Abstract
Bone morphogenic proteins (BMPs) are growth factors important for skeletal development and bone growth. Noggin, one of the soluble BMP antagonists, regulates the action of BMPs on mesenchymal precursor cells, partially through a feedback type of inhibition. In this study, we constructed a novel BMP2/7 ‘fusion gene’ that encodes both BMP2 and BMP7 genes in tandem by a linker. Polymerase chain reaction (PCR) and Western blotting showed that the BMP2/7 fusion gene construct led to the production of BMP2/7 heterodimers in A549 ‘producer’ cells. When applied to C2C12 myoblastic cells, BMP2/7 heterodimers increased alkaline phosphatase (ALP) activity and osteocalcin (OCN) expression (markers of osteoblastic differentiation) more effectively than either BMP2 or BMP7 homodimers. Moreover, this heterodimer induced significantly lower levels of Noggin expression in C2C12 cells than respective homodimers at similar doses. The addition of Noggin did not affect the heterodimer’s activities in increasing osteoblastic differentiation in C2C12 cells. In contrast, BMP2 and BMP7 homodimers were largely inhibited by Noggin. Our finding suggests that the ‘fusion gene’ construct led to the production of bioactive BMP2/7 heterodimers, which were not antagonized by Noggin as effectively as it to BMP homodimers. The weaker Noggin antagonism on BMP heterodimers compared to homodimers may contribute to increased osteogenic potency of heterodimers in vitro and in vivo.
Keywords: Bone morphogenetic protein, Fusion gene, Homodimer and heterodimer, Noggin, Osteoblastic differentiation
Introduction
Bone morphogenic proteins (BMPs), originally identified for their ability to induce de novo ectopic bone formation, are a group of proteins critically important for embryonic skeletal development and post-natal bone healing [1-16]. While a number of soluble antagonists regulate BMP activity in vivo [4,17-21], one of these antagonists, Noggin, is particularly important in regulating BMP-mediated differentiation of mesenchymal precursor cell differentiation [18,22,23]. Secreted as a glycoprotein containing a similar cystine knot structure to that of BMP family, Noggin binds to BMPs. The Noggin/BMP interaction prevents BMPs from binding to their cell surface receptors, thus disabling the initiation of BMP signal transduction in target cells [22-25]. Noggin secretion in mesenchymal cells is stimulated by BMPs, but declines once cells undergo differentiation [18,22,26,27]. This suggests that Noggin may regulate BMP activity in undifferentiated cells, at least in part, through a negative feedback type of mechanism to control the rate of cell differentiation. Moreover, addition of Noggin completely diminishes BMP-induced osteoblastic differentiation in mesenchymal cells, further supporting Noggin’s antagonizing effect on BMP activities [17,18,21,22,26,27].
Although recombinant BMP (rBMP) homodimers have been used in experimental models and clinical trials to enhance bone formation, the effective doses are extremely high [1,2,6,7,9,10,28-34]. High doses are not only costly; they may be associated with potential drawbacks such as the stimulation of bone resorption, excessive bone formation or nerve cell reactions in unintended areas [35-40]. One approach to address these potential issues in current BMP therapy is to apply a more potent form of BMP, which may be effective at lower doses in inducing osteogenesis. Previous studies including ours have found that BMP heterodimers, resulting from co-expression of two different BMP genes in vitro, are more potent than relative homodimers in inducing osteoblastic differentiation in mesenchymal precursor cells [41-44]. Recently, we have shown that combined BMP2 and BMP7 gene transfer enhances allograft spine fusion in rats more effectively than either single BMP gene transfer [44].
The mechanisms that underlie the increased potency of BMP heterodimers are largely unknown. In this study, we examined whether it is related to differences in Noggin antagonism of heterodimer versus homodimer. By utilizing a fusion gene strategy [45], we synthesized a novel gene construct containing BMP2 and BMP7 cDNAs in tandem, but separated by a linker for the generation of a single BMP2/7 heterodimer transcript. Here we report that the BMP2/7 fusion gene construct leads to the production of bioactive BMP2/7 heterodimers and that the activities of this heterodimer are not antagonized by Noggin as effectively as those of BMP2 or BMP7 homodimers in inducing osteoblastic differentiation.
Material and methods
BMP2/7 fusion gene construction
To construct the “BMP2/7 fusion gene” fragment, serial polymerase chain reactions (PCR) were performed. To amplify BMP2 cDNA without the stop codon and BMP7 cDNA without the signal peptide, two pairs of PCR primers were designed. One pair was composed of a 5′BMP2 primer (5′-atggtgg ccgggacccg ctgtctt-3′) and a 3′BMP2 primer tagged with a (Gly4Ser)4 linker (3′BMP2 + linker, 5′-gttgtggagggttgtgggtgtcgc + ggtggtggaggaagtggaggtggaggtagtggaggaggtggtagtggtggaggtggaagt-3′). The other pair includes 5′BMP7 primer preceded by the linker (linker + 5′BMP7, 5′-ggtggtggaggaagtggaggtggaggtagtggaggaggtggtagtggtggaggtggaagt + gacttcagcctggacaacgaggtg-3′), and 3′BMP7 primer (5′-gtccgggcctgtggctgccactag-3′).
Amplification generated one fragment consisting of BMP2 (minus stop codon) and linker, and also another fragment containing linker followed by BMP7 (minus the signal peptide). These BMP2 and BMP7 cDNAs were then fused in tandem at the linker by PCR reactions using 5′BMP2 primer and 3′ BMP7 primer. This BMP2/7 fusion gene fragment was cloned into an expression vector (pShuttleCMV, Stratagene) under a cytomegalovirus (CMV) promoter and the recombinant plasmid is designated pSCMV-BMP2/7.
Transient expression of BMP2/7 heterodimers
A549 cells (American Type Culture Collection) were used as the “producer” cell line, as described in our previous study [44]. Cells were maintained in “complete media” (DMEM with 10% FBS and 1% penicillin–streptomycin; all from Gibco). Approximately 80% confluent wells of A549 cells were transfected by pSCMV-BMP2/7 (Polyfect, Qiagen). As controls, cells were transfected with a control plasmid encoding green fluorescent protein (pCMV-GFP, a gift from Bishnu Dee, Ph.D., Weill Medical College of Cornell University), or no DNA (mock-transfection, medium only). Supernatants and cells were collected 2 days after transfection for measurement of BMP levels and in vitro bioactivity assays.
To detect the expression of BMP2/7 fusion gene, total RNA was extracted from transfected cells by using Trizol Reagent (Sigma), reverse transcribed (RT, Applied Biosystems), and then tested for the 2.6-kb fragment, which is the expected size for BMP2/7 fusion gene, by PCR using 5′BMP2 and 3′BMP7 primers. As controls, total RNA of samples were amplified in RT-PCR by using 5′BMP2 and 3′BMP7 without the reverse transcriptase in the RT reaction. As additional controls, 5′BMP2 and 3′BMP2 primer including the stop codon; 5′ BMP7 including signal peptide and 3′BMP7 primer were also used to amplify the total RNA of samples to examine whether the transfection with fusion gene will lead to the expression of BMP2 (1.2 kb) or BMP7 (1.4 kb) cDNA alone.
To detect the expression of BMP2/7 heterodimer protein, Western blotting was performed (Nupage Bis-Tris gel systems, Invitrogen) under both reducing and non-reducing conditions [44]. The supernatants from transfected cells were precipitated with 10% (v/v) of trichloroacetic acid solution (TCA, Sigma) and deglycosylated by N-Glycanase (10 mg glycoprotein per unit of N-Glycanase, Prozyem) at 37°C for overnight. The BMP2/7 heterodimer protein in transfected cells were detected with mouse anti-human BMP2 or BMP7 primary antibodies followed by Horseradish peroxidase conjugated goat anti mouse IgG (all from R&D Systems).
To confirm that only BMP2/7 heterodimers were synthesized, supernatants from transfected cells were immunoprecipitated with anti-BMP7 antibody, then detected by anti-BMP2 antibody by Western blot, or vice versa [44]. Briefly, anti-BMP2 or anti-BMP7 antibodies were pre-coated on a spin column containing immobilized protein G (Pierce), and the fraction of samples containing BMP2 or BMP7 antigens entrapped by respective antibodies as “immunoprecipitates”. The unbound portion would flow through the column and served as negative controls in Western Blotting experiments (see above).
Amounts of BMPs in A549 supernatants were quantified by using a commercially available enzyme linked immunosorbent assay (ELISA) kit for BMP2 (R&D Systems) or direct ELISA for BMP7 [44]. To quantify the amount of BMP2/7 heterodimers, supernatants were immunoprecipitated with antibody against one BMP and the amount of the other BMP measured by ELISA [44]. The amount of heterodimer in cell supernatants was estimated to be equivalent to the amount of BMP7 detectable in the anti-BMP2 antibody immunoprecipitates or the amount of BMP2 detectable in the anti-BMP7 antibody immunoprecipitates [44].
Determination of BMP2/7 bioactivity in vitro
Time course and dose–response studies were performed to compare the potencies of BMP2/7 heterodimers versus BMP2 or BMP7 homodimers for inducing the osteoblastic differentiation of C2C12 mouse myoblastic cell line (American Type Culture Collection). Cells were seeded at a density of 2 × 105 cells/well in 6-well cell culture plates, and were maintained in “low growth media” consisting of DMEM with 2% FBS and 1% penicillin–streptomycin [44].
For dose–response studies, pre-confluent cells were stimulated with supernatants of pSCMV-BMP2/7-stimulated A549 cells diluted with “low growth medium” at ratios from 1:80 to 1:1 for 5 days. These dilutions contained approximately between 125 pg/ml and 5 ng/ml of BMP2/7. For comparison, cells were also stimulated with recombinant (r) BMP2 or rBMP7 (all from R&D Systems) at doses ranging from 0 to 1000 ng/ml. As additional controls, cells were stimulated with supernatants of A549 cells transfected with pCMV-GFP or medium only without stimulation.
For the time course studies, C2C12 cells were stimulated with supernatants of A549 cells containing approximately 5 ng/ml of BMP2/7 heterodimers and assayed on days 1, 4, 7, and 12. For comparison, cells were also stimulated with rBMP2 or rBMP7 at 200 ng/ml or A549 supernatants transfected from pCMV-GFP or medium only.
Osteocalcin (OCN) expression, detected in cell medium by ELISA (Biomedical Technologies), and alkaline phosphatase (ALP) activity in cell lysates, determined by colorimetric assay (Sigma), were used as markers of osteoblastic differentiation as described in our previous studies [44]. ALP activity was normalized to total cellular protein as detected by bicinchoninic acid (BCA) protein assay (Pierce).
Determination of BMP-induced Noggin gene expression
C2C12 cells were stimulated with A549 supernatants containing approximately 5 ng/ml of BMP2/7 heterodimer. For comparison, cells were also stimulated with rBMP2 or rBMP7 at 1, 5, 50 and 100 ng/ml. At 6 and 24 h after stimulation, BMP-induced Noggin expression was then detected by quantitative Real Time PCR, which was performed on a My iQ Single-color Real Time PCR detection system (BioRad). Primers were designed using the Primer Express software version 2.0.0 (Applied Biosystems) using published mouse Noggin sequences [22]. The forward primer sequences are 5′-cgagcgagatcaaagggct-3′; and the reverse primer sequences are 5′-tcctcctcagcttcttgctca-3′. Relative Noggin expression levels were calculated based on ΔCT numbers, which is the difference in number of threshold cycle between the Noggin gene and the house keeping gene glyceraldehyde phosphate dehydrogenase (GAPDH).
Determination of Noggin antagonism on BMPs in vitro
BMP2/7 heterodimer-containing supernatants, which were generated from A549 cells transfected with pSCMV-BMP2/7, were diluted in DMEM medium containing 2% FBS and 1% penicillin–streptomycin (low growth medium), to reach a final concentration of 5 ng/ml. Heterodimers were then co-incubated with Noggin protein (PeproTech) in ratios of 1:1, 1:10 and 1:40 (for ALP staining only) in low growth medium for 1 h at room temperature, as indicated in previous studies [24,46]. For comparison, 200 ng/ml of rBMP2 or rBMP7 homodimer proteins (R&D Systems) were co-incubated with Noggin protein at the same ratios in low growth medium for 1 h at room temperature. C2C12 cells were then stimulated with low growth culture medium containing premixed BMP heterodimer or homodimer with Noggin. As controls, cells were stimulated with low growth medium containing BMP2/7 alone at 5 ng/ml or rBMP2, rBMP7 alone at 200 ng/ml. As additional controls, cells were incubated in low growth medium containing Noggin protein only at 50, 200 and 2000 ng/ml or without stimulation (medium only).
Four and 7 days after stimulation, cell supernatants were assayed for the level of OCN expression [44]. At 5 days after stimulation, cells were also assessed for ALP activity (Alkaline Phosphatase Histochemical Staining kit, Sigma). After staining for ALP, cells were imaged by inverted light microscopy (Olympus LH50A) and a Nikon E4500 digital camera (Nikon). For each culture well, 4–6 fields were acquired at 20× magnification. The proportion of ALP positively stained area within the total cellular area in each field was quantified based on the color intensity by an observer blinded to the treatment groups, using a Bioquant Nova 2000 software (R&M Biometrics).
Statistical analysis
Results for OCN expression and ALP activity (n = 6 per group in duplicate measurements), histochemical staining grading (n = 3–4 per group in duplicate measurements) were expressed as a mean ± SE. Statistical analysis for Noggin gene expression (n = 3–4 per group in duplicate measurements) was based on the calculation for ‘relative expression’ by the ΔCT numbers (difference between cycle numbers for Noggin and GAPDH). Groups were compared by ANOVA with post hoc testing where differences were detected. Significance was accepted where P < 0.05.
Results
Expression of BMP2/7 fusion gene and heterodimer protein
Two days after transfection, a single gene product of 2.6 kb, which is the expected size for the BMP2/7 ‘fusion gene’, was detected by RT-PCR in cells transfected with pSCMV-BMP2/7, but not in cells transfected with pCMV-GFP or medium only control (Fig. 1A). Furthermore, no BMP2 cDNA at expected size of 1.2 kb and BMP7 cDNA at expected size of 1.4 kb were detected in pSCMV-BMP2/7 transfected cells by RT-PCR reactions, indicating that pSCMV-BMP2/7 transfection only resulted in the production of BMP2/7 fusion gene but not BMP2 or BMP7 transcripts alone (data not shown). Moreover, there was no BMP2/7 fusion gene amplified from RNA of cells transfected with pSCMV-BMP2/7 without the addition of reverse transcriptase, which excluded the possibility that the amplified BMP2/7 fusion gene product in these cells was contributed directly by plasmid DNA of pSCMV-BMP2/7 (data not shown).
Fig. 1.
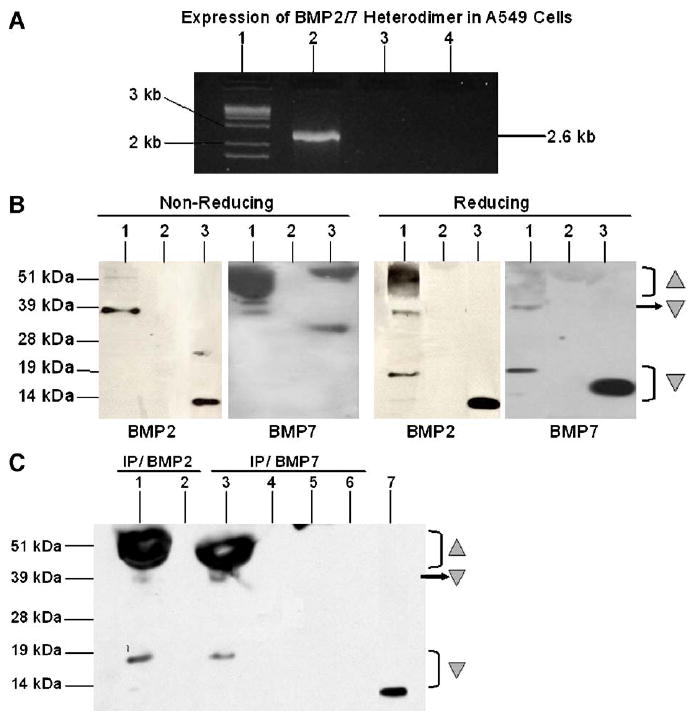
(A) Expression of BMP2/7 fusion gene in A549 cells. A549 epithelial cells were transfected with pSCMV-BMP2/7, or as controls, pCMV-GFP or medium only without plasmid. The expression of the BMP2/7 fusion gene was detected by RT-PCR using 5′BMP2 primer and 3′BMP7 primer. Lane 1, DNA Ladder; Lane 2, A549 transfected with pSCMV-BMP2/7; Lane 3, A549 transfected with pCMV-GFP; Lane 4, A549 transfected with medium only. (B and C) Expression of BMP2/7 heterodimer protein in A549 cells. A549 epithelial cells were transfected with pSCMV-BMP2/7, or with medium only without plasmid. The expression of BMP2/7 fusion gene protein was detected byWestern blot using anti-BMP2 antibody or anti-BMP7 antibody under non-reducing (left panel, B) and reducing conditions (right panel, B). Lane 1, A549 transfected with pSCMV-BMP2/7; Lane 2, A549 transfected with medium only; Lane 3, recombinant (r) BMP2 or rBMP7 positive control. The presence of BMP2/7 heterodimer was further confirmed by immunoprecipitation (IP) with either anti-BMP2 or anti-BMP7 antibody, and followed by detection of BMP2 under reducing conditions (C). Lanes 1 and 2, A549 transfected with pSCMV-BMP2/7 IP with anti-BMP2 antibody and the ‘flow-through’ fraction (unbound fraction from the immunoprecipitation columns of the sample), respectively; Lanes 3 and 4, A549 transfected with pSCMV-BMP2/7 IP with anti-BMP7 antibody and the ‘flow-through’ fraction, respectively; Lanes 5 and 6, A549 transfected with medium only IP with anti-BMP7 antibody and the ‘flow-through’ fraction, respectively; Lane 7, rBMP2 positive control. Shaded ‘△’ pro-peptides; ‘▽’: mature peptides.
Western blot detected with either anti-BMP2 or anti-BMP7 antibody indicated that the majority of the mature BMP proteins in the supernatants, from cells transfected with pSCMV-BMP2/7, migrated as an immunoreactive band at approximately 39 kDa under non-reducing conditions, which is the expected size for the mature BMP2/7 proteins (Fig. 1B, left panel). Under reducing conditions, with the addition of β-mecaptoethanol, a major portion of these mature peptides further migrated as smaller products with molecular mass between approximately 13 to 18 kDa detected by anti-BMP2 antibody or 15 to 19 kDa detected by anti-BMP7 antibody (Fig. 1B, right panel), indicating that these mature peptides were disulfide-linked proteins. The generation of a broad range of small peptides (Fig. 1B, right panel), which migrated slightly slower than the rBMP2 positive control (~13 kDa) or than the rBMP7 positive control (~15 kDa, all from R&D Systems), might be due to the N-terminal heterogeneity during the multiple-site proteolytic cleavage of pro-peptides, as indicated in previous studies [41,47], which possibly resulted in multiple cleavages of the BMP2 and BMP7 pro-peptides. Also possibly due to that, the processed mature form of BMP2/7 was detected at approximately 39 kDa, which is bigger than the mathematical size of the heterodimer that is calculated to be between the size of BMP2 and BMP7 homodimers.
Moreover, similar pattern of mature BMP peptides migrating at approximately 39 kDa and similar size of small peptides migrating at approximately 13 to 18 kDa were detected by BMP2 Western blotting in supernatants from cells transfected with pSCMV-BMP2/7, which had been immunoprecipitated with either anti-BMP7 antibody or anti-BMP2 antibody (Fig. 1C). This suggests that BMP2/7 heterodimers were synthesized in transfected cells. In contrast, no BMP2 signal was detected in medium only control or in unbound ‘flow-through’ proportion of these samples collected from the immunoprecipitation columns, further confirming that there were no BMP2 or BMP7 homodimers, but only BMP2/7 heterodimer proteins, produced in transfected cells (Fig. 1C). In agreement with previous studies which found that BMP2 and BMP7 were processed as pro-peptides during protein synthesis [41,47-49], a broad band running between 45 kDa to 60 kDa was also detected in cells transfected with pSCMV-BMP2/7 (Fig. 1C), indicating the presence of pro-forms of BMP2/7 proteins that might contain pro-domains of BMP2 and BMP7 and the linker.
After pSCMV-BMP2/7 transfection, similar amounts of total BMP2 (10 ± 1.2 ng/ml) and BMP7 (12 ± 0.2 ng/ml) were detected in A549 supernatants (P > 0.05), while no BMP secretion was found in cells transfected with control plasmid pCMV-GFP or with medium (Fig. 2A). Moreover, after immunoprecipitation by using anti-BMP2 antibody, BMP7 was only detected by ELISA in immunoprecipitates from pSCMV-BMP2/7 transfected cells, but not from controls (Fig. 2B). The fact that BMP2 antigen was also immunoadsorbed by BMP7 antibodies suggested the co-presence of BMP2 and BMP7 in a heterodimeric complex. Similarly, when supernatants were immunoprecipitated with anti-BMP7, ELISA for BMP2 was positive in pSCMV-BMP2/7-transfected, but not in control cells (data not shown).
Fig. 2.
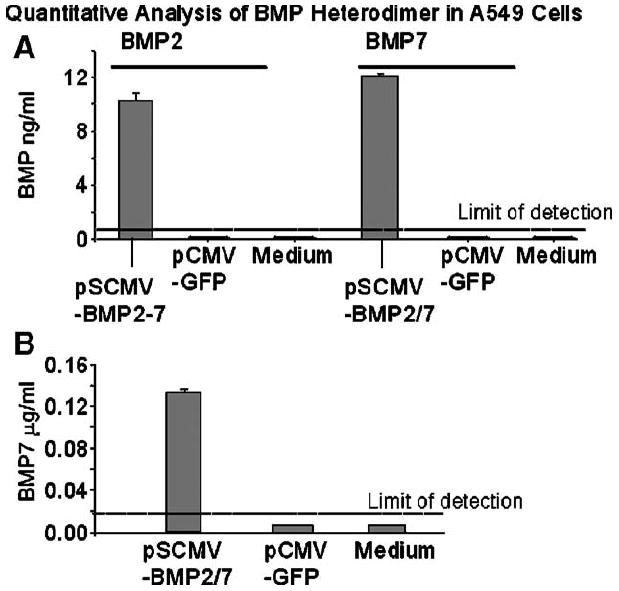
(A and B) Quantitative analysis of total BMP2 or BMP7 in A549 cells. The amount of total BMP2 or BMP7 in cell supernatants was quantified by ELISA (A) before and (B) after immunoprecipitation with anti-BMP2 antibody. Results are expressed as mean ± SE for six culture wells in each treatment group.
BMP2/7 heterodimer increased osteoblastic differentiation in C2C12 myoblastic cells
After 5 days of stimulation, both BMP2/7 heterodimer-containing supernatants and rBMP2 or rBMP7 homodimers increased OCN expression in C2C12 cells in a dose-dependent manner, with a threshold dose for heterodimer activity at approximately 1 ng/ml (1:10 dilution, Fig. 3A) and for homodimer activity at 100 ng/ml (Fig. 3B). The level of OCN expression (5 ± 0.2 ng/ml) induced by a 1:5 dilution (~2 ng/ml) of heterodimer-containing supernatant was comparable to levels induced by rBMP2 or rBMP7 at 200 ng/ml and 300 ng/ml (P > 0.05, Figs. 3A and B) respectively, which also represented half-maximal activity of BMP homodimers in C2C12 cells (Fig. 3B). Moreover, the level of OCN expression induced by a 1:1 dilution (~5 ng/ml) of heterodimer-containing supernatant was 1.5-fold greater than that induced by rBMP2 or rBMP7 at 200 ng/ml (P < 0.05, Figs. 3A and B). There was no detectable OCN expression in C2C12 cells stimulated with pCMV-GFP transfected supernatant or medium only (Figs. 3A and B).
Fig. 3.
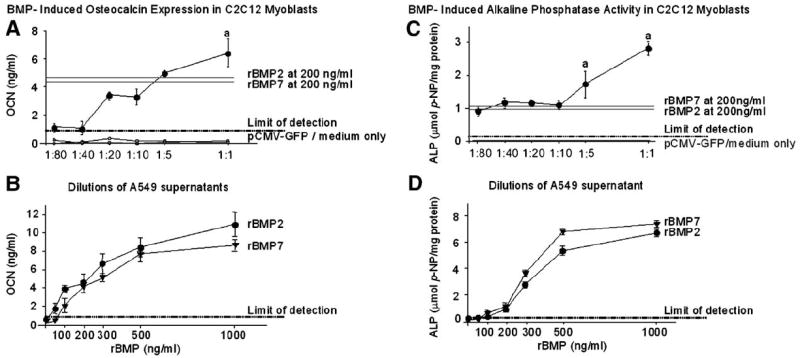
Dose–response expression of osteocalcin (OCN) and alkaline phosphatase (ALP) induced in BMP-stimulated C2C12 myoblasts. Supernatants of A549 transfected with pSCMV-BMP2/7 diluted from 1:80 to 1:1 (approximately between 125 pg/ml and 5 ng/ml BMP2/7) were used to stimulate C2C12 cells. For comparison, C2C12 cells were stimulated with recombinant (r) BMP2 or rBMP7 ranging from 0 to 1000 ng/ml. Five days after stimulation, OCN expression (A and B) and ALP activity that normalized by total protein (C and D) were measured as markers of osteoblastic differentiation. Each data point represents the mean ± SE for six culture wells at each dose in each treatment group. aP < 0.05 vs. rBMP2 or rBMP7 at 100 ng/ml (threshold dose) and 200 ng/ml (dose for approximately half-maximal activity).
A similar dose-dependent pattern of ALP activity was also detected in C2C12 cells with a threshold dose for heterodimer at approximately 1 ng/ml (1:10 dilution, Fig. 3C) and for homodimers (rBMP2 and rBMP7) at 100 ng/ml (Fig. 3D). The ALP activity (1.6 ± 0.4 μmol/mg) induced by a 1:10 dilution (~1 ng/ml) of heterodimer-containing supernatant was at comparable levels to that induced by rBMP2 or rBMP7 at 200 ng/ml (Figs. 3C and D). Moreover, the ALP activity (1.6 ± 0.4 μmol/mg) induced by a 1:5 dilution (~2 ng/ml) and ALP activity (2.7 ± 0.2 μmol/mg) induced by a 1:1 dilution (~5 ng/ml) of heterodimer-containing supernatants were 1.5-fold and 2.5-fold greater than that induced by rBMP7 and rBMP2 at 200 ng/ml, respectively (P < 0.05, Figs. 3C and D). As expected, there was no detectable ALP activity in control groups (Figs. 3C and D).
During a 12-day time course, supernatant of pSCMV-BMP2/7 at 1:1 dilution, which contained approximately 5 ng/ml of BMP2/7, increased the ALP activity and OCN expression in C2C12 cells in a time-dependent manner. pSCMV-BMP2/7 stimulation induced comparable levels of ALP activity and OCN expression at each time point (days 1, 4, 7 and 12) as that induced by rBMP7 and rhBMP2 at 200 ng/ml, respectively (data not shown). As expected, there was no detectable ALP activity and OCN expression in cells stimulated with supernatants of pCMV-GFP or medium controls during the entire time course (data not shown).
BMP2/7 heterodimer induced a lower level of Noggin gene expression in C2C12 cells
Real time PCR data showed that Noggin gene expression induced by 5 ng/ml of rBMP2 or 5 ng/ml of rBMP7 was 50% higher (P < 0.05) than that in cells induced by medium only control at 6 h after stimulation (Fig. 4A). In contrast, BMP2/7 did not result in a significant increase in Noggin expression when compared to the medium control (P > 0.05, Fig. 4A) at this time point. After 24-h stimulation, 5 ng/ml of rBMP2 continued to induce a 50% increase of Noggin expression relative to medium control (P < 0.05) while either rBMP7 or BMP2/7 heterodimer did not induce significant increase in Noggin expression when compared to that in medium control (P > 0.05, Fig. 4B).
Fig. 4.
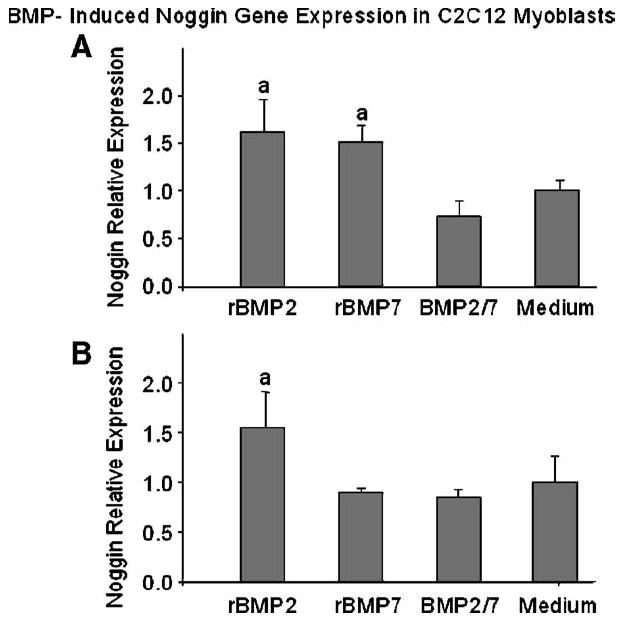
BMP-induced Noggin gene expression in C2C12 myoblasts (A) 6 h and (B) 24 h after stimulation. C2C12 cells were stimulated with supernatants of pSCMV-BMP2/7 transfected A549 cells containing approximately 5 ng/ml of BMP2/7 heterodimer. For comparison, cells were stimulated with 5 ng/ml of recombinant (r) BMP2, 5 ng/ml of rBMP7 or medium only without BMP stimulation. The Noggin expression was quantified by real time PCR and relative expression level was calculated by the ΔCT numbers, which were the difference in number of threshold cycle between the Noggin gene and the house keeping gene glyceraldehyde phosphate dehydrogenase (GAPDH). Each data point represents the mean ± SE of ΔCT numbers for 3 or 4 culture wells at each dose in each treatment group. aP < 0.05 vs. medium only control.
Our data also found a dose-dependent increase of Noggin gene expression induced by rBMP2 or rBMP7 at 6 and 24 h after stimulation (data not shown). In agreement with our data that BMP2/7 heterodimers at approximately 5 ng/ml were more potent than homodimers at 100 ng/ml or 200 ng/ml in increasing OCN expression (Figs. 3A and B) and ALP activity (Figs. 3C and D), we found that the level of Noggin gene expression induced by this dose of BMP2/7 heterodimer was actually 4- and 5-fold lower (P < 0.05) than that induced by rBMP2 or rBMP7 at 100 ng/ml, respectively, at both time points (data not shown). This indicated that in terms of supporting osteoblastic differentiation, BMP2/7 significantly induced lower levels of Noggin antagonist expression than that by either homodimers.
Weaker Noggin antagonizing effects on BM2/7 heterodimer activity
The OCN expression (4.2 ± 0.9 ng/ml) in C2C12 cells after 4 days of stimulation by BMP2/7 at 5 ng/ml was not affected by pre-mixing with Noggin at either a 1: 1 or 1:10 ratio (P > 0.05 for all comparisons, Fig. 5). Similarly, at 7 days, amounts of OCN expression (4.8 ± 0.7 ng/ml) in cells stimulated with BMP2/7 alone was also found at comparable level to that in cells stimulated with BMP2/7 and Noggin premixed at 1:1 and 1:10 ratios (P > 0.05, data not shown).
Fig. 5.
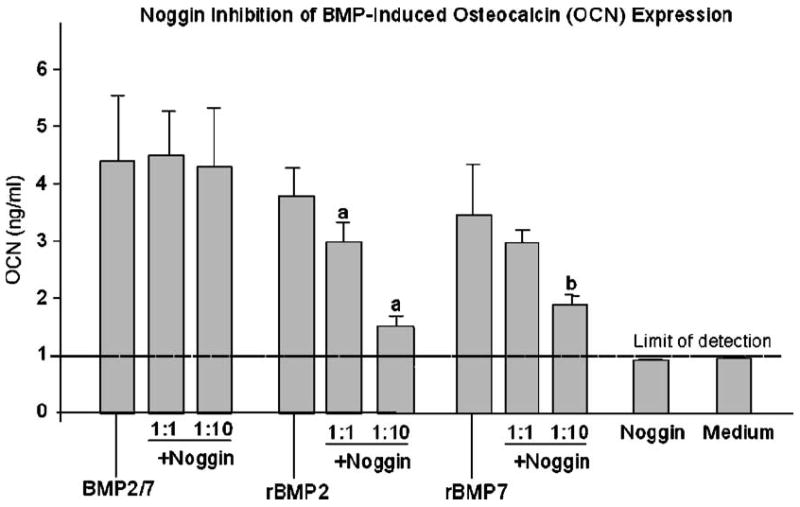
Noggin inhibition of BMP-induced osteocalcin (OCN) expression. BMP2/7 heterodimers or recombinant (r) BMP 2 or -7 homodimers were pre-incubated with Noggin at 1:1 and 1:10 ratios for 1 h at room temperature. C2C12 myoblasts were stimulated with Noggin and BMP premix or BMP alone for 4 days. For comparison, cells were stimulated with Noggin alone or medium only. As a measure of osteoblastic differentiation, OCN expression was measured. Each data point represents the mean ± SE for six culture wells at each dose in each treatment group. aP < 0.05 vs. rBMP2 alone; bP < 0.05 vs. rBMP7 alone.
In contrast, rBMP2/Noggin premix at the ratio of 1:1 and 1:10 reduced the level of OCN expression by 25% and 60%, respectively (P < 0.05, both comparisons), when compared to the OCN level induced by rBMP2 alone on day 4 (3.8 ± 0.6 ng/ml, Fig. 5). This reduction in OCN expression was also observed on day 7 with 50% and 30% decrease in cells by premixing with Noggin at 1:1 and 1:10, respectively, when compared to that in cells induced by rBMP2 alone (P < 0.05 for both comparisons, data not shown).
Similarly, rBMP7/Noggin premix at the ratio of 1:10 also reduced OCN expression in C2C12 cells by 40% (P < 0.05), when compared to the level induced by the rBMP7 alone (3.6 ± 0.8 ng/ml) on day 4 (Fig. 5). Similar results were also found at day 7, in which there was a 50% (P < 0.05) decrease in OCN expression detected in cells stimulated with the rBMP7/Noggin premix at a ratio of 1:10 compared to the level induced by rBMP7 alone (4.2 ± 0.8 ng/ml). Therefore, stimulation with the rBMP7/Noggin premix at the ratio of 1:1 for 4 or 7 days did not significantly reduced OCN expression (Fig. 5).
As expected, no significant OCN expression was detected in cells stimulated with Noggin alone at 50 ng/ml, 200 ng/ml or 2000 ng/ml for 4 or 7 days when compared to that in cells maintained in the medium only (Fig. 5).
Consistent with our OCN results, premix of BMP2/7 and Noggin at 1:1 and 1:10 ratios did not affect the BMP2/7-increased ALP activity in these cells when compared to the extent of ALP staining (Fig. 6A) in cells stimulated with BMP2/7 alone (24 ± 0.7% of cells ALP-positive, Fig. 6B). Premix of BMP2/7 and Noggin at 1:40 ratio (5 ng/ml of BMP2/7 and 200 ng/ml of Noggin) reduced the ALP activity by 75% when compared to that in cells stimulated with BMP2/7 alone (P < 0.05, Fig. 6B). In marked contrast, histochemical staining for ALP after stimulation with premixing of rBMP2 or rBMP7 with Noggin at 1:1 and 1:10 ratios reduced the extent of ALP staining in C2C12 cells to undetectable levels when compared to that in cells stimulated by rBMP2 or rBMP7 alone (P < 0.05, Figs. 6A and B). There was no detectable ALP staining was found in cells stimulated with Noggin alone at 50 ng/ml, 200 ng/ml and 2000 ng/ml, or in cells maintained in medium only (data not shown).
Fig. 6.
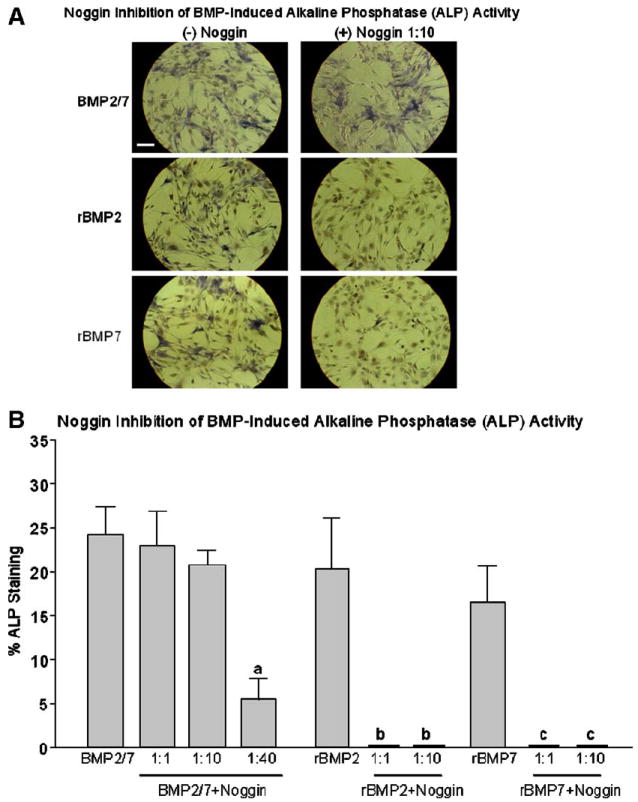
Noggin inhibition of BMP-induced alkaline phosphatase (ALP) activity in C2C12 cells. BMP2/7 heterodimers or recombinant (r) BMP 2 or -7 homodimers were pre-incubated with Noggin at 1:1 and 1:10 ratios for 1 h at room temperature. C2C12 myoblasts were stimulated with Noggin and BMP premix or BMP alone for 5 days. For comparison, cells were stimulated with Noggin alone or medium only without stimulation. As a marker of osteoblastic differentiation ALP activity was detected by histochemical staining (A) and the percentage of ALP positively area (B) were quantified by color intensity by using a BioQuant software. Each data point represents the mean ± SE for 3 to 4 culture wells. aP < 0.05 vs. BMP2/7 alone; bP < 0.05 vs. rBMP2 alone; cP < 0.05 vs. rBMP7 alone. Length of the bar equals to 100 μm.
Discussion
In this study, we have found that BMP 2/7 heterodimers generated from a BMP 2/7 ‘fusion gene’ construct are bioactive and are more potent than either BMP 2 or BMP 7 homodimers for increasing osteoblastic differentiation in C2C12 myoblastic cells. At similar doses, this heterodimer induced Noggin expression in C2C12 cells at a significantly lower level than that induced by their homodimers. Moreover, excessive Noggin did not antagonize heterodimer’s osteoinductive activity in these precursor cells as effectively as it to that of either homodimers.
Production of BMP heterodimers
In previous studies, BMP heterodimers were synthesized by transfection of a producer cell line with two different BMP genes [41-43]. Consistent with that, we previously used two adenovirus vectors (AdBMP2 and AdBMP7) to stimulate A549 cells and found that BMP 2/7 heterodimers synthesized by these producer cells were in approximately 80% of total amount of BMPs [44]. Apparently, by using this co-transfection approach, the possibilities of simultaneous production of homodimers could not be excluded.
The fusion gene strategy has previously been used to connect two subunits of a Calcineurin protein by a linker [45]. In that study, folding pathways and biological functions of Calcineurin heterodimers generated from the fusion gene construct were not affected and the authors reported that only heterodimer was produced [45]. Similarly, instead of using two BMP genes, we designed a BMP2/7 fusion gene to generate BMP 2/7 heterodimers in A549 producer cell line.
Crystal structure analysis studies of BMP2 and BMP7 homodimers suggest they could share a ‘head to tail’ folding configuration during dimerization [50,51]. The interactions that would be responsible for dimer formation were postulated to be between the alpha-helix domain of one monomer and the beta-sheet domain of the other monomer, resulting two separated beta-sheets from different monomers formed in an anti-parallel position and a four-turn alpha-helix approximately perpendicular to the strands [50 51]. Based on this information, we constructed a BMP2/7 ‘fusion gene’ with the stop codon of BMP2 gene and the signal peptide of BMP7 gene replaced by a linker. We anticipated that heterodimers could be produced by using this approach, as the fusion gene peptides may also fold at the linker to follow a similar “head to tail” configuration.
Our RT-PCR results showed that only a single transcript which encodes both BMP2 and BMP7 cDNA was expressed in cells transfected with the BMP2/7 fusion gene expression plasmid (pSCMV-BMP2/7), while individual BMP2 or BMP7 genes were not expressed. Moreover, the presence of BMP2/7 fusion protein was detected by a combination of immunoprecipitation and Western blotting, suggesting that only BMP2/7 heterodimers were produced in producer cells. The data also showed that the fusion gene protein contained a disulfide bond peptide, which dissociated under reducing conditions. Furthermore, the presence of BMP 2/7 heterodimers in fusion gene-transfected cells was also confirmed by immunoprecipitation and ELISA methods similar to that by which BMP heterodimers generated from transfection with two BMP genes was shown in previous studies [41,44].
However, while we have shown the protein expression of BMP2/7 heterodimers by “producer” cells, the post-translational processing of pro-peptides of the fusion gene protein is largely unknown. In addition, the folding pattern of the fusion gene protein that leads to the formation of mature heterodimers will need further investigation to determine whether it follows a “head to tail” configuration as we may expect. Thus, the future protein crystal structure of purified heterodimer proteins will need to be determined in order to analyze the folding topology of the fusion gene protein. Moreover, sequencing and peptide mass spectrometry will need to be performed on purified heterodimer proteins to precisely characterize physical compositions and chemical properties of the pro-form and mature form of fusion gene protein.
Osteogenic potency of BMP heterodimers
By testing a variety of BMP heterodimers produced by co-transfection of Chinese hamster ovary cells with two different BMP genes, Israel et al. [41] found that heterodimers consisting of one monomer of either BMP 2 or BMP 4 with another monomer of BMP 5, -6 or -7 were all more osteogenic than their relative homodimers in vitro [41]. Of all the heterodimers, BMP 2/7 appeared to be most potent and was shown to form more ectopic bone than BMP 2 in rats [41]. Similarly, the xenopus BMP 4/7 (xBMP 4/7) heterodimer has also been reported with a greater potency than either xBMP 4 or 7 homodimer for alkaline phosphatase (ALP) activity induction, and, in contrast to recombinant human (rh) BMP2 homodimers, up-regulated osteoblastic-specific transcriptional activator Runx2 (Runt-related transcription factor 2, also known as core binding factor or cbfa-1) in murine mesenchymal cells [42,43].
In this study, we consistently found that BMP2/7 heterodimer produced from a BMP 2/7 ‘fusion gene’ was more potent than rBMP2 or rBMP7 homodimers for increasing ALP activity and OCN expression in C2C12 myoblastic cells. Furthermore, when compared to the BMP2/7 heterodimer generated in our previous study by Ad-mediated BMP2 and BMP7 co-transfection in A549 cells [44], we found that BMP2/7 heterodimer generated from this fusion gene construct was equally potent for inducing osteoblastic differentiation in C2C12 cells.
Noggin antagonism on BMP activities
During early embryogenesis and postnatal bone development, BMP expression and activity are precisely regulated by a number of antagonists which includes Noggin, Gremlin, Dan family members such as Sclerostin, which leads to functional BMP gradients and overall bone homeostasis [4,17-23]. While all these antagonists may play a specific role in osteogenesis, previous studies suggest that Noggin may specifically inhibit BMP activity in mesenchymal precursor cells [17-23].
In long-term human and murine mesenchymal cell cultures, BMP stimulation induced Noggin expression, and addition of exogenous Noggin completely inhibited BMP-induced osteoblastic differentiation in mesenchymal precursor cells [17,18,21,22,26,27]. These studies suggest that Noggin regulates the BMP response of undifferentiated cells, at least in part, through a negative feedback mechanism that may serve to maintain cells in a less differentiated state or control the rate of maturation [17,18,21,22,26,27]. In our current study, we found that BMP2/7 stimulation resulted in a significantly lower level of Noggin expression than that by similar doses of BMP2 or BMP7 homodimers in C2C12 cells. Our results are in consensus with previous findings that heterodimer more effectively induced osteoblastic differentiation in these precursor cells than homodimer [41-44], because less Noggin production may contribute to a greater rate of differentiation at the site of BMP2/7 stimulation.
Alternatively, while a lower level of Noggin expression may be beneficial to osteogenesis at sites where bone formation are desired, the loss of balance in the regulation of BMP-antagonist feedback loop may have undesirable effects under some situations. Previous studies have detected a dramatically attenuated expression of Noggin and Gremlin in response to BMP4 stimulation in lymphoblastoid cells derived from fibrodysplasia ossificans progressiva (FOP) patients when compared to cells cultured from healthy donors [52]. Moreover, administration of an engineered Noggin mutein or viral delivery of Noggin gene prevented BMP4-induced heterotopic ossification in mice [53,54]. Although the genetic defects of FOP and the mechanisms underlying heterotopic ossification are largely unknown, this information underscores the importance of Noggin regulation on BMP activities.
While the co-expression of two different BMP genes have been detected in developing limb and during fracture healing, and co-purification of BMP2 and BMP7 proteins have also been reported in previous studies [6,55-58], the physiological occurrence of BMP heterodimers and their activities potentially regulated by BMP antagonists during skeletal development and bone healing are largely unknown. Whereas Noggin is an important regulator of BMP action in immature precursor cells such as C2C12 myoblastic cells, previous studies have suggested that Sclerostin, another soluble BMP antagonist, may be more significant in regulating BMP activities in mature bone cells. Sclerostin has been reported to be expressed specifically by osteocytes, where they regulated bone deposition activities of mature osteoblasts at vicinity sites [59-61]. Similarly, loss of Sclerostin leads to Sclerosis, a skeletal disorder characterized by progressive bone overgrowth [59-61]. When comparing these two BMP antagonists, an interesting observation was found, there was a high affinity between recombinant Sclerostin and Noggin. The formation of a Noggin/Sclerostin complex lost the ability to bind with any BMPs, therefore, the interaction between Noggin and Sclerostin attenuated each other’s BMP-antagonizing activity [22]. These observations suggest that Noggin and Sclerostin may not be expressed in an overlapping manner when regulating BMP activity. Alternatively, it may suggest that BMP activities are precisely co-regulated by a set of antagonists during cell differentiation and maturation. As there are no reports yet on regulation of BMP heterodimer activities by Sclerostin or other BMP antagonists, future studies on the biological roles of heterodimers and their modulation by antagonists during bone formation and regeneration may result in interesting findings.
Mechanisms underlying Noggin antagonizing effects on BMPs
The suggested mechanism for the Noggin inhibition of BMP2 and BMP7 activities is by Noggin binding to BMPs and preventing BMPs from interacting with their cell surface receptors, and thus disabling the signal transduction of BMPs on target cells [24,25,62]. In agreement with this mechanism, we found that premixing of BMP2 homodimer or BMP7 homodimer with Noggin, largely decreased their activity in increasing ALP activity and OCN expression in C2C12 myoblastic cells when compared to controls. Moreover, while premixing BMP2 with Noggin at 1:1 ratio decreased its activity in inducing OCN expression, premixing BMP7 with Noggin at the same ratio did not significantly affect BMP7-induced OCN expression. This suggests a higher noggin inhibition effect on BMP2 than that on BMP7, and these data are also consistent with findings from previous studies that Noggin binds BMP2 with higher affinity than it binds BMP7 [24,25,50,51].
Furthermore, our finding suggests a weaker Noggin antagonizing effect on heterodimer versus homodimer, which may contribute, at least in part, to the increased osteogenic potency of heterodimer [44]. It is possible that at a site of BMP2/7 stimulation, lower Noggin induction may be related to relatively greater differentiation in local mesenchymal precursor cell populations, which may further favor the formation of new bone as we previously reported in a rat spine fusion model [44]. Future studies using purified BMP2/7 heterodimers will need to be performed to test whether the increased potency of heterodimer would be associated with their binding capacity with BMP receptors, and also look at the efficiency of activation of intracellular Smad proteins, which consequently act in BMP signal transduction pathway [11-16,24,25,62].
A limitation in the current study is the lack of purified heterodimers. Thus, a definitive conclusion on comparing bioactivities of heterodimer with that of homodimer, as well as the differential Noggin antagonizing effects on heterodimer from that on homodimer still lies ahead. Moreover, by using conditioned medium containing heterodimers as oppose to purified heterodimers, we cannot exclude the possibility that other factors that exist in the cell supernatants may also be involved in the BMP and Noggin interactions. However, in this study, we have supplied indirect evidence of a lower Noggin binding affinity to heterodimer as compared to homodimer, in which our results shows fewer osteoblastic activity after premixing Noggin with BMP2/7 heterodimer in comparison to premixing Noggin with BMP homodimers under the same culture conditions. Although the current understanding of Noggin and BMP crystal structure are limited and of the study of BMP heterodimer protein structure has not been looked at, however, the similarity of the homodimeric crystal structure of Noggin to that of BMP2 and BMP7 homodimers suggests that symmetrical Noggin may not be able to bind as well to asymmetrical heterodimeric BMP2/7 as it binds to either BMP2 or BMP7 homodimers, due to the conformational discrepancy between homodimer and heterodimer proteins [24,25,50,51,62]. This hypothesis needs to be confirmed by additional crystal structure analysis of heterodimers and the measurement of binding affinity of heterodimer to Noggin.
Although in this study we examined two markers of osteoblastic differentiation, ALP and OCN, future studies will need to be performed to examine a broader range of markers, such as Runx2, genes expressed during the earlier stages of differentiation, the synthesis of type I collagen and the formation of mineralization module [26,27,41-43]. These studies would further help elucidate the mechanisms of differential effects of Noggin modulation on BMP heterodimer and homodimer into a broader type of bone cells, which may potentially participate in an in vivo ossification process.
In summary, our findings suggest that a fusion gene approach led to the production of bioactive BMP2/7 heterodimers. These heterodimers were more potent than relative homodimers for inducing C2C12 myoblastic cells to undergo osteoblastic differentiation, and that was partially due to less Noggin antagonizing effects. The development of a more potent form of BMP heterodimer will have clinical potentials, as administration of BMP heterodimers may enhance bone formation and regeneration at lower and more effective dosages when compared to BMP heterodimers, such as those used clinically. This would be beneficial to orthopaedic patients by supplying more cost-efficient and effective therapies for the treatments of bone repair and regeneration diseases.
Acknowledgments
This work is funded by a grant from the Orthopaedic Research and Education Foundation, a grant from Medtronic Sofamor Danek, and a NIH core facility grant to Hospital for Special Surgery (P30-AR046121). CH is supported, in part, by the Arthritis Foundation, and the Institute for Sports Medicine Research.
References
- 1.Urist MR. Bone: formation by autoinduction. Science. 1965;150:S893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, et al. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990;87(24):9843–7. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helder MN, Ozkaynak E, Sampath KT, Luyten FP, Latin V, Oppermann H, et al. Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J Histochem Cytochem. 1995;43(10):1035–44. doi: 10.1177/43.10.7560881. [DOI] [PubMed] [Google Scholar]
- 4.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16(3):247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 5.Reddi AH. Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: noggin, chordin and DAN. Arthritis Res. 2001;3(1):1–5. doi: 10.1186/ar133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampath TK, Coughlin JE, Whetstone RM, Banach D, Corbett C, Ridge RJ, et al. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J Biol Chem. 1990;265(22):13198–205. [PubMed] [Google Scholar]
- 7.Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267(28):20352–62. [PubMed] [Google Scholar]
- 8.Schmitt JM, Hwang K, Winn SR, Hollinger JO. Bone morphogenetic proteins: an update on basic biology and clinical relevance. J Orthop Res. 1999;17(2):269–78. doi: 10.1002/jor.1100170217. [DOI] [PubMed] [Google Scholar]
- 9.Wozney JM. Overview of bone morphogenetic proteins. Spine. 2002;27(16 Suppl 1):S2–8. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 10.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop. 1998;346:26–37. [PubMed] [Google Scholar]
- 11.Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, et al. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J Bone Miner Res. 2002;17(10):1785–94. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]
- 12.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 13.Kawabata M, Miyazono K. Bone morphogenetic proteins. In: Canalis E, editor. Skeletal Growth Factors. Philadelphia: Lippincott, Williams and Wilkins; 2000. pp. 269–90. [Google Scholar]
- 14.Kretzschmar M, Massague J. Smads: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev. 1998;8:103–11. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 15.Miyazono K. Signal transduction by bone morphogenetic protein receptors: functional roles of Smads proteins. Bone. 1999;25:91–3. doi: 10.1016/s8756-3282(99)00113-1. [DOI] [PubMed] [Google Scholar]
- 16.Nohe A, Hassel S, Enrlich M, Neubauer F, Sebald W, Henis YI, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP2 signaling pathways. J Biol Chem. 2002;277:5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 17.Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102(12):2106–14. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brien CA, Economides AN, et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15(4):663–73. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 19.Hanaoka E, Ozaki T, Nakamura Y, Moriya H, Nakagawara A, Sakiyama S. Overexpression of DAN causes a growth suppression in p53-deficient SAOS-2 cells. Biochem Biophys Res Commun. 2000;278(1):20–6. doi: 10.1006/bbrc.2000.3758. [DOI] [PubMed] [Google Scholar]
- 20.Pereira RC, Economides AN, Canalis E. Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts. Endocrinology. 2000;141(12):4558–63. doi: 10.1210/endo.141.12.7851. [DOI] [PubMed] [Google Scholar]
- 21.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250(2):231–50. [PubMed] [Google Scholar]
- 22.Winkler DG, Yu C, Geoghegan JC, Ojala EW, Skonier JE, Shpektor D, et al. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem. 2004;279(35):36293–8. doi: 10.1074/jbc.M400521200. [DOI] [PubMed] [Google Scholar]
- 23.Avsian-Kretchmer O, Hsueh AJ. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrinol. 2004;18(1):1–12. doi: 10.1210/me.2003-0227. [DOI] [PubMed] [Google Scholar]
- 24.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420(6916):636–42. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 25.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, et al. Structural basis of BMP signaling inhibition by Noggin, a novel twelve-membered cystine knot protein. J Bone Jt Surg, Am. 2003;85-A(Suppl 3):52–8. doi: 10.2106/00004623-200300003-00010. [DOI] [PubMed] [Google Scholar]
- 26.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127(6 Pt 1):1755–66. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh LC, Tsai AD, Lee JC. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem. 2002;87:292–304. doi: 10.1002/jcb.10315. [DOI] [PubMed] [Google Scholar]
- 28.Zlotolow DA, Vaccaro AR, Salamon ML. The role of human bone morphogenetic proteins in spinal fusion. J Am Acad Orthop Surg. 2000;8:3–9. doi: 10.5435/00124635-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22(7):669–71. [PubMed] [Google Scholar]
- 30.Boden SD, Hair GA, Viggeswarapu M, Liu Y, Titus L. Gene therapy for spine fusion. Clin Orthop. 2000;379(Suppl):S225–33. doi: 10.1097/00003086-200010001-00030. [DOI] [PubMed] [Google Scholar]
- 31.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27(23):2662–73. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Khan SN, Hidaka C, Sandhu HS, Girardi FP, Cammisa FP, Jr, Diwan AD. Gene therapy for spine fusion. Orthop Clin North Am. 2000;31(3):473–84. doi: 10.1016/s0030-5898(05)70165-6. [DOI] [PubMed] [Google Scholar]
- 33.Khan SN, Sandhu HS, Lane JM, Cammisa FP, Jr, Girardi FP. Bone morphogenetic proteins: relevance in spine surgery. Orthop Clin North Am. 2002;33(2):447–63. doi: 10.1016/s0030-5898(01)00010-4. [DOI] [PubMed] [Google Scholar]
- 34.Vaccaro AR, Anderson DG, Toth CA. Recombinant human osteogenic protein-1 (bone morphogenetic protein-7) as an osteoinductive agent in spinal fusion. Spine. 2002;27(16 Suppl 1):S59–65. doi: 10.1097/00007632-200208151-00013. [DOI] [PubMed] [Google Scholar]
- 35.Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, et al. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorping activity. J Bone Miner Res. 1995;10(11):1681–90. doi: 10.1002/jbmr.5650101110. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2002;27(4):479–86. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 37.Paramore CG, Lauryssen C, Rauzzino MJ, Wadlington VR, Palmer CA, Brix A, et al. The safety of OP-1 for lumbar fusion with decompression — A canine study. Neurosurgery. 1999;44(5):1151–5. doi: 10.1097/00006123-199905000-00134. [DOI] [PubMed] [Google Scholar]
- 38.Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, et al. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15(16):2094–110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yabe T, Samuels I, Schwartz JP. Bone morphogenetic proteins BMP-6 and BMP-7 have differential effects on survival and neurite outgrowth of cerebellar granule cell neurons. J Neurosci Res. 2002;68(2):161–8. doi: 10.1002/jnr.10210. [DOI] [PubMed] [Google Scholar]
- 40.Yanagisawa M, Takizawa T, Ochiai W, Uemura A, Nakashima K, et al. Fate alteration of neuroepithelial cells from neurogenesis to astrocytogenesis by bone morphogenetic proteins. Neurosci Res. 2001;41(4):391–6. doi: 10.1016/s0168-0102(01)00297-8. [DOI] [PubMed] [Google Scholar]
- 41.Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13(3–4):291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 42.Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, et al. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995;210(3):670–7. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 43.Hazama M, Aono A, Ueno N, Fujisawa Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun. 1995;209(3):859–66. doi: 10.1006/bbrc.1995.1578. [DOI] [PubMed] [Google Scholar]
- 44.Zhu W, Rawlins BA, Boachie-Adjei O, Myers EJ, Arimizu J, Choi E, et al. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004;19(12):2021–32. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]
- 45.Qin YL, Yu DY, Wei Q. Function and structure of recombinant single chain calcineurin. Biochem Biophys Res Commun. 2003;308(1):87–93. doi: 10.1016/s0006-291x(03)01340-8. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86(4):599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 47.Israel DI, Nove J, Kerns KM, Moutsatsos IK, Kaufman RJ. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors. 1992;7(2):139–50. doi: 10.3109/08977199209046403. [DOI] [PubMed] [Google Scholar]
- 48.Wang EA, Rosen V, D’Alessandro JS, Bauduy M, Cordes P, Harada T, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87(6):2220–4. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones WK, Richmond EA, White K, Sasak H, Kusmik W, Smart J, et al. Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth Factors. 1994;11(3):215–25. doi: 10.3109/08977199409046919. [DOI] [PubMed] [Google Scholar]
- 50.Griffith DL, Keck PC, Sampath TK, Rueger DC, Carlson WD. Three-dimensional structure of recombinant human osteogenic protein1: structural paradigm for the transforming growth factor beta superfamily. Proc Natl Acad Sci U S A. 1996;93:878–83. doi: 10.1073/pnas.93.2.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheufler C, Sebald W, Hulsmeyer M. Crystal structure of human bone morphogenetic protein-2 at 2.7A resolution. J Mol Biol. 1999;287:103–15. doi: 10.1006/jmbi.1999.2590. [DOI] [PubMed] [Google Scholar]
- 52.Ahn J, Serrano de la Pena L, Shore EM, Kaplan FS. Paresis of a bone morphogenetic protein-antagonist response in a genetic disorder of heterotopic skeletogenesis. J Bone Jt Surg, Am. 2003;85-A(4):667–74. doi: 10.2106/00004623-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, et al. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Jt Surg, Am. 2003;85-A(12):2332–42. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Weber FE, Schmokel H, Oelgeschlager M, Nickel J, Maly FE, Hortschansky P, et al. Deletion mutants of BMP folding variants act as BMP antagonists and are efficient inhibitors for heterotopic ossification. J Bone Miner Res. 2003;18(12):2142–51. doi: 10.1359/jbmr.2003.18.12.2142. [DOI] [PubMed] [Google Scholar]
- 55.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1998;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 56.Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- 57.Katagiri T, Boorla S, Frendo JL, Hogan BL, Karsenty G. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet. 1998;22:340–8. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 58.Solloway MJ, Robertson EJ. Early embryonic lethality in BmpBmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126(5):1753–68. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- 59.Ohyama Y, Nifuji A, Maeda Y, Amagasa T, Noda M. Spatiotemporal association and BMP regulation of SOST and Osterix expression during embryonic osteogenesis. Endocrinology. 2004;145(10):4685–92. doi: 10.1210/en.2003-1492. [DOI] [PubMed] [Google Scholar]
- 60.Van Bezooijen RL, Roelen BA, Visser A, Van Der Wee-Pals L, De Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–14. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nickel J, Dreyer MK, Kirsch T, Sebald W. The crystal structure of the BMP-2: BMPR-IA complex and the generation of BMP-2 antagonists. J Bone Jt Surg, Am. 2001;83-A(Suppl 1 (Pt 1)):S7–14. [PubMed] [Google Scholar]


