Abstract
The serotonin (5-HT) system in the brain has been studied more than any other neurotransmitter for its role in the neurobiological basis of aggression. However, which mechanisms modulate the 5-HT system to promote escalated aggression is not clear. We here explore the role of GABAergic modulation in the raphé nuclei, from which most 5-HT in the forebrain originates, on escalated aggression in male mice. Pharmacological activation of GABAB, but not GABAA, receptors in the dorsal raphé nucleus (DRN) escalated aggressive behaviors. In contrast, GABA agonists did not escalate aggressive behaviors after microinjection into the median raphé nucleus. The aggression-heightening effect of the GABAB agonist baclofen depended on the activation of 5-HT neurons in the DRN because it was blocked by coadministration of the 5-HT1A agonist 8-OH-DPAT [((±)-8-hydroxy-2-(di-n-propylamino)tetralin) hydrobromide] (DPAT), which acts on autoreceptors and inhibits 5-HT neural activity. In vivo microdialysis showed that GABAB activation in the DRN increased extracellular 5-HT level in the medial prefrontal cortex. This may be attributable to an indirect action via presynaptic GABAB receptors. The presynaptic GABAB receptors suppress Ca2+ channel activity and inhibit neurotransmission, and the coadministration of N-type Ca2+ channel blocker facilitated the effect of baclofen. These findings suggest that the indirect disinhibition of 5-HT neuron activity by presynaptic GABAB receptors on non-5-HT neurons in the DRN is one of the neurobiological mechanisms of escalated aggression.
Introduction
Aggressive behavior is phylogenetically conserved, typically enhancing an individual's survival and reproductive success. However, excessive levels of aggression become maladaptive (Miczek et al., 2007). Escalated aggression has been an enormous problem in public health as highlighted by 2.3 million violence-related injury visits to emergency departments annually in the United States (Pan American Health Organization, 2007).
Serotonin (5-HT) is one of the major neurotransmitters that has been linked to escalated aggression in species ranging from invertebrates to humans (Miczek et al., 2004; Olivier, 2004; de Boer and Koolhaas, 2005; Coccaro et al., 1997). 5-HT in the mammalian CNS derives mainly from the midbrain raphé nuclei. Especially, the dorsal raphé nucleus (DRN) contains the largest accumulation of 5-HT neuronal cell bodies (Dahlström and Fuxe, 1964), which project to several targets, including the limbic structures and cortex (Azmitia and Segal, 1978; Michelsen et al., 2007). Lesions, pharmacological manipulations, and electrophysiological recordings link the DRN to aggressive and defensive behaviors in rodents, cats, and tree shrews (Jacobs and Cohen, 1976; Vergnes et al., 1986; Koprowska and Romaniuk, 1997; Sijbesma et al., 1991; Bannai et al., 2007; Faccidomo et al., 2008; van der Vegt et al., 2003; Mos et al., 1993; Walletschek and Raab, 1982). The DRN–5-HT system is modulated by other amines, acids, peptides, and steroids (Adell et al., 2002), but the nature of the neural systems modulating 5-HT neuronal activity to promote escalated aggression has not yet been determined.
Many GABA interneurons and distal GABAergic afferents can be found in the DRN (Nanopoulos et al., 1982; Belin et al., 1983; Gervasoni et al., 2000; Wang et al., 1992), and both GABAA and GABAB receptors are expressed in the DRN (Bowery et al., 1987). Activation of both the GABAA and GABAB receptors inhibits 5-HT cell firing (Innis and Aghajanian, 1987; Gallager and Aghajanian, 1976; Judge et al., 2004; Colmers and Williams, 1988). In vivo microdialysis studies have shown, however, that the GABAA and GABAB receptors in the DRN differentially modulate 5-HT release depending on the projection sites (Tao et al., 1996). GABAB receptor agonist microinjection in the DRN can induce either increases or decreases of 5-HT neuronal activity (Tao et al., 1996; Abellán et al., 2000). Therefore, 5-HT neurons in the DRN are differentially modulated by GABAA and GABAB receptors.
Here, we examine the GABAergic modulation of the DRN underlying escalated aggression. We demonstrate that the pharmacological activation by GABAB receptors, but not GABAA receptors, in the DRN, but not median raphé nucleus (MRN), escalates aggressive behaviors in male mice. Our data suggest that presynaptic GABAB receptors on non-5-HT neurons are responsible for the escalation of aggressive behaviors. The prefrontal cortex (PFC), one of the projection sites of DRN 5-HT neurons, has been implicated in impulsive aggression (Davidson et al., 2000), and we found that GABAB receptor activation in the DRN increased 5-HT release in the medial PFC (mPFC). Our results provide evidence that the GABAB receptor modulation in the DRN is one of the neurobiological mechanisms underlying escalated aggression in mice.
Materials and Methods
Mice.
Male CFW mice (Charles River Laboratories), weighing 21–23 g on arrival, were housed in pairs with females in a clear polycarbonate cage (28 × 17 × 14 cm) with pine shavings as bedding material. Additional males used as intruders were housed in groups of 7–10 per cage (48 × 26 × 14 cm) with corncob bedding. All animals were maintained in our vivarium with controlled humidity and temperature (35–40%, 21 ± 1°C) on a reversed 12 h light/dark cycle (lights off at 7:00 AM) with water and food (Purina) available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Tufts University. For the antagonist experiment, mice of the ICR strain (CLEA Japan), weighing 21–23 g on arrival, were tested in the National Institute of Genetics (Mishima, Japan). The animals were cared for according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Resident-intruder test training.
After being housed with a female for 3 weeks, the resident males were studied for their aggression toward the same intruder (Miczek and O'Donnell, 1978). Immediately after the female and pups were removed, an intruder was introduced into the home cage of the resident male. Their behaviors were recorded for 5 min after the first attack bite, or the intruder was removed after 5 min if no attack occurred. This encounter occurred once every other day until animals showed a stable number of attack bites, and stability was reached within 7–16 encounters [coefficient of variation (σ/μ) in the last three confrontations reached <0.2 for each animal]. All behavioral tests were performed in the dark period (11:00 A.M. to 2:00 P.M., in the reversed light/dark cycle).
Surgery and cannulation.
Once aggressive behavior had stabilized, residents were anesthetized by intraperitoneal injection of a mixture of 100 mg/kg ketamine HCl and 10 mg/kg xylazine and stereotaxically implanted with a 26 gauge guide cannula (Plastics One) aimed 2 mm above the DRN [anteroposterior (AP), −4.2 mm; mediolateral (ML), ±1.5 mm; dorsoventral (DV), −1.9 mm to bregma; angled 26° to the vertical] or MRN (AP, −4.2 mm; ML, ±1.2 mm; DV, −3.0 mm to bregma; angled 14° to the vertical) as calculated from a mouse brain atlas (Paxinos and Franklin, 2001). A 33 gauge obdurator (Plastics One) that extended 0.5 mm beneath the guide cannula tip was inserted after surgery. The obdurator was moved daily to prevent blockage and scarring and also to habituate the animals to handling. After surgery, animals were housed individually for 5 d to recover and then pair housed with the same female. To prevent gnawing by the female, the obdurator and head mount were covered with a quinine preparation (Bite it). One week after surgery, residents were assessed for fighting at least three times before starting the microinjection experiment. Most of the animals showed similar levels of aggressive behavior after the cannulation surgery. Animals that stopped fighting after the surgery were excluded from the microinjection test (Table 1).
Table 1.
Number of animals used in this study
| Group | Target | Experiment | Drugs | Vehicle | Total animals used for analysis | Missed placements | Excluded a |
|---|---|---|---|---|---|---|---|
| 1 | DRN | Mixture of muscimol and baclofen | Muscimol (0.006 nmol) + baclofen (0.06 nmol) | Saline | 12 | 0 | 1 |
| 2 | DRN | Muscimol or baclofen | Muscimol (0.06 nmol) | Saline | 9 | 0 | 1 |
| Baclofen (0.06 nmol) | |||||||
| 3 | DRN | Baclofen dose effect | Baclofen (0.01, 0.06, 0.10 nmol) | Saline | 12 | 3 | 2 |
| 4 | DRN | Antagonist (phaclofen) | Baclofen (0.06 nmol) + phaclofen (0, 0.15, 0.3 nmol) | Saline | 10 | 0 | 3 |
| 5 | DRN | Antagonist (CGP54626) | Baclofen (0.06 nmol) + CGP54626 (0, 0.06, 0.60 pmol) | Saline | 9 | 2 | 4 |
| 6 | DRN | ωGVIA dose effect | ωGVIA (0.1, 0.3, 3.0 pmol) | Artificial CSF | 15 (11 DRN, 4 aqueduct) | 1 | 5 |
| 7 | DRN | ωGVIA and baclofen | ωGVIA (0.1 pmol) + baclofen (0.01 nmol) | Artificial CSF | 13 | 2 | 3 |
| 8 | DRN | 8-OH-DPAT and baclofen | 8-OH-DPAT (0.9 nmol) + baclofen (0.06 nmol) | Artificial CSF | 15 | 2 | 1 |
| 9 | DRN | Baclofen temporal pattern | Baclofen (0.06 nmol), 10, 40, 100 min interval | Saline | 9 | 1 | 0 |
| 10 | DRN | Intra-DRN baclofen | Baclofen (0.06 nmol) | Saline | 6 | 3 | 0 |
| mPFC | 5-HT microdialysis in mPFC | ||||||
| 11 | MRN | Muscimol or baclofen | Muscimol (0.06 nmol) | Saline | 10 | 5 | 9 |
| Baclofen (0.06 nmol) | |||||||
| 12 | DRN | Antagonist only (CGP54626) | Baclofen (0.06 nmol) | Saline | 10 | 4 | 1 |
| CGP54626 (0.6, 6.0 pmol) |
aAnimals were excluded from this study because they died after surgery (15), stopped fighting after surgery (7), or could not complete all injections as a result of guide cannula problems (7). In the MRN, three animals continued the turning behavior 1 d after the muscimol injection.
Microinjection and aggression test.
The obdurator was removed, and a 33 gauge microinjector (Plastics One) attached to polyethylene tubing (Intramedic PE-50) was inserted into the guide cannula. The microinjector extended 2 mm below the end of the guide to reach the DRN or MRN. The other end of the tubing was connected to a 1 μl Hamilton syringe placed into an infusion pump (CMA Microdialysis). The drug was infused in a volume of 0.2 μl over 2 min, and the microinjector was left in place for 1 min after the infusion to allow the drug to diffuse completely. Ten minutes after the microinjection, an intruder male was introduced and aggressive behaviors were recorded for 5 min after the first attack bites. A resident male received a total of four to six microinjections, including two vehicle injections, in an irregular order. The drugs, doses, and number of animals for each experiment are summarized in Table 1.
Histology.
At the end of the experiment, mice were deeply anesthetized (ketamine and xylazine mixture) and intracardially perfused with 0.9% saline, followed by 4% paraformaldehyde (PFA) in PBS. After postfixation in 4% PFA for at least 24 h, brains were placed into 15% sucrose solution. A freezing microtome was used to slice the brains into 60 μm sections, which were stained with cresyl violet to verify the placements of the cannula and microdialysis probe. The injection site for each animal is depicted in Figure 1 and supplemental Figures S1 and S2 (available at www.jneurosci.org as supplemental material).
Figure 1.
GABAA and GABAB receptor activation in the DRN and MRN on escalated aggression. Representative mouse brain coronal sections (20×) that were stained with cresyl violet to visualize the injection sites in the DRN (A) and MRN (B). C, Schematic representation of the distribution of injection site in the DRN (white circles) and MRN (gray triangles). The GABAA receptor agonist muscimol (0.06 nmol), the GABAB receptor agonist baclofen (0.06 nmol), or vehicle (saline) was microinjected into the DRN or MRN 10 min before the aggression test. Intra-DRN microinjection of baclofen, but not muscimol, escalated frequency of attack bites (D). No effect of either muscimol or baclofen was observed when microinjected into the MRN (E). Values are means ± SEM; *p < 0.05 compared with vehicle control.
Drugs.
Muscimol (5-aminomethyl-3-hydroxy-isoxazole), baclofen [(±)-β-(aminomethyl)-4-chlorobenzenepropanoic acid], phaclofen [3-amino-2-(4-chlorophenyl)propanephosphonic acid], 8-OH-DPAT [((±)-8-hydroxy-2-(di-n-propylamino)tetralin) hydrobromide], and ω-conotoxin GVIA (ωGVIA) were purchased from Sigma-Aldrich. CGP54626 ([S-(R*,R*)]-[3-[[1-(3,4-dichlorophenyl)ethyl]amino]-2-hydroxypropyl] (cyclohexylmethyl) phosphinic acid) was obtained from Tocris Bioscience. All drugs were dissolved in 0.9% saline except 8-OH-DPAT and ω-conotoxin GVIA, which were dissolved in artificial CSF.
Extracellular 5-HT measurement in the mPFC.
Nine male mice were housed in pairs with a female for 3 weeks before the microdialysis experiment. Males were implanted with a CMA/7 guide cannula (CMA Microdialysis) aimed 2 mm above the mPFC (AP, ±2.6 mm; ML, −0.3 mm; DV, −0.8 mm to bregma) and a 26 gauge guide cannula (Plastics One) aimed 2 mm above the DRN (AP, −4.2 mm; ML, ±1.5 mm; DV, −1.9 mm to bregma; angled 26° to the vertical). After 1 week of recovery with handling, a CMA/7 probe of 2 mm active membrane was inserted into the mPFC under isoflurane inhalation anesthesia. The probe was infused overnight with artificial CSF at a flow rate of 0.5 μl/min using an infusion pump (CMA Microdialysis). On the following morning, the flow rate was increased to 1.5 μl/min, and a sample was collected every 20 min after 1 h stabilization. A total of 7.5 μl of stabilizing agent consisting of 20 mm phosphate buffer including 25 mm EDTA and 0.5 mm ascorbic acid was added to each vial to prevent aliquot degradation. The animals were housed in their home cage during the sample collection without water and food. Five samples were collected to measure the baseline level of 5-HT in the mPFC. Then, a microinjector was inserted and saline was microinfused into the DRN in a volume of 0.2 μl over 2 min. Ten minutes after the saline injection, three additional samples were collected. One hour after the saline injection, a microinjector was reinserted, and the animal received 0.06 nmol of baclofen into the DRN. Six samples were collected 10 min after the baclofen injection. The changes in 5-HT concentrations were expressed as the percentage change from the average baseline samples for each individual.
Microdialysates were measured using an HPLC system equipped with an electrochemical detector. 5-HT was determined using a cation-exchange column (1.5 × 250 mm, 5 μm inner diameter; Shiseido) with column temperature set at 30°C. The mobile phase consisted of 150 mm ammonium acetate, 50 mm citric acid, 27 μm EDTA, 10% methanol, and 1% acetonitrile with pH adjusted to 4.6, at a rate of 0.2 ml/min. The concentrations of 5-HT levels were determined by using standard curves by adding known amount of 5-HT in each vial in the range of 2.1–17 fmol. Under this condition, the correlation coefficient for the standard solutions exceeded 0.99. The limit of detection for 5-HT was 0.11 fmol.
Statistics.
Detailed behavior analysis from videotapes of resident–intruder encounters was performed by a trained observer using the Observer software (Observer XT 8.0; Noldus) to quantify aggressive behaviors (attack bites, sideways threats, pursuit, and tail rattles) and non-aggressive behaviors (walking, rearing, auto-grooming, and contacts) (Grant and Mackintosh, 1963; Miczek and O'Donnell, 1978). Frequencies were assessed for attack bites and sideways threats because these are point events, whereas duration was used for the other behaviors. Repeated-measures one-way ANOVA was performed to examine the effect of the drug treatment on aggressive behaviors and non-aggressive behaviors. In case of significant F values, post hoc Holm–Sidak tests were conducted to determine which doses of the drug differed significantly from vehicle (α = 0.05). For the antagonism experiment and 8-OH-DPAT experiment, we performed Holm–Sidak tests on all pairs to determine the effect of drug compared with vehicle and baclofen (α = 0.05). In the microdialysis analysis, repeated-measures one-way ANOVA was performed for 14 data points (five baseline samples, three samples after the saline microinfusion, and six samples after the baclofen microinfusion). If the F value was significant, Holm–Sidak test was conducted to compare baseline with each subsequent sample (three samples after the saline and six samples after the baclofen microinfusion).
Results
Activation of DRN GABAB receptors escalates intermale aggression in mice
GABAergic modulation is involved in escalated aggression
Local administration of GABAA receptor agonist muscimol and GABAB receptor agonist baclofen mixture (bac–mus) has been used for the reversible inactivation of target brain areas (McFarland and Kalivas, 2001), and thus we used this method to examine the effect of overall GABAergic modulation in the DRN on aggressive behaviors. The ratio of bac–mus mixture (10:1) was based on previous studies (McFarland and Kalivas, 2001), and the doses used in this study (0.06 and 0.006 nmol of baclofen and muscimol, respectively) were based on a pilot study. Intra-DRN administration of bac–mus mixture significantly escalated the frequency of attack bites (F (1,11) = 17.62, p < 0.001) and sideways threats (F (1,11) = 8.66, p = 0.013) but did not have any effect on non-aggressive behaviors (Table 2). Therefore, GABAergic receptor stimulation of the DRN selectively escalated aggressive behaviors.
Table 2.
The effect of GABA receptor agonists mixture (bac–mus) into the DRN on aggressive and non-aggressive behaviors
| Control | Baclofen–muscimol | |
|---|---|---|
| Sideways threat | 25.9 ± 3.6 | 35.8 ± 3.4* |
| Attack bite | 20.1 ± 4.8 | 36.0 ± 2.7* |
| Tail rattle (s) | 18.8 ± 1.1 | 13.0 ± 2.4* |
| Pursuit (s) | 2.0 ± 1.1 | 2.6 ± 0.8 |
| Grooming (s) | 21.6 ± 6.0 | 32.3 ± 3.2 |
| Rearing (s) | 48.4 ± 4.1 | 44.6 ± 6.7 |
| Walking (s) | 67.4 ± 7.8 | 75.7 ± 8.3 |
| Contact (s) | 9.1 ± 1.3 | 3.7 ± 4.6 |
Frequencies of attack bites and sideways threats and durations of tail rattle, pursuit, and non-aggressive behaviors. Values are means ± SEM.
*p < 0.05 compared with control.
Baclofen, but not muscimol, in the DRN escalates aggressive behaviors
We next investigated which type of GABA receptor in the DRN was responsible for this aggression-heightening effect. Animals received microinjections of either muscimol or baclofen separately into the DRN. Baclofen significantly increased attack bites and sideways threats compared with vehicle (Fig. 1 D). In contrast, muscimol significantly reduced tail rattles but did not change attack bites or sideways threat (Table 3). There were significant effects of drug on the frequency of attack bites (F (2,20) = 13.30, p < 0.001), sideways threats (F (2,20) = 6.00, p = 0.009), and duration of tail rattles (F (2,20) = 7.76, p = 0.003). A significant effect on the duration of walking was also observed (F (2,20) = 4.40, p = 0.026), and post hoc analysis showed that baclofen increased walking compared with vehicle. We also examined the lower dose of muscimol (0.006 nmol) used for bac–mus mixture, but there was no effect of muscimol on any behaviors (data not shown).
Table 3.
Effects of the intra-DRN and MRN microinjection of muscimol (0.06 nmol) and baclofen (0.06 nmol) on aggressive and non-aggressive behaviors
| DRN |
MRN |
|||||
|---|---|---|---|---|---|---|
| Control | Muscimol | Baclofen | Control | Muscimol | Baclofen | |
| Sideways threat | 13.6 ± 1.8 | 13.7 ± 2.0 | 27.5 ± 3.9* | 25.8 ± 4.3 | 18.5 ± 4.2 | 19.7 ± 3.6 |
| Attack bite | 18.7 ± 1.8 | 18.3 ± 3.8 | 40.7 ± 5.2* | 31.3 ± 3.3 | 25.2 ± 3.8 | 29.8 ± 4.5 |
| Tail rattle (s) | 10.1 ± 0.9 | 5.6 ± 0.9* | 7.2 ± 1.0 | 11.2 ± 2.5 | 6.6 ± 1.7 | 4.9 ± 2.1* |
| Pursuit (s) | 2.6 ± 1.7 | 5.0 ± 3.5 | 4.7 ± 2.1 | 0.8 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.2 |
| Grooming (s) | 24.1 ± 5.7 | 45.0 ± 13.9 | 28.0 ± 6.3 | 22.2 ± 3.4 | 18.1 ± 4.7 | 13.8 ± 2.6 |
| Rearing (s) | 21.0 ± 5.1 | 38.1 ± 9.6 | 24.4 ± 6.6 | 22.1 ± 3.7 | 25.6 ± 4.5 | 28.6 ± 7.6 |
| Walking (s) | 50.3 ± 4.0 | 59.2 ± 5.3 | 71.5 ± 7.4* | 55.1 ± 5.6 | 124.7 ± 23.8* | 63.1 ± 9.6 |
| Contact (s) | 3.1 ± 0.9 | 1.7 ± 1.1 | 3.0 ± 1.0 | 18.7 ± 6.7 | 24.3 ± 9.1 | 19.1 ± 4.9 |
Frequencies of attack bites and sideways threats and durations of tail rattle, pursuit, and non-aggressive behaviors. Values are means ± SEM.
*p < 0.05 compared with control.
Anatomical specificity: median raphé nucleus
The MRN also supplies 5-HT to the forebrain areas and has significant influence on 5-HT-related behaviors (Li et al., 2005; Adell et al., 2002). For an anatomical comparison, we investigated the effect of muscimol and baclofen into the MRN on aggressive behaviors. Neither muscimol nor baclofen had any effect on attack bites when infused into the MRN (Fig. 1 E). A significant main effect of drug was observed on tail rattles (F (2,18) = 3.86, p = 0.04), and baclofen reduced tail rattling compared with vehicle (Table 3). Also, we found a significant increase in walking behavior after intra-MRN muscimol but not baclofen (F (2,18) = 5.59, p = 0.013). We noticed that, in some animals, muscimol induced stereotypic-like turning behaviors toward the contralateral direction from cannula insertion and therefore induced hyperactivity.
During the histological verification of the cannula placement, we found that a total of two animals of all groups received their baclofen injection into the aqueduct. There was a slight but not significant increase of attack bites between vehicle (30.5 ± 4.5) and 0.06 nmol baclofen (33.5 ± 6.5) when the drug was microinjected into the aqueduct. Six animals that received the 0.06 nmol baclofen injection into regions outside of the DRN (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) were combined, and there was no change in the frequency of attack bites after the baclofen injection (20.0 ± 4.2) compared with vehicle (25.3 ± 4.7).
Bidirectional effect of GABAB receptor activation on aggressive behaviors
Intra-DRN baclofen (0.01, 0.06, and 0.1 nmol) resulted in an inverse U-shaped dose effect on aggressive behaviors. Baclofen at 0.06 nmol caused a significant increase in attack bites and sideways threats, whereas lower (0.01 nmol) or higher (0.1 nmol) doses of baclofen did not differ from vehicle (Fig. 2 A, Table 4). Significant main effects of drug were observed on the frequency of attack bites (F (3,33) = 5.33, p = 0.004), sideways threats (F (3,33) = 4.81, p = 0.007), and the duration of tail rattling (F (3,33) = 5.16, p = 0.005). The reduction of aggressive behaviors at the highest dose of baclofen was not attributable to sedation or motor incoordination; there was no significant effect of baclofen on non-aggressive behaviors (Fig. 2 B, Table 4).
Figure 2.
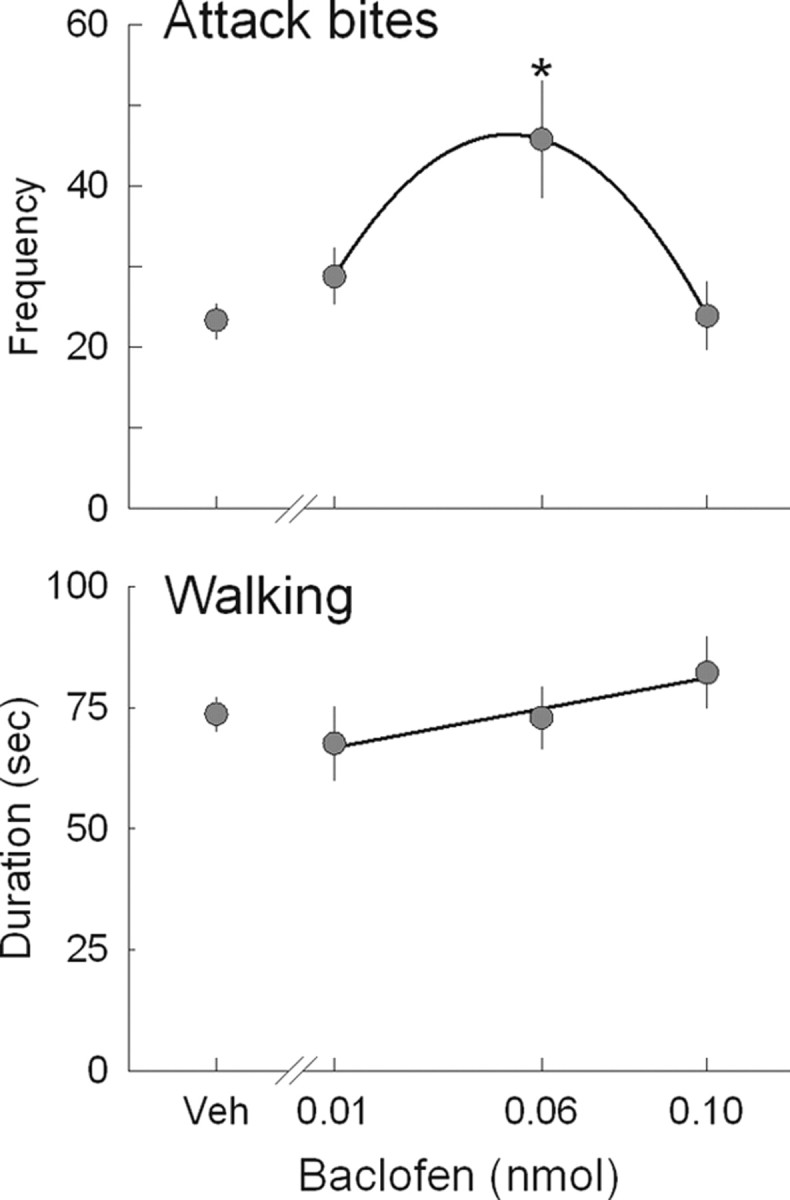
Dose effect of baclofen on aggressive behaviors. Three doses of baclofen (0.01, 0.06, and 0.10 nmol) or saline was injected into the DRN 10 min before the aggression test. Top, Inverted U-shape dose–effect pattern of baclofen was observed on the frequency of attack bites. Bottom, No effect of baclofen was observed on the duration of walking. Values are means ± SEM; *p < 0.05 compared with vehicle control.
Table 4.
Dose effects of intra-DRN baclofen on aggressive and non-aggressive behaviors
| Control | Baclofen |
|||
|---|---|---|---|---|
| 0.01 nmol | 0.06 nmol | 0.10 nmol | ||
| Sideways threat | 51.3 ± 4.3 | 51.8 ± 3.4 | 73.9 ± 9.1* | 50.4 ± 8.1 |
| Attack bite | 24.5 ± 2.1 | 28.0 ± 3.0 | 42.6 ± 6.5* | 24.8 ± 3.6 |
| Tail rattle (s) | 15.9 ± 3.0 | 11.8 ± 3.5 | 6.1 ± 0.9* | 5.5 ± 1.3* |
| Pursuit (s) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.8 ± 0.5 | 0.7 ± 0.4 |
| Grooming (s) | 10.7 ± 2.0 | 9.3 ± 2.3 | 16.8 ± 4.1 | 11.2 ± 3.5 |
| Rearing (s) | 28.3 ± 5.3 | 29.8 ± 7.8 | 23.0 ± 4.3 | 25.2 ± 7.9 |
| Walking (s) | 73.1 ± 4.6 | 64.3 ± 7.2 | 67.0 ± 6.7 | 76.3 ± 7.4 |
| Contact (s) | 7.2 ± 2.6 | 10.8 ± 3.8 | 10.8 ± 4.7 | 12.8 ± 5.5 |
Frequencies of attack bites and sideways threats and durations of tail rattle, pursuit, and non-aggressive behaviors. Values are means ± SEM.
*p < 0.05 compared with control.
GABAB receptor antagonists blocked the aggression-heightening effect of baclofen
The escalated aggression induced by intra-DRN microinjection of a moderate dose of baclofen (0.06 nmol) was antagonized by coadministration of GABAB receptor antagonists CGP54626 or phaclofen (Fig. 3). The doses of antagonists were based on a pilot study (phaclofen) and by referring to previous studies using cultured cells (CGP54626) (White et al. 2000). Again, baclofen itself significantly increased attack bites compared with vehicle in both groups. Coadministration of either CGP54626 or phaclofen with baclofen dose dependently blocked baclofen-heightened aggression, and all doses of CGP54626 (0.06 and 0.6 pmol) and the highest dose of phaclofen (0.3 nmol) significantly reduced attack bites to the same level as control (Fig. 3). Repeated-measures ANOVA indicated a significant effect of drug on attack bites for both CGP54626 (F (3,24) = 6.17, p = 0.003) and phaclofen (F (3,24) = 6.13, p = 0.003). No significant effect of drug was observed for the other behaviors (Table 5).
Figure 3.
GABAB receptor antagonists blocked aggression-heightening effect of baclofen. The effect of baclofen on attack bites was antagonized by coadministration of CGP54626 (left) or phaclofen (right) into the DRN. Values are means ± SEM; *p < 0.05 compared with vehicle; + p < 0.05 compared with baclofen treatment.
Table 5.
The effects of two antagonists (CGP54626 and phaclofen) on baclofen-heightened aggression
|
CGP54626 |
Phaclofen |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline | Saline + 0.06 nmol baclofen | 0.06 pmol CGP54626 + 0.06 nmol baclofen | 0.6 pmol CGP54626 + 0.06 nmol baclofen | Saline + saline | Saline + 0.06 nmol baclofen | 0.15 nmol phaclofen + 0.06 nmol baclofen | 0.3 nmol phaclofen + 0.06 nmol baclofen | |
| Sideways threat | 19.6 ± 1.9 | 21.7 ± 3.2 | 20.2 ± 3.0 | 22.4 ± 5.1 | 19.5 ± 1.5 | 26.4 ± 2.9 | 25.5 ± 4.1 | 24.7 ± 2.7 |
| Attack bite | 24.8 ± 1.9 | 41.0 ± 5.4* | 30.1 ± 4.2 † | 27.7 ± 4.8 † | 22.8 ± 1.9 | 40.0 ± 4.5* | 32.9 ± 4.3 | 30.0 ± 2.9 |
| Tail rattle (s) | 10.9 ± 2.6 | 5.0 ± 1.6 | 7.1 ± 1.5 | 5.7 ± 3.3 | 10.9 ± 1.6 | 8.7 ± 1.5 | 9.3 ± 2.4 | 11.6 ± 2.9 |
| Pursuit (s) | 0.9 ± 0.3 | 1.7 ± 0.5 | 1.1 ± 0.3 | 0.4 ± 0.2 † | 0.6 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.6 | 1.4 ± 0.5 |
| Grooming (s) | 19.7 ± 4.2 | 25.6 ± 7.7 | 27.4 ± 7.8 | 33.8 ± 8.7 | 28.5 ± 5.2 | 31.8 ± 7.8 | 38.0 ± 10.9 | 30.4 ± 6.8 |
| Rearing (s) | 50.4 ± 7.8 | 42.3 ± 9.4 | 39.6 ± 9.3 | 36.2 ± 9.6 | 42.3 ± 6.2 | 44.4 ± 8.6 | 35.6 ± 7.6 | 43.1 ± 6.8 |
| Walking (s) | 63.6 ± 7.7 | 73.6 ± 9.4 | 67.2 ± 7.4 | 68.1 ± 10.9 | 53.9 ± 2.6 | 60.8 ± 7.5 | 62.6 ± 9.1 | 69.5 ± 4.9 |
| Contact (s) | 4.2 ± 1.9 | 8.9 ± 3.1 | 8.8 ± 3.1 | 5.2 ± 3.0 | 4.1 ± 0.9 | 4.3 ± 1.5 | 5.4 ± 3.1 | 7.8 ± 2.9 |
Frequencies of attack bites and sideways threats and durations of tail rattle, pursuit, and non-aggressive behaviors. Values are presented as mean ± SEM.
*p < 0.05 versus vehicle control;
† p < 0.05 versus baclofen alone.
The effect of the antagonist by itself on species-typical aggression was examined using CGP54626 (0.6 and 6 pmol). Again, we confirmed the effect of 0.06 nmol baclofen in ICR mouse strains (supplemental Table 1, available at www.jneurosci.org as supplemental material). We found a slight reduction of attack bites after the highest dose of CGP54626 compared with control, but the effect was not statistically significant. There was no significant effect of CGP54626 on any behavior except contact duration. Repeated-measures ANOVA indicated a significant effect of drug on attack bites (F (3,27) = 7.09, p = 0.001), sideways threat (F (3,27) = 3.95, p = 0.019), pursuit (F (3,27) = 3.142, p = 0.042) caused by the effect of baclofen, and contact (F (3,27) = 5.162, p = 0.006). A pilot study had shown that phaclofen by itself (0.3 nmol) also did not change any of the aggressive behaviors (data not shown). Therefore, the blockade of the GABAB receptor in the DRN did not change the species-typical aggressive behaviors.
The role of the presynaptic N-type Ca2+ channel in baclofen-heightened aggression
Intra-DRN ω-conotoxin GVIA escalates aggressive behaviors
Presynaptic GABAB receptors have a higher sensitivity to low doses of baclofen than the postsynaptic GABAB receptors in several brain areas, including the DRN (Cruz et al., 2004; Howe et al., 1987; Abellán et al., 2000; Davies et al., 1990). The inverse U-shaped dose–effect curve for baclofen suggested that the presynaptic rather than postsynaptic GABAB receptors may be the target of baclofen-escalated aggressive behaviors. Therefore, we examined the possible involvement of presynaptic GABAB receptors. Presynaptic GABAB receptors inhibit neurotransmitter release by suppressing Ca2+ currents via voltage-gated Ca2+ channels (Kamatchi and Ticku, 1990; Couve et al., 2000). One subtype of Ca2+ channels, the N-type Ca2+ channel, is localized exclusively on presynaptic terminals in cultured cerebral cortex neurons (Timmermann et al., 2002). To examine the involvement of N-type Ca2+ channels in the DRN on aggressive behaviors, the N-type Ca2+ channel blocker ωGVIA was microinjected into the DRN. The higher dose of ωGVIA was chosen based on the previous study by Kim et al. (2009), showing that 3 pmol of ωGVIA increased isolation-induced aggression in C57BL/6J mice. We found that ωGVIA had effects that varied across individual animals: some animals showed strong sedation, whereas some animals showed escalated aggression. Histological analysis showed that animals with a placement inside the aqueduct (n = 1) or with placements in the dorsal area of DRN with injector breaking through the aqueduct (n = 3), with possibility of ωGVIA leakage into aqueduct, all showed sedation (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Thus, we separated the data from the aqueduct group (n = 4) from those of the DRN group (n = 11) for statistical analysis. An inverse U-shaped dose–effect curve was observed in the DRN group, and a significant increase in attack bites was observed after microinjection of 0.3 pmol of ωGVIA compared with vehicle (Fig. 4 A). A significant main effect of drug was observed on attack bites (F (3,30) = 5.81, p = 0.003), sideways threats (F (3,30) = 3.34, p = 0.032), and pursuit (F (3,30) = 3.60, p = 0.025). In contrast, in the aqueduct group, significant dose-dependent decreases in aggressive behaviors (F (3,9) ≥ 4.28, p ≤ 0.039) and non-aggressive behaviors (F (3,9) ≥ 4.61, p ≤ 0.032) were observed (Fig. 4 A, Table 6). Therefore, it is possible that the reduction of fighting at the highest dose of intra-DRN ωGVIA is attributable to the effect of the drug after diffusion into the aqueduct.
Figure 4.

Interaction between N-type Ca2+ channel and GABAB receptor in the DRN. A, Microinjection of N-type Ca2+ channel blocker ωGVIA into the DRN enhanced the frequency of attack bites with a moderate dose (filled circles). Animals who received injection into the aqueduct and in the dorsal area of DRN with injector breaking through the aqueduct showed dose-dependent sedation (triangles). B, Coadministration of low doses of baclofen (0.01 nmol) and ωGVIA (0.1 pmol) escalated aggressive behaviors. Either baclofen or ωGVIA alone did not cause a significant increase of aggressive behaviors. Values are means ± SEM; *p < 0.05 compared with vehicle control.
Table 6.
Effects of N-type Ca2+ channel blocker ω-conotoxin GVIA in the DRN on aggressive and non-aggressive behaviors
| Control | ωGVIA |
|||
|---|---|---|---|---|
| 0.1 pmol | 0.3 pmol | 3 pmol | ||
| Intra-DRN | ||||
| Sideways threat | 31.6 ± 3.6 | 26.5 ± 3.5 | 38.3 ± 4.8 | 27.3 ± 6.2 |
| Attack bite | 29.3 ± 3.9 | 29.3 ± 4.3 | 39.8 ± 5.5* | 23.6 ± 5.2 |
| Tail rattle (s) | 12.1 ± 2.7 | 8.4 ± 2.0 | 13.9 ± 3.0 | 15.5 ± 6.0 |
| Pursuit (s) | 1.2 ± 0.5 | 0.5 ± 0.2 | 1.5 ± 0.7 | 0.4 ± 0.2 |
| Grooming (s) | 27.8 ± 5.9 | 37.5 ± 8.5 | 31.1 ± 5.4 | 40.2 ± 8.4 |
| Rearing (s) | 42.9 ± 7.2 | 47.5 ± 7.1 | 41.5 ± 7.5 | 47.5 ± 10.3 |
| Walking (s) | 61.7 ± 5.3 | 60.4 ± 4.8 | 62.1 ± 4.8 | 64.7 ± 9.4 |
| Contact (s) | 14.6 ± 5.1 | 12.3 ± 3.0 | 13.2 ± 6.2 | 7.7 ± 2.8 |
| Near aqueduct | ||||
| Sideways threat | 31.3 ± 7.8 | 31.5 ± 10.3 | 15.5 ± 9.7 | 0.8 ± 0.5* |
| Attack bite | 24.5 ± 5.3 | 26.5 ± 4.9 | 13.5 ± 7.8 | 0.3 ± 0.3* |
| Tail rattle (s) | 16.1 ± 4.8 | 32.0 ± 15.1 | 2.5 ± 1.9 | 0.3 ± 0.3 |
| Pursuit (s) | 2.0 ± 0.8 | 1.5 ± 0.9 | 0.8 ± 0.5 | 0.0 ± 0.0 |
| Grooming (s) | 19.4 ± 5.7 | 31.5 ± 7.0 | 6.3 ± 5.6 | 0.0 ± 0.0 |
| Rearing (s) | 18.5 ± 5.0 | 26.5 ± 9.0 | 4.8 ± 2.8 | 0.3 ± 0.3 |
| Walking (s) | 51.1 ± 3.8 | 61.3 ± 6.7 | 24.8 ± 8.2 | 4.5 ± 2.3* |
| Contact (s) | 17.3 ± 7.3 | 12.3 ± 4.2 | 8.8 ± 2.8 | 3.3 ± 2.0* |
Frequencies of attack bites and sideways threats and durations of tail rattle, pursuit, and non-aggressive behaviors. Values are presented as mean ± SEM.
*p < 0.05 versus control.
Interaction between GABAB and N-type Ca2+ channels
To examine the interaction between N-type Ca2+ channels and GABAB receptors, we coadministered a low dose of ωGVIA (0.1 pmol) with a low dose of baclofen (0.01 nmol), which did not induce a significant effect on attack bites by itself (Figs. 2, 4 A), into the DRN. We found that coadministration of baclofen and ωGVIA significantly increased attack bites compared with vehicle (Fig. 4 B; Table 7, left). Baclofen itself also tended to increase aggressive behaviors, but this effect was not statistically significant. A significant main effect of drug was detected on the measure of attack bites (F (3,36) = 6.40, p < 0.001). Together, there may be a functional interaction between GABAB receptor activation and N-type Ca2+ channel underlying the baclofen-induced escalated aggression.
Table 7.
Interactions between GABAB receptors and N-type Ca2+ channels or 5-HT1A receptors in the DRN on aggressive and non-aggressive behaviors
| N-type Ca2+ channels |
5-HT1A receptors |
|||||||
|---|---|---|---|---|---|---|---|---|
| aCSF | aCSF + 0.01 nmol baclofen | 0.1 pmol ωGVIA + 0.01 nmol baclofen | 0.1 pmol ωGVIA + aCSF | aCSF | aCSF + 0.06 nmol baclofen | 0.9 nmol 8-OH-DPAT + 0.06 nmol baclofen | 0.9 nmol 8-OH-DPAT + aCSF | |
| Sideways threat | 30.2 ± 2.9 | 32.6 ± 6.1 | 34.6 ± 3.8 | 27.1 ± 3.9 | 27.5 ± 3.3 | 34.5 ± 2.8 | 25.4 ± 2.6 | 23.9 ± 3.3 |
| Attack bite | 29.9 ± 2.7 | 36.3 ± 6.2 | 43.7 ± 5.3* | 27.5 ± 2.8 | 22.0 ± 2.5 | 38.7 ± 2.4* | 30.0 ± 4.6* , † | 19.1 ± 3.4 |
| Tail rattle (s) | 8.9 ± 1.3 | 7.4 ± 1.8 | 6.9 ± 2.2 | 9.5 ± 2.5 | 6.9 ± 1.4 | 5.7 ± 1.4 | 7.1 ± 2.2 | 7.6 ± 1.6 |
| Pursuit (s) | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.9 ± 0.5 | 1.5 ± 0.6 | 0.6 ± 0.2 | 2.4 ± 0.7* | 1.3 ± 0.6 | 0.6 ± 0.3 |
| Grooming (s) | 21.1 ± 2.7 | 19.8 ± 4.2 | 18.8 ± 3.4 | 21.0 ± 3.1 | 20.5 ± 3.2 | 22.2 ± 4.5 | 23.1 ± 5.2 | 18.1 ± 3.1 |
| Rearing (s) | 44.0 ± 4.9 | 36.3 ± 5.8 | 32.7 ± 4.8 | 46.9 ± 7.0 | 39.4 ± 8.7 | 36.2 ± 5.9 | 38.2 ± 5.5 | 30.6 ± 4.6 |
| Walking (s) | 67.7 ± 3.5 | 58.5 ± 4.2 | 73.1 ± 6.1 | 64.5 ± 6.0 | 78.4 ± 5.5 | 80.7 ± 8.8 | 79.6 ± 9.3 | 97.4 ± 8.1 |
| Contact (s) | 16.2 ± 4.1 | 22.7 ± 5.4 | 20.7 ± 9.4 | 10.1 ± 2.5 | 12.6 ± 6.9 | 18.9 ± 6.7 | 12.8 ± 6.8 | 11.0 ± 3.4 |
Frequencies of attack bites and sideways threats and durations of tail rattle, pursuit, and non-aggressive behaviors. Values are presented as mean ± SEM.
*p < 0.05 versus vehicle control;
† p < 0.05 versus baclofen alone. aCSF, Artificial CSF.
Indirect modulations of DRN 5-HT neurons by GABAB receptors
Intact 5-HT neuronal activity is required for baclofen to promote escalated aggression
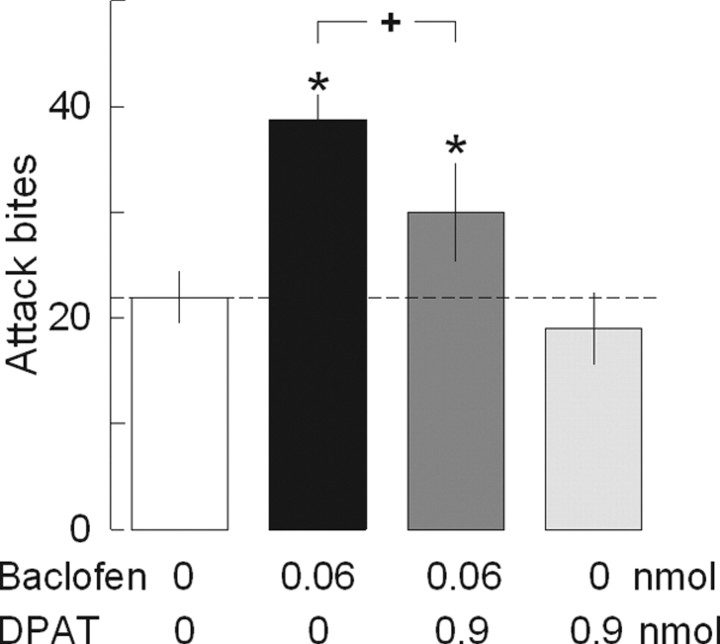
5-HT1A receptors are mainly localized on the somata and dendrites of 5-HT neurons and inhibit serotonergic activity (Kia et al., 1996; Miquel et al., 1992; Will et al., 2004). The 5-HT1A receptor agonist DPAT acts on the autoreceptors on 5-HT neurons and selectively inhibits 5-HT neurons (Bortolozzi et al., 2004; Bonvento et al., 1992; Sprouse and Aghajanian, 1987). Therefore, intra-raphé DPAT injection has been used for pharmacological inhibition of 5-HT neurons within the DRN (Will et al., 2004). In this study, we examined how inhibition of 5-HT impulse flow affects baclofen-heightened aggression by coadministering DPAT with baclofen. We used a low dose of DPAT (0.9 nmol) that does not produce any motor effect when it is microinjected into the DRN of mice but inhibits alcohol-heightened aggression (I. M. Quadros, L. S. Hwa, J. F. DeBold, and K. A. Miczek, unpublished observation). Baclofen significantly increased attack bites, and coadministration of DPAT and baclofen significantly reduced attack bites compared with baclofen treatment (Fig. 5; Table 7, right). Injection of DPAT (0.9 nmol) alone reduced attack bites slightly but not significantly. Repeated-measures ANOVA indicated a significant effect of drug on attack bites (F (3,42) = 16.09, p < 0.001) and pursuit (F (3,42) = 4.20, p = 0.011). Therefore, transient inactivation of 5-HT neurons by DPAT disrupted the effect of baclofen. This result suggests that intact impulse flow in 5-HT neurons is necessary for baclofen to induce escalated aggressive behaviors.
Figure 5.
Interaction between 5-HT1A receptor and GABAB receptor in the DRN. Coadministration of 5-HT1A receptor agonist DPAT (0.9 nmol) blocked the effect of baclofen (0.06 nmol) on frequency of attack bites. Baclofen itself significantly increased the frequency of attack bites in these animals, whereas DPAT itself did not change attack bites. Values are means ± SEM; *p < 0.05 compared with vehicle control; + p < 0.05 compared with baclofen treatment.
Modulation of prefrontal 5-HT release by intra-DRN baclofen
To examine the effect of an aggression-heightening dose of baclofen (0.06 nmol) on 5-HT neuronal activities in vivo, we measured the extracellular 5-HT concentrations in one of the projection areas of the DRN, the mPFC, by microdialysis. A probe was inserted in the mPFC of mice in addition to a guide cannula aimed at the DRN. We found that the microinjection of baclofen (0.06 nmol) into the DRN increased the extracellular 5-HT in the mPFC (Fig. 6 A). The elevated 5-HT level gradually recovered and returned to baseline after 90 min. A significant increase of 5-HT was observed in the first sample (10–30 min) after the intra-DRN baclofen microinjection compared with baseline (one-way repeated measures ANOVA; F (13, 64) = 2.63, p = 0.005). In contrast, no change of 5-HT level was observed after the saline injection.
Figure 6.
Extracellular 5-HT concentration in the mPFC of mice after GABAB receptor activation in the DRN. A, Baclofen microinjected into the DRN increased the 5-HT level in the mPFC, whereas saline injection did not change the 5-HT level. Twenty-minute samples were collected: five samples for baseline, three samples after saline injection, and six samples after baclofen (0.06 nmol) injection. Data are means ± SEM expressed as percentage of baseline (n = 7); *p < 0.05 compared with baseline. B, Histological representation of probe placement in the mPFC for the microdialysis (vertical bars: 2 mm probe membrane) and drug injection site in the DRN (circles). C, The effect of 0.06 nmol of baclofen (black bars) or saline (gray bars) on attack bites at different postinjection intervals (10, 40, and 100 min, corresponding to fractions 9, 11, and 14 in the microdialysis, respectively). Escalated attack bites were observed both 10 and 40 min after the intra-DRN baclofen injection. Values are means ± SEM; *p < 0.05 compared with corresponding vehicle control.
To examine the relationship of this temporal change of mPFC 5-HT with behavioral manifestations, we examined the aggressive behaviors at various intervals after intra-DRN baclofen treatment. Aggressive behaviors were assessed only one time per day at different intervals (10, 40, and 100 min) after either baclofen (0.06 nmol) or saline injection into the DRN. We found that the animals showed significantly higher frequencies of attack bites at 10 and 40 min after baclofen injection compared with the corresponding saline treatment (Fig. 6 C). This result shows that the effect of baclofen is long lasting, and the animals showed escalated aggressive behaviors when baclofen enhanced 5-HT levels in the mPFC.
Discussion
GABAB, but not GABAA, receptor modulation in the DRN escalated aggression
The current results highlight differential modulation between GABAA and GABAB receptors in the DRN on aggressive behavior in mice. Activation of GABAB, but not GABAA, receptors in the DRN reliably escalates aggressive behaviors. Previous studies have consistently shown that GABAA agonists inhibit 5-HT neuron firing in the DRN and reduce 5-HT release and synthesis in the projection areas (Tao et al., 1996; Tao and Auerbach, 2000; Nishikawa and Scatton, 1985), whereas the effect of GABAB receptor agonists on 5-HT neurons is more complex. The GABAB agonist baclofen increases 5-HT release during the light but decreases it in the dark (Tao et al., 1996; Abellán et al., 2000). Also, local infusion of baclofen modulated 5-HT release bidirectionally in the DRN; low doses of baclofen increased 5-HT release in the DRN, whereas high doses of baclofen suppressed it (Abellán et al. 2000). This bidirectional effect is probably attributable to activation of presynaptic versus postsynaptic GABAB receptors in the DRN (Serrats et al., 2003). Presynaptic GABAB receptors have a higher sensitivity to low doses of baclofen than the postsynaptic GABAB receptors in the frontal cortex, hippocampus, ventral tegmental area (VTA), and DRN (Cruz et al., 2004; Howe et al., 1987; Abellán et al., 2000; Davies et al., 1990). Previous studies of the VTA showed that the presynaptic GABAB receptors on GABA neurons had higher sensitivity than the postsynaptic GABAB receptors as a result of different coupling efficiency with the G-protein-coupled inwardly rectifying potassium (GIRK) channels mediated by Rgs2. A lower concentration of baclofen inhibits the GABA neurons and consequently disinhibits the postsynaptic dopamine neural activities, whereas a high concentration of baclofen inhibits the dopamine neurons through direct action (Labouèbe et al., 2007; Cruz et al., 2004). The current data show that a moderate dose of baclofen (0.06 nmol) increased attack bites, but a higher dose (0.10 nmol) did not. It is possible that this inverted U-shaped dose effect of baclofen on aggressive behaviors corresponds to the bidirectional effect on 5-HT neuronal activity.
Here, we did not observe any effect of GABAB antagonists on species-typical aggression. Although the systemic administration of GABAB receptor antagonist interacts with 5-HT system (Slattery et al., 2005), the intra-raphé administration of GABAB antagonists had no effect on raphé and forebrain 5-HT (Tao et al., 1996). Along with the result of the 8-OH-DPAT experiment, these results indicate that the change of 5-HT neuron activity is necessary to induce escalated aggression.
Presynaptic GABAB receptors as a target of baclofen-induced escalated aggression
In this study, a neuropharmacological approach identified which subpopulations of GABAB receptors, presynaptic or postsynaptic, are involved in the aggression-heightening effect of baclofen. Presynaptic GABAB receptor activation inhibits neurotransmitter release by suppressing Ca2+ influx via voltage-activated Ca2+ channels, whereas postsynaptic GABAB receptors are coupled mainly to inwardly rectifying K+ (GIRK) channels and inhibit 5-HT neurons in the DRN (Cryan and Kaupmann, 2005; Bowery et al., 2002). In postsynaptic 5-HT neurons, both GABAB heteroreceptor and 5-HT1A autoreceptor activate the same GIRK channels through the pertussis toxin-sensitive G-protein (Innis and Aghajanian, 1987; Innis et al., 1988; Williams et al., 1988; Costa et al., 2005; Cornelisse et al., 2007). If postsynaptic GABAB receptors are involved in escalated aggression, activation of the 5-HT1A receptor should also have an aggression-heightening effect similar to that of baclofen. Contrary to baclofen, local administration of 5-HT1A receptor agonists into the DRN reduced aggressive behaviors in rats and mice (Mos et al., 1993; van der Vegt et al., 2003; Faccidomo et al., 2008). Coadministration of a low dose of 8-OH-DPAT, a 5-HT1A receptor agonist, together with baclofen did not further enhance, but rather prevented, the aggression-heightening effect of baclofen. Therefore, it is likely that the aggression-heightening effect does not depend on postsynaptic GABAB receptors. The bidirectional effect of baclofen on aggressive behaviors also suggests the presynaptic GABAB autoreceptors as a target for escalated aggression.
Presynaptic GABAB receptors inhibit Ca2+ current by inactivating voltage-gated Ca2+ channels of N type or P/Q type (Kamatchi and Ticku, 1990; Couve et al., 2000). In this study, we focused on N-type Ca2+ channels because of the reported involvement of this subtype on aggressive behaviors (Murakami et al., 2007; Kim et al., 2009). In the present experiments, intra-DRN administration of the N-type Ca2+ channel blocker ω-conotoxin GVIA escalated intermale aggression similarly to baclofen. Coadministration of a low dose of ωGVIA with a non-effective dose of baclofen into the DRN significantly increased attack bites relative to control. These data suggest a functional interaction between the presynaptic GABAB receptor and N-type Ca2+ channel in the DRN to promote escalated aggression. We propose that the aggression-heightening effects of baclofen are mediated by the activation of the presynaptic GABAB receptors, which in turn inhibit N-type Ca2+ channels to suppress neurotransmission from the presynaptic terminals in the DRN (Fig. 7). In vivo microdialysis showed that intra-DRN baclofen increased the release of 5-HT in the medial prefrontal cortex. This suggests that the activation of the GABAB receptors on inhibitory neurons, possibly GABAergic, suppress the inhibitory neurotransmission on 5-HT, which may increase 5-HT release in cortical projection areas. ωGVIA (1 μm) application into the DRN slices inhibited GABA-mediated IPSCs in 5-HT neurons and consequently disinhibited 5-HT neuronal activities (Kim et al. 2009). We hypothesize that GABAB receptors in GABA neurons, or on glutamatergic neurons that activate GABA neurons in the DRN, may be the target of baclofen-escalated aggression.
Figure 7.
Simple scheme of presynaptic and postsynaptic GABAB receptor modulation in the DRN. Presynaptic GABAB receptors inhibit voltage-dependent Ca2+ channel and inhibit neurotransmitter release. Postsynaptic GABAB receptors are coupled with GIRK channels and inhibit postsynaptic neuronal activity; the same GIRK channels are activated by 5-HT1A autoreceptors. Our results suggest that baclofen activates presynaptic GABAB receptors to inhibit neurotransmitter release via N-type Ca2+ channels and escalates aggressive behaviors in male mice.
5-HT in the medial prefrontal cortex and escalated aggression
The PFC has been implicated in behavioral inhibition and emotion regulation that is pertinent to impulsive aggression. In humans, increased antisocial behavior was associated with the functional reductions of some subareas of the PFC (Yang and Raine, 2009). It is not clear how the 5-HT projections to the PFC regulate cellular activity necessary for escalating aggression. We demonstrated that intra-DRN microinjection of an aggression-escalating dose of baclofen increased extracellular 5-HT level in the mPFC. Mice that increased aggressive behavior after repeated aggressive encounters show elevated 5-HT tissue level in the mPFC (Caramaschi et al., 2008). Activation of 5-HT1B receptors in the mPFC potentiated alcohol-heightened aggression in mice (Faccidomo et al., 2008). In contrast, a reduced extracellular 5-HT level in the mPFC was observed during and after species-typical aggressive behaviors in rats (van Erp and Miczek, 2000).
The PFC contains several types of 5-HT receptors with a particular abundance of the 5-HT1A and 5-HT2A subtypes. Physiological concentrations of 5-HT can either activate or inhibit pyramidal cells because of its action on those subtypes; 5-HT1A receptors inhibit and 5-HT2A receptors excite the firing rates of pyramidal neurons in the mPFC (Araneda and Andrade, 1991; Ashby et al., 1994; Aghajanian and Marek, 1997; Puig et al., 2005; Amargós-Bosch et al., 2004). Electric stimulation of the DRN inhibited most of the pyramidal neurons (66%) but also activated some neurons (13%) in the mPFC (Puig et al., 2005). The higher release of 5-HT in the mPFC increased the magnitude of the inhibition and turned some excitation into inhibition (Puig et al., 2005; Gartside et al., 2000). A certain amount of 5-HT can excite pyramidal neurons, but, in contrast, an excessive release of 5-HT inhibits the mPFC activity. In this study, intra-DRN baclofen microinjection nearly doubled 5-HT release in the mPFC. It is possible that the abundant 5-HT release by baclofen caused the inhibition of mPFC activity and consequently induced escalated aggressive behaviors.
DRN, MRN, and escalated aggression in mice
Activation of GABA receptors in the MRN did not escalate aggressive behaviors. Thus, 5-HT projections from the DRN and the MRN have different effects on aggressive behaviors. Both the DRN and MRN constitute the primary sources for 5-HT in forebrain structures, but these two nuclei also have differential projections areas in rats (Azmitia and Segal, 1978). The MRN provides major 5-HT projections into medial septal nucleus and dorsal hippocampus, whereas the DRN projects to the dorsal striatum, ventral hippocampus, amygdala, nucleus accumbens, and cerebral cortex (Mokler et al., 2009). Lesions of the DRN, but not MRN, were sufficient to escalate some types of aggressive and defensive behaviors in rats (Jacobs and Cohen, 1976; Waldbillig, 1979). Conversely, involvement of the MRN in aggressive behaviors was also suggested in the cat (Koprowska and Romaniuk, 1997) and female rats (De Almeida and Lucion, 1997). It is now recognized that the role of 5-HT on aggressive behaviors may vary depending on brain regions and also types of aggressive behaviors (Mos et al., 1993; Faccidomo et al., 2008; Bannai et al., 2007; De Almeida and Lucion, 1997; Ferris et al., 1999; Cologer-Clifford et al., 1997). Our results indicate that the increased 5-HT release in the brain areas that receive the ascending projections from the DRN can promote the escalation of aggressive behavior.
In conclusion, our data point to GABAB receptors in the DRN as a new neurobiological target in the mechanisms mediating escalated aggression. The disinhibition of 5-HT projections from the DRN that are indirectly modulated by presynaptic GABAB receptors are involved in escalated aggression in male mice.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA13983 and a Tufts University Center for Neuroscience Research grant. We thank Andrea T. Henry and Jisoo Kim for their great help.
References
- Abellán MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphé serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellán MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphé nuclei. Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphé nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphé nucleus of mice. Psychopharmacology. 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF. Immunohistochemical evidence for the presence of γ-aminobutyric acid and serotonin in one nerve cell. A study on the raphé nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Scatton B, Claustre Y, Rouquier L. Effect of local injection of 8-OH-DPAT into the dorsal or median raphé nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett. 1992;137:101–104. doi: 10.1016/0304-3940(92)90308-t. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Amargós-Bosch M, Toth M, Artigas F, Adell A. In vivo efflux of serotonin in the dorsal raphé nucleus of 5-HT1A receptor knockout mice. J Neurochem. 2004;88:1373–1379. doi: 10.1046/j.1471-4159.2003.02267.x. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acidB receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, de Vries H, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189:263–272. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry. 1997;154:1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- Colmers WF, Williams JT. Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphé nucleus in vitro. Neurosci Lett. 1988;93:300–306. doi: 10.1016/0304-3940(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Cologer-Clifford A, Simon NG, Lu SF, Smoluk SA. Serotonin agonist-induced decreases in intermale aggression are dependent on brain region and receptor subtype. Pharmacol Biochem Behav. 1997;58:425–430. doi: 10.1016/s0091-3057(97)00295-5. [DOI] [PubMed] [Google Scholar]
- Cornelisse LN, Van der Harst JE, Lodder JC, Baarendse PJ, Timmerman AJ, Mansvelder HD, Spruijt BM, Brussaard AB. Reduced 5-HT1A and GABAB receptor function in dorsal raphé neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol. 2007;98:196–204. doi: 10.1152/jn.00109.2007. [DOI] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Stoffel M, Scott-McKean JJ. G-protein-gated potassium (GIRK) channels containing the GIRK2 subunit are control hubs for pharmacologically induced hypothermic responses. J Neurosci. 2005;25:7801–7804. doi: 10.1523/JNEUROSCI.1699-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. Don't worry “B” happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. 8-OH-DPAT in the median raphé, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology. 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek KA. Escalated aggression after alcohol drinking in male mice: dorsal raphé and prefrontal cortex serotonin and 5-HT1B receptors. Neuropsychopharmacology. 2008;33:2888–2899. doi: 10.1038/npp.2008.7. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus) Behav Neurosci. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphé cells. II. Reversal by picrotoxin. Eur J Pharmacol. 1976;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Hajós-Korcsok E, Bagdy E, Hársing LG, Jr, Sharp T, Hajós M. Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience. 2000;98:295–300. doi: 10.1016/s0306-4522(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphé serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–295. [Google Scholar]
- Howe JR, Sutor B, Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Innis RB, Nestler EJ, Aghajanian GK. Evidence for G protein mediation of serotonin- and GABAB-induced hyperpolarization of rat dorsal raphé neurons. Brain Res. 1988;459:27–36. doi: 10.1016/0006-8993(88)90282-x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Cohen A. Differential behavioral effects of lesions of the median or dorsal raphé nuclei in rats: open field and pain-elicited aggression. J Comp Physiol Psychol. 1976;90:102–108. doi: 10.1037/h0077262. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Ingram CD, Gartside SE. GABA receptor modulation of 5-HT neuronal firing: characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem Int. 2004;45:1057–1065. doi: 10.1016/j.neuint.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kamatchi GL, Ticku MK. Functional coupling of presynaptic GABAB receptors with voltage-gated Ca2+ channel: regulation by protein kinases A and C in cultured spinal cord neurons. Mol Pharmacol. 1990;38:342–347. [PubMed] [Google Scholar]
- Kia HK, Brisorgueil MJ, Hamon M, Calas A, Vergé D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kim C, Jeon D, Kim YH, Lee CJ, Kim H, Shin HS. Deletion of N-type Ca2+ channel Cav2.2 results in hyperaggressive behaviors in mice. J Biol Chem. 2009;284:2738–2745. doi: 10.1074/jbc.M807179200. [DOI] [PubMed] [Google Scholar]
- Koprowska M, Romaniuk A. Behavioral and biochemical alterations in median and dorsal raphé nuclei lesioned cats. Pharmacol Biochem Behav. 1997;56:529–540. doi: 10.1016/s0091-3057(96)00297-3. [DOI] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Lüscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Li S, Varga V, Sik A, Kocsis B. GABAergic control of the ascending input from the median raphé nucleus to the limbic system. J Neurophysiol. 2005;94:2561–2574. doi: 10.1152/jn.00379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphé nucleus: from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O'Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and l-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, De Almeida RM, Bannai M, Fish EW, Debold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel MC, Doucet E, Riad M, Adrien J, Vergé D, Hamon M. Effect of the selective lesion of serotoninergic neurons on the regional distribution of 5-HT1A receptor mRNA in the rat brain. Brain Res Mol Brain Res. 1992;14:357–362. doi: 10.1016/0169-328x(92)90104-j. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Dugal JR, Hoffman JM, Morgane PJ. Functional interrelations between nucleus raphé dorsalis and nucleus raphé medianus: a dual probe microdialysis study of glutamate-stimulated serotonin release. Brain Res Bull. 2009;78:132–138. doi: 10.1016/j.brainresbull.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H. The effects of dorsal raphé administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol. 1993;238:411–415. doi: 10.1016/0014-2999(93)90877-k. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakagawasai O, Yanai K, Nunoki K, Tan-No K, Tadano T, Iijima T. Modified behavioral characteristics following ablation of the voltage-dependent calcium channel beta3 subunit. Brain Res. 2007;1160:102–112. doi: 10.1016/j.brainres.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphé dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982;232:375–389. doi: 10.1016/0006-8993(82)90281-5. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy; 1996. [Google Scholar]
- Nishikawa T, Scatton B. Inhibitory influence of GABA on central serotonergic transmission. Raphé nuclei as the neuroanatomical site of the GABAergic inhibition of cerebral serotonergic neurons. Brain Res. 1985;331:91–103. doi: 10.1016/0006-8993(85)90718-8. [DOI] [PubMed] [Google Scholar]
- Olivier B. Serotonin and aggression. Ann N Y Acad Sci. 2004;1036:382–392. doi: 10.1196/annals.1330.022. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization. Health in the Americas: 2007. Vol II. Washington, DC: Pan American Health Organization; 2007. [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 2001. [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphé stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Serrats J, Artigas F, Mengod G, Cortés R. GABAB receptor mRNA in the raphé nuclei: co-expression with serotonin transporter and glutamic acid decarboxylase. J Neurochem. 2003;84:743–752. doi: 10.1046/j.1471-4159.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-l. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Ther. 2005;312:290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphé neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphé nuclei and forebrain of rats. Br J Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann DB, Westenbroek RE, Schousboe A, Catterall WA. Distribution of high-voltage-activated calcium channels in cultured γ-aminobutyric acidergic neurons from mouse cerebral cortex. J Neurosci Res. 2002;67:48–61. doi: 10.1002/jnr.10074. [DOI] [PubMed] [Google Scholar]
- van der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martínez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Depaulis A, Boehrer A. Parachlorophenylalanine-induced serotonin depletion increases offensive but not defensive aggression in male rats. Physiol Behav. 1986;36:653–658. doi: 10.1016/0031-9384(86)90349-5. [DOI] [PubMed] [Google Scholar]
- Waldbillig RJ. The role of the dorsal and median raphé in the inhibition of muricide. Brain Res. 1979;160:341–346. doi: 10.1016/0006-8993(79)90429-3. [DOI] [PubMed] [Google Scholar]
- Walletschek H, Raab A. Spontaneous activity of dorsal raphé neurons during defensive and offensive encounters in the tree-shrew. Physiol Behav. 1982;28:697–705. doi: 10.1016/0031-9384(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphé nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- White JH, McIllhinney RA, Wise A, Ciruela F, Chan WY, Emson PC, Billinton A, Marshall FH. The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proc Natl Acad Sci U S A. 2000;97:13967–13972. doi: 10.1073/pnas.240452197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, Pepin JL, Watkins LR, Maier SF. Electrolytic lesions and pharmacological inhibition of the dorsal raphé nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology. 2004;171:191–198. doi: 10.1007/s00213-003-1572-1. [DOI] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphé neurons in vitro . J Neurosci. 1988;8:3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]