of TRPV4. Representative blots are shown from 4 independent experiments. Data presented as bar graphs show mean ± S.E. from 4 independent experiments. Statistical analysis was carried out using one-way ANOVA with Bonferroni post test.
FIGURE 4.
AIP4 is required for angiotensin-induced ubiquitination of TRPV4, and β-arrestin 1 acts as an adaptor for AIP4. A, HEK-293 cells expressing AT1aR and TRPV4 were transfected with either control or siRNA against AIP4. Cells were preincubated with MG132 (10 μm) for 2 h and then stimulated with angiotensin (0.1 μm) for 1 h. Subsequently, the cells were lysed and immunoprecipitated with TRPV4 antibody followed by Western blot using anti-ubiquitin antibody. The blots were reprobed with anti-TRPV4 antibody, and lysate was probed with anti-AIP4 antibody. CTL, control siRNA; Ub, ubiquitin. B, densitometry analysis of angiotensin-induced TRPV4 ubiquitination (shown in panel A). Data are presented as -fold increase over the basal ubiquitination of TRPV4. C, HEK-293 cells expressing AT1aR and TRPV4 were transfected with either control or siRNA against β-arrestin 1 (β-arr). Cells were stimulated with angiotensin (0.1 μm) for 2 min. Subsequently, the cells were lysed and immunoprecipitated with AIP4 antibody followed by Western blot using anti-TRPV4 antibody. The blots were reprobed with anti-AIP4 antibody, and lysate was probed with anti-TRPV4 and anti-AIP4 antibody. D, densitometry analysis of angiotensin-induced coimmunoprecipitation of TRPV4 and AIP4 (shown in panel C). Data are presented as -fold increase over the basal interaction between TRPV4 and AIP4. Representative blots are shown from at 3 independent experiments, and the data presented as bar graphs show mean ± S.E. from at least 3 independent experiments. Statistical analysis was carried out using one-way ANOVA with Bonferroni post test.
FIGURE 5.
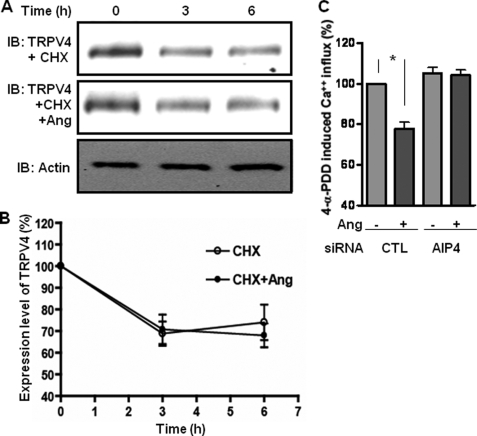
Angiotensin-induced ubiquitination of TRPV4 promotes internalization but not degradation. A, HEK-293 cells expressing AT1aR and TRPV4 were incubated with cycloheximide (CHX) (10 μm) in the absence or presence of angiotensin (Ang) (0.1 μm) for the indicated time points. Cells were lysed, and the expression levels of TRPV4 in the lysate were detected by Western blot using mouse monoclonal anti-FLAG antibody. An anti-actin blot is shown as the loading control. B, densitometry analysis of angiotensin-induced degradation of TRPV4 normalized with levels of actin. Data are presented as the percentage of decrease in TRPV4 level over the basal level of TRPV4. C, 4-α-PDD-induced Ca2+ influx measured in HEK-293 cells transfected with control (CTL) or AIP4 siRNA. Cells were prestimulated with angiotensin (0.1 μm) for 1 h, and subsequently 4-α-PDD-induced Ca2+ influx was measured using the Fluo-4 NW kit. The data are presented as the percentage of control cells, i.e. cells prestimulated with saline. The data presented in panels B and C show mean ± S.E. from 3 independent experiments. Statistical analysis was carried out using one-way ANOVA with Bonferroni post test.
Ca2+ Influx Assay
4-α-PDD-induced Ca2+ influx was essentially carried out as described in the Fluo-4 NW calcium assay kit manual (Invitrogen) (62). The readings were recorded using the NovoStar multiwavelength plate reader (BMG Labtech). The Ca2+ influx data are normalized with respect to a 4-α-PDD-induced but angiotensin-non-stimulated sample (100%).
Ubiquitination Assay
For ubiquitination experiments, cells were serum-starved for 4–6 h and incubated with 10 μm MG132 for time intervals as mentioned in the legends for Figs. 1–5. Subsequently, the cells were stimulated with 0.1–1 μm angiotensin for appropriate time points and lysed using the lysis buffer. TRPV4 was immunoprecipitated using anti-TRPV4 goat polyclonal antibody overnight, and subsequently, ubiquitinated proteins were detected using anti-ubiquitin mouse monoclonal antibody.
Densitometry and Statistical Analysis
The bands on the Western blots were quantified using the GeneTool software (Syngene Inc.) as per software guidelines, and data were incorporated in GraphPad for subsequent analysis. To calculate the -fold increase in the densitometry analysis, the data are normalized with respect to the non-stimulated sample (1 or 100%). The raw intensity values obtained by densitometry analysis for the non-stimulated sample after background correction were considered as “1-fold” or 100%. The raw intensity values for treated samples were divided by the raw values of the non-stimulated sample, and the number was represented as “-fold response” or “percentage of response.” Statistical significance and p values in bar graphs were determined by one-way ANOVA with Bonferroni post test using the PRISM software as indicated in the legends for Figs. 1–5. p values were calculated p value (*, p < 0.05; **, p < 0.01; ***, p < 0.001) of <0.05 was considered statistically significant.
RESULTS
Identification and Validation of TRPV4 as an Interaction Partner of β-Arrestin 1
We identified TRPV4 as a specific interaction partner of β-arrestins using a global interactomics analysis (11). To further confirm this interaction and explore the regulatory outcome, we chose rVSMCs as our model system. These cells, isolated from rat aorta, endogenously express AT1aR, TRPV4, and β-arrestins and represent an excellent, physiologically relevant model system. First, we stimulated rVSMCs with angiotensin for different times, immunoprecipitated TRPV4, and subsequently detected β-arrestins by Western blot. As shown in Fig. 1, A and B, angiotensin stimulation led to a robust but transient interaction between TRPV4 and β-arrestin 1. We also carried out a reciprocal immunoprecipitation experiment where we stimulated rVSMCs with angiotensin, immunoprecipitated β-arrestins, and detected TRPV4 by Western blot. Again, we observed a robust interaction between TRPV4 and β-arrestin 1 at the 2-min time point (Fig. 1, C and D). rVSMCs express very low levels of β-arrestin 2, and β-arrestin 1 is the predominant isoform of β-arrestins in these cells. Thus, to confirm the specificity of interaction of TRPV4 with β-arrestin 1 versus β-arrestin 2, we used HEK-293 cells expressing endogenous β-arrestins 1 and 2 but transfected with HA-AT1aR and FLAG-TRPV4. Co-immunoprecipitation experiments revealed a selective interaction between TRPV4 and β-arrestin 1 that was transient in nature, similar to that observed in rVSMCs (supplemental Fig. S1, A–D). These findings confirm our initial observation based on the proteomic screen that β-arrestin 1 and TRPV4 interact with each other upon activation of AT1aR and provide the first evidence of a direct link between TRPV4 and β-arrestins.
AT1aR and TRPV4 Interact with Each Other and Form a Multiprotein Complex with β-Arrestin 1
Next, we asked whether AT1aR and TRPV4 are involved in a direct cross-talk. As rVSMCs express very low levels (20 fmol/mg) of AT1aR, which is insufficient for coimmunoprecipitation, we transfected these cells with HA-AT1aR to probe a direct interaction between AT1aR and TRPV4. First, we immunostained AT1aR and TRPV4 and examined the localization of these two proteins by confocal microscopy. Both AT1aR and TRPV4 were expressed at the cell surface and exhibited clear colocalization indicating a potential interaction (Fig. 1E). Subsequently, we immunoprecipitated either HA-AT1aR or endogenous TRPV4 and detected the respective partner by Western blotting. As shown in Fig. 1, F and G, we detected a constitutive interaction between the AT1aR and TRPV4, and this interaction is not modulated by angiotensin stimulation during the time course examined in this experiment. We also observed a similar pattern of constitutive interaction of AT1aR and TRPV4 in HEK-293 cells (supplemental Fig. S2, A and B). Taken together with the data presented in Fig. 1, A–D, these findings establish the formation of a previously unsuspected multiprotein complex involving TRPV4, AT1aR, and β-arrestin 1 in response to angiotensin stimulation.
Angiotensin Stimulation Leads to Internalization and Functional Down-regulation of TRPV4
What are the functional outcomes of this physical interaction among TRPV4, AT1aR, and β-arrestin 1? One of the critical roles of β-arrestins that has emerged in the context of 7TMR regulation is their ability to support receptor internalization by recruiting a number of endocytotic proteins such as clathrin, AP2 (adaptor-related protein complex), N-ethylmaleimide-sensitive fusion protein, and ARF6 (ADP-ribosylation factor 6) (40). Thus, it is tempting to speculate that β-arrestin 1 might play a similar role in angiotensin-induced internalization of TRPV4. To test this hypothesis, we first examined whether TRPV4 undergoes internalization in response to angiotensin stimulation. We stimulated rVSMCs with angiotensin and examined the localization pattern of TRPV4 by confocal microscopy. As shown in Fig. 2A, TRPV4 was localized at the cell surface in non-stimulated cells, and stimulation by angiotensin led to robust internalization as evident by targeting of TRPV4 to intracellular vesicles. To further confirm this finding, we used a surface biotinylation assay to get a quantitative assessment of angiotensin-induced internalization of TRPV4. Again, in response to angiotensin stimulation for 1 h, we observed ∼50% decrease in the cell surface TRPV4, indicating TRPV4 internalization (Fig. 2, B and C). In yet another approach to confirm internalization of TRPV4 and to investigate whether internalization of TRPV4 translates into a functional down-regulation, we measured 4-α-PDD-induced Ca2+ influx in rVSMCs. 4-α-PDD is a potent and specific agonist for TRPV4, which leads to robust Ca2+ influx in cells expressing TRPV4. We treated the cells with either PBS or angiotensin for 1 h and subsequently measured 4-α-PDD-induced Ca2+ influx. As shown in Fig. 2D and supplemental Fig. S3, 4-α-PDD stimulation resulted in a strong Ca2+ influx, as expected, in PBS-treated cells; however, angiotensin prestimulation resulted in significant inhibition of 4-α-PDD-induced Ca2+ influx. These observations reveal for the first time that activation of AT1aR can trigger internalization and functional down-regulation of TRPV4, and the data uncover an entirely novel functional attribute for the heterodimerization between AT1aR and TRPV4. It is also possible that decreased Ca2+ influx upon prestimulation with angiotensin results, in part, from cross-desensitization of TRPV4.
β-Arrestin 1 Is Required for TRPV4 Internalization and Functional Down-regulation
What, if any, is the involvement of β-arrestins in TRPV4 internalization? To answer this question, we used two parallel approaches. First, we used a β-arrestin-biased agonist of AT1aR called SII-angiotensin to probe TRPV4 internalization. Similar to angiotensin, SII-angiotensin leads to recruitment of β-arrestin to the AT1aR. However, unlike angiotensin, SII-angiotensin selectively activates β-arrestin-dependent signaling without activating any detectable G protein-dependent signaling (41). Thus, an effect elicited by both angiotensin and SII-angiotensin stimulation would suggest the potential involvement of β-arrestins. Indeed, we found that pretreatment with SII-angiotensin also results in a qualitatively and quantitatively similar decrease in 4-α-PDD-induced Ca2+ influx, suggesting a critical involvement of β-arrestins in TRPV4 internalization (Fig. 2D). In a second approach, we used siRNA-directed knockdown of β-arrestins and measured its effect on TRPV4 internalization. As shown in Fig. 2E, knocking down β-arrestin 1, but not β-arrestin 2, blocks the effect of angiotensin prestimulation on 4-α-PDD-induced Ca2+ influx. Similarly, β-arrestin 1 knockdown, but not β-arrestin 2 knockdown, inhibits angiotensin-induced internalization of TRPV4 as detected by surface biotinylation (Fig. 2, F and G). These findings not only provide the first evidence of a critical role of β-arrestin 1 in angiotensin-induced internalization and down-regulation of TRPV4 but also imply that the physical interaction between TRPV4 and β-arrestin translates into a functional outcome. The cells transfected with β-arrestin 1 siRNA also exhibited lower 4-α-PDD-induced Ca2+ influx when compared with control siRNA-transfected (CTL) cells, which probably results from lower surface expression of TRPV4 in these cells.
Angiotensin Stimulation Promotes Ubiquitination of TRPV4, Which Requires β-Arrestin 1
What is the mechanism of angiotensin-induced and β-arrestin 1-dependent internalization of TRPV4? Ubiquitination and deubiquitination of membrane proteins are conserved mechanisms underlying the regulation of their surface expression. Thus, we first checked whether angiotensin stimulation results in ubiquitination of TRPV4 and whether β-arrestins are involved in this process. We pretreated rVSMCs with MG132 (to inhibit proteasomal degradation) followed by angiotensin stimulation and subsequent immunoprecipitation of TRPV4. Then, we probed the immunoprecipitated sample by Western blot using a ubiquitin-specific antibody (P4D1). As shown in Fig. 3, A and B and supplemental Fig. S4, A and B, angiotensin stimulation promotes significant ubiquitination of TRPV4 in both rVSMCs and HEK-293 cells. These data demonstrate for the first time that activation of a 7TMR can lead to ubiquitination of an ion channel and underscore a previously unknown functional implication of the intricate networks of 7TMRs in the membrane. Moreover, stimulation by SII-angiotensin also promotes ubiquitination of TRPV4 to a similar extent as angiotensin, strongly indicating the involvement of β-arrestins in this process.
To further probe the involvement of β-arrestins in angiotensin-induced ubiquitination of TRPV4, we knocked down either β-arrestin 1 or β-arrestin 2 using siRNA and studied its effect on TRPV4 ubiquitination. As shown in Fig. 3, C and D, knocking down β-arrestin 1 completely inhibits angiotensin-induced ubiquitination of TRPV4. Surprisingly, knocking down β-arrestin 2 led to an increase in angiotensin-induced ubiquitination of TRPV4. This reciprocal role of the β-arrestin isoforms, i.e. β-arrestins 1 and 2, in ubiquitination of TRPV4 highlights an intriguing example of functional antagonism/divergence of the two isoforms.
How do β-arrestins regulate angiotensin-induced ubiquitination of TRPV4? A novel aspect of β-arrestin functions that has emerged recently in the context of 7TMRs is their ability to bind and act as adaptors for E3 ubiquitin ligases (6). Thus, one possibility is that β-arrestin 1 serves as an adaptor for an E3 ubiquitin ligase required for TRPV4 ubiquitination. AIP4 (atrophin 1-interacting protein 4), a member of the HECT (homologous to E6-AP carboxyl terminus) family of ubiquitin ligases, was recently identified as the relevant ligase for TRPV4 ubiquitination (42). AIP4 is a member of HECT family of E3 ubiquitin ligases where the ligase-substrate interaction is mediated by their WW domains and PPXY motif. However, TRPV4 does not contain a consensus PY motif, and therefore, it was proposed that an adaptor protein may facilitate the recruitment of AIP4 to TRPV4. Thus, to examine our hypothesis of β-arrestin 1 being an adaptor for AIP4, we first checked the interaction between β-arrestins and AIP4. Consistent with a previous report (42), we observed a constitutive interaction between β-arrestins and AIP4 (supplemental Fig. S5). The interaction of β-arrestins and AIP4 was not altered by angiotensin stimulation.
We next examined the effect of AIP4 knockdown on angiotensin-induced TRPV4 ubiquitination. As shown in Fig. 4, A and B and supplemental Fig. S6, knockdown of AIP4 in HEK-293 cells using three different siRNAs completely blocked angiotensin-induced ubiquitination of TRPV4, suggesting that AIP4 is required for angiotensin-induced ubiquitination of TRPV4. To check whether β-arrestin 1 acts as an adaptor for AIP4, we examined the interaction between TRPV4 and AIP4 in response to angiotensin stimulation. As shown in Fig. 4, C and D, angiotensin stimulation promoted a significant interaction between TRPV4 and AIP4. Interestingly, this interaction between TRPV4 and AIP4 was dependent on the presence of β-arrestin 1 as siRNA-mediated knockdown of β-arrestin 1 inhibits the angiotensin-induced increase in the interaction between TRPV4 and AIP4. Thus, our data identify β-arrestin 1 as a previously unknown adaptor linking AIP4 to the TRPV4 and provide the first demonstration that β-arrestins can act as E3 ligase adaptors for ion channels.
Angiotensin-induced Ubiquitination Does Not Promote Degradation but Is Required for Internalization of TRPV4
The primary purpose of ubiquitination of proteins is proteasomal degradation to maintain an optimal level in the cells. Therefore, we checked whether angiotensin-induced TRPV4 ubiquitination enhances the rate or extent of its degradation. As shown in Fig. 5, A and B, incubation of HEK-293 cells with cycloheximide (to block new protein synthesis) led to a substantial decrease in the TRPV4 levels in the cells. However, the addition of angiotensin in addition to cycloheximide did not lead to any further decrease in TRPV4 levels in the cells. These data suggest that angiotensin-induced ubiquitination does not lead to TRPV4 degradation in these cells. We observed a similar pattern of TRPV4 degradation in rVSMCs.
So what role does angiotensin-induced ubiquitination of TRPV4 play in its regulation? It has been proposed that in addition to directing proteins for proteasomal degradation, ubiquitination might also serve as a signal for non-degradative internalization of membrane proteins (43). In fact, our observation that siRNA against β-arrestin 1, which inhibits TRPV4 ubiquitination, also blocks angiotensin-induced TRPV4 internalization provides an indication for such a possibility. To further confirm this, we investigated whether AIP4 knockdown blocks TRPV4 internalization. Again, we measured a 4-α-PDD-induced Ca2+ influx assay to test this possibility. As shown in Fig. 5C, after depletion of AIP4, angiotensin-induced internalization was completely blocked. These data, taken together with the data presented in Fig. 2, conclusively demonstrate that angiotensin-induced ubiquitination of TRPV4 serves as a signal for its internalization but not its degradation.
DISCUSSION
Cross-talk between AT1aR and TRPV4
A direct or indirect communication among proteins in the cell membrane is an efficient mode of regulating cellular processes. Considering the diverse range of cellular events mediated by 7TMRs, it is not surprising that they form intricate networks of interactions in the membrane. In particular, communication between 7TMRs and ion channels is of great interest. Here, we report for the first time that the AT1aR and TRPV4 form a constitutive heterodimer in the membrane, and their interaction remains unchanged in response to angiotensin stimulation at least during the time course examined in this study. So what purpose does this constitutive interaction of AT1aR and TRPV4, or any other 7TMR with an ion channel, serve? One can envisage several possibilities. For example, the presence of such multiprotein complexes might ensure rapid responses to stimuli because the key players are already in close proximity, thus overcoming the requirement for lateral diffusion or the need to achieve a specific concentration of the components. Additionally, preassembled and localized signaling complexes with specific partners may reduce the nonspecific effects arising from random collision events and will be energetically more efficient for the cells. Although there exist some examples of heterodimer formation between ion channels and 7TMRs (44–47), our data not only provide the first evidence of direct cross-talk between AT1aR and TRPV4 but also report the first example of formation of a multiprotein complex involving β-arrestins, a 7TMR, and a TRP channel. The findings described here clearly indicate that assembly of multiprotein complexes around 7TMRs can be a general mechanism to mediate efficient signaling and to exert precise control over their function via formation of localized signaling domains.
Expanding Network of β-Arrestin Interactions Includes TRPV4
Since the original discovery of β-arrestins in the context of 7TMR desensitization, the list of β-arrestin-interacting proteins has grown rapidly. β-Arrestins are now well known to interact with proteins involved in clathrin-coated endocytosis to support 7TMR internalization, to scaffold a number of MAP kinases to mediate G protein-independent signaling, and to act as adaptors for E3 ubiquitin ligases to promote ubiquitination of 7TMRs (40). Interestingly, in contrast to the original assumption that β-arrestins evolved solely to regulate 7TMRs, we identify a novel functional link between β-arrestins and an ion channel. We report that β-arrestin 1 interacts with TRPV4 and fine-tunes the level of functional TRPV4 at the cell surface. This represents an entirely novel mechanism to exert precise control over TRPV4-mediated Ca2+ influx in cells to avoid the unwanted consequences of excessive Ca2+ signaling. Moreover, this finding also suggests that the functional capabilities of β-arrestins might be much broader than currently anticipated.
Critical Involvement of β-Arrestins in Ubiquitination of TRPV4
The very first indication of any involvement of β-arrestins in ubiquitination of any membrane protein was reported in the context of the human β2AR, a 7TMR (48). Isoproterenol stimulation led to robust ubiquitination of the β2AR in wild type mouse embryonic fibroblasts but not in β-arrestin 2-null mouse embryonic fibroblasts, suggesting a potential role of β-arrestin 2 in this process. It was proposed that β-arrestin 2 can act as an adaptor for an E3 ubiquitin ligase required for ubiquitination of β2AR. Although the specific E3 ligase for β2AR was not known at that time, it was subsequently found that β-arrestin 2 indeed acts as an adaptor for an E3 ubiquitin ligase Nedd 4 to promote β2AR ubiquitination and degradation (35). Since then, a critical requirement of β-arrestins in ubiquitination and degradation of several 7TMRs has been reported (49). Here, we report the first example of involvement of β-arrestin 1 in ubiquitination of an ion channel, TRPV4. Moreover, our data also demonstrate that β-arrestin 1 acts as an adaptor for the E3 ubiquitin ligase, AIP4, to facilitate TRPV4 ubiquitination and thus identify β-arrestin 1 as a previously unknown adaptor linking AIP4 to the TRPV4. However, unlike 7TMRs, β-arrestin 1-mediated ubiquitination of TRPV4 does not result in degradation of TRPV4. Thus, our findings are also unique in the sense that in this case, β-arrestins are involved in promoting non-degradative ubiquitination of the target protein. It is intriguing that the E3 ubiquitin ligase adaptor function of β-arrestin 1 for TRPV4 is analogous to that reported for 7TMRs, and therefore, highlights an evolutionarily conserved principle. These data also suggest that some of the paradigms originally discovered for 7TMRs, and anticipated to be functional solely for 7TMRs, might be applicable to other integral membrane proteins, and therefore, provide hints at novel regulatory mechanisms implicated more generally in cellular signaling. Interestingly, this emerging adaptor function of β-arrestins appears to be operative not only in mammalian cells (50, 51) but also in Drosophila (52) and in yeast (53, 54). Our findings raise the interesting possibility that β-arrestins can be global mediators of ubiquitination of ion channels and transporters, and therefore, open up a new avenue of research in this rapidly evolving area. It should be noted that β-arrestins may not necessarily act as E3 ligase adaptors in every case but might also influence the process of ubiquitination in other ways such as acting as E2 ubiquitin-conjugating enzyme adaptors or through downstream signaling events.
A somewhat unexpected observation in this study was the increased ubiquitination of TRPV4 upon β-arrestin 2 knockdown. A similar reciprocal effect of β-arrestins 1 and 2 was reported for AT1aR-mediated activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) (55). In transfected HEK-293 cells, β-arrestin 1 knockdown increased the levels of activated ERK1/2, whereas β-arrestin 2 knockdown decreased (or completely abolished) the levels of activated ERK1/2. It was proposed that β-arrestin 1 is much weaker than β-arrestin 2 in scaffolding the ERK activation cascade. As both β-arrestin isoforms (1 and 2) appear to bind AT1aR with similar affinity, at physiological levels, β-arrestin 1 competitively inhibits the amount of β-arrestin 2 recruited to the activated receptor. Knocking down β-arrestin 1 leads to increased recruitment of β-arrestin 2 and in turn results in higher levels of activated ERK1/2. A similar mechanism might explain the increased ubiquitination of TRPV4 upon β-arrestin 2 knockdown. Thus, it is plausible that lowering the levels of β-arrestin 2 by siRNA results in quantitative increase in β-arrestin 1 recruitment to the AT1aR-TRPV4 complex, which in turn results in increased ubiquitination of TRPV4.
A Potential Global Role of β-Arrestins in Regulation of Ion Channels and Its Physiological Implications
Since their discovery in the context of G protein-coupled receptor desensitization, the repertoire of functions that β-arrestins can perform has increased tremendously. Not only can β-arrestins mediate G protein-independent signaling, but several of the paradigms originally discovered with reference to 7TMRs appear to be applicable to other receptor systems. Even more intriguing is the involvement of β-arrestins in the regulation of non-receptor membrane proteins such as ion channels and transporters. Our studies establish a functional link between β-arrestin 1 and TRPV4 and provide a novel mechanism for regulation of TRPV4 (a schematic representation is shown in Fig. 6). It is possible that β-arrestins interact with and play a similar role in regulation of other TRP channels as described here for TRPV4. However, it would not be surprising if there exist other mechanisms through which β-arrestins can regulate other members of the TRP channel family. Interestingly, we have also identified a number of other ion channels such as the cyclic nucleotide-gated ion channel (CNGA3), the nuclear chloride channel 27, the potassium channel Kvβ1, and the nucleotide-sensitive chloride channel 1A in our β-arrestin interactomics screen (11). Moreover, there are also some reports of potential involvement of β-arrestins in the regulation of other ion channels (56–59). Thus, our data, taken together with the previous findings, strongly suggest a new paradigm in which β-arrestins interact with and regulate ion channels and transporters in the cell. These data also indicate that β-arrestins play broader roles in promoting the endocytosis of integral plasma membrane proteins than previously suspected.
FIGURE 6.
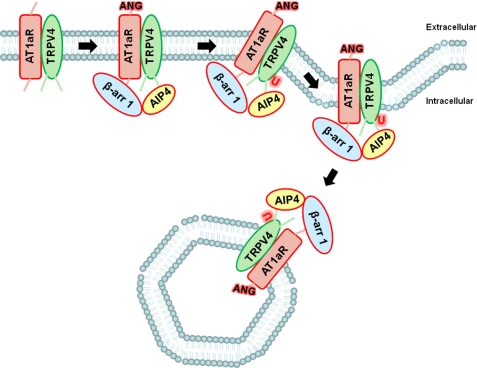
Schematic representation of β-arrestin 1-dependent ubiquitination and functional down-regulation of TRPV4 upon activation of AT1aR. This illustrative scheme shown for TRPV4 can be potentially applicable to β-arrestin-mediated down-regulation of other TRP channels as well as other ion channels and transporters. ANG, angiotensin; U, ubiquitin; β-arr 1, β-arrestin 1.
An appropriate balance of different ions inside the cells is extremely important for various cellular and physiological processes. Perturbation of proper functioning and tight regulation of ion channels may disturb the balance of various ions in the cells, and this can lead to various disease conditions. In particular, a variety of cellular signaling pathways depend on Ca2+, and therefore, a precise control of Ca2+ concentrations in the cells is required. Thus, it is tempting to speculate that by interacting with ion channels and maintaining appropriate levels at the cell surface, β-arrestins might play a crucial role in avoiding ionic imbalance in the cells. For example, myocardial apoptosis has been proposed to be a major contributing factor in heart failure in animal models and in human subjects. Although apoptosis can be triggered by a number of different stimulants, elevated intracellular Ca2+ is believed to be a key initiator of apoptotic signaling (60). It is interesting that β-arrestin-dependent signaling has been described to be cardioprotective under conditions of chronic catecholamine stress (61). One mechanism underlying this cardioprotective effect of β-arrestins appears to be β-arrestin-dependent transactivation of the EGF receptor (61). However, our data describe another novel mechanism, i.e. fine-tuning of the levels of TRP channels in the plasma membrane to maintain a balance of intracellular Ca2+ levels. Moreover, 7TMR-dependent regulation of ion channels via β-arrestin-dependent mechanisms also has potential for designing novel 7TMR ligands to modulate the function of ion channels, thereby addressing channelopathies.
In conclusion, our data provide the first evidence of β-arrestin interaction with TRPV4 and describe a novel regulatory mechanism governing the functional down-regulation of TRPV4. Moreover, our findings also suggest that some of the paradigms originally discovered for 7TMRs might also be applicable to other receptor systems, ion channels, and transporters.
Acknowledgments
We thank Donna Addison and Elizabeth Hall for excellent secretarial assistance. The technical support of Prachi Tripathi is greatly acknowledged.
This work was supported, in whole or in part, by National Institutes of Health Grants HL16037 and HL70631 (to R. J. L.).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- 7TMR
- seven-transmembrane receptors
- TRP
- transient receptor potential
- AIP4
- atrophin 1-interacting protein 4
- 4-α-PDD
- 4-α-phorbol 12, 13-didecanoate
- rVSMC
- rat vascular smooth muscle cell
- ANOVA
- analysis of variance
- IB
- immunoblotting
- IP
- immunoprecipitation
- SII-Ang II
- ([sarcosine1,IIe4,IIe8]Angiotensin II)
- β2AR
- beta 2 adrenergic receptor.
REFERENCES
- 1.Takeda S., Kadowaki S., Haga T., Takaesu H., Mitaku S. (2002) FEBS Lett. 520, 97–101 [DOI] [PubMed] [Google Scholar]
- 2.Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Nat. Rev. Mol. Cell Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz R. J. (1998) J. Biol. Chem. 273, 18677–18680 [DOI] [PubMed] [Google Scholar]
- 4.Pitcher J. A., Freedman N. J., Lefkowitz R. J. (1998) Annu. Rev. Biochem. 67, 653–692 [DOI] [PubMed] [Google Scholar]
- 5.Shenoy S. K., Lefkowitz R. J. (2003) Biochem. J. 375, 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 7.Chen W., Ren X. R., Nelson C. D., Barak L. S., Chen J. K., Beachy P. A., de Sauvage F., Lefkowitz R. J. (2004) Science 306, 2257–2260 [DOI] [PubMed] [Google Scholar]
- 8.Chen W., ten Berge D., Brown. J., Ahn S., Hu L. A., Miller W. E., Caron M. G., Barak L. S., Nusse R., Lefkowitz R. J. (2003) Science 301, 1391–1394 [DOI] [PubMed] [Google Scholar]
- 9.Lin F. T., Daaka Y., Lefkowitz R. J. (1998) J. Biol. Chem. 273, 31640–31643 [DOI] [PubMed] [Google Scholar]
- 10.Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., Blobe G. C. (2003) Science 301, 1394–1397 [DOI] [PubMed] [Google Scholar]
- 11.Xiao K., McClatchy D. B., Shukla A. K., Zhao Y., Chen M., Shenoy S. K., Yates J. R., 3rd, Lefkowitz R. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montell C., Birnbaumer L., Flockerzi V. (2002) Cell 108, 595–598 [DOI] [PubMed] [Google Scholar]
- 13.Wissenbach U., Niemeyer B. A., Flockerzi V. (2004) Biol. Cell 96, 47–54 [DOI] [PubMed] [Google Scholar]
- 14.Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000) Cell 103, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strotmann R., Harteneck C., Nunnenmacher K., Schultz G., Plant T. D. (2000) Nat. Cell Biol. 2, 695–702 [DOI] [PubMed] [Google Scholar]
- 16.Liedtke W., Friedman J. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alessandri-Haber N., Yeh J. J., Boyd A. E., Parada C. A., Chen X., Reichling D. B., Levine J. D. (2003) Neuron. 39, 497–511 [DOI] [PubMed] [Google Scholar]
- 18.Gevaert. T., Vriens J., Segal A., Everaerts W., Roskams T., Talavera K., Owsianik G., Liedtke W., Daelemans D., Dewachter I., Van Leuven F., Voets T., De Ridder D., Nilius B. (2007) J. Clin. Invest. 117, 3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Köhler R., Heyken W. T., Heinau P., Schubert R., Si H., Kacik M., Busch C., Grgic I., Maier T., Hoyer J. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1495–1502 [DOI] [PubMed] [Google Scholar]
- 20.Tian W., Salanova M., Xu H., Lindsley J. N., Oyama T. T., Anderson S., Bachmann S., Cohen D. M. (2004) Am. J. Physiol. Renal. Physiol. 287, F17–F24 [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Fernández J. M., Andrade Y. N., Arniges M., Fernandes J., Plata C., Rubio-Moscardo F., Vázquez E., Valverde M. A. (2008) Pflugers Arch. 457, 149–159 [DOI] [PubMed] [Google Scholar]
- 22.Denda M., Sokabe T., Fukumi-Tominaga T., Tominaga M. (2007) J. Invest. Dermatol. 127, 654–659 [DOI] [PubMed] [Google Scholar]
- 23.Liedtke W., Kim C. (2005) Cell Mol. Life Sci. 62, 2985–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedtke W. (2008) Ann. N.Y. Acad. Sci. 1144, 42–52 [DOI] [PubMed] [Google Scholar]
- 25.Hartmannsgruber V., Heyken W. T., Kacik M., Kaistha A., Grgic I., Harteneck C., Liedtke W., Hoyer J., Köhler R. (2007) PLoS One 2, e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loot A. E., Popp R., Fisslthaler B., Vriens J., Nilius B., Fleming I. (2008) Cardiovasc. Res. 80, 445–452 [DOI] [PubMed] [Google Scholar]
- 27.Earley S., Pauyo T., Drapp R., Tavares M. J., Liedtke W., Brayden J. E. (2009) Am. J. Physiol. Heart. Circ. Physiol. 297, H1096–H1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao F., Sui D., Garavito R. M., Worden R. M., Wang D. H. (2009) Hypertension 53, 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jian M. Y., King J. A., Al-Mehdi A. B., Liedtke W., Townsley M. I. (2008) Am. J. Respir. Cell Mol. Biol. 38, 386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willette R. N., Bao W., Nerurkar S., Yue T. L., Doe C. P., Stankus G., Turner G. H., Ju H., Thomas H., Fishman C. E., Sulpizio A., Behm D. J., Hoffman S., Lin Z., Lozinskaya I., Casillas L. N., Lin M., Trout R. E., Votta B. J., Thorneloe K., Lashinger E. S., Figueroa D. J., Marquis R., Xu X. (2008) J. Pharmacol. Exp. Ther. 326, 443–452 [DOI] [PubMed] [Google Scholar]
- 31.Matsusaka T., Ichikawa I. (1997) Annu. Rev. Physiol. 59, 395–412 [DOI] [PubMed] [Google Scholar]
- 32.Patel P. A., Tilley D. G., Rockman H. A. (2008) Circ. J. 72, 1725–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel P. A., Tilley D. G., Rockman H. A. (2009) J. Mol. Cell Cardiol. 46, 300–308 [DOI] [PubMed] [Google Scholar]
- 34.Shukla A. K., Violin J. D., Whalen E. J., Gesty-Palmer D., Shenoy S. K., Lefkowitz R. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9988–9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shenoy S. K., Xiao K., Venkataramanan V., Snyder P. M., Freedman N. J., Weissman A. M. (2008) J. Biol. Chem. 283, 22166–22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn S., Nelson C. D., Garrison T. R., Miller W. E., Lefkowitz R. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn S., Kim J., Hara M. R., Ren X. R., Lefkowitz R. J. (2009) J. Biol. Chem. 284, 8855–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhandari D., Trejo J., Benovic J. L., Marchese A. (2007) J. Biol. Chem. 282, 36971–36979 [DOI] [PubMed] [Google Scholar]
- 39.Kim J., Ahn S., Rajagopal K., Lefkowitz R. J. (2009) J. Biol. Chem. 284, 11953–11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefkowitz R. J., Whalen E. J. (2004) Curr. Opin. Cell Biol. 16, 162–168 [DOI] [PubMed] [Google Scholar]
- 41.Wei H., Ahn S., Shenoy S. K., Karnik S. S., Hunyady L., Luttrell L. M., Lefkowitz R. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wegierski T., Hill K., Schaefer M., Walz G. (2006) EMBO J. 25, 5659–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill L. A. (2009) J. Biol. Chem. 284, 8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J. Y., Saffen D. (2005) J. Biol. Chem. 280, 32035–32047 [DOI] [PubMed] [Google Scholar]
- 45.Hannan M. A., Kabbani N., Paspalas C. D., Levenson R. (2008) Biochim. Biophys. Acta 1778, 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kisilevsky A. E., Mulligan S. J., Altier C., Iftinca M. C., Varela D., Tai C., Chen L., Hameed S., Hamid J., Macvicar B. A., Zamponi G. W. (2008) Neuron 58, 557–570 [DOI] [PubMed] [Google Scholar]
- 47.Davare M. A., Avdonin V., Hall D. D., Peden E. M., Burette A., Weinberg R. J., Horne M. C., Hoshi T., Hell J. W. (2001) Science 293, 98–101 [DOI] [PubMed] [Google Scholar]
- 48.Shenoy S. K., McDonald P. H., Kohout T. A., Lefkowitz R. J. (2001) Science 294, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 49.Shenoy S. K. (2007) Circ. Res. 100, 1142–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girnita L., Shenoy S. K., Sehat B., Vasilcanu R., Girnita A., Lefkowitz R. J., Larsson O. (2005) J. Biol. Chem. 280, 24412–24419 [DOI] [PubMed] [Google Scholar]
- 51.Lakshmikanthan V., Zou L., Kim J. I., Michal A., Nie Z., Messias N. C., Benovic J. L., Daaka Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9379–9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee A., Veraksa A., Bauer A., Rosse C., Camonis J., Artavanis-Tsakonas S. (2005) Nat. Cell Biol. 7, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 53.Lin C. H., MacGurn J. A., Chu T., Stefan C. J., Emr S. D. (2008) Cell 135, 714–725 [DOI] [PubMed] [Google Scholar]
- 54.Nikko E., Sullivan J. A., Pelham H. R. (2008) EMBO Rep. 9, 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn S., Wei H., Garrison T. R., Lefkowitz R. J. (2004) J. Biol. Chem. 279, 7807–7811 [DOI] [PubMed] [Google Scholar]
- 56.Shui Z., Khan I. A., Haga T., Benovic J. L., Boyett M. R. (2001) J. Biol. Chem. 276, 11691–11697 [DOI] [PubMed] [Google Scholar]
- 57.Lipsky R., Potts E. M., Tarzami S. T., Puckerin A. A., Stocks J., Schecter A. D., Sobie E. A., Akar F. G., Diversé-Pierluissi M. A. (2008) J. Biol. Chem. 283, 17221–17226 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Puckerin A., Liu L., Permaul N., Carman P., Lee J., Diversé-Pierluissi M. A. (2006) J. Biol. Chem. 281, 31131–31141 [DOI] [PubMed] [Google Scholar]
- 59.Szabó E. Z., Numata M., Lukashova V., Iannuzzi P., Orlowski J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2790–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verkhratsky A. (2007) Subcell Biochem. 45, 465–480 [DOI] [PubMed] [Google Scholar]
- 61.Noma T., Lemaire A., Naga Prasad S. V., Barki-Harrington L., Tilley D. G., Chen J., Le Corvoisier P., Violin J. D., Wei H., Lefkowitz R. J., Rockman H. A. (2007) J. Clin. Invest. 117, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Invitrogen (2006) Fluo-4 NW calcium assay kit manual, Carlsbad, CA [Google Scholar]