Abstract
β-Arrestins are crucial regulators of G-protein coupled receptor (GPCR) signaling, desensitization, and internalization. Despite the long-standing paradigm that agonist-promoted receptor phosphorylation is required for β-arrestin2 recruitment, emerging evidence suggests that phosphorylation-independent mechanisms play a role in β-arrestin2 recruitment by GPCRs. Several PDZ proteins are known to interact with GPCRs and serve as cytosolic adaptors to modulate receptor signaling and trafficking. Na+/H+ exchange regulatory factors (NHERFs) exert a major role in GPCR signaling. By combining imaging and biochemical and biophysical methods we investigated the interplay among NHERF1, β-arrestin2, and the parathyroid hormone receptor type 1 (PTHR). We show that NHERF1 and β-arrestin2 can independently bind to the PTHR and form a ternary complex in cultured human embryonic kidney cells and Chinese hamster ovary cells. Although NHERF1 interacts constitutively with the PTHR, β-arrestin2 binding is promoted by receptor activation. NHERF1 interacts directly with β-arrestin2 without using the PTHR as an interface. Fluorescence resonance energy transfer studies revealed that the kinetics of PTHR and β-arrestin2 interactions were modulated by NHERF1. These findings suggest a model in which NHERF1 may serve as an adaptor, bringing β-arrestin2 into close proximity to the PTHR, thereby facilitating β-arrestin2 recruitment after receptor activation.
Keywords: Adaptor Proteins, Cyclic AMP (cAMP), G Protein-coupled Receptor (GPCR), Peptide Hormones, Protein-Protein Interactions, Signal Transduction, NHERF, PDZ, Arrestin, Parathyroid Hormone Receptor
Introduction
Scaffold proteins play an increasingly recognized role in the regulation and the signal transduction of G protein coupled receptors (GPCRs).2 The interaction of GPCRs with β-arrestins results in several distinct effects: (i) signal termination by homologous receptor desensitization, (ii) recruitment of elements of the internalization machinery thereby promoting GPCR endocytosis, and (iii) switching GPCR signaling to nonclassical pathways such as the mitogen-activated protein kinase pathway (1–3). Despite their limited sequence homology, nearly all GPCRs bind to β-arrestins, and β-arrestin recruitment is considered to be a universal mechanism for GPCR regulation. Rather than binding to a specific motif, β-arrestins appear to interact with several parts of the receptor involving the second and third intracellular loops and the C terminus (4–6). It is generally thought that β-arrestin-receptor interaction involves an initial weak interaction of cytosolic β-arrestins with an active and phosphorylated receptor conformation, followed by conformational rearrangements of β-arrestins that further stabilize the receptor·arrestin complex (7, 8).
The receptor for parathyroid hormone (PTH) and PTH-related protein is involved in regulating calcium homeostasis and bone remodeling (9). Agonist occupancy of the PTH/PTH-related protein receptor (PTHR) leads to Gs-mediated stimulation of adenylyl cyclase, resulting in cAMP production and PKA activation, and Gq/11-mediated phosphatidylinositol-specific phospholipase Cβ stimulation, leading to inositol 1,4,5-trisphosphate formation, calcium mobilization, and PKC activation (10–12). PTH-induced activation of the PTHR results in rapid phosphorylation of several serine residues in the receptor C terminus with subsequent internalization of the PTH·receptor complex via the clathrin-coated pit pathway involving β-arrestin2 recruitment (13, 14). Our previous work revealed that the G protein-activating conformation of the PTHR and that leading to β-arrestin interaction and receptor internalization may differ (15). Moreover, we showed that the initial association of β-arrestin with the PTHR is promoted by determinants in the receptor C terminus, which are independent of receptor phosphorylation (16–18), thus suggesting that other elements in the C terminus may mediate the initial steps of β-arrestin interaction.
Recently, a group of scaffold proteins has been discovered containing PDZ (PSD95/Drosophila discs large/ZO-1) protein interaction domains and a C-terminal ezrin-radixin-moesin-merlin (ERM)-binding domain. Two members of this group, the Na+/H+-exchanger regulatory factors 1 and 2 (NHERF1 and 2) interact with several GPCRs (19–23). NHERF proteins bind with either of their two PDZ domains to a specific recognition motif consisting of the last 4 amino acids of the receptor C terminus (18, 24). The second PDZ domain of NHERF enables the formation of signaling complexes. The ERM domain of NHERF tethers the receptor complex to the actin cytoskeleton (23, 25). The PTHR interacts with both NHERF proteins (24, 26). Although NHERF2 has been reported to recruit phospholipase Cβ to the PTHR and thereby to modulate its signaling properties (24), NHERF1 has been shown mainly to affect PTHR internalization and distribution at the cell surface (26, 27).
In the present study, we investigated the role of NHERF1 in the recruitment of β-arrestin2 by the PTHR. We show that NHERF1 forms a ternary complex with β-arrestin2 and PTHR, which increases the association kinetics of arrestin to the receptor.
EXPERIMENTAL PROCEDURES
Materials
Effectene transfection reagent was from Qiagen, Lipofectamine 2000 was from Invitrogen, dithiobis[succinimidyl-propionate] (DSP) was from Pierce. Human [Nle8,18,Tyr34]PTH(1–34), abbreviated as PTH(1–34), was from Bachem and human [Aib1,3,M]PTH(1–14), abbreviated as PTH(1–14), was a kind gift from Dr. Thomas Gardella (Massachusetts General Hospital, Boston, MA). Monoclonal HA.11 anti-hemagglutinin (HA) antibody was purchased from Covance, and anti-HA and anti-FLAG affinity-agarose were from Sigma-Aldrich. Anti-NHERF1 (EBP50) antibodies were from Affinity BioReagents, and anti-GFP antibodies were purchased from Abcam. Anti-mouse and anti-rabbit peroxidase-conjugated secondary antibodies were obtained from Dianova. Cy3-conjugated anti-mouse antibody was from Jackson ImmunoResearch Labs. All other reagents were from Sigma-Aldrich or Applichem.
cDNA Constructs
HA-tagged human PTHR and C-terminally truncated PTHR (PTHR-T480) (28) were subcloned into pcDNA3 (Invitrogen) vector using the restriction sites EcoRI and XhoI. To generate a PTHR-CFP construct, enhanced CFP preceded by a 6-amino acid linker sequence was fused to the human HA-PTHR by PCR. Human M3 muscarinic acetylcholine receptor was purchased from UMR cDNA Resource Center, and murine α2A-adrenergic receptor has been described previously (29). To generate pcDNA3.1(+)-sEYFP-NHERF1-HA, sEYFP with KpnI and XhoI restriction sites at 5′ and 3′ was amplified via PCR using pcDNA3.1-sEYFP vector as template. The fragment of hNHERF1-HA with incorporation of XhoI and XbaI restriction sites at 5′ and 3′ was amplified via PCR using pcDNA3.0-hNHERF1-HA (a generous gift from Mark von Zastrow, University of California, San Francisco) as a template. After digestion with KpnI and XhoI, XhoI and XbaI, or KpnI and XbaI, PCR products of sEYFP and hNHERF1-HA were subcloned into pcDNA3.1(+) vector. An expression plasmid for wild type human NHERF1 was generated by PCR amplification of the coding sequence of NHERF1 incorporating restrictions sites for EcoRI and XhoI and subcloning into pcDNA3. Expression plasmids for bovine β-arrestin2-CFP and β-arrestin2-YFP fusion proteins have been described (30). N-terminally FLAG-tagged β-arrestin2 was generated by PCR amplification of bovine β-arrestin2 using a sense primer with a HindIII restriction site and an antisense primer with an XbaI restriction site, followed by subcloning into pFLAG-CMV2 vector (Sigma-Aldrich). The accuracy of all constructs was confirmed by sequencing.
Cell Culture and Transfection
The generation and cultivation of CHO-N10 and CHO-N10-R3 cells have been described elsewhere (26, 27). HEK-293T cells were maintained in Dulbecco's modified Eagle's medium containing 10% (v/v) fetal calf serum. All tissue culture media were supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2, 95% air. For transient transfections, cells were plated 12 h prior to transfection. Transient transfection was carried out with Effectene for HEK-293T cells or with Lipofectamine 2000 for CHO cells according to the manufacturer's instructions using 1–3 μg of plasmid DNA/well of a 6-well dish. Expression of the transfected genes was analyzed 36 h after transfection.
Coimmunoprecipitation and Western Blotting
After stimulation, cells were washed in PBS, and proteins were cross-linked with 0.25 mm DSP in PBS for 30 min at room temperature. Subsequently, cells were scraped into and lysed in ice-cold radioimmune precipitation assay buffer (10 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% (w/v) Nonidet P-40, 0.1% (w/v) SDS, 0.5% (w/v) sodium deoxycholate, 5 mm EDTA) supplemented with a mix of protease inhibitors (10 μg/ml soybean trypsin inhibitor, 30 μg/ml benzamidine, 1 mg/ml leupeptin, 100 μm PMSF). Lysates were cleared by centrifugation at 20,000 × g for 30 min at 4 °C, and the supernatant was incubated for 2 h with anti-HA affinity-agarose, anti-FLAG affinity-agarose, or anti-EBP50 antibodies bound to protein A-Sepharose (GE Healthcare). Precipitates were collected by gentle centrifugation and washed three times in cold radioimmune precipitation assay buffer. Proteins were eluted with SDS-sample buffer and subjected to SDS-PAGE, and proteins were transferred onto PVDF membranes (Millipore). The blots were incubated with primary antibodies as indicated, and bound antibody was visualized with secondary antibodies and ECL Plus Western blotting Detection Reagent (GE Healthcare) in accordance with the manufacturer's instructions.
Membrane Preparation and Adenylyl Cyclase Activity
Cells were scraped into 5/2 buffer (5 mm Tris (pH 7.4), 2 mm EDTA) and disrupted with an Ultra-Turrax cell disrupter. Crude cell membrane preparations were obtained by centrifugation at 100,000 × g. Pellets were resuspended in 50 mm Tris (pH 7.4) at a final protein concentration of 1–2 mg/ml. Determination of adenylyl cyclase activity in cell membranes in the absence or presence of 100 nm PTH(1–14) using [α-32P]ATP as the substrate was essentially performed as described in Ref. 31.
Confocal Imaging and FRET Measurements
Confocal images were taken using a Leica TCS SP5 system (30). For immunostaining of the HA-PTHR, cells were washed twice with PBS, incubated for 5 min with monoclonal mouse anti-HA antibody (1:500) at room temperature, and washed again twice with PBS. Subsequently, cells were incubated for 5 min with Cy3-conjugated goat anti-mouse antibody (1:250) and washed twice with PBS.
Dynamic FRET between CFP- and YFP-labeled proteins was measured in single cells as described previously (4, 30, 32). Briefly, HEK-293T or CHO-N10 cells were transiently cotransfected with HA-PTHR-CFP and βArr2-YFP or HA-PTHR, YFP-NHERF1, and βArr2-CFP, respectively. NHERF1 expression in CHO-N10 cells was induced by adding 50 ng/ml tetracycline 12 h prior to the experiment. Cells were observed on an Axiovert200 inverted microscope, and upon excitation at 436 ± 10 nm fluorescence intensities at 480 ± 20 and 535 ± 15 nm were measured with a dual emission photometric system (Till Photonics).
FRET was also determined by donor dequenching after acceptor photobleaching as described previously (32). For this purpose FCFP was recorded (436-nm excitation) followed by 5-min illumination at 480 nm (YFP bleaching ≈ 90%). Subsequently, FCFP was recorded again, and the increase of FCFP after bleaching of YFP was determined. Direct bleaching of CFP was determined in cells solely expressing HA-PTHR-CFP (2.57%), βArr2-CFP (1.4%), membrane-anchored CFP (0.4%), or cytosolic CFP (0.3%), respectively, and all data were corrected for this effect. To determine agonist-induced changes in FRET, cells were preincubated for 5 min in FRET buffer (137 mm NaCl, 5.4 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES (pH 7.3)) containing 1 μm PTH(1–34), and donor dequenching after photobleaching was measured thereafter.
Data Processing
Fluorescence intensities were acquired using CLAMPEX (Axon Instruments) or LAS AF Software (Leica). Values are given as mean ± S.E. of n independent experiments. Statistical analyses and curve fitting were performed using Prism 4.0 (GraphPad) or Origin (OriginLab).
RESULTS
Formation of a Ternary β-Arrestin2, NHERF1, PTHR Complex
It has previously been shown that β-arrestin2 and NHERF1 interact with intracellular parts of the PTHR (14, 24). Although β-arrestin2 interaction with the PTHR has been demonstrated in vitro and in living cells, NHERF1 binding to the PTHR C terminus so far has only been shown in in vitro experiments. To extend these findings to living cells, we first monitored the distribution of YFP-tagged NHERF1 alone or in combination with PTHR or other GPCRs in living cells by confocal microscopy. In HEK-293T cells transiently transfected with YFP-NHERF1 alone, NHERF1 was distributed throughout the cytoplasm. Coexpression of PTHR redistributed NHERF1 to the plasma membrane (Fig. 1, A versus B), suggesting a strong and constitutive association of NHERF1 with the PTHR. Conversely, coexpression of YFP-NHERF1 and α2A-adrenergic receptor or M3 muscarinic receptor, two GPCRs that do not contain a C-terminal PDZ binding motif, and have not been reported to bind to NHERF1, did not result in significant translocation of NHERF1 to the plasma membrane (Fig. 1, C and D). Similarly, coexpression of membrane-anchored CFP (mCFP) did not influence the localization of YFP-NHERF1 (Fig. 1E). Next, we examined whether agonist-induced translocation of β-arrestin2 to the PTHR affected the association of NHERF1 with the receptor. CFP-βArr2, YFP-NHERF1, and HA-PTHR were coexpressed in HEK-293T cells. HA-PTHR was stained as described under “Experimental Procedures,” and cells were stimulated with PTH(1–34) for 5 min as indicated. CFP-βArr2, YFP-NHERF1, and HA-PTHR were visualized by confocal microscopy. YFP-NHERF1 association with the PTHR was not altered significantly by the capacity of PTH(1–34) to mediate CFP-βArr2 translocation to the plasma membrane (Fig. 1F). A distinct colocalization of YFP-NHERF1, CFP-βArr2, and HA-PTHR after stimulation with PTH(1–34) was observed, suggesting that β-arrestin2 and NHERF1 can reside at the PTHR simultaneously (Fig. 1F, overlay).
FIGURE 1.
Constitutive recruitment of NHERF1 and ligand-dependent recruitment of β-arrestin2 to the PTHR. A–E, HEK-293T cells were transiently transfected with YFP-NHERF1 alone (A) or in combination with PTHR (B), α2A-adrenergic receptor (α2A-AR) (C), M3 muscarinic acetylcholine receptor (M3-AchR) (D), or mCFP (E). NHERF was visualized by confocal microscopy. F, HEK-293T cells were cotransfected with YFP-NHERF1, βArr2-CFP, and HA-PTHR. PTHR was stained with mouse anti-HA antibody followed by Cy3-conjugated anti-mouse secondary antibody. β-Arrestin2 (cyan), NHERF1 (yellow), and PTHR (red) were visualized by confocal microscopy. Where indicated, cells were treated with 100 nm PTH(1–34) for 5 min before imaging. Results are representative of an entire population of cells from three independent experiments.
As suggested from our microscopy experiments, NHERF1 and β-arrestin2 may associate with the PTHR at the same time. To confirm these results, HA-PTHR was expressed in HEK-293T cells together with NHERF1 and FLAG-βArr2, and cells were stimulated with PTH(1–34) for 10 min. HA-PTHR was immunoprecipitated, and NHERF1 and β-arrestin2 were detected in Western blots using antibodies against NHERF1 and the FLAG epitope, respectively. In line with the above findings, NHERF1 immunoprecipitated with the PTHR independently of receptor activation, and binding was not significantly altered by PTH(1–34). In contrast, β-arrestin2 was only barely detectable in unstimulated cells but showed a strong immunoreactive band upon stimulation with PTH(1–34) (Fig. 2A). To confirm these results, FLAG-βArr2 was precipitated from cells expressing HA-PTHR, NHERF1, and FLAG-βArr2, and HA-PTHR and NHERF1 were detected in Western blots. Similarly, HA-PTHR was precipitated from unstimulated cells, indicating a basal interaction of β-arrestin2 with PTHR. Stimulation with PTH(1–34) further increased the coprecipitation of HA-PTHR (Fig. 2B, upper panel). NHERF1 coprecipitated with FLAG-βArr2 in stimulated as well as in unstimulated cells. Notably, NHERF1 also coprecipitated with FLAG-βArr2 from cells not expressing HA-PTHR, indicating a receptor-independent interaction between FLAG-βArr2 and NHERF1 (Fig. 2B, second lane versus third and fourth lanes).
FIGURE 2.
NHERF1 forms a ternary complex with PTHR and β-arrestin2. A, HEK-293T cells were transiently transfected with HA-PTHR, NHERF1, and FLAG-βArr2 as indicated. Cells were stimulated with 100 nm PTH(1–34) for 10 min, cross-linked with DSP, and the PTHR was precipitated (IP) with anti-HA affinity beads. Western blots of cell lysates and immunoprecipitates were probed for FLAG, NHERF1, and HA. B, HEK-293T cells were transiently transfected with NHERF1, FLAG-βArr2, and HA-PTHR as indicated. Cells were stimulated with 100 nm PTH(1–34) for 10 min, cross-linked with DSP, and FLAG-βArr2 was precipitated with anti-FLAG affinity beads. Western blots of cell lysates and immunoprecipitates were probed for FLAG, NHERF1, and HA.
NHERF1 Interacts Directly with β-Arrestin2
Accepting that NHERF1 and β-arrestin2 interact simultaneously with the PTHR and that interaction sites most likely involve the receptor C terminus resulting in a close proximity of both proteins, we hypothesized that NHERF1 and β-arrestin2 interact directly. To test this idea, NHERF1 and FLAG-βArr2 were coexpressed in HEK-293T cells. Subsequent immunoprecipitation of FLAG-βArr2 resulted in a strong coprecipitation of NHERF1 (Fig. 3, upper panel). Likewise, FLAG-βArr2 could be specifically coprecipitated by using a NHERF1 antibody for immunoprecipitation (Fig. 3, lower panel). Collectively, these findings demonstrate that NHERF1 forms a complex with β-arrestin2 independently of the presence of PTHR.
FIGURE 3.
NHERF1 forms a constitutive complex with β-arrestin2. HEK-293T cells were transiently transfected with NHERF1 and FLAG-βArr2 as indicated, and β-arrestin2 (upper panel) or NHERF1 (lower panel) was precipitated with anti-FLAG affinity beads and anti-NHERF antibodies, respectively, subsequent to DSP cross-linking. Western blots of cell lysates and immunoprecipitates (IP) were probed for FLAG and reprobed for NHERF1.
PTH Induces a Conformational Change in the Ternary Complex
We independently assessed the interaction of the individual proteins by FRET using donor dequenching after acceptor photobleaching to measure constitutive as well as agonist-driven interactions. PTHR-CFP and βArr2-CFP were used as donors, and YFP-NHERF1 and βArr2-YFP served as acceptors. Combinations of each donor protein with each acceptor protein were expressed in HEK-293T cells as indicated in Fig. 4. Note that in Fig. 4C, nonfluorescent PTHR was cotransfected, allowing recording of agonist-dependent FRET changes between βArr2-CFP and YFP-NHERF1. To assess nonspecific signals, βArr2-CFP and PTHR-CFP were replaced by enhanced CFP and mCFP, respectively (Fig. 4, white bars). For every combination performed in unstimulated cells, we detected increases of FCFP after acceptor photobleaching compared with the control groups indicating constitutive interactions of (i) PTHR and β-arrestin2, (ii) PTHR and NHERF1, and (iii) NHERF1 and β-arrestin2 (Fig. 4, gray bars versus white bars). Receptor activation by PTH(1–34) caused a further increase of FCFP for all FRET pairs tested (Fig. 4, black bars), indicating an increased interaction or a rearrangement of preformed complexes among PTHR, β-arrestin2, and NHERF1. Together with the biochemical findings, these results suggest that β-arrestin2, NHERF1, and PTHR exist as a preformed complex, which undergoes a reorganization upon receptor activation.
FIGURE 4.
PTH induces a conformational change in the ternary complex. HEK-293T cells were transfected with FRET donors PTHR-CFP, βArr2-CFP, CFP, or mCFP and FRET acceptors YFP-NHERF1 or βArr2-YFP as indicated. Recovery of CFP emission was measured after photobleaching of YFP in unstimulated cells (n.s.) and cells which were stimulated with 1 μm PTH(1–34). Changes in FRET are presented as relative increase of FCFP after photobleaching of YFP at 480 nm for 5 min. White bars indicate the relative increase of FCFP after acceptor photobleaching of mCFP (A and B) or CFP (C). Data represent the mean ± S.E. (error bars) from 10–15 experiments. The statistical significance was determined by one-way ANOVA followed by Newman-Keuls test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
NHERF1 Accelerates the Recruitment of β-Arrestin2 to the PTHR
Next, we asked whether NHERF1 directly influenced β-arrestin2 recruitment to the PTHR. Therefore, we analyzed the kinetics of PTH-induced interaction among PTHR, NHERF1, and β-arrestin2 using FRET in living cells. Here, we used a PTHR with a C-terminal CFP fusion because several previous studies evidenced that measuring protein-protein interactions with intracellular parts of a GPCR by FRET required an intracellular fusion of a fluorophore to receptor (33). To test whether this receptor was still able to bind to NHERF1, coimmunoprecipitation experiments were performed. NHERF1 binding of PTHR-CFP was only slightly impaired compared with wild type PTHR, whereas no NHERF1 binding was detected with a receptor lacking the C-terminal tail (PTHR-T480) (supplemental Fig. 1).
First, we examined the ligand-induced β-arrestin2 recruitment of the PTHR in the absence and presence of NHERF1. Here, we took advantage of CHO cells harboring a tetracycline-inducible expression system for NHERF1 (27). These cells have no significant NHERF1 expression in the absence of tetracycline, whereas treatment with tetracycline for 12 h resulted in a concentration-dependent increase of NHERF1 expression (Fig. 5A). Cells were transiently transfected with PTHR-CFP and βArr2-YFP. Single-cell fluorescence was measured while the cell was superfused with buffer or agonist. Application of PTH(1–14) led to a decrease in FCFP and an increase in FYFP, resulting in an increase in the FRET ratio (Fig. 5B). Tetracycline-induced overexpression of NHERF1 provoked an acceleration of β-arrestin2 recruitment (Fig. 5C), whereas maximal FRET remained equal in both groups (Fig. 5C, inset). These experiments were best fitted with two-component fits, which gave half-lives of ∼5 s and ∼90 s, respectively. Overexpression of NHERF1 resulted in an increase of the fast component and a decrease of the slow component without changes in the respective half-lives (Fig. 5D).
FIGURE 5.
NHERF1 accelerates the β-arrestin2 recruitment to the PTHR. A, CHO-N10 cells were treated with tetracycline as indicated to induce NHERF1 expression. 12 h later, cells were lysed, and NHERF1 was detected by Western blotting. B, to measure agonist-dependent β-arrestin recruitment to the PTHR, CHO-N10 cells were transiently transfected with PTHR-CFP and βArr2-YFP. Cells were superfused with FRET buffer and 1 μm PTH(1–14) (black bar). Shown are the emissions of a representative experiment at 480 nm (cyan) and 535 nm (yellow) as well as the ratiometric FRET change (red). C, 12 h prior to the experiment NHERF1 expression was induced by 50 ng/ml tetracycline. FRET was measured between PTHR-CFP and βArr2-YFP. Shown are averaged curves indicating the change in FRET ratio (F535/F480) based on n = 13–14 measurements. The inset depicts an extended time scale showing the maximal FRET signal. D, to determine the maximal speed of β-arrestin recruitment to the PTHR, the data were best fit to a two-component model that gave the following parameters (values with NHERF1 expressed in parentheses): fast component, amplitude 0.09 ± 0.01 (0.17 ± 0.01); t½ = 4.7s, 95% confidence interval (CI) = 3–11 (4.9 s, 3.4–8.3); slow component, amplitude 0.39 ± 0.01 (0.31 ± 0.01); t½ = 89 s, 95% CI = 81–98 (96 s, 82–115). Shown are the amplitudes of the fast component. Statistical significance was determined using an unpaired t test (***, p < 0.001).
We then investigated whether PTHR activation also led to a rearrangement of NHERF1 and β-arrestin2 as had been suggested by an increased FCFP after acceptor photobleaching (Fig. 4C). For these experiments, HEK-293T cells were cotransfected with PTHR, βArr2-CFP, and YFP-NHERF1, and FRET was measured as described above. Again, application of the agonist PTH(1–14) led to a decrease in FCFP and an increase in FYFP and thus to an increase in the FRET ratio (Fig. 6A). Half-maximal FRET was detected after 65.7 ± 5.9 s (n = 13; Fig. 6B). Taken together, these results imply that β-arrestin2 interacts with the PTHR with biphasic kinetics. NHERF1 increases the fast component thereby accelerating the association of β-arrestin2 with the PTHR.
FIGURE 6.
PTH induces a rearrangement of the NHERF1 interaction with β-arrestin2. A, HEK-293T cells were transiently transfected with βArr2-CFP, YFP-NHERF1, and HA-PTHR. β-Arrestin2-NHERF1 interaction was measured after activation of PTHR with 1 μm PTH(1–14) as described above. Shown are the emissions of a representative experiment at 480 nm (cyan) and 535 nm (yellow) as well as the ratiometric FRET (red). B, kinetics of β-arrestin2-NHERF interaction are shown as averaged curves indicating the change in FRET ratio (F535/F480) based on n = 13 measurements.
NHERF1 Reduces PTH-stimulated Adenylyl Cyclase Activity
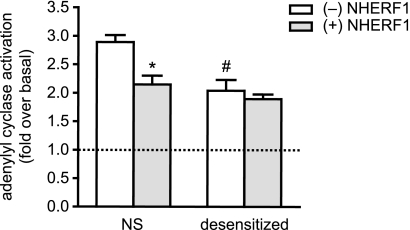
To investigate whether the NHERF1-dependent complex formation of β-arrestin2 at the PTHR had an effect on receptor signaling, we assessed the ability of the PTHR to activate adenylyl cyclase after receptor activation. Here, we used a CHO cell line with constitutive PTHR and tetracycline-inducible NHERF1 expression (26). Adenylyl cyclase activity was assessed in crude membranes. In membranes from cells not expressing NHERF1, we observed a robust ∼3-fold stimulation of adenylyl cyclase activity by PTH(1–14). In contrast, when NHERF1 was present, the stimulation was only ∼2-fold (Fig. 7). When cells were desensitized by stimulation with 10 nm PTH(1–14) for 15 min prior to membrane preparation, the stimulation by PTH was also reduced to ∼2-fold; the presence of NHERF1 caused only very little further reduction of desensitized adenylyl cyclase stimulation (Fig. 7).
FIGURE 7.
NHERF inhibits adenylyl cyclase activity. NHERF1 expression was induced in CHO-N10-R3 cells as indicated. Cells were left untreated (NS), or PTHR desensitization was provoked by prestimulation with 10 nm PTH(1–14). Adenylyl cyclase activity was measured in the absence and presence of 100 nm PTH(1–14). Given are the results of five independent experiments. Statistical significance was determined using an unpaired t test: *, p < 0.01 versus cells without NHERF1 expression; #, p < 0.01 versus nondesensitized cells without NHERF1 expression. Error bars, S.D.
DISCUSSION
Scaffold proteins are important regulators of GPCR function, and several distinct functions of scaffold proteins have been identified in recent years. However, whether multiple scaffold proteins may reside at the same receptor and how they affect receptor function have not been clarified.
In this report, we analyzed the role of the PDZ protein NHERF1 on the interaction of β-arrestin with the PTHR. It has been reported previously that both proteins are able to interact with the PTHR. Although NHERF1 constitutively interacts with the PTHR C terminus (24), β-arrestin interaction is primarily ligand-dependent (34). Using microscopic and biochemical techniques we show that β-arrestin2 and NHERF1 can bind to the PTHR without affecting the binding of each other, suggesting that a single receptor may interact with both scaffold proteins simultaneously.
The comparison of the biochemical data with the data obtained from FRET measurements revealed interesting differences regarding the nature of the protein complexes: in accordance with previous reports (24), our biochemical data did not reveal a ligand-dependent change in PTHR-NHERF1 interaction. However, when measuring FRET after acceptor photobleaching between PTHR and NHERF1, we observed a signal increase after receptor activation with PTH. We thus conclude that even though there is no additional recruitment of NHERF1 to the PTHR, a conformational rearrangement between both proteins may occur after receptor activation. In coimmunoprecipitation assays, β-arrestin2 interaction with the PTHR was low in unstimulated cells and was strongly increased upon receptor activation by PTH. However, when measuring FRET after acceptor photobleaching we found a significant interaction of both proteins already at nonstimulated conditions, and the signal increase after receptor activation was not as pronounced as in coimmunoprecipitation. These differences can be well explained by differently stable protein complexes. With the relatively harsh conditions of coimmunoprecipitation, predominantly strong protein-protein interactions will be detected, such as β-arrestin2 tightly bound to the phosphorylated PTHR after PTH stimulation. FRET also allows the detection of weak and transient interactions. Therefore, a weak complex of β-arrestin2 and the inactivated PTHR may exist in the presence of NHERF1, which would explain the already strong FRET signal at basal conditions and the slight increase after receptor activation. These assumptions are further strengthened by the identification of a direct interaction between NHERF1 and β-arrestin2 detected by both coimmunoprecipitation and FRET. As evidenced by FRET measurements, activation of the PTHR induced a rearrangement of NHERF1 and β-arrestin2. Interestingly, NHERF1 overexpression also accelerated the β-arrestin2 recruitment to the PTHR. We found that the association kinetics of β-arrestin2 could be best described by a two-component model. Although the half-lives of both phases remained equal, NHERF1 overexpression increased the extent of the fast component of β-arrestin2 recruitment to the PTHR by about 2-fold.
In summary, our findings indicate that under basal conditions NHERF1 may serve as an adaptor for β-arrestin2 and the PTHR, thereby assembling a ternary complex and bringing parts of the cellular β-arrestin2 pool into close proximity to the PTHR. Upon receptor activation, a conformational change within the complex then allows a rapid association of β-arrestin2 with the PTHR, which would explain the increased extent of the rapid association kinetics between the receptor and β-arrestin2 in the presence of NHERF1. These accelerated kinetics appear to occur with a half-life of ∼5 s, whereas the slow phase, which most likely represents recruitment of β-arrestin2 from the cytosol, proceeds with a half-life of ∼90 s in our model.
The present findings support a cooperative effect between NHERF1 and β-arrestin2 with the PTHR. In contrast, in a previous work Wang et al. described a competitive action between NHERF1 and β-arrestin2 with the PTHR which correlated with an inhibition of PTHR desensitization by NHERF1 (34). It appears that actions of NHERF1 depend on the ratio of the adaptor to that of PTHR, and the presence or absence of other PDZ proteins affects the biological actions of NHERF1. Based on our model, a high excess of NHERF1 compared with β-arrestin2 and PTHR could restrict access of β-arrestin2 to the PTHR. In contrast, increased PTHR or β-arrestin2 expression would preferentially lead to a NHERF1-mediated precoupling of β-arrestin2 to PTHR. Moreover, the expression of different PDZ- and PDZ-binding proteins interfering with NHERF could also be responsible for the differential effects of NHERF1 that have been observed. Thus, cell-specific actions, in particular differences between bone and kidney cells, need to be considered when interpreting the integrated actions of NHERF1.
The competitive effects were observed in COS-7 cells, which do not express NHERF1, whereas the present work was conducted with HEK-293 cells with endogenous NHERF expression or CHO cells with tetracycline-induced NHERF1 expression. It is becoming increasingly clear that the cellular context plays an important role for the integration of many signal transduction processes. Several studies indicate that the extent and also the quality of a receptor response are highly dependent on the cellular composition and stoichiometry of intracellular interaction partners (35–38). Also, the functional implications of NHERF interaction with the PTHR are complex and probably highly cell type-dependent. In osteoblastic-like ROS 17/2.8 cells, NHERF1 increases PTH-stimulated cAMP production whereas it decreases cAMP response in kidney OKH cells (39, 40). In renal proximal tubule cells, NHERF1 expression, however, does not alter PTH-induced cAMP levels (41, 42). In the present study, we found an increased β-arrestin2 recruitment of the PTHR when NHERF1 was expressed. In line with this, adenylyl cyclase activity was significantly reduced in crude membranes expressing NHERF1 upon receptor activation. This could be explained by a NHERF1-induced precoupling of β-arrestin to the PTHR or by a rapid recruitment of β-arrestin in the crude membrane preparation leading to reduced PTHR signaling to adenylyl cyclase.
β-Arrestin recruitment to GPCRs is commonly thought to be a process dependent on the phosphorylation of distinct residues in the C terminus and/or in intracellular loops of the receptor. However, recently we demonstrated that the interaction of β-arrestins with the PTHR may occur independently of receptor phosphorylation and that other yet unidentified elements in the C terminus of the receptor may be responsible for the initial interaction (16, 17). Here, we provide evidence that NHERF1 brings β-arrestin into close proximity with the C terminus of the nonactivated PTHR and thus might promote phosphorylation-independent interactions of β-arrestin with the receptor. Whether the described mechanism of NHERF1-facilitated, β-arrestin2 recruitment may also be operative in other cell types or play a more general role in other receptors that have been shown to interact with NHERF1 will be the subject of further investigation.
Supplementary Material
Acknowledgments
We thank Michaela Hoffmann for technical assistance and Dr. Thomas Gardella (Massachusetts General Hospital, Boston) for providing human [Aib1,3,M]PTH (1–14).
This work was supported, in whole or in part, by grants of the Deutsche Forschungsgemeinschaft and the European Research Council (to M. J. L.). This work was also supported by National Institutes of Health Grants DK54171 and DK069998 (to P. A. F.) and DK087688 (to J.-P. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- GPCR
- G-protein coupled receptor
- βArr2
- β-arrestin2
- CFP
- cyan fluorescent protein
- mCFP
- membrane-anchored CFP
- DSP
- dithiobis[succinimidyl-propionate]
- NHERF
- Na+/H+-exchanger regulatory factor
- PTH
- parathyroid hormone
- PTH(1–14)
- [Aib1,3,M]PTH(1–14)
- PTH(1–34)
- [Nle8,18,Tyr34]PTH(1–34)
- PTHR
- PTH receptor type 1.
REFERENCES
- 1.Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. (1990) Science 248, 1547–1550 [DOI] [PubMed] [Google Scholar]
- 2.DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 3.Goodman O. B., Jr., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. (1996) Nature 383, 447–450 [DOI] [PubMed] [Google Scholar]
- 4.Krasel C., Zabel U., Lorenz K., Reiner S., Al-Sabah S., Lohse M. J. (2008) J. Biol. Chem. 283, 31840–31848 [DOI] [PubMed] [Google Scholar]
- 5.Raman D., Osawa S., Weiss E. R. (1999) Biochemistry 38, 5117–5123 [DOI] [PubMed] [Google Scholar]
- 6.Krupnick J. G., Gurevich V. V., Schepers T., Hamm H. E., Benovic J. L. (1994) J. Biol. Chem. 269, 3226–3232 [PubMed] [Google Scholar]
- 7.Han M., Gurevich V. V., Vishnivetskiy S. A., Sigler P. B., Schubert C. (2001) Structure 9, 869–880 [DOI] [PubMed] [Google Scholar]
- 8.Kovoor A., Celver J., Abdryashitov R. I., Chavkin C., Gurevich V. V. (1999) J. Biol. Chem. 274, 6831–6834 [DOI] [PubMed] [Google Scholar]
- 9.Gardella T. J., Jüppner H. (2000) Rev. Endocr. Metab. Disord. 1, 317–329 [DOI] [PubMed] [Google Scholar]
- 10.Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pines M., Fukayama S., Costas K., Meurer E., Goldsmith P. K., Xu X., Muallem S., Behar V., Chorev M., Rosenblatt M., Tashjian A. H., Jr., Suva L. J. (1996) Bone 18, 381–389 [DOI] [PubMed] [Google Scholar]
- 12.Offermanns S., Iida-Klein A., Segre G. V., Simon M. I. (1996) Mol. Endocrinol. 10, 566–574 [DOI] [PubMed] [Google Scholar]
- 13.Ferrari S. L., Bisello A. (2001) Mol. Endocrinol. 15, 149–163 [DOI] [PubMed] [Google Scholar]
- 14.Ferrari S. L., Behar V., Chorev M., Rosenblatt M., Bisello A. (1999) J. Biol. Chem. 274, 29968–29975 [DOI] [PubMed] [Google Scholar]
- 15.Vilardaga J. P., Frank M., Krasel C., Dees C., Nissenson R. A., Lohse M. J. (2001) J. Biol. Chem. 276, 33435–33443 [DOI] [PubMed] [Google Scholar]
- 16.Vilardaga J.-P., Krasel C., Chauvin S., Bambino T., Lohse M. J., Nissenson R. A. (2002) J. Biol. Chem. 277, 8121–8129 [DOI] [PubMed] [Google Scholar]
- 17.Dicker F., Quitterer U., Winstel R., Honold K., Lohse M. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5476–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sneddon W. B., Syme C. A., Bisello A., Magyar C. E., Rochdi M. D., Parent J.-L., Weinman E. J., Abou-Samra A. B., Friedman P. A. (2003) J. Biol. Chem. 278, 43787–43796 [DOI] [PubMed] [Google Scholar]
- 19.Hall R. A., Ostedgaard L. S., Premont R. T., Blitzer J. T., Rahman N., Welsh M. J., Lefkowitz R. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 8496–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirakawa T., Galet C., Kishi M., Ascoli M. (2003) J. Biol. Chem. 278, 49348–49357 [DOI] [PubMed] [Google Scholar]
- 21.Hall R. A., Premont R. T., Chow C. W., Blitzer J. T., Pitcher J. A., Claing A., Stoffel R. H., Barak L. S., Shenolikar S., Weinman E. J., Grinstein S., Lefkowitz R. J. (1998) Nature 392, 626–630 [DOI] [PubMed] [Google Scholar]
- 22.Trifilieff A., Walker C., Keller T., Kottirsch G., Neumann U. (2002) Br. J. Pharmacol. 135, 1655–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinman E. J., Hall R. A., Friedman P. A., Liu-Chen L.-Y., Shenolikar S. (2006) Annu. Rev. Physiol. 68, 491–505 [DOI] [PubMed] [Google Scholar]
- 24.Mahon M. J., Donowitz M., Yun C. C., Segre G. V. (2002) Nature 417, 858–861 [DOI] [PubMed] [Google Scholar]
- 25.Voltz J. W., Weinman E. J., Shenolikar S. (2001) Oncogene 20, 6309–6314 [DOI] [PubMed] [Google Scholar]
- 26.Wang B., Bisello A., Yang Y., Romero G. G., Friedman P. A. (2007) J. Biol. Chem. 282, 36214–36222 [DOI] [PubMed] [Google Scholar]
- 27.Wheeler D., Sneddon W. B., Wang B., Friedman P. A., Romero G. (2007) J. Biol. Chem. 282, 25076–25087 [DOI] [PubMed] [Google Scholar]
- 28.Castro M., Dicker F., Vilardaga J.-P., Krasel C., Bernhardt M., Lohse M. J. (2002) Endocrinology 143, 3854–3865 [DOI] [PubMed] [Google Scholar]
- 29.Bünemann M., Bücheler M. M., Philipp M., Lohse M. J., Hein L. (2001) J. Biol. Chem. 276, 47512–47517 [DOI] [PubMed] [Google Scholar]
- 30.Krasel C., Bünemann M., Lorenz K., Lohse M. J. (2005) J. Biol. Chem. 280, 9528–9535 [DOI] [PubMed] [Google Scholar]
- 31.Klotz K. N., Hessling J., Hegler J., Owman C., Kull B., Fredholm B. B., Lohse M. J. (1998) Naunyn-Schmiedeberg's Arch. Pharmacol. 357, 1–9 [DOI] [PubMed] [Google Scholar]
- 32.Bünemann M., Frank M., Lohse M. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohse M. J., Nikolaev V. O., Hein P., Hoffmann C., Vilardaga J. P., Bünemann M. (2008) Trends Pharmacol. Sci. 29, 159–165 [DOI] [PubMed] [Google Scholar]
- 34.Wang B., Yang Y., Abou-Samra A. B., Friedman P. A. (2009) Mol. Pharmacol. 75, 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arslan G., Kull B., Fredholm B. B. (1999) Naunyn-Schmiedeberg's Arch Pharmacol. 359, 28–32 [DOI] [PubMed] [Google Scholar]
- 36.Clark R. B., Rich T. C. (2003) Mol. Pharmacol. 64, 1015–1017 [DOI] [PubMed] [Google Scholar]
- 37.Duzic E., Lanier S. M. (1992) J. Biol. Chem. 267, 24045–24052 [PubMed] [Google Scholar]
- 38.Watson C., Chen G., Irving P., Way J., Chen W. J., Kenakin T. (2000) Mol. Pharmacol. 58, 1230–1238 [DOI] [PubMed] [Google Scholar]
- 39.Mahon M. J., Cole J. A., Lederer E. D., Segre G. V. (2003) Mol. Endocrinol. 17, 2355–2364 [DOI] [PubMed] [Google Scholar]
- 40.Wheeler D., Garrido J. L., Bisello A., Kim Y. K., Friedman P. A., Romero G. (2008) Mol. Endocrinol. 22, 1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham R., E X., Steplock D., Shenolikar S., Weinman E. J. (2005) Am. J. Physiol. Renal Physiol. 289, F933–F938 [DOI] [PubMed] [Google Scholar]
- 42.Cunningham R., Steplock D., Wang F., Huang H., E X., Shenolikar S., Weinman E. J. (2004) J. Biol. Chem. 279, 37815–37821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.