Abstract
Background
Both depression and cognitive impairment are common in hemodialysis patients, are associated with adverse clinical outcomes, and place an increased burden on health care resources.
Study Design
Cross-sectional cohort
Setting & Participants
241 maintenance hemodialysis patients in the Boston area
Predictor
Depressive symptomatology, defined by a Center for Epidemiological Studies Depression Scale (CES-D) score of 16 or higher
Outcome
Performance on a detailed neurocognitive battery
Results
Mean age was 63.8 years, 49.0% were female, 21.6% were African American, and median dialysis duration was 13.8 months. There were 57 (23.7%) participants with significant depressive symptoms. In multivariable analysis adjusting for age, sex, education and other comorbid conditions, participants with and without depressive symptoms performed similarly on the Mini-Mental State Examination (p=0.4) and tests of memory. However, participants with greater depressive symptoms performed significantly worse on tests assessing processing speed, attention, and executive function, including Trails Making Test B (p=0.02) and Digit-Symbol Coding (p=0.01). Defining depression using a CES-D score ≥18 did not substantially change results.
Limitations
Cross-sectional design, absence of brain imaging
Conclusions
Hemodialysis patients with a greater burden of depressive symptoms perform worse on tests of cognition related to processing speed and executive function. Further research is needed to assess the effects of treating depressive symptoms on cognitive performance in dialysis patients.
Keywords: Depression, cognitive function, dementia, ESRD, chronic kidney disease
Depression and cognitive impairment are prevalent in patients with kidney disease. Estimated rates of clinical depression among hemodialysis patients range from 20 to 30% with as many as 42% of hemodialysis patients exhibiting some form of depressive affect [1-6]. These rates are substantially higher than those found in the general population, where rates of depression are between 3% and 6% [7], and those found in older adults, where rates are between 6% and 10% [8]. Recent estimates project that, by 2050, rates of depression will increase by 35% in adults and more than double in older adults [9].
Depression has significant effects on both individual patient well-being as well as delivery of medical care. Hemodialysis patients with depression have a lower quality of life, more functional impairments, a greater occurrence of co-morbid conditions and psychopathology, lower adherence to drug treatment, and an increased likelihood of long-term body pain [3, 10-12]. Hemodialysis patients with depression also have higher rates of hospitalization, emergency room visits, cardiovascular disease events and mortality [4-6, 13, 14].
Similarly, cognitive impairment is associated with negative outcomes, including non-adherence to drug treatment [15, 16] and increased costs of care [17]. In hemodialysis patients, medication non-adherence may be as high as 58.2% in those with cognitive impairment (versus 25% in the general population with intact cognition) [16, 18]. Mild cognitive impairment is likely under-diagnosed but highly prevalent in individuals with end-stage renal disease (ESRD) [19-21], with Murray et al. describing mild cognitive impairment in nearly 64% of hemodialysis patients [22]. The nature of these deficits is under debate, with some research highlighting global declines and others noting particular impairment in executive function in individuals with kidney disease [20, 22-24].
Though there is ongoing debate regarding the temporal relationship of cognitive decline with depression in the general population, it appears that there is strong support for at least a reciprocal association between depression and cognitive impairment [25-28], particularly influencing executive function, processing speed, and episodic memory [29]. This pattern is seen in late-life depression, a process related to cerebrovascular and white matter disease and often associated with impairments of executive function and processing speed [30].
In the current study, we examine the relationship between depression and cognitive function in hemodialysis patients. Given the high prevalence of vascular disease in hemodialysis patients and the proposed underlying role of vascular disease in late-life depression, we hypothesized that executive functioning and processing speed domains may be most affected in hemodialysis patients with depression.
Methods
Participants
All patients receiving hemodialysis at 5 Dialysis Clinic Inc. (DCI) units in the greater Boston area were considered for the Cognition and Dialysis Study. Eligibility criteria include English fluency, sufficient visual and hearing acuity to complete cognitive testing, absence of pre-existing advanced dementia or confusion (based on provider testimony or medical chart review), medically stable condition without urgent non-access-related hospitalization within 1 month, receipt of maintenance hemodialysis for at least 1 month, and spKt/V (single pool Kt/V, a measure of dialysis dose) >1.0. Demographic information was obtained through participant report, medical charts, and the DCI database. The Tufts Medical Center Human Investigation Review Board approved the study, and all participants signed informed consent and research authorization forms.
Assessment of Cognitive Function and Symptoms of Depression
Subjects were administered a battery of cognitive tests by trained research assistants; to assure quality and inter-rater reliability, reassessment of research assistants by the study neuropsychologist with either mock training sessions or witnessed testing of study participants occurred at 3-6 month intervals. To limit subject fatigue, all testing, including the CES-D, was completed during the 1st hour of hemodialysis. The neuropsychological battery included well validated and commonly used cognitive tests that possess high inter- and intra-rater reliability and have established age, gender, and education-matched normative scores. Tests included the MMSE [31], the North American Adult Reading Test (NAART) [32], the Wechsler Memory Scale-III (WMS-III) Word List Learning Subtest [33], the Wechsler Adult Intelligence Scale-III (WAIS-III) Block Design and Digit Symbol-Coding tasks [33], and Trail Making Tests A and B [34] (Table 1). The overall battery assesses a broad range of functioning including global ability, verbal intelligence, supraspan learning, auditory retention, visual retention, attention/mental processing speed, visual construction/fluid reasoning, and motor speed.
Table 1.
Components of the neuropsychiatric battery in the Dialysis and Cognition Study
| Function Assessed | Cognitive Test | Scoring | Test Details |
|---|---|---|---|
| Cognitive Screen | Mini-Mental State Exam |
Number Correct | 30-point questionnaire that samples abilities such as arithmetic, memory, and orientation. |
| Intelligence | North American Adult Reading Test |
128.7 - (0.89 × # of pronunciation errors) |
Estimation of verbal intelligence quotient that requires subjects to read a list of 61 words out loud. |
| Supraspan Learning & Word Recall |
Subjects attempt to memorize a list of 12 words read out loud over 4 trials. After a delay of 25 to 35 minutes, percent retention is the percentage of words correctly recalled compared to immediate recall and delayed recognition is the number of words correctly identified as familiar or non-familiar from a list of 24. |
||
| Immediate Recall* | Total initially correct | ||
| Percent Retention* | Percent recall after delay |
||
| Delayed Recognition* | Number of correctly identified words |
||
| Visual Construction & Fluid Reasoning |
Block Design^ | Number completed weighted for time |
Subjects are required to reproduce depicted patterns using a set of colored blocks. |
| Attention, Mental Processing Speed, & Executive Function |
|||
| Digit Symbol-Coding^ | Number of copied symbols in 2 minutes |
Symbols are decoded by matching a given symbol to a digit provided in an answer key |
|
| Trail Making Test A | Time to Completion | “Connect-the-dots” for a consecutive number sequence from 1 to 25. |
|
| Trail Making Test B | Time to Completion | “Connect-the-dots” alternating between numbers (1 to 13) and letters (A to L) (ex: 1-A-2-B-3-C). |
Derived from the Word List Learning subtest of the Wechsler Memory Scale - III (WMS-III)
From the Weschler adult intelligence scale III.
Depression screening was performed using the CES-D. The CES-D is a validated self-reporting questionnaire composed of 20 questions (Item S1; available as online supplementary material associated with this article at www.ajkd.org); in the general population, a score greater than or equal to 16 is consistent with the presence of major depressive symptoms [35], with one analysis of hemodialysis patients showing greater diagnostic accuracy at a CES-D cutpoint of 18 [3].
Statistical Analysis
All analyses were performed using SAS, version 9.1 (www.sas.com). Depression was defined by a score of 16 or greater on the CES-D. Baseline characteristics of eligible dialysis patients who consented and did not consent to participate were compared using chi-square tests, t-tests and ANOVA as appropriate. Similarly, baseline characteristics of participants with and without depression were also compared. Linear regression was used to evaluate the association between depressive symptoms (dichotomized at CES-D≥16) and cognitive performance with cognitive outcomes being the raw scores on cognitive tests. Parsimonious models further adjusted for age, education, and sex, while all fully-adjusted models adjusted for age, education, and sex as well as Table 1 variables with a p-value <0.20 in parsimonious models, with the exception of history of hypertension and diastolic blood pressure due to collinearity with systolic blood pressure, and history of diabetes due to collinearity with cause of kidney disease. This results in different covariates in each fully adjusted model. Non-linear relationships were assessed through testing risk factors quartiles and squared terms and were not noted. Analyses performed on the Trails B test used Tobit regression, censoring for failure to complete the task within 5 minutes [36]. To conceptualize the association between cognitive performance and depressive symptoms, we also transformed individual test scores into standardized Z-scores (Z-score=(test score-mean test score)/standard deviation) and generated compound scores for psychomotor speed and attention (average of z-scores for Trail Making Test A, Block Design, and Digit Symbol-Coding) and memory (average of z-scores for recognition and delayed recall); the z-score for executive function was based solely on Trail Making Test B). Trail Making test z-scores were multiplied by –1 prior to inclusion.
Because some cognitive testing is dependant on manual dexterity that may be hindered during hemodialysis due to arteriovenous access in the dominant arm, we performed a sensitivity analysis limiting the study population to those individuals who performed testing using their dominant hand in an unencumbered manner. Additionally, to confirm the consistency of the results, we also varied our depression cutpoint to a CES-D score of ≥18 as suggested in prior research [3]. Finally, to better assess the interaction among cerebrovascular disease, depressive symptoms and cognitive performance, we tested an interaction term for stroke and depression in parsimonious models.
Results
A total of three-hundred and eighty nine individuals were eligible for the study and 250 (64.3%) were consented, with 241 (96.4%) completing cognitive testing, including the CES-D. Eligible patients who were not enrolled were similar to those enrolled across all measured characteristics, including age, dialysis adequacy, serum albumin, and dialysis vintage, except for levels of intact parathyroid hormone (data not shown). The mean age of participants was 63.8 ± 16.6 years, 49.0% were female, 21.6% were African American, 37.3% had diabetes as the primary cause of kidney failure, 90.1% graduated high school, and median dialysis vintage was 13.8 months (Table 2). There were 57 (23.7%) participants with CES-D scores ≥16. Demographics and patient characteristics overall were similar between individuals with and without significant depressive symptoms (Table 2).
Table 2.
Characteristics of participants with and without depressive symptoms.
| Total | Not depressed (CES-D <16) |
Depressed (CES-D ≥16) |
P-value | |
|---|---|---|---|---|
| All participants | 241 | 184 (76.4%) | 57 (23.7%) | |
| Age (years) | 63.8 ± 16.6 | 63.7 ± 16.4 | 64.0 ± 17.7 | 0.9 |
| Female | 118 (49.0%) | 90 (48.9%) | 28 (49.1%) | 0.9 |
| African American | 52 (21.6%) | 41 (22.3%) | 11 (19.3%) | 0.7 |
| Education (%) | 0.5 | |||
| <12th grade | 24 (10.0%) | 16 (8.7%) | 8 (14.0%) | |
| High school graduate | 146 (60.6%) | 112 (60.9%) | 34 (59.7%) | |
| 2+ Years college | 71 (29.5%) | 56 (30.4%) | 15 (26.3%) | |
| Medical History (%) | ||||
| PVD | 55 (22.9%) | 40 (21.9%) | 15 (26.3%) | 0.5 |
| Hypertension | 212 (88.0%) | 162 (88.0%) | 50 (87.7%) | 0.9 |
| Stroke | 45 (18.7%) | 34 (18.5%) | 11 (19.3%) | 0.9 |
| Diabetes | 119 (49.4%) | 90 (48.9%) | 29 (50.9%) | 0.9 |
| Heart Failure | 88 (36.5%) | 61 (33.2%) | 27 (47.4%) | 0.06 |
| CAD | 84 (34.9%) | 63 (4.9%) | 21 (36.8%) | 0.8 |
| Primary cause of ESRD (%) | 0.02 | |||
| Diabetes | 90 (37.3%) | 66 (35.9%) | 24 (42.1%) | |
| Glomerulonephritis | 40 (16.6%) | 37 (20.1%) | 3 (5.3%) | |
| Hypertension | 49 (20.3%) | 39 (21.2%) | 10 (17.5%) | |
| Other | 45 (18.7%) | 33 (17.9%) | 12 (21.1%) | |
| Unknown | 17 (7.1%) | 9 (4.9%) | 8 (14.0%) | |
| Smoking History (%) | 0.5 | |||
| Never | 92 (40.0%) | 74 (42.3%) | 18 (32.7%) | |
| Past | 120 (52.2%) | 88 (50.3%) | 32 (58.2%) | |
| Current | 18 (7.8%) | 13 (7.4%) | 5 (9.1%) | |
| Vascular Access (%) | 0.7 | |||
| Fistula | 143 (60.9%) | 110 (61.5%) | 33 (58.9%) | |
| Graft | 12 (5.1%) | 10 (5.6%) | 2 (3.6%) | |
| Catheter | 80 (34.0%) | 59 (33.0%) | 21 (37.5%) | |
| Systolic BP (mm Hg) | 143.8 ± 26.3 | 143.4 ± 25.0 | 145.0 ± 30.4 | 0.7 |
| Diastolic BP (mm Hg) | 73.5 ± 16.0 | 73.6 ± 15.1 | 73.2 ± 18.9 | 0.9 |
| BMI (kg/m2) | 28.5 ± 6.8 | 28.2 ± 6.6 | 29.3 ± 7.5 | 0.3 |
| Intradialytic weight gain (kg) | 2.6 ± 1.1 | 2.5 ± 1.1 | 2.7 ± 1.0 | 0.2 |
| Hematocrit (%) | 35.7 ± 3.4 | 35.9± 3.4 | 35.3 ± 3.3 | 0.2 |
| Serum Albumin (g/dL) | 3.8 ± 0.4 | 3.8 ± 0.3 | 3.8 ± 0.4 | 0.1 |
| Phosphate (mg/dL) | 5.4 ± 1.4 | 5.3 ± 1.4 | 5.6 ± 1.6 | 0.3 |
| Dialysis Vintage (months) | 13.8 (7.0, 33.5) | 13.5 (7.0, 32.9) | 14.9 (6.4, 35.3) | 0.9 |
| PTH (pg/ml) | 292 ± 254 | 285 ± 240 | 315 ± 294 | 0.4 |
| spKt/V | 1.53 ± 0.26 | 1.54 ± 0.27 | 1.49 ± 0.24 | 0.2 |
| CES-D | 10.1 ± 8.0 | 6.4 ± 4.4 | 21.9 ± 5.3 | <0.001 |
Continuous data are mean ± standard deviation except vintage which is median (25th percentile, 75th percentile); categorical data are presented as no. (%).
Conversion factors for units: albumin in g/dL to g/L, ×10; phosphate in mg/dL to mmol/L, ×0.3229. No conversion necessary for PTH in pg/mL and ng/L.
Abbreviations: BMI, body mass index; BP, Blood Pressure; CAD, Coronary Artery Disease; PVD, Peripheral Vascular Disease; spKt/V, single-pool Kt/V; ESRD, End-Stage Renal Disease; PTH, parathyroid hormone; CES-D, Center for Epidemiological Studies Depression Scale
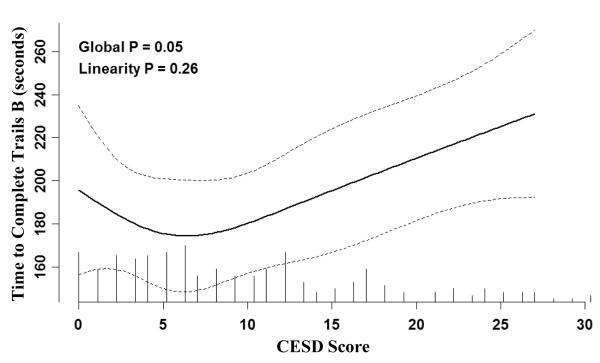
The results of cognitive testing are presented in Table 3. There were no differences in Mini Mental State Exam (MMSE) performance or tests of verbal intelligence or memory between groups. Parsimonious analysis adjusting for only age, sex and education demonstrated that participants with greater depressive symptoms performed significantly worse on Block Design, Digit-Symbol Coding, and Trail Making Test B (Figure 1). Multivariable models demonstrated similar findings, such that higher CES-D scores were associated with significantly poorer performance on Block Design, Digit-Symbol Coding and Trails B and a trend to worse performance on Trails A. Analyses utilizing z-scores to assess the influence of depressive symptoms on cognitive performance revealed significantly worse performance on tasks assessing executive function and tasks assessing psychomotor speed and processing (Table 4).
Table 3.
Results of cognitive testing stratified by depression status.
| Function Tested/Cognitive Test |
Test Scores | Univariate | Parsimonious* | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|
| CES-D <16 | CES-D ≥16 | β coefficient | p-value | β coefficient | p-value | β coefficient | p-value | |
| Screen | ||||||||
| MMSE | 26.7 ± 2.8 | 26.4 ± 2.8 | −0.2 | 0.6 | −0.2 | 0.7 | −0.3 | 0.4 |
| Intelligence | ||||||||
| NAART** | 101.7 ± 12.0 | 101.9 ± 12.0 | 0.2 | 0.9 | 1.0 | 0.6 | 2.0 | 0.2 |
| Primarily Cortical (memory) |
||||||||
| Percent Retention | 54.5 ± 28.4 | 47.6 ± 28.0 | −6.8 | 0.1 | −6.7 | 0.1 | −7.7 | 0.07 |
| Recognition | 20.8 ± 3.2 | 20.5 ± 2.7 | −0.3 | 0.6 | −0.3 | 0.5 | −0.4 | 0.3 |
| Primarily Subcortical (executive function & processing speed) |
||||||||
| Block Design | 26.8 ± 10.5 | 23.7 ± 10.5 | −3.0 | 0.06 | −2.7 | 0.06 | −2.9 | 0.03 |
| Digit Symbol Coding | 41.4 ± 17.2 | 34.2 ± 14.7 | −7.2 | 0.008 | −6.8 | 0.002 | −5.5 | 0.01 |
| Trails A | 58.8 ± 33.4 | 67.9 ± 47.2 | 8.5 | 0.1 | 7.5 | 0.2 | 9.2 | 0.09 |
| Trails B | 135.9 ± 65.8a | 157.9 ± 62.0b | 40.3 | 0.02 | 37.3 | 0.01 | 34.6 | 0.02 |
Scores are provided as mean ± standard deviation. All tests represent number or percent correct except Trails A and B, which are reported in seconds required to complete the task. Trails B analyses were performed using Tobit regression to account for failure to complete the task within 5 minutes. A negative B coefficient is consistent with poorer performance on all tests except the Trails A and B, where a positive coefficient identifies worse performance.
Abbreviations: BMI, body mass index; CAD, coronary artery disease ; CES-D, Center for Epidemiological Studies Depression Scale ; CHF, congestive heart failure ; ESRD, end-stage renal disease; MMSE, Mini-Mental State Exam ; NAART, North American Adult Reading Test; PTH, parathyroid hormone; PVD, peripheral vascular disease; SBP, systolic blood pressure
Parsimonious models adjust for age, education, and sex.
full multivariable models adjust for age, education, and sex as well as additional variables that vary by cognitive test: MMSE (cause of ESRD, smoking status, race, BMI, PTH, Kt/V, and phosphate); NAART (cause of ESRD, race, PTH, hematocrit, and PVD); Percent Retention (CAD, CHF, race, and BMI); Recognition (smoking status, race, BMI, SBP, and Kt/V); Block Design (CAD, vintage, race, BMI, Kt/V, and PVD); Digit Symbol Coding (CAD, CHF, cause of ESRD, race, SBP, weight change, stroke, and PVD); Trails A (smoking status and vintage); and Trails B (CAD, cause of ESRD, race, BMI, Kt/V, and PVD).
For those who completed; 19.9% did not complete.
For those who completed; 29.6% did not complete.
Figure 1.
Estimated performance on the Trail Making Part B task as a function of the CES-D score adjusted for age, sex and educational achievement generated using restricted cubic splines with 4 knots. Tick marks along the x-axis indicate the number of individuals with a given CES-D score. Dashed lines represent the 95% confidence interval. The p-value for linearity indicates that the association is not significantly different from a linear relationship.
Table 4.
The influence of greater depressive symptoms on cognitive performance in multiple domains
| Cognitive Domain | Tests Included | Univariate | Parsimonious* | Multivariable** | |||
|---|---|---|---|---|---|---|---|
| β coefficient | p-value | β coefficient | p-value | β coefficient | p-value | ||
| Executive Function | Trails B | −0.37 | 0.02 | −0.33 | 0.01 | −0.30 | 0.03 |
| Psychomotor Speed and Processing |
Trails A; Digit Symbol Coding; Block Design |
−0.27 | 0.03 | −0.25 | 0.02 | −0.24 | 0.02 |
| Memory | Delayed Recall; Recognition |
−0.17 | 0.2 | −0.17 | 0.2 | −0.21 | 0.08 |
Greater depressive symptoms are considered to be CES-D scores ≥16; cognitive domains were quantified using z-scores. Coefficients represent the effect of having a CES-D score of ≥16 versus < 16 on cognitive performance. A negative coefficient is consistent with worse performance.
Parsimonious models adjust for age, education, and sex.
For the executive function cognitive domain, full multivariable models additionally adjust for cause of ESRD, race, and peripheral vascular disease; for Psychomotor Speed and Processing, multivariable models additionally adjust for race, Kt/V, and stroke; and for memory, multivariable models additionally adjust for race, stroke and peripheral vascular disease.
Abbreviation: CES-D, Center for Epidemiological Studies Depression Scale
Of the 241 participants, 18 performed testing with dialysis access in their dominant arm and 39 were missing data on handedness. Among the remaining 184 participants, results on digit symbol coding and Trails B were similar to primary multivariable analyses (p=0.05 and p=0.08, respectively), while performance on the block design task no longer differed between groups (p=0.3). In a second sensitivity analyses where depression was defined by CES-D ≥18, 40 (16.6%) of patients were defined as having depression. Using this alternate definition, in multivariable analyses greater depressive symptoms remained significantly associated with poorer performance on Trails B (p=0.009 for both) and again trended toward significance on the Block Design, Digit-Symbol Coding and Trails A tests (p=0.08, 0.2 and 0.1, respectively). Finally, in models assessing the interaction between stroke and depression (CES-D ≥16), stroke only achieved statistical significance when evaluating percent retention (data not shown). In this model, p (for interaction) was 0.1, consistent with a borderline synergistic effect of depression and diagnosed stroke for performance on this test of memory.
Discussion
In maintenance hemodialysis patients, we demonstrate worse performance on cognitive tests that assess processing speed, attention and executive functioning among patients with greater depressive symptoms. Performance on tests of memory did not significantly differ by depression status. These deficits are consistent with subcortical brain pathology and may indicate underlying cerebrovascular disease [37, 38]. Similar to prior observations, our study also confirms a high prevalence of depressive symptoms in hemodialysis patients [2-5]. This approximately 1/3rd of a standard deviation poorer cognitive performance among individuals with greater depressive symptomatology may have subtle clinical manifestations.
Dialysis patients with concurrent depression and cognitive impairment may comprise an especially vulnerable population. These individuals may require greater health care resources and be more resistant to treatment, consistent with prior studies in non-dialysis patients demonstrating that individuals with both depression and impaired executive function have comparatively poorer responses to anti-depressive medications and are at greater risk of relapse [39, 40]. In the non-dialysis population, older adults with concurrent depression and cognitive deficits have benefited from more comprehensive treatment strategies [41, 42]. This has not been evaluated in the dialysis population.
Depression in hemodialysis patients may be similar to depression occurring in older adults, both in etiology and presentation. Late-life depression is associated with a greater risk of cognitive decline, an increase in illness and medical burden (including cardiovascular disease), distinct morphological changes in the brain, and an absence of a family history of mood disorders [43, 44]. Similar to our findings in hemodialysis patients, Rapp et al. noted that cognitive deficits associated with late-life depression were more likely to be found in attention and executive function, whereas those accompanying recurrent depression were primarily related to memory [45].
Although the current study does not directly address the underlying neuropathology of depression, prior research suggests an overlap in morphological changes consistent with maintenance hemodialysis and depression in older adults. Previous studies have found a greater incidence of white-matter hyperintensities and subcortical small-vessel disease in patients during all stages of chronic kidney disease [24, 46-49]; similar subcortical white matter abnormalities are a defining characteristic of depression in older adults [50] and are associated with both cardiovascular disease risk factors and poorer performance on tests of processing speed and executive function [51]. As such, the increased risk of vascular disease in hemodialysis patients likely puts them at an increased risk of white matter disease that potentially leads to both cognitive decline and depression [52]. Future studies that incorporate neuroimaging with quantification of white matter disease will provide greater insight towards the underlying etiology of depression and cognitive impairment in dialysis patients.
There are several limitations to our study. Our cross-sectional design is unsuited for determining causality or the temporal relationship between depression and cognitive declines. Accordingly, while we hypothesize that both depression and worse cognitive performance may be a function of cerebrovascular disease, it is possible that cognitive impairment may cause depression or depression may cause cognitive impairment, historically referred to as pseudodementia [53]. These relationships cannot be discerned using cross-sectional data. A second important limitation was the lack of a gold standard for identifying depression. While the CES-D provides reasonable screening data, it identifies symptoms of depression and not depression itself, and several of these symptoms, including sleep abnormalities, are common in the dialysis population. Recent analyses of the CES-D and other depression scales in CKD patients noted similar or slightly higher cutpoints to those identified in the general population [3, 54]. Accordingly, it is important to note that a sensitivity analysis using the higher cutpoint of 18 on the CES-D had nearly identical results. Third, we performed testing during hemodialysis. While testing in this environment may result in worse test performance and limit generalizability of tests to other populations, it has the beneficial effect of assessing hemodialysis patients in the same environment where they are likely to receive most of the medical counseling from physician, nurse, and nutritionist providers. Furthermore, we do not believe the relationship between depression and cognitive function should be altered by the time of testing. Fourth, we only included individuals with English fluency, potentially limiting the generalizability of results for non-English speakers. Importantly, this should not affect the association between cognitive function and symptoms of depression. Finally, it is important to note that our study examined the association between depressive affect and cognitive function rather than a current or prior diagnosis of “depression” per se. As such, although anti-depressant medications may affect depressive symptoms, we did not control for medication intake, including anti-depressants.
Our study has several strengths as well. We used a detailed neurocognitive evaluation that facilitated the identification of specifically affected cognitive domains. Additionally, we were able to enroll a substantial number of maintenance hemodialysis patients with characteristics and distribution of causes of ESRD similar to those seen in the prevalent US dialysis population [55]. Furthermore, exclusion criteria were few, and overall, enrolled participants were similar to those who refused, further improving generalizability.
In summary, in the setting of a relatively high prevalence of depressive symptoms in hemodialysis patients, we demonstrated that patients with more depressive symptoms performed worse across measures of executive function, attention, and processing speed than non-depressed counterparts. We hypothesize that depression in hemodialysis patients may be similar to depression in older adults. Future studies should address the longitudinal relationship between depression and cognitive function in addition to conducting neuroimaging to explore the etiology of concurrent depression and cognitive impairment. The association between cognition and depressive symptoms in dialysis patients suggests that healthcare providers must be vigilant towards detecting depression in individuals with cognitive deficits and vice versa.
Supplementary Material
Acknowledgements
We would like to acknowledge the tremendous assistance of Dialysis Clinic, Inc. and, in particular, the staff of the five DCI units in the Boston area, without whose cooperation the study would not have been successful.
Support: The study was funded through grants R21 DK068310, K23 DK71636, K24 DK078204 and R01 DK078204.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplementary Material Item S1: Center for Epidemiologic Studies Depression (CES-D) Scale questionnaire.
Note: The supplementary material accompanying this article (doi: ) is available at www.ajkd.org.
) is available at www.ajkd.org.
Descriptive Text for Online Delivery
Hyperlink: Supplementary Item S1 (PDF)
About: Center for Epidemiologic Studies Depression (CES-D) Scale questionnaire.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript was presented in abstract form at the American Society of Nephrology Annual Meeting in San Diego in 2009.
References
- 1.Kimmel PL, Peterson RA, Weihs KL, et al. Behavioral compliance with dialysis prescription in hemodialysis patients. J Am Soc Nephrol. 1995;5(10):1826–1834. doi: 10.1681/ASN.V5101826. [DOI] [PubMed] [Google Scholar]
- 2.Cukor D, Coplan J, Brown C, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(3):484–490. doi: 10.2215/CJN.00040107. [DOI] [PubMed] [Google Scholar]
- 3.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69(9):1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 4.Hedayati SS, Bosworth HB, Briley LP, et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008;74(7):930–936. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62(1):199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 6.Riezebos RK, Nauta KJ, Honig A, Dekker FW, Siegert CE. The association of depressive symptoms with survival in a Dutch cohort of patients with end-stage renal disease. Nephrol Dial Transplant. 2010;25(1):231–236. doi: 10.1093/ndt/gfp383. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 8.Weyerer S, Eifflaender-Gorfer S, Kohler L, et al. Prevalence and risk factors for depression in non-demented primary care attenders aged 75 years and older. J Affect Disord. 2008;111(2-3):153–163. doi: 10.1016/j.jad.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Heo M, Murphy CF, Fontaine KR, Bruce ML, Alexopoulos GS. Population projection of US adults with lifetime experience of depressive disorder by age and sex from year 2005 to 2050. Int J Geriatr Psychiatry. 2008;23(12):1266–1270. doi: 10.1002/gps.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutner NG, Brogan D, Hall WD, Haber M, Daniels DS. Functional impairment, depression, and life satisfaction among older hemodialysis patients and age-matched controls: a prospective study. Arch Phys Med Rehabil. 2000;81(4):453–459. doi: 10.1053/mr.2000.3878. [DOI] [PubMed] [Google Scholar]
- 11.Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75(11):1223–1229. doi: 10.1038/ki.2009.51. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Hayashino Y, Akiba T, et al. Depressive symptoms predict the subsequent risk of bodily pain in dialysis patients: Japan Dialysis Outcomes and Practice Patterns Study. Pain Med. 2009;10(5):883–889. doi: 10.1111/j.1526-4637.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 13.Abbas T Seyed, Mehdi E, Navvab S, Assari S. Effect of depression on health care utilization in patients with end-stage renal disease treated with hemodialysis. Eur J Intern Med. 2009;20(4):411–414. doi: 10.1016/j.ejim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1(3):496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 15.Hayes TL, Larimer N, Adami A, Kaye JA. Medication adherence in healthy elders: small cognitive changes make a big difference. J Aging Health. 2009;21(4):567–580. doi: 10.1177/0898264309332836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hain DJ. Cognitive function and adherence of older adults undergoing hemodialysis. Nephrol Nurs J. 2008;35(1):23–29. [PubMed] [Google Scholar]
- 17.Claesson L, Linden T, Skoog I, Blomstrand C. Cognitive impairment after stroke - impact on activities of daily living and costs of care for elderly people. The Goteborg 70+ Stroke Study. Cerebrovasc Dis. 2005;19(2):102–109. doi: 10.1159/000082787. [DOI] [PubMed] [Google Scholar]
- 18.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30(1):41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 20.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodial Int. 2007;11(3):309–314. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 22.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 23.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56(11):2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53(3):438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63(3):273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 26.Cui X, Lyness JM, Tu X, King DA, Caine ED. Does depression precede or follow executive dysfunction? Outcomes in older primary care patients. Am J Psychiatry. 2007;164(8):1221–1228. doi: 10.1176/appi.ajp.2007.06040690. [DOI] [PubMed] [Google Scholar]
- 27.Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry. 2006;63(2):153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 29.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1-3):1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychol Med. 2007;37(12):1693–1702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Blair J, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3(2):129–136. [Google Scholar]
- 33.Tulsky D, Zhu J, Lebetter M. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III), Wechsler Memory Scale-Third Scale (WMS-III): Technical Manual. Harcourt Brace and Company; San Antonio: 1997. [Google Scholar]
- 34.Heaton R, Grant I, Matthews C. Comprehensive Norms for an Expanded Halstead-Reitan Battery. Psychological Assessment Resources, Inc; Odessa, Florida: 1991. [Google Scholar]
- 35.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 36.Tobin J. Estimation for relationships with limited dependent variables. Econometrica. 1958;26(1):24–36. [Google Scholar]
- 37.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004;226(1-2):3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Roman GC. Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc. 2003;51(5 Suppl Dementia):S296–304. doi: 10.1046/j.1532-5415.5155.x. [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29(12):2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 40.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos GS, Raue PJ, Sirey JA, Arean PA. Developing an intervention for depressed, chronically medically ill elders: a model from COPD. Int J Geriatr Psychiatry. 2008;23(5):447–453. doi: 10.1002/gps.1925. [DOI] [PubMed] [Google Scholar]
- 42.Bogner HR, Bruce ML, Reynolds CF, 3rd, et al. The effects of memory, attention, and executive dysfunction on outcomes of depression in a primary care intervention trial: the PROSPECT study. Int J Geriatr Psychiatry. 2007;22(9):922–929. doi: 10.1002/gps.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luijendijk HJ, Stricker BH, Hofman A, Witteman JC, Tiemeier H. Cerebrovascular risk factors and incident depression in community-dwelling elderly. Acta Psychiatr Scand. 2008;118(2):139–148. doi: 10.1111/j.1600-0447.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 44.Wilkins CH, Mathews J, Sheline YI. Late life depression with cognitive impairment: Evaluation and treatment. Clin Interv Aging. 2009;4(1):51–57. [PMC free article] [PubMed] [Google Scholar]
- 45.Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162(4):691–698. doi: 10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- 46.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134(1-2):83–88. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki M, Wada A, Isaka Y, Maki K, Inoue T, Fukuhara Y. Cerebral magnetic resonance T2 high intensities in end-stage renal disease. Stroke. 1997;28(12):2528–2531. doi: 10.1161/01.str.28.12.2528. [DOI] [PubMed] [Google Scholar]
- 48.Seliger SL, Longstreth WT, Jr., Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16(12):3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 49.Kim CD, Lee HJ, Kim DJ, et al. High prevalence of leukoaraiosis in cerebral magnetic resonance images of patients on peritoneal dialysis. Am J Kidney Dis. 2007;50(1):98–107. doi: 10.1053/j.ajkd.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Nebes RD, Reynolds CF, 3rd, Boada F, et al. Longitudinal increase in the volume of white matter hyperintensities in late-onset depression. Int J Geriatr Psychiatry. 2002;17(6):526–530. doi: 10.1002/gps.635. [DOI] [PubMed] [Google Scholar]
- 51.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 52.Seliger SL, Sarnak MJ. Subclinical vascular disease of the brain in dialysis patients. Am J Kidney Dis. 2007;50(1):8–10. doi: 10.1053/j.ajkd.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 53.Caine ED. Pseudodementia. Current concepts and future directions. Arch Gen Psychiatry. 1981;38(12):1359–1364. doi: 10.1001/archpsyc.1981.01780370061008. [DOI] [PubMed] [Google Scholar]
- 54.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54(3):433–439. doi: 10.1053/j.ajkd.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kidney Dis. 2010;55(Suppl 1):S1–S420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.