Abstract
The E2 polyubiquitin-conjugating enzyme Ubc13 is a mediator of innate immune reactions. Ubc13 mediates the conjugation of keratin (K)63-linked polyubiquitin chains onto TNF receptor-associated factor 6 and IKKγ during NF-κB activation. In contrast to K48-linked polyubiquitin chains, K63-linked polyubiquitin chains function in nonproteasomal biological processes. Although Ubc13 has been shown to be critical for Toll-like receptor (TLR) and IL-1 receptor signaling, the function of Ubc13 in the epidermis has not been studied. We generated keratinocyte-specific Ubc13-deficient mice (Ubc13flox/floxK5-Cre). At birth, the skin of the Ubc13flox/floxK5-Cre mice was abnormally shiny and smooth; in addition, the mice did not grow and died by postnatal day 2. Histological analysis showed atrophy of the epidermis with keratinocyte apoptosis. Immunohistochemical analyses revealed reduced proliferation, abnormal differentiation, and apoptosis of keratinocytes in the Ubc13flox/floxK5-Cre mouse epidermis. In culture, Ubc13flox/floxK5-Cre keratinocyte growth was impaired, and spontaneous cell death occurred. Moreover, the deletion of Ubc13 from cultured Ubc13flox/flox keratinocytes by means of an adenoviral vector carrying Cre recombinase also resulted in spontaneous cell death. Therefore, Ubc13 is essential for keratinocyte growth, differentiation, and survival. Analyses of intracellular signaling revealed that the IL-1 and TNF-induced activation of JNK, p38, and NF-κB pathways was impaired in Ubc13flox/floxK5-Cre keratinocytes. In conclusion, Ubc13 appears to be essential for epidermal integrity in mice.
Keywords: Apoptosis, NF-κB, Signal Transduction, Skin, Ubiquitin-conjugating Enzyme (Ubc), Keratinocyte
Introduction
The NF-κB, JNK, and p38 intracellular signaling cascades mediate innate immune or pro-inflammatory responses such as TLR, IL-1 receptor, and TNF receptor signaling (1, 2). Upon stimulation, TNF receptor-associated factor 6 is polyubiquitinated, which induces it to phosphorylate TGF-β-activated kinase 1 (TAK1)3 (3, 4). The polyubiquitin chains on TNF receptor-associated factor 6 are generated by the E2 ubiquitin-conjugating enzyme Ubc13 (3, 5). Ubc13 conjugates keratin (K)63-linked polyubiquitin chains to TNF receptor-associated factor 6 and IKK-γ. In contrast to K48-linked polyubiquitin chains, K63-linked polyubiquitin chains function in nonproteasomal biological processes, such as stress responses, rather than protein destruction. However, the role of these signaling molecules varies by cell type and stimulus.
Epidermal keratinocytes proliferate at the basal cell layer and then differentiate to form the multilayered epidermis, which serves as a physical barrier against the external environment. The proliferation and differentiation of keratinocytes are regulated by intracellular signaling pathways, including those mediated by NF-κB, MAPK, and PI3K (6–8). In this study, we focused on the NF-κB pathway. Genetic studies have shown that mutations in NEMO/IKK-γ cause incontinentia pigmenti or Bloch-Sulzberger syndrome in humans (9). The disruption of NEMO/IKK-γ leads to hyperproliferation and increased apoptosis in keratinocytes (10, 11). The functional blockade of NF-κB in vivo by the expression of a dominant-negative mutant of NF-κB in mouse epidermis resulted in a hyperplastic epithelium (6). Similarly, a deficiency in the p65 subunit of NF-κB caused hyperplasia of the epidermis (12). In addition to the regulation of differentiation and cell growth, NF-κB protects keratinocytes from apoptosis. The blockade of NF-κB function in the epidermis by the expression of a dominant-negative mutant of IκBα provoked premature spontaneous cell death (13). TAK1 is a critical mediator of NF-κB activation. Previously, we generated keratinocyte-specific TAK1-deficient mice and showed that along with IKKs, TAK1 regulates keratinocyte growth, differentiation, and apoptosis (14).
Because Ubc13 functions in the NF-κB signaling pathway, it might be expected to regulate keratinocyte growth, differentiation, and apoptosis. However, the function of Ubc13 in epidermal keratinocytes has not been studied. To address this issue, we generated keratinocyte-specific Ubc13-deficient mice by breeding Ubc13flox/flox mice (15) with mice carrying the Cre transgene under the control of the keratin-5 promoter (K5-Cre) (16).
EXPERIMENTAL PROCEDURES
Generation of Keratinocyte-specific Ubc13-deficient Mice Using Gene Targeting with the Cre Transgene
The targeting construct was described previously (15). Ubc13flox/flox mice were bred with K5-Cre mice (generous gift from Junji Takeda, Osaka University, Osaka, Japan) (16) to generate K5-Cre/Ubc13flox/+ mice. Subsequently, the K5-Cre/Ubc13flox/+ mice were bred with Ubc13flox/flox mice to generate K5-Cre/Ubc13flox/flox mice. This protocol was approved by the Institutional Review Board of the Ehime University Graduate School of Medicine.
The genotype was confirmed by Southern and Western blotting as described previously (15). Genomic DNA was extracted from the tails of the mice, digested with NcoI and ScaI, electrophoresed, and hybridized with a radiolabeled probe (15). For Western blot analysis, newborn mouse keratinocytes were cultured overnight, and adherent keratinocytes were harvested for analysis.
Histological Analysis
To analyze the expression of the differentiation markers K5, K14, K1, K10, and loricrin, paraffin-embedded sections were deparaffinized, blocked with 10% goat serum, and reacted with primary antibodies overnight at 4 °C. After washing, the antibodies were detected using a peroxidase staining kit (ImmPRESS; Vector Laboratories, Burlingame, CA) and visualized with aminoethyl carbazole. For K15 and Ki67 staining, the deparaffinized sections were boiled in 10 mm citrate buffer, pH 6.0, for 40 min and cooled at room temperature for 20 min for antigen retrieval.
TUNEL
Keratinocyte apoptosis was detected using the TUNEL method as described previously (14) using an in situ detection kit (Roche Applied Science).
Antibodies
The following antibodies were used: Covance); Ki67 (MM1; Novo Castra); β-actin (AC-15; Abcam); IKK-γ (sc-8330; Santa Cruz Biotechnology); ubiquitin (P4D1; Santa Cruz Biotechnology); mouse TNF-α (goat; R & D Systems); and Ubc13 (4E11; Zymed Laboratories); cIAP-2 (mouse; R & D Systems); and caspase-3 (rabbit; Cell Signaling Technology). Antibodies specific for the phosphorylation forms of ERK (9101), JNK (9251), and p38 (9211) were purchased from Cell Signaling Technology.
Preparation of the Ax
An Ax encoding Cre-recombinase (Ax-Cre) was prepared as described previously (14). We infected the keratinocytes with the Ax at a multiplicity of infection of 100. Ax carrying LacZ (Ax-LacZ) was used as a control.
Keratinocyte Culture
Primary mouse keratinocytes were isolated from newborn mouse skin and cultured as described previously (14) using CnT-07 medium (CellnTec, Bern, Switzerland).
For the analysis of cell growth, freshly isolated newborn mouse keratinocytes were allowed to adhere to the culture dishes for several hours, and the nonadherent cells were removed by washing. The number of adherent cells was counted using a Coulter Counter (Beckman Coulter); this was denoted day 0. Cell culture continued for 3 days. The number of cells at day 0 was referred to as 100%.
For cytotoxic analysis, freshly isolated newborn mouse keratinocytes were stimulated with mouse TNF-α (Kamiya Biomedical Company; 10 ng/ml) or anti-mouse TNF-α (5 μg/ml). At 48 h, the supernatant was harvested for use in the LDH assay. Next, Ubc13 was deleted in Ubc13flox/flox keratinocytes by transfection of Ax-Cre at a multiplicity of infection of 100. Finally, 24 h after transfection, keratinocytes were stimulated with TNF-α or anti-TNF-α for 48 h. For analysis of intracellular signaling, freshly isolated newborn mouse keratinocytes were stimulated soon after adherence to cell culture dishes with IL-1β (PeproTech EC, London, UK; 10 ng/ml), TNF-α (10 ng/ml), or HB-EGF (R & D Systems; 10 ng/ml).
Western Blotting
Keratinocytes were harvested on ice with lysis buffer, and Western blotting was performed as described previously (8) using a FluoroImager (Molecular Dynamics). Phosphorylation of TAK1 was analyzed as described previously (1).
Immunoprecipitation
The cell lysates were precleaned with protein G-Sepharose (Amersham Biosciences) for 2 h and then incubated with protein G-Sepharose containing 1.0 μg of anti-IKK-γ for 12 h with rotating at 4 °C. After washing, the sample was eluted by boiling with sample buffer and then subjected to Western blot analysis using anti-ubiquitin as described previously (15).
LDH Assay
Cell death was quantitated by measuring LDH release using an LDH assay kit (Kyokutokogyo, Tokyo, Japan) according to the manufacturer's instructions. LDH from the living cells was obtained by cell lysis with 0.1% Tween 20. LDH release was expressed as a percentage of the total LDH, which was obtained by summing the LDH released and the LDH of the living cells. The data are expressed as the means ± S.E.
EMSA
Nuclear proteins were isolated, and 5 μg of proteins were applied for EMSA analyses as described previously (17) using a Light Shift Chemiluminescent EMSA kit (Pierce) according to the manufacturer's instructions. Specific NF-κB probe sets (biotin-labeled and unlabeled probes) were obtained from Panomics (Redwood City, CA). Protein-DNA complexes were separated and transferred to Biodyne B nylon membranes (Pierce). In competition experiments, unlabeled probes were added at 200-fold molar excess. The biotin-labeled molecules in the membranes were detected using a Chemiluminescent nucleic acid detection module (Pierce) and exposed to x-ray film.
Real Time PCR
Newborn mouse epidermis was separated from the dermis by heat treatment at 60 °C for 30 s. mRNA was extracted from the epidermis and was subjected to real time PCR analyses as described previously (18). The primers and the probes used for GAPDH, IL-1β, and TNF-α were obtained from Applied Biosystems (Branchburg, NJ). Gene expression levels were quantified using the comparative CT method and were normalized against GAPDH (18). The mean value of Ubc13flox/flox mice was referred to as 1.0 unit.
Luciferase Assay
pNFκB-TA-Luc (Clontech) was transfected to the keratinocytes. To normalize the transfection efficiency, pRL-TK (Promega) was included in the assay. The reporter plasmids were introduced into keratinocytes using FuGENE 6 (Roche Applied Science) according to the manufacturer's instructions. After treatment, the same number of cells was harvested with 250 μl of lysis buffer (Promega), and the luciferase activity was measured using the dual luciferase reporter assay system (Promega) with a luminometer (Luminescencer JNR AB-2100; Atto, Japan). The relative luciferase activity was calculated by normalizing to the level of Renilla luciferase activity.
Statistical Analysis
Statistical significance was determined using a paired Student's t test. A difference of p < 0.01 (*) or p < 0.05 (**) was considered statistically significant.
RESULTS
Generation of Keratinocyte-specific Ubc13-deficient Mice
Because the germ line deletion of Ubc13 results in embryonic lethality (15), we generated keratinocyte-specific Ubc13-deficient mice by breeding Ubc13flox/flox mice (15) with K5-Cre mice (16). Southern and Western blot analyses showed the efficient deletion of Ubc13 in keratinocytes collected from Ubc13flox/flox K5-Cre mice at birth (Fig. 1).
FIGURE 1.
Generation of keratinocyte-specific Ubc13-deficient mice. Keratinocyte-specific Ubc13-deficient mice were generated by breeding Ubc13flox/flox mice (15) with K5-Cre mice (16). Southern (A) and Western (B) blotting confirmed the genotype. A, genomic DNA was extracted from mouse tails, digested with NcoI and ScaI, electrophoresed, and hybridized with a radiolabeled probe (15). 4.0-kb band, wild-type allele; 2.0-kb band, floxed allele; 1.1-kb band, deleted allele. B, newborn mouse keratinocytes were cultured overnight, and the adherent keratinocytes were harvested for Western blot analysis. β-Actin was used as an internal standard.
Skin Phenotypes of the Ubc13flox/floxK5-Cre Mice at Birth
At birth, the skin of the Ubc13flox/flox K5-Cre mice was abnormally shiny and smooth (Fig. 2A). In addition, the mice did not grow and died by postnatal day 2. In comparison, Ubc13flox/+ and Ubc13+/+mice with or without K5-Cre showed no pathological phenotypes.
FIGURE 2.
Skin phenotypes of the Ubc13flox/flox K5-Cre mice. A, appearance of the Ubc13flox/flox K5-Cre mice at postnatal day 1. The skin of the mice was abnormally shiny and smooth. B, histological analysis of Ubc13flox/flox K5-Cre mouse skin sections by H&E staining, Ki67 staining, and TUNEL. H&E staining showed atrophy of the epidermis in the Ubc13flox/flox K5-Cre mice. Ki67 staining showed decreased numbers of positive cells in the epidermis of the Ubc13flox/floxK5-Cre mice. The epidermis also contained a few apoptotic cells as shown by H&E staining and TUNEL. The dotted lines indicate the basement membrane in the Ki67 section and the basement membrane and surface of the epidermis in the TUNEL section. Ubc13flox/flox represents the undeleted controls. Scale bar, 100 μm. C, mRNA expression of TNF-α, IL-1α, and IL-1β in the epidermis. Newborn mouse epidermis was separated from the dermis, and mRNA expression was analyzed by real time PCR. mRNA expression levels were normalized against GAPDH, and the mean value of Ubc13flox/flox mice was referred to as 1.0 unit. n = 17 (Ubc13flox/flox mice). n = 28 (Ubc13flox/flox K5-Cre mice). *, p < 0.01. **, p < 0.05.
Histological analysis revealed atrophy of the epidermis in the Ubc13flox/flox K5-Cre mice (Fig. 2B), indicating that cell growth is impaired by the deletion of Ubc13. Ki67 staining showed decreased keratinocyte proliferation, whereas H&E staining and TUNEL revealed the presence of a small number of apoptotic cells, in the epidermis of each Ubc13flox/flox K5-Cre mouse (Fig. 2B). Ki67-positive cells/basal cells were 90.5 and 40% (p < 0.01, n = 4) in Ubc13flox/flox mice and Ubc13flox/flox K5-Cre mice, respectively. Apoptotic cells/basal cells were 0 and 2.9% (p < 0.01, n = 4) in Ubc13flox/flox mice and Ubc13flox/flox K5-Cre mice, respectively. Taken together, these results indicate that Ubc13 regulates keratinocyte growth and apoptosis in mice.
Because disruption of the NF-κB pathway causes inflammation in the skin (10, 11, 14, 19), the expression of pro-inflammatory cytokines in the epidermis was studied. Real time PCR analysis revealed that TNF-α mRNA expression was decreased, whereas IL-1α expression was increased in Ubc13flox/floxK5-Cre mouse epidermis (Fig. 2C).
Abnormal Keratinocyte Differentiation in Ubc13flox/floxK5-Cre Mouse Epidermis
To analyze the differentiation status of the epidermal keratinocytes, we performed immunohistochemical analyses using skin sections treated with antibodies specific for various differentiation markers (Fig. 3). According to our data, the suprabasal keratinocytes in the epidermis of the Ubc13flox/flox K5-Cre mice expressed K14, which is normally confined to the basal layer. Loricrin is a marker of late phase keratinocyte differentiation and is normally expressed in the upper epidermis, as shown in the control mice. Loricrin expression was absent from the viable epidermal keratinocytes of the Ubc13flox/flox K5-Cre mice. K15 is a marker of the bulge area in adult mice and is also expressed at the basal cell layer in neonatal mice (20), as shown in Fig. 2. However, K15 expression was absent from the epidermis of the Ubc13flox/flox K5-Cre mice. These results indicate that keratinocyte differentiation is disrupted by the deletion of Ubc13 in keratinocytes.
FIGURE 3.
Abnormal expression of differentiation markers in the epidermis of Ubc13flox/floxK5-Cre mice. The expression of various differentiation markers in the epidermis was analyzed immunohistochemically. The expression of K5 and K14 is normally confined to the basal cell layer. The expression of K1, K10, and loricrin marks suprabasal and late phase differentiation, whereas K15 is expressed at the basal cell layer in neonatal mouse epidermis. The dotted lines indicate the basement membrane. Ubc13flox/flox represents the undeleted controls. Scale bar, 100 μm.
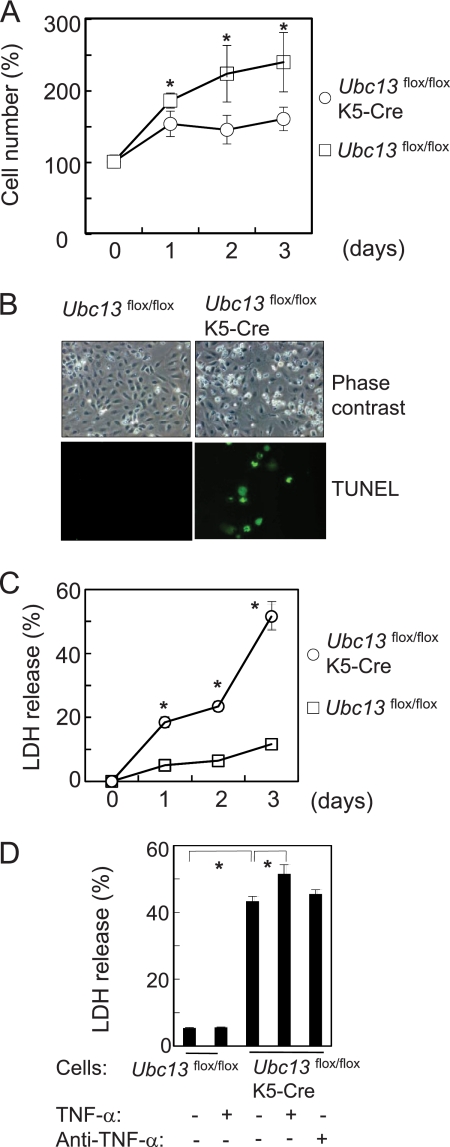
Impaired Cell Growth and Spontaneous Cell Death by Ubc13 Deletion
Keratinocytes were isolated from newborn mouse epidermis and cultured for cellular function analyses. Ubc13-deficient keratinocytes had growth impairments as shown in Fig. 4A. Furthermore, spontaneous cell death occurred, and the dead cells were positive for TUNEL (Fig. 4B). Analysis of LDH release revealed that cell death began within 1 day of adherence to the culture dishes and increased over 3 days (Fig. 4C). Because blockade of NF-κB pathway in keratinocytes enhances susceptibility to apoptosis by TNF-α (21), the cells were treated with TNF-α. The spontaneous cell death was slightly enhanced by TNF-α but was not blocked by anti-TNF-α antibodies (Fig. 4D).
FIGURE 4.
Impaired cell growth and spontaneous cell death of Ubc13flox/floxK5-Cre keratinocytes. A, cell growth analysis. Freshly isolated newborn mouse keratinocytes were cultured for 3 days, and the number of cells was counted each day using a Coulter counter (n = 6). The number of cells at day 0 was referred to as 100%. B, cell morphology was examined by phase contrast microscopy, and apoptotic cells were detected using TUNEL after 2 days of culturing. C, cell death was quantified by measuring LDH release (n = 6). D, cytotoxic effects of TNF-α. Freshly isolated mouse keratinocytes were stimulated with mouse TNF-α (10 ng/ml) or goat anti-mouse TNF-α antibody (5 μg/ml). After 48 h, the supernatant was harvested for LDH assay. The data are expressed as the means ± S.E. (n = 6). *, p < 0.01.
Next, Ubc13 was deleted in cultured keratinocytes derived from Ubc13flox/flox mice, and Ax-Cre was transfected into cultured keratinocytes from Ubc13flox/flox or Ubc13+/+ mice using Ax-LacZ as a control. Ubc13 expression began to decrease at 36 h after transfection with Ax-Cre (Fig. 5A). The deletion of Ubc13 in the cultured keratinocytes resulted in cell death, and the dead cells were positive for TUNEL (Fig. 5B). Similar to Fig. 4D, cell death was slightly enhanced by TNF-α but was not blocked by anti-TNF-α antibodies (Fig. 5D). These data indicate that Ubc13 is essential for keratinocyte survival. To further study the mechanisms of spontaneous cell death, the expression of anti-apoptotic protein cIAP-2 and the activation of caspase-3 (22) were analyzed by Western blot (Fig. 5E). The expression of cIAP-2 was reduced by Ubc13 deletion, which was correlated with the activation of caspase-3.
FIGURE 5.
Spontaneous cell death by deletion of Ubc13 using Ax-Cre. Ubc13 was deleted from cultured keratinocytes derived from Ubc13flox/flox mice. Ax-Cre was transfected into the cultured keratinocytes of Ubc13flox/flox mice or Ubc13+/+ mice at an multiplicity of infection of 100. Ax-LacZ was used as a control. A, the expression of Ubc13 in keratinocytes was analyzed by Western blotting using β-actin as an internal standard. B, cell morphology was examined by phase contrast microscopy, whereas apoptotic cells were detected using TUNEL at 72 h post-transfection. C, cell death was quantified by measuring LDH release. Ax was transfected into the cultured keratinocytes, and the culture supernatant was harvested for LDH assay at the indicated time (n = 5). D, cytotoxic effects of TNF-α. Freshly isolated Ubc13flox/flox keratinocytes were transfected with Ax at a multiplicity of infection of 100. After 24 h, the cells were stimulated with mouse TNF-α (10 ng/ml) or goat anti-mouse TNF-α antibody (5 μg/ml). After 48 h, the supernatant was harvested for use in the LDH assay (n = 6). The data are expressed as the means ± S.E. *, p < 0.01. E, the expression of c-IAP-2 and caspase-3 were analyzed by Western blot using β-actin as an internal standard.
Impaired Activation of p38, JNK, and NF-κB in Ubc13-deficient Keratinocytes
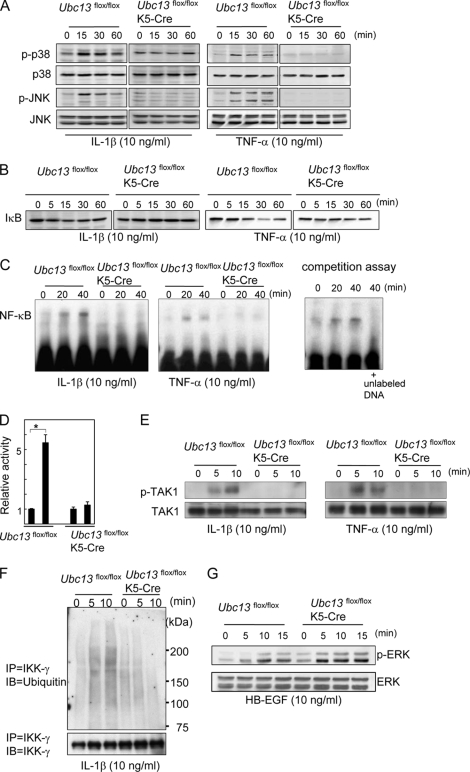
To study the cause of these functional defects of Ubc13-deficient keratinocytes, intracellular signals were analyzed. Because spontaneous cell death occurs, freshly isolated keratinocytes were stimulated soon after adherence to the culture dishes (Fig. 6). Neither p38 nor JNK was phosphorylated in the Ubc13-deficient keratinocytes by IL-1β or TNF-α, although both were phosphorylated within 15 min in the control keratinocytes (Fig. 6A). Activation of NF-κB pathway by IL-1β or TNF-α was also impaired in Ubc13-deficient keratinocytes as shown by Western blotting of IκB (Fig. 6B) and EMSA (Fig. 6C). Next, to study whether TAK1 is involved in this impaired NF-κB activation, the phosphorylation of TAK1 was analyzed. Although TAK1 was phosphorylated by IL-1β or TNF-α within 10 min in control keratinocytes, TAK1 was not phosphorylated in Ubc13-deficient keratinocytes (Fig. 6E). Furthermore, luciferase assay confirmed the impaired activation of NF-κB pathway (Fig. 6D). Because Ubc13 is a polyubiquitin-conjugating enzyme, ubiquitination of IKK-γ was studied using immunoprecipitation. As shown in Fig. 6F, ubiquitination of IKK-γ by IL-1β was impaired in Ubc13-deficient keratinocytes. Thus, p38, JNK, and NF-κB pathways were impaired in Ubc13-deficient keratinocytes.
FIGURE 6.
Impaired activation of p38, JNK, and NF-κB in Ubc13-deficient keratinocytes. Freshly isolated mouse keratinocytes were stimulated with IL-1β (10 ng/ml), TNF-α (10 ng/ml) (A–E), or HB-EGF (10 ng/ml) (F). Intracellular signals were then analyzed by Western blotting (A, B, E, and G), EMSA (C), luciferase assay (D), and immunoprecipitation (F, IP). Ubc13flox/flox represents the undeleted controls. A, phosphorylation of p38 and JNK (p-p38 and p-JNK, respectively). p38 and JNK were used as standards. B, Western blotting of IκB. C, NF-κB activity was analyzed by EMSA. Protein-DNA complexes were separated and transferred to nylon membranes. In competition assay, Ubc13flox/flox keratinocytes were stimulated with IL-1β (10 ng/ml), and unlabeled probe (200-fold molar excess) was added to the sample of 40 min. The shift by IL-1β was prevented by the unlabeled probe, indicating that the shift was the result of specific protein-DNA interaction. D, luciferase assay. After transfection of pNFκB-TA-Luc, the keratinocytes were stimulated with IL-1β (10 ng/ml) for 24 h. The relative luciferase activity was calculated by normalizing to the level of Renilla luciferase activity. The data are expressed as the means ± S.E. *, p < 0.01. n = 3. E, phosphorylation of TAK1 (p-TAK1) was analyzed by Western blotting. TAK1 was the standard. F, ubiquitination of IKK-γ. The samples were first immunoprecipitated with anti-IKK-γ and then immunoblotted (IB) with anti-ubiquitin. In control cells, broad bands were detected at 5 and 10 min after the IL-1β stimulation. G, phosphorylation of ERK (ERK was the standard). These studies (A–G) were performed more than three times, and the representative data are shown.
Heparin-binding EGF-like growth factor (HB-EGF) is a ligand for the EGF receptor and is a potent mitogen for keratinocytes (23). We tested whether HB-EGF-induced ERK activation was also impaired in Ubc13flox/floxK5-Cre keratinocytes. We found that phosphorylation of ERK by HB-EGF was not impaired (Fig. 6G), indicating that overall cellular signaling was not impaired in Ubc13flox/floxK5-Cre keratinocytes.
DISCUSSION
In the present study, we found that Ubc13 is essential for the growth, differentiation, and survival of mouse keratinocytes. Although Ubc13 regulates NF-κB signaling, the skin phenotype of the Ubc13flox/flox K5-Cre mice was different from that of TAK1- or IKK-γ-deficient epidermis. Epidermis-specific deletion of TAK1 or IKK-γ causes inflammation, abnormal keratinocyte differentiation, and keratinocyte apoptosis in the mouse skin (14, 24). The development of similar skin phenotypes among these mice indicates the disruption of a common cascade in the keratinocytes of these mice. Although an epidermal phenotype is not apparent in TAK1- or IKK-γ-deficient mice at birth, the skin of our Ubc13-deficient mice was already abnormal at birth. The number of apoptotic cells in Ubc13-deficient epidermis was much lower than that in TAK1-deficient epidermis (14). Furthermore, the microabscesses seen in TAK1- or IKK-γ-deficient epidermis were not observed in our Ubc13-deficient mice. Although Ubc13 has been shown to regulate the NF-κB pathway in keratinocytes (Fig. 6), the phenotype produced by Ubc13 deficiency compared with that of TAK1 or IKK-γ deficiency indicates that the signaling controlled by Ubc13 is not identical to that of TAK1 or IKK-γ.
The role of Ubc13 and TAK1 in the activation of JNK, p38, and NF-κB varies by cell type and stimulus. TAK1 is indispensable for the activation of NF-κB and MAPK by TLRs, IL-1 receptor, and TNF receptor (1, 2). However, TAK1-deficient B cells can still activate NF-κB in response to B cell receptor stimulation (1). Thymocytes from TAK1-deficient mice display severely defective NF-κB activation in response to anti-CD3/CD28 stimulation (1, 25, 26); however, NF-κB activation is normal in the peripheral T cells of such animals (26). In vivo analyses of mice lacking Ubc13 in their B cells, myeloid cells, and embryonic fibroblasts have shown nearly normal NF-κB activation during BCR-, IL-1 receptor-, TLR, or CD40-mediated signal transduction (15), indicating that Ubc13 plays a minor role in these signaling pathways (15). Although NF-κB activation was modestly affected, JNK and p38 activation was impaired in Ubc13-deficient thymocytes (27). During IL-1 receptor-mediated signaling in these cell types, Ubc13 is more important for the activation of p38 and JNK than of NF-κB (27). However, in this study we found that in keratinocytes, Ubc13 is essential for the IL-1 and TNF-induced activation of the NF-κB, JNK, and p38 pathways. The impaired activation of these signaling pathways may cause the functional defect of Ubc13-deficient keratinocytes.
Ubc13 deficiency causes spontaneous cell death that is associated with decreased cIAP-2 and the activation of caspase-3 (Fig. 5E). Similarly, in TAK1-deficient keratinocytes, the expression of cIAP-2 is down-regulated and caspase-3 is activated, which causes TRAIL-induced cell death (22). These data suggest that the TAK1 deletion facilitates TRAIL-induced cell death by activating caspase through down-regulating cIAP (22). Therefore, it is most likely that decreased cIAP-2 enhanced the susceptibility to cell death in Ubc13-deficient keratinocytes, as well as TAK1. Because cIAP-2 is a target molecule of NF-κB, the down-regulation of cIAP-2 may be due to the impaired NF-κB pathway in Ubc13-deficient keratinocytes. This spontaneous cell death is enhanced slightly by TNF-α. Because TNF-α mRNA expression in the epidermis of Ubc13flox/floxK5-Cre mice was decreased (Fig. 2C) and cell death was not blocked by anti-TNF-α antibodies (Figs. 4D and 5D), spontaneous cell death is not likely to be due to endogenous TNF-α. In studies of IKKs-deficient keratinocytes, the cells were also susceptible to TNF-induced cell death (28–30), as well as TAK1 (21). However, the effects are limited compared with the TAK1 deletion. In Ubc13-deficient keratinocytes, the cytotoxic effects of TNF are also limited (Figs. 4D and 5D). The low sensitivity to TNF in Ubc13-deficient cells may be partially due to the spontaneous cell death. Because spontaneous cell death occurs via the deletion of Ubc13 alone, susceptible cells may undergo cell death without TNF. The remaining cells may be somewhat resistant to cell death.
During embryogenesis, the NF-κB pathway is activated at the placode via binding of EdaA1 to its receptor, EdaR (31). This EdaA1/EdaR/NF-κB pathway is essential for the development of ectodermal appendages such as hair follicles, teeth, and sweat glands (32, 33). In addition, mutations of these genes can cause reduced or absent ectodermal appendages. In Ubc13flox/flox K5-Cre mice, the skin was abnormally shiny and smooth. Because the NF-κB pathway is impaired in these mice, this phenotype may be partially due to the anomaly of ectodermal appendages; however, this point should be studied further.
Because the NF-κB pathway regulates pro-inflammatory responses, the disruption of this pathway likely has a negative effect on epithelial inflammation. However, the deletion of TAK1, IKK-β, or IKK-γ results in severe inflammation (abscess formation) in the skin (10, 11, 14, 19). Similarly, a lack of NF-κB signaling caused by the conditional ablation of IKKγ or IKKα and IKKβ in the intestinal epithelium causes severe chronic intestinal inflammation in mice (34). Continuous NF-κB activation at the basal level may be required to maintain the homeostasis of the epithelium. However, the deletion of Ubc13 in epidermal keratinocytes does not cause inflammation. One possible explanation for this is the early mortality of the Ubc13flox/flox K5-Cre mice by postnatal day 2, before the occurrence of epidermal inflammation. In conclusion, Ubc13 in keratinocytes appears to be essential for maintaining epidermal integrity in mice.
Acknowledgments
We thank Teruko Tsuda and Eriko Tan for providing significant technical assistance.
This work was supported by grants from the Ministries of Education, Culture, Sports, Science, and Technology of Japan; by Health and Labor Sciences Research Grants (Research on Intractable Diseases) from the Ministry of Health, Labor, and Welfare of Japan; and by grants from the Takeda Science Foundation, Mishima Kaiun Memorial Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Naito Foundation, Kowa Life Science Foundation, and the Yasuda Medical Foundation.
- TAK1
- TGF-β-activated kinase 1
- K
- keratin
- Ax
- adenoviral vector
- LDH
- lactate dehydrogenase
- HB-EGF
- heparin-binding EGF-like growth factor
- TLR
- toll-like receptor
- TRAIL
- TNF-related apoptosis-inducing ligand.
REFERENCES
- 1.Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 2.Shim J. H., Xiao C., Paschal A. E., Bailey S. T., Rao P., Hayden M. S., Lee K. Y., Bussey C., Steckel M., Tanaka N., Yamada G., Akira S., Matsumoto K., Ghosh S. (2005) Genes Dev. 19, 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z. J. (2000) Cell 103, 351–361 [DOI] [PubMed] [Google Scholar]
- 4.Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 5.Hofmann R. M., Pickart C. M. (1999) Cell 96, 645–653 [DOI] [PubMed] [Google Scholar]
- 6.Seitz C. S., Lin Q., Deng H., Khavari P. A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2307–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayama K., Yamasaki K., Hanakawa Y., Shirakata Y., Tokumaru S., Ijuin T., Takenawa T., Hashimoto K. (2002) J. Biol. Chem. 277, 40390–40396 [DOI] [PubMed] [Google Scholar]
- 8.Sayama K., Hanakawa Y., Shirakata Y., Yamasaki K., Sawada Y., Sun L., Yamanishi K., Ichijo H., Hashimoto K. (2001) J. Biol. Chem. 276, 999–1004 [DOI] [PubMed] [Google Scholar]
- 9.Smahi A., Courtois G., Vabres P., Yamaoka S., Heuertz S., Munnich A., Israël A., Heiss N. S., Klauck S. M., Kioschis P., Wiemann S., Poustka A., Esposito T., Bardaro T., Gianfrancesco F., Ciccodicola A., D'Urso M., Woffendin H., Jakins T., Donnai D., Stewart H., Kenwrick S. J., Aradhya S., Yamagata T., Levy M., Lewis R. A., Nelson D. L. (2000) Nature 405, 466–472 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Supprian M., Bloch W., Courtois G., Addicks K., Israël A., Rajewsky K., Pasparakis M. (2000) Mol. Cell 5, 981–992 [DOI] [PubMed] [Google Scholar]
- 11.Makris C., Godfrey V. L., Krähn-Senftleben G., Takahashi T., Roberts J. L., Schwarz T., Feng L., Johnson R. S., Karin M. (2000) Mol. Cell 5, 969–979 [DOI] [PubMed] [Google Scholar]
- 12.Zhang J. Y., Green C. L., Tao S., Khavari P. A. (2004) Genes Dev. 18, 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seitz C. S., Freiberg R. A., Hinata K., Khavari P. A. (2000) J. Clin. Invest. 105, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayama K., Hanakawa Y., Nagai H., Shirakata Y., Dai X., Hirakawa S., Tokumaru S., Tohyama M., Yang L., Sato S., Shizuo A., Hashimoto K. (2006) J. Biol. Chem. 281, 22013–22020 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M., Okamoto T., Takeda K., Sato S., Sanjo H., Uematsu S., Saitoh T., Yamamoto N., Sakurai H., Ishii K. J., Yamaoka S., Kawai T., Matsuura Y., Takeuchi O., Akira S. (2006) Nat. Immunol. 7, 962–970 [DOI] [PubMed] [Google Scholar]
- 16.Takeda J., Sano S., Tarutani M., Umeda J., Kondoh G. (2000) J. Dermatol. Sci. 23, 147–154 [DOI] [PubMed] [Google Scholar]
- 17.Dai X., Sayama K., Shirakata Y., Tokumaru S., Yang L., Tohyama M., Hirakawa S., Hanakawa Y., Hashimoto K. (2008) J. Dermatol. Sci. 50, 53–60 [DOI] [PubMed] [Google Scholar]
- 18.Dai X., Yamasaki K., Shirakata Y., Sayama K., Hashimoto K. (2004) J. Invest. Dermatol. 123, 1078–1085 [DOI] [PubMed] [Google Scholar]
- 19.Stratis A., Pasparakis M., Markur D., Knaup R., Pofahl R., Metzger D., Chambon P., Krieg T., Haase I. (2006) J. Invest. Dermatol. 126, 614–620 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Lyle S., Yang Z., Cotsarelis G. (2003) J. Invest. Dermatol. 121, 963–968 [DOI] [PubMed] [Google Scholar]
- 21.Omori E., Matsumoto K., Sanjo H., Sato S., Akira S., Smart R. C., Ninomiya-Tsuji J. (2006) J. Biol. Chem. 281, 19610–19617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morioka S., Omori E., Kajino T., Kajino-Sakamoto R., Matsumoto K., Ninomiya-Tsuji J. (2009) Oncogene 28, 2257–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto K., Higashiyama S., Asada H., Hashimura E., Kobayashi T., Sudo K., Nakagawa T., Damm D., Yoshikawa K., Taniguchi N. (1994) J. Biol. Chem. 269, 20060–20066 [PubMed] [Google Scholar]
- 24.Nenci A., Huth M., Funteh A., Schmidt-Supprian M., Bloch W., Metzger D., Chambon P., Rajewsky K., Krieg T., Haase I., Pasparakis M. (2006) Hum. Mol. Genet. 15, 531–542 [DOI] [PubMed] [Google Scholar]
- 25.Sato S., Sanjo H., Tsujimura T., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Takeuchi O., Akira S. (2006) Int. Immunol. 18, 1405–1411 [DOI] [PubMed] [Google Scholar]
- 26.Liu H. H., Xie M., Schneider M. D., Chen Z. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11677–11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M., Sato S., Saitoh T., Sakurai H., Uematsu S., Kawai T., Ishii K. J., Takeuchi O., Akira S. (2006) J. Immunol. 177, 7520–7524 [DOI] [PubMed] [Google Scholar]
- 28.Rudolph D., Yeh W. C., Wakeham A., Rudolph B., Nallainathan D., Potter J., Elia A. J., Mak T. W. (2000) Genes Dev. 14, 854–862 [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka M., Fuentes M. E., Yamaguchi K., Durnin M. H., Dalrymple S. A., Hardy K. L., Goeddel D. V. (1999) Immunity 10, 421–429 [DOI] [PubMed] [Google Scholar]
- 30.Li Q., Van Antwerp D., Mercurio F., Lee K. F., Verma I. M. (1999) Science 284, 321–325 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Ullrich R., Tobin D. J., Lenhard D., Schneider P., Paus R., Scheidereit C. (2006) Development 133, 1045–1057 [DOI] [PubMed] [Google Scholar]
- 32.Mikkola M. L., Thesleff I. (2003) Cytokine Growth Factor Rev. 14, 211–224 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Ullrich R., Paus R. (2005) Bioessays 27, 247–261 [DOI] [PubMed] [Google Scholar]
- 34.Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., Gumucio D., Neurath M. F., Pasparakis M. (2007) Nature 446, 557–561 [DOI] [PubMed] [Google Scholar]