Abstract
Despite recognition as a significant stressor in childhood cancer, illness-related uncertainty from the perspective of children remains under-studied. We tested a conceptual model of uncertainty, derived from Mishel’s uncertainty in illness theory, in 68 school-aged children and adolescents with cancer. As hypothesized, uncertainty was significantly related to psychological distress, but only one hypothesized antecedent (parental uncertainty) significantly predicted children’s uncertainty. An alternative model incorporating antecedent developmental factors (age and illness-specific expertise) explained 21% of the variance in child uncertainty; controlling for stage of treatment, uncertainty was higher in children with shorter time since diagnosis, older age, lower cancer knowledge, and higher parental uncertainty. These findings provide the foundation for further studies to understand children’s management of uncertainty and its contribution to psychological adjustment to illness.
Keywords: Uncertainty, parent-child relationship, cancer, growth and development, adolescence
Cancer remains the leading cause of disease-related mortality in children and adolescents (Gurney & Bondy, 2006). Although significantly improving overall survival, the utilization of increasingly intensive treatments has created an illness trajectory characterized by frequent, repetitive courses of intensive treatment alternating with periods of relative good health and normalcy. The outcome for any individual child remains unpredictable, such that hope for long term survival is accompanied by enduring uncertainty.
Uncertainty has long been recognized as a significant issue in childhood cancer (Cohen, 1993; Koocher & O’Malley, 1981). It consistently emerges as a major source of distress in interviews with parents and families of children with cancer (De Graves & Aranda, 2008; Stewart & Mishel, 2000). Potential sources of children’s uncertainty include fears of death, whether or not they choose to acknowledge them to parents and staff (Bearison, 1991; Stewart, 2003b), unpredictable severity of treatment side effects, non-specific symptoms that could be attributed to either worsening illness or treatment toxicity, unpredictable interruptions in school and peer relationships, and the risk for long-term sequelae. However, only a few descriptive studies have specifically addressed illness-related uncertainty from the perspective of children and adolescents.
Qualitative studies of children and adolescents completing cancer therapy document lingering misconceptions about their illness, persistent fears about possible recurrence, and use of coping strategies such as selective attention and distraction to actively focus their thoughts away from enduring uncertainty (Haase & Rostad, 1994; Weekes & Kagan, 1994). Uncertainty was reported in nearly half of young adults’ retrospective descriptions of the emotional impact of childhood cancer (Novakovic et al., 1996), and was strongly correlated with posttraumatic stress symptoms in young adult survivors an average of 14 years after completion of cancer treatment (Santacroce & Lee, 2006). In the single study of uncertainty in newly diagnosed adolescents with cancer, it was strongly correlated with psychological distress and negatively correlated with perceived social support (Neville, 1998). This limited set of studies offers compelling evidence for uncertainty as a major contributor to the childhood cancer experience, but they predominantly reflect the perspectives of adolescents and young adults looking back on their earlier experiences and thus provide only limited insight into what children experience while they are undergoing cancer treatment.
As an initial step towards systematically studying children’s uncertainty, Stewart (2003b) asked children aged 9 to 12 years old undergoing cancer treatment to describe illness situations in which they felt “unsure.” Their rich descriptions yielded a conceptualization of uncertainty consistent with Mishel’s (1988) classic categorization of uncertainty as novelty, complexity, ambiguity, and unpredictability. Uncertainty led to children’s negative emotional arousal, interfered with their ability to cope successfully with illness-related stressors and aversive events, and challenged their determination to view themselves as normal children living an ordinary life. These findings underscore the importance of studying children’s uncertainty as a critical aspect of their adjustment to cancer.
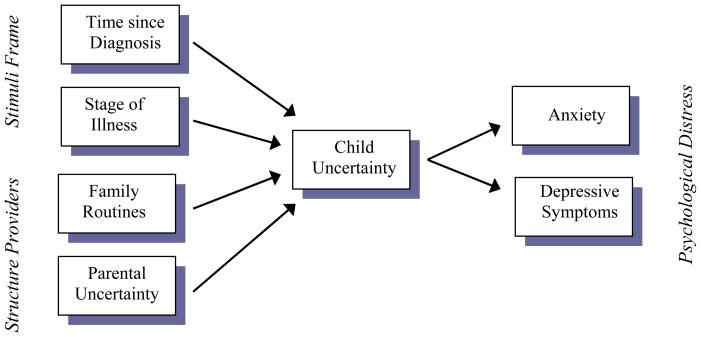
The current study was the second in a planned program of research into uncertainty in children with cancer designed to provide the foundation for theory-driven interventions to promote children’s adjustment to illness. The purpose was to test a conceptual model of uncertainty in school-aged children and adolescents with cancer (Figure 1) adapted from a well-tested theoretical model of uncertainty in adults. A secondary goal was to explore the relationship of uncertainty to two indicators of development, age and expertise.
Figure 1.
Conceptual Model of Uncertainty in Children with Cancer
Theoretical Framework
Mishel’s (1988) uncertainty in illness theory has guided extensive study of uncertainty in ill adults (Bailey & Stewart, 2009; Mishel, 1997a, 1999) and numerous descriptive studies with parents of children with serious illnesses (Stewart & Mishel, 2000). The theory posits that uncertainty results when an adequate cognitive schema cannot be formed with which to interpret the meaning of illness-related events, and uncertainty leads to psychological distress if coping responses are insufficient to resolve uncertainty or to manage negative emotional arousal when uncertainty cannot be resolved.
Mishel identified two primary antecedents to uncertainty. Stimuli frame reflects the degree to which the illness trajectory is patterned, familiar, and congruent with expectations. Structure providers are the individuals in the patient’s social and professional caregiving networks who serve as resources in interpreting the stimuli frame. Together these factors support formulation of a cognitive schema with which to interpret the significance of subsequent illness events, thereby reducing uncertainty. Barriers to cognitive schema development, in the form of unpatterned, unfamiliar, and/or incongruent stimuli or ineffective support from structure providers, lead to uncertainty.
The literature on cognitive development and childhood illness, although not specifically addressing illness-related uncertainty, suggests that Mishel’s model could apply to children. By early school age children formulate and rely on cognitive schemata in the context of illness (Hergenrather & Rabinowitz, 1991); however, the novelty and ambiguity associated with cancer treatment has been shown to interfere with children’s capacity to form a sufficient schema within which to interpret subsequent illness experiences (Bearison, 1991). Work by Weisz and colleagues (Weisz, 1990; Weisz, McCabe, & Dennig, 1994) demonstrated that ill children’s inability to determine the controllability of events, a condition referred to by the theorists as contingency uncertainty, places them at increased risk for psychological distress.
Figure 1 represents a proposed extension of Mishel’s model to the study of uncertainty in children. Time since diagnosis and phase of treatment, representing potential changes in the patterning of treatment and therefore symptoms, are used as indices of the interpretability of the stimuli frame. As children’s most significant source of illness information and security, parents would be expected to act as the predominant structure providers for ill children. Parents’ uncertainty about their child’s illness has been well-described (Stewart & Mishel, 2000) and may undermine their confidence in accurately appraising their child’s health, interfere with family routines, and limit their capacity to provide the information and support that would promote their child’s illness schema formation, thereby increasing children’s uncertainty. Parents also help manage childhood illness by reinforcing the normalcy of family life through the maintenance of family routines and rituals (Clarke-Steffen, 1997; Knafl, Deatrick, & Gallo, 2008). Parents’ contributions as structure providers are therefore represented in the model by two constructs: parental uncertainty and family routines. The outcome portion of the model, psychological distress, is operationalized as anxiety and depressive symptoms, the two most commonly studied emotional outcomes in children with cancer.
This conceptual model of uncertainty in children and adolescents with cancer directed two specific hypotheses which were tested in the current study. Longer time since diagnosis, continuous remission (vs. presence of newly diagnosed or progressive disease), higher parental uncertainty, and lower parental endorsement of family routines were hypothesized to predict higher uncertainty in child subjects. Higher child uncertainty was hypothesized to predict higher levels of child anxiety and depressive symptoms.
There was limited guidance from the literature as to how age or developmental maturation might influence children’s perception of uncertainty. Based on developmental stage theories, younger children would be expected to have less sophisticated understanding of illness processes than older children (e.g., Bibace & Walsh, 1980). Younger children might not be given the same amount and complexity of information about their illness by parents and clinicians. Domain-specific theories suggest that whereas maturation places constraints on children’s thinking, knowledge within a specific domain such as illness can drive children’s cognitive development (Crisp, Ungerer, & Goodnow, 1996). Thus any explanation about how children respond to uncertainty based on age must be qualified by an appreciation of how illness-specific knowledge might accelerate their cognition. A secondary goal of the current study was to explore the relationship of uncertainty to two indicators of development, age and illness-specific knowledge.
Methods
Settings and Participants
Data were collected in 2002–2003 at four pediatric cancer centers affiliated with the Children’s Oncology Group, a cooperative study group representing institutions across the United States participating in cancer clinical trials research with children and adolescents. Eligible participants were 8 to 18 years old, currently undergoing treatment for any form of cancer, with a parent available and willing to participate. Potential participants were excluded if they did not speak English, could not read at the second grade level, or their treatment team deemed them too ill to participate. Treatment team members were asked to identify and approach all eligible subjects; children and parents who chose not to participate in the study were asked to provide basic demographic information (age, ethnicity, gender) and their reasons for not participating.
Seventy two children and their parents participated in the study. Ten eligible children and three mothers of eligible children or adolescents declined to participate, yielding a refusal rate of 15.3%. Nine children who refused said they were not interested in participating in research. The remaining child subject and the mothers who declined participation cited issues related to uncertainty specifically or research participation in general. There were no significant differences in age, race, or sex between participants and those who refused.
The child sample characteristics are presented in Table 1. The sample was predominantly Caucasian and male. Most of the children had been diagnosed with leukemia or lymphoma, were in remission, and were being treated with chemotherapy. The mean time since diagnosis was positively skewed by two subjects with long illness durations (100 months and 166 months); therefore the natural logarithm of the number of months since diagnosis was calculated to create the variable “time since diagnosis,” which more closely approximated a normal distribution (M= 2.41, SD = 1.1).
Table 1.
Sample Characteristics (Child)
| Mean | Standard Deviation | Median | Range | |
|---|---|---|---|---|
| Age in years | 13.0 | 2.9 | 13 | 8 – 18 |
| Grade in school | 8 | 2.7 | 8 | 1st – 12th |
| Months since diagnosis | 18.6 | 24.2 | 11.9 | .2 – 166 |
| n | % | |||
| Sex | ||||
| Male | 42 | 58.3 | ||
| Female | 30 | 41.7 | ||
| Race/ethnicity | ||||
| African American | 15 | 20.8 | ||
| Asian American | 2 | 2.8 | ||
| Caucasian | 52 | 72.2 | ||
| Hispanic | 2 | 2.8 | ||
| Native American | 1 | 1.4 | ||
| Diagnoses | ||||
| Leukemia/Lymphoma | 48 | 66.7 | ||
| Solid tumor | 17 | 23.6 | ||
| Central Nervous System | 6 | 8.5 | ||
| Stage of illness | ||||
| Newly diagnosed | 11 | 15.3 | ||
| Remission | 49 | 68.1 | ||
| Relapsed | 12 | 16.7 | ||
| n | % | |||
| Type of treatment | ||||
| Chemotherapy | 68 | 95.8 | ||
| Radiation | 18 | 25.4 | ||
| Surgery | 12 | 16.7 | ||
| Stem Cell Transplantation | 8 | 11.3 | ||
The parent participants (Table 2) were predominantly mothers and married or living with a partner. Four parents submitted incomplete instrument data and were removed from further analyses, yielding a sample size of 68 for testing the hypothesized model.
Table 2.
Sample Characteristics (Parent/Family)
| Mean | Standard Deviation | Median | Range | |
|---|---|---|---|---|
| Parent education in yrs | 13.2 | 2.1 | 12.0 | 9 – 18 yrs |
| # of family members | 4.5 | 1.6 | 4.0 | 2 – 10 |
| n | % | |||
| Relationship to Child | ||||
| Mother | 53 | 74.6 | ||
| Father | 12 | 16.7 | ||
| Grandparent | 3 | 4.2 | ||
| Stepparent | 2 | 2.8 | ||
| Custodial | 2 | 2.8 | ||
| Marital status | ||||
| Married/Living with partner | 53 | 73.6 | ||
| Not married | 19 | 26.4 | ||
| Annual family income | ||||
| < $20,000 | 13 | 18.1 | ||
| $20 – 40,000 | 14 | 19.4 | ||
| $40 – 60,000 | 15 | 20.8 | ||
| $60 – 80,000 | 5 | 6.9 | ||
| > $80,000 | 15 | 20.8 | ||
| Preferred not to answer | 10 | 13.9 | ||
Procedures
Approval for the study was obtained from each participating site’s Institutional Review Board. Informed consent was obtained from parents and assent from children and adolescents, who were assured they did not have to participate in the study even if their parent had given consent. Children’s responses were not shared with parents, and neither children’s nor parents’ responses were shared with treatment team members.
Data collection took place at the location most convenient for the family, with all but one family completing study instruments in the inpatient or outpatient setting. Questionnaires were read to all children aged 10 and younger. When instruments were read out loud, the investigator met with the child privately. No discussion of responses took place in front of the parent to ensure children’s responses remained confidential.
Variables and Measures
Child-Report Measures
Anxiety
The Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds & Richmond, 1978) is a 28-item, dichotomous self-report measure of children’s anxiety and worry, with higher scores indicating higher levels of anxiety/worry. Reliability for the anxiety/worry scale is supported by KR-20 estimates ranging from .83 to .85, and stability by 3-week test-retest correlation of .98 (Pela & Reynolds, 1982). The scale discriminates between anxious and non-anxious samples (Perrin & Last, 1992; Seligman, Ollendick, Langley, & Baldacci, 2004), and has demonstrated adequate reliability and validity in samples of children with acute and chronic illness (DeMaso et al., 2000; Loney, 2008; Reynolds, 1980). The KR-20 estimate of reliability for the current sample was .87.
Child uncertainty
The Uncertainty Scale for Kids (USK; Stewart, Lynn, & Mishel, in press) is a 22-item, 4-point ordinal self-report scale which indexes the frequency with which children with cancer experience illness-related uncertainty. In its initial psychometric evaluation with the current sample, the USK demonstrated strong internal consistency (Cronbach’s alpha = .95), 1-week test-retest reliability (r = .64, p = .005), and its discriminant validity was supported with lower scores among children treated with less complex regimens (chemotherapy alone, M = 44.4, SD = 14.2, vs. in combination with surgery and/or radiation, M = 55.8, SD = 15.0, t(63) = 3.06, p = .003).
Depressive symptoms
The Children’s Depression Inventory (CDI; Kovacs, 1985) is a 27-item self-report scale that elicits children’s depressive symptomatology for the previous 2 weeks, with higher scores indicating greater symptom intensity. The CDI has accumulated considerable evidence for reliability and construct, predictive, and discriminant validity in non-ill and chronically ill children (Kovacs; Phipps & Srivastava, 1997; White et al., 2005; Wood et al., 2007). The Cronbach’s alpha for the current sample was .80.
Illness-specific knowledge
The investigator-developed Cancer Knowledge Scale (CKS) (Stewart, 2003c) was created for use in the current study, based on published cancer educational materials designed for children. It consists of 12 true-false statements representing general information about childhood cancer and common side effects to treatment (e.g., hair loss, risk of infection, radioactivity). Content validity was established with review by advanced practice pediatric oncology nurses. CKS scores were positively correlated with age (r = .36, p < .01), indicating that older children had more knowledge. In this study sample, the KR-21 was .44, indicating limited internal consistency.
Parent-Report Measures
Demographic data
Parents provided the following information: child’s date of birth, race/ethnicity, type and date of cancer diagnosis, stage of treatment (newly diagnosed, remission, recurrence), treatment previously or currently received (chemotherapy, radiation, surgery to debulk or remove tumor tissue, hematopoetic stem cell transplant/HCST, other), as well as their relationship to the child subject, marital status, highest grade in school attained, and family income.
Family routines
The Family Routines Inventory (FRI; Jensen, James, Boyce, & Hartnett, 1983) is a 28-item scale parent-report scale that measures the stability and predictability of family routines. The FRI has demonstrated adequate test-retest reliability (r = .74 –.79), as well as convergent validity (Jensen et al.). In the current study, scores were averaged across the items endorsed by each parent rather than summed, due to considerable missing data corresponding to items that did not pertain to all families (e.g., caring for pre-school children). Cronbach’s alpha for the study sample was .79.
Parent uncertainty
The Parent Perceptions of Uncertainty Scale (PPUS; Mishel, 1983) is a 32 item, 5-point Likert-type scale measuring parents’ uncertainty about their child’s illness. The scale has four factors (Ambiguity, Lack of Clarity, Lack of Information, and Unpredictability). Coefficient alphas have ranged from .86 –.93, and factor analysis with combined samples was consistent with the theoretical basis of the scale (Mishel, 1997b). In the current sample, two of the subscales, Lack of Information and Unpredictability, yielded inadequate internal consistency estimates (.37 and .65, respectively), and therefore the largest of the two reliable subscales (Ambiguity, 13 items, alpha = .88), was used as the measure of parent uncertainty.
Data Analyses
Multiple regression analyses were used to test the fit of the conceptual model to the study data. To test the first hypothesis, the four independent variables (time since diagnosis, stage of illness, parent uncertainty, and family routines) were entered into a multiple regression equation with uncertainty as the dependent variable. To test the second hypothesis, uncertainty was treated as the independent variable in two separate regression equations with anxiety and depressive symptoms as the dependent variables. Standardized beta coefficients (β) are presented to facilitate comparison of the paths within the model.
Bivariate correlations were calculated for age, illness-specific knowledge, and uncertainty to examine the relationship of uncertainty to children’s development. To examine the multivariate relationships, age and illness-specific knowledge were then entered as predictors into a regression equation with uncertainty as the dependent variable. The potential interaction effect between the two predictor variables was tested by centering each around its mean, calculating their product, and including this interaction term as a third predictor of uncertainty.
Post-hoc analyses of model fit were performed using AMOS structural equation modeling software. With all model variables observed (i.e., represented by a single measure) rather than latent, the program replicates the linear regression techniques commonly employed in path analyses, but provides goodness-of-fit indices including the adjusted goodness-of-fit index (AGFI), normed fit index (NFI), relative fit index (RFI), and root mean square error of approximation (RMSEA). The AGFI, NFI, and RFI are reported as coefficients ranging zero to one, with values closer to 1.0 indicating superior fit; for the RMSEA, values less than .05 are considered to indicate good fit, 08 to .10 mediocre fit, and greater than .10 an inadequate fit. A narrower confidence interval and a non-significant close fit test (pclose) increase the interpretability of a favorable RMSEA (Byrne, 2001).
Results
Hypothesized Model
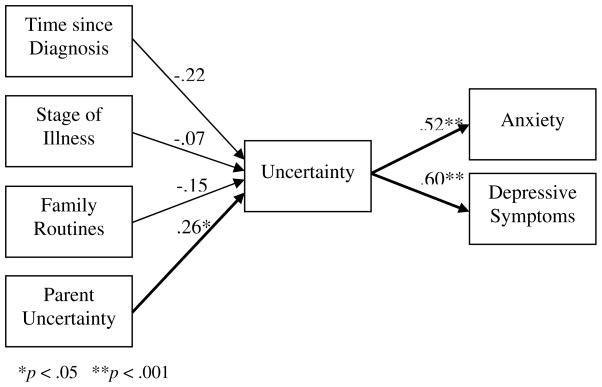
Bivariate correlations among the model variables are presented in Table 3. The regression equation for the antecedent model with the four predictors of uncertainty (Figure 2) was non-significant (Table 4) and explained 5.4% of the variance in child uncertainty. Only the path from parental uncertainty to child uncertainty was significant, such that as parental uncertainty increased, child uncertainty also increased. None of the pathways of the other antecedent variables to child uncertainty were significant, although the path from time since diagnosis was in the expected direction. Child uncertainty had significant paths to both anxiety (F[1,66] = 30.34, β = .56, p < .001) and depressive symptoms (F[1,66] = 36.86, β = .60, p < .001), such that as child uncertainty increased, psychological distress also increased. Child uncertainty explained 30% of the variance in anxiety and 36% of the variance in depressive symptoms.
Table 3.
Zero-Order Correlation Coefficients, Means, and Standard Deviations for Study Variables
| Time Dx | Stage | Age | CKS | PPUS | USK | ANX | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|
| Time Dx | 2.4 (1.1) | |||||||
| Stage a | −.27* | |||||||
| Age | −.01 | .06 | 12.8 (2.8) | |||||
| CKS | −.06 | −.10 | .36** | 9.5 (1.7) | ||||
| PPUS | −.13 | .30* | .19 | .09 | 32.2 (16.9) | |||
| USK | −.23 | .06 | .21 | −.15 | .26* | 49.9 (15.7) | ||
| ANX | −.02 | −.10 | −.10 | −.20 | .18 | .56** | 10.0 (6.2) | |
| DEPR | −.13 | −.07 | .17 | −.09 | .20 | .59** | .59** | 7.4 (5.4) |
p < .05
p < .001
Time Dx = natural logarithm of months since diagnosis; Stage = stage of illness (a1 = remission, 0 = not in remission); CKS = illness-specific knowledge; FRI = family routines; PPUS = parent uncertainty (ambiguity subscale); USK = child uncertainty; ANX = anxiety; DEPR = depressive symptoms
Figure 2.
Statistical Representation of Hypothesized Model with Standardized Beta Coefficients
Table 4.
Ordinary Least Squares Parameter Estimates, Standard Errors, and t-Tests for Child Uncertainty Regressed on Hypothesized Model Antecedents
| Unstandardized Beta | Std. Error | Standardized Beta | t | Sig. | |
|---|---|---|---|---|---|
| (Constant) | 48.70 | 18.78 | 2.592 | <.001 | |
| Time since diagnosis | −3.06 | 1.84 | −.22 | −1.66 | .10 |
| Stage of illness | −2.29 | 4.35 | −.07 | −.53 | .60 |
| Parent uncertainty | .41 | .207 | .26 | 2.0 | .04 |
| Family routines | −.55 | 5.34 | −.01 | −.10 | .92 |
F(4,63) = 1.93, p = .12, Adj R2 = .054
Relationship of Age and Expertise to Uncertainty
Neither age nor illness-specific knowledge was significantly correlated with uncertainty. In the multivariate regression model both age and knowledge significantly predicted uncertainty (Table 5), such that lower illness-specific knowledge and older age were associated with higher uncertainty, and accounted for 9% of its variance. Adding the interaction term retained the model’s significance, but the interaction term was not a significant predictor.
Table 5.
Ordinary Least Squares Parameter Estimates, Standard Errors, and t-Tests for Child Uncertainty Regressed on Age, Illness-specific Knowledge, and their Interaction Term
| Unstandardized Beta | Std. Error | Standardized Beta | t | Sig. | |
|---|---|---|---|---|---|
| (Constant) | 53.38 | 11.54 | 4.62 | <.001 | |
| Age | 1.68 | .66 | .31 | 2.54 | .01 |
| Knowledge | −2.65 | 1.15 | −.28 | −2.31 | .02 |
| Age × Knowledge | .23 | .41 | .08 | .61 | .54 |
F(4,66) = 4.17, p = .03, Adj R2 = .09
Alternative Model
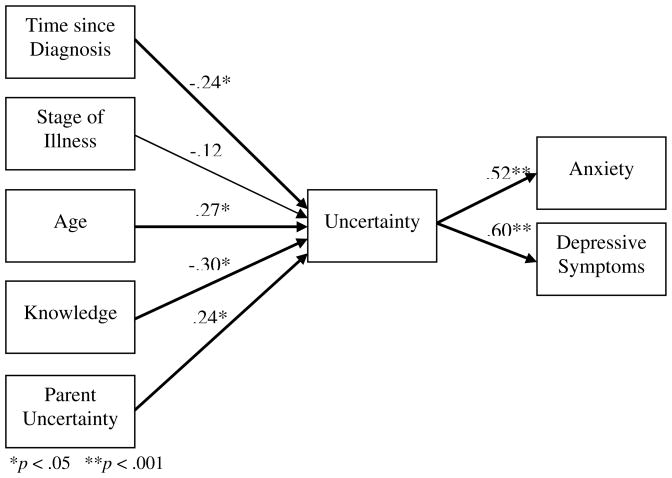
An alternative to the hypothesized model was constructed with additional antecedent variables suggested by the exploratory analysis of developmental factors and child uncertainty. Age and domain-specific expertise were added as indicators of cognitive development, time since diagnosis and stage of treatment were retained as indicators of strength of the stimuli frame, and parental uncertainty was retained as the significant structure provider variable. These five independent variables were entered into a multiple regression equation with uncertainty as the dependent variable (Table 6, Figure 3). All of the proposed paths in this model were significant except the path between stage of treatment and child uncertainty. Together the antecedent variables predicted 21% of the variance in child uncertainty. Removing the non-significant antecedent (stage of treatment) changed the strength of the paths for time since diagnosis and parental uncertainty to child uncertainty slightly and rendered them non-significant.
Table 6.
Ordinary Least Squares Parameter Estimates, Standard Errors, and t-Tests for Child Uncertainty Regressed on Alternative Model Antecedents
| Unstandardized Beta | Std. Error | Standardized Beta | t | Sig. | |
|---|---|---|---|---|---|
| (Constant) | 58.00 | 14.54 | 3.986 | .00 | |
| Age | 1.55 | 0.71 | .27 | 2.20 | .03 |
| Knowledge | −2.81 | 1.17 | −.30 | −2.41 | .02 |
| Time since diagnosis | −3.51 | 1.74 | −.28 | −2.02 | .05 |
| Stage of illness | −3.94 | 4.19 | −.12 | −.94 | .35 |
| Parent uncertainty | .39 | .20 | .24 | 1.99 | .05 |
F(4,63) = 3.28, p = .101, Adj R2 = .21
Figure 3.
Statistical Representation of Alternative Model with Standardized Beta Coefficients
Post-hoc Analyses of Model Fit
For the hypothesized model, the goodness of fit indices suggested a marginally adequate fit to the data (AFGI = .90, NFI = .91, RFI = .80). An RMSEA value of zero [90% CI .0–.10, pclose = .79]) likely represents an overadjustment for parsimony given the small number of parameters in the model. The alternative model demonstrated a somewhat better and more interpretable fit to the data (AFGI = .92, NFI = .99, RFI = .97, RMSEA = .05 [90% CI .0–.15, pclose = .44). Removing the non-significant antecedent (stage of treatment) did not substantially change the fit indices for the stronger alternative model.
Discussion
The consistent characterization in the literature of uncertainty as a major stressor in childhood cancer, informed by the limited empirical literature from the perspective of adolescents and young adults, provided the foundation for this detailed examination of uncertainty from the perspective of ill children. The validity of a conceptual model of uncertainty in children undergoing treatment for cancer derived from Mishel’s uncertainty in illness theory was partially supported by the present study, most importantly in the strong relationship between children’s uncertainty and their level of psychological distress. This relationship provides compelling evidence for the importance of further study in this area.
The previous literature on children’s and adolescents’ uncertainty in the context of cancer treatment and survivorship, while providing compelling evidence that uncertainty is stressful for children as well as adults, has not yielded a comprehensive representation of children’s uncertainty or the mechanisms by which it influences their psychological adjustment. Several exploratory studies document the retrospective insights of adolescents and young adults who have completed treatment (Haase & Rostad, 1994; Novakovic et al., 1996; Santacrocce & Lee, 2006), and the single study documenting the association between uncertainty and psychological distress during active treatment was conducted with a sample between 14 and 22 years old. (Neville, 1998). Thus the perspectives of younger children and those currently undergoing treatment have been largely unrepresented. The systematic study of children’s uncertainty requires a valid, developmentally-sensitive conceptual framework to guide the careful examination of the processes by which children react to uncertainty and ultimately support the development of interventions to reduce the impact of uncertainty on children’s psychological outcomes.
The risk that serious illness poses to children’s psychological adjustment, particularly in the form of internalizing symptoms such as anxiety and depression, has been supported across studies that compare ill children to their non-ill peers (Bennett, 1994; Lavigne & Faier-Routman, 1992), but there has been less understanding about what factors might account for the variability evident within groups of ill children. In this sample of children with cancer, much of that variability was accounted for by their level of uncertainty, providing support for the perspective that cognitive processes account for individual differences in adjustment to life-threatening illness among children (Thompson & Gustafson, 1996; Wallander & Thompson, 1995). The application of Mishel’s theory to children with cancer therefore contributes important insight into children’s responses to serious illness and explains in part the process by which life-threatening illness contributes to children’s psychological burden. The striking positive relationship between children’s uncertainty and their anxiety and depressive symptoms underscores the importance of identifying children who are at greatest risk for uncertainty, as helping children ameliorate and manage their uncertainty may have real benefit in reducing their psychological distress during treatment.
The hypothesized model provided limited understanding about factors that might contribute to the variability in children’s uncertainty, with only parental uncertainty emerging as a significant predictor. The complex, multivariate relationships among age, illness-specific knowledge, and uncertainty prompt a return to the original theory to consider how these developmental factors might be incorporated. Mishel’s theory stipulates that an individual’s cognitive capacities, conceptualized in adults as arising from their information processing abilities and limited by demands on attentional capacity by the illness, influence the interpretation of the stimuli frame, thereby influencing their level of uncertainty. In children, the conceptualization of cognitive capacity must incorporate developmental changes that take place as children mature. The alternative model with age and illness-specific knowledge included as antecedent developmental factors fit the data well, with all of the antecedent factors except stage of treatment having significant paths to child uncertainty in the expected direction.
The antecedent portion of this superior alternative model can be interpreted thus: controlling for stage of illness, children’s uncertainty lessens with increasing duration of illness, and with accumulating illness-specific knowledge. Their level of uncertainty increases in direct relationship to their parents’ level of uncertainty, and older children report higher levels of uncertainty. The positive relationship between age and uncertainty requires careful consideration. If complexity of the cognitive schema were associated with lower levels of uncertainty, then maturation would be expected to reduce uncertainty, all other things being equal. However, Mishel’s theory addresses not the complexity of the illness schema but its sufficiency: uncertainty arises when the schema is not sufficient to support interpretation of illness-related events. Possibly the relative simplicity of a younger child’s schema means that it is more durable in the face of unpatterned or incongruent experiences, whereas the inherent unpredictability and ambiguity of the illness experience poses a greater threat to an older child’s higher expectations for being able to understand, predict, and determine the significance of illness-related symptoms and events.
Two parent factors were hypothesized to influence children’s uncertainty: parents’ own uncertainty, as it might interfere with their ability to support their child’s interpretation of illness related events, and parents’ endorsement of family routines, which might influence the degree to which families are able to reinforce children’s sense of the illness as familiar, patterned, and congruent with expectations. The FRI was chosen as a structure provider measure based on the assumption that children’s sense of routine would likely be stronger in families that endorsed and encouraged the structure of daily routines despite the demands of cancer treatment. However, parent subjects found that some of the items on this established instrument did not pertain to families of school aged and adolescent children. Although the scale was scored by averaging the item scores endorsed by each parent and demonstrated adequate reliability, its limited applicability undermined its validity in this context. It is possible that families promote stability and routine for their older children in ways not indexed on the FRI, and therefore parents’ role as structure providers within the context of child uncertainty deserves further study.
Limitations of the Study
The sample size of 68 represented available subjects from 4 major pediatric cancer centers and exceeds the 10 subjects per estimated model parameter recommended for model testing (Kline, 1998). However, post-hoc analysis revealed a power of only 25% to detect an effect size of .05 (the calculated effect size of the hypothesized antecedent model); obtaining a power of 80% would require a sample size of 244 (Faul, Erdfelder, Buchner, & Lang, 2009). Therefore Type II error cannot be ruled out in the failure to confirm several of the hypothesized antecedents to uncertainty. Despite the broad inclusion criteria used in obtaining the sample, the limited variability on several key constructs further constrained the analysis. For example, most of the children in the study were in remission and had received chemotherapy, such that the power to detect difference based on these parameters was limited by the small number of children in the other conditions. Likewise, the limited demographic variability of the sample, which was largely Caucasian, prevents any examination of the relationship of uncertainty to potentially influential cultural factors. Two alternative design approaches would be to limit the sample to a particular diagnosis, phase of illness, or treatment modality in order to control some of the variability, or to intentionally sample children and stratify them based on pertinent factors included in the analyses, which would require a much larger sample.
Operationalizing the conceptual model posed several measurement challenges. The investigator-developed measure of children’s illness-specific knowledge demonstrated limited reliability. Items that might best distinguish between “novice” and “expert” relate to management of treatment side effects, such as bone marrow suppression from chemotherapy, but items that did not potentially pertain to all subjects were excluded from consideration. The resulting knowledge items offered limited discrimination between the highest and lowest scoring subjects and therefore the estimation of scale reliability. Even with these limitations, scores on the knowledge scale did correlate negatively with children’s uncertainty when age was controlled, enabling a developmental exploration of children’s uncertainty that contributed to the substantially improved fit of the alternative conceptual model.
An additional limitation of the current study design was the inclusion of only one parent subject per child. Most of the parents who participated in the study were mothers, as they most frequently accompanied their children to clinical visits at which most data were collected. This limits the interpretation of findings especially as it relates to the relationship of parent uncertainty to child uncertainty at a single point in time. Again this limitation could be addressed with more purposeful sampling, by including both parents, and by a more deliberate analysis of the variability among individual and dyadic levels of uncertainty.
Directions for Future Research
The alternative conceptual model provides compelling evidence that children and adolescents, like adults, are affected by the uncertainty inherent to serious illness such as cancer, and warrants further research into children’s uncertainty. The interplay of uncertainty for children and their parents suggests that parents’ uncertainty, in addition to affecting their own psychological outcomes, could limit their confidence in carrying out parental roles such as decision making and planning for children’s futures, which could have consequences for children’s emotional and social functioning. Likewise, the importance of parents as structure providers for children attempting to make sense of their illness experience suggests that additional parent- and family-level variables be considered as potential influences on children’s uncertainty.
The current study also provides preliminary evidence for the developmental nature of the uncertainty construct in children. The relationships among age, domain-specific knowledge, and uncertainty yielded some interesting insights into the developmental factors that might influence children’s adjustment to life-threatening illness, but the problems with measurement of knowledge and the use of age as the only indicator of maturation limit their interpretability. A more stringent test would include a measure of cognitive development that would allow for the comparison of children at distinctly different maturational levels, as well as a more discriminating measure of domain-specific knowledge. It is certainly possible that maturation could affect the dimensionality of children’s uncertainty, that different experiences trigger uncertainty for children at different developmental stages, or that the relationship between uncertainty and psychological distress changes based on age or developmental level. A much larger sample than available in the current study would be required to compare the fit of the model in separate age groups. Given that the co-occurrence of illness and developmental demands can pose unique challenges for children and adolescents with chronic illnesses (Charron-Prowchownik, 2002; Stewart, 2003a), it would be particularly enlightening to examine more carefully the interactions among development, specific illness stressors, uncertainty, and psychological outcomes.
The ultimate goal of further research into children’s uncertainty is to develop interventions to reduce the negative impact of illness on children’s quality of life. The current study suggests two possible targets for ameliorating children’s uncertainty: increasing children’s illness-specific knowledge, and helping parents manage their own uncertainty in order to function more effectively to provide structure for their child’s illness experience. However, to a large degree uncertainty is inherent to the illness experience, and therefore the goal of intervention is not only to resolve uncertainty when possible but to assist children in learning to manage it so that its impact on their psychological adjustment is reduced. The literature on children’s coping suggests children’s appraisal and coping response to illness-related stressors can be impaired when those stressors are uncontrollable or their controllability is in question (Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001; Weisz et al., 1994). Therefore understanding more about how children appraise uncertainty and their capacity to mobilize strategies to cope with it are critical to planning interventions to assist children in managing their uncertainty and improving their psychological adjustment to illness.
Acknowledgments
We wish to thank the pediatric oncology teams at Duke University Children’s Hospital, Durham NC; Wake Forest University Baptist Medical Center, Winston-Salem, NC; Children’s Hospital of Atlanta, GA; and University of North Carolina Hospitals, Chapel Hill, NC, for their assistance with participant recruitment. This study was conducted as part of Dr. Stewart’s doctoral dissertation at the University of North Carolina at Chapel Hill School of Nursing and supported by a research grant from the Oncology Nursing Society Foundation/Amgen, Inc., pre-doctoral fellowships from the National Cancer Institute (NCI R25 CA57726-07), the National Institute of Child Health and Human Development (NIH 1 T32 HD07376-15), and the National Institute of Nursing Research (NIH 1 T32 NR07091-01), and the American Cancer Society’s Doctoral Scholarship in Cancer Nursing.
Contributor Information
Janet L. Stewart, University of Pittsburgh School of Nursing, PA.
Merle H. Mishel, University of North Carolina at Chapel Hill School of Nursing.
Mary R. Lynn, University of North Carolina at Chapel Hill School of Nursing.
Lauren Terhorst, University of Pittsburgh School of Nursing, PA.
References
- Bailey DE, Jr, Stewart JL. Merle Mishel: Uncertainty in illness. In: Tomey AM, Alligood MR, editors. Nursing theorists and their work. 7. St. Louis, MO: Mosby; 2009. pp. 623–642. [Google Scholar]
- Bearison DJ. They never want to tell you. Cambridge, MA: Harvard University Press; 1991. [Google Scholar]
- Bennett DS. Depression among children with chronic medical problems: A meta-analysis. Journal of Pediatric Psychology. 1994;19:149–169. doi: 10.1093/jpepsy/19.2.149. [DOI] [PubMed] [Google Scholar]
- Bibace R, Walsh ME. Development of children’s concepts of illness. Pediatrics. 1980;66:912–917. [PubMed] [Google Scholar]
- Byrne BM. Structural equation modeling with AMOS. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Charron-Prochownik D. Special needs of the chronically ill child during middle childhood: Application of a stress-coping paradigm. Journal of Pediatric Nursing. 2002;17:407–413. doi: 10.1053/jpdn.2002.129792. [DOI] [PubMed] [Google Scholar]
- Clarke-Steffen L. Reconstructing reality: Family strategies for managing childhood cancer. Journal of Pediatric Nursing. 1997;12:278–287. doi: 10.1016/S0882-5963(97)80045-0. [DOI] [PubMed] [Google Scholar]
- Cohen MH. The unknown and the unknowable - Managing sustained uncertainty. Western Journal of Nursing Research. 1993;15:77–96. doi: 10.1177/019394599301500106. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127:87–127. [PubMed] [Google Scholar]
- Crisp J, Ungerer JA, Goodnow JJ. The impact of experience on children’s understanding of illness. Journal of Pediatric Psychology. 1996;21:57–72. doi: 10.1093/jpepsy/21.1.57. [DOI] [PubMed] [Google Scholar]
- De Graves S, Aranda S. Living with hope and fear – the uncertainty of childhood cancer after relapse. Cancer Nursing. 2008;31:292–301. doi: 10.1097/01.NCC.0000305745.41582.73. [DOI] [PubMed] [Google Scholar]
- DeMaso DR, Spratt EG, Vaughan BL, D’Angelo EJ, Van der Feen JR, Walsh E. Psychological functioning in children and adolescents undergoing radiofrequency catheter ablation. Psychosomatics. 2000;41:134–139. doi: 10.1176/appi.psy.41.2.134. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5. Philadelphia: J B Lippincott; 2006. pp. 1–12. [Google Scholar]
- Haase JE, Rostad M. Experiences of completing cancer therapy: Children’s perspectives. Oncology Nursing Forum. 1994;21:1483–1492. [PubMed] [Google Scholar]
- Hergenrather JR, Rabinowitz M. Age-related differences in the organization of children’s knowledge of illness. Developmental Psychology. 1991;27:952–959. [Google Scholar]
- Jensen EW, James SA, Boyce WT, Hartnett SA. The Family Routines Inventory: Development and validation. Social Science & Medicine. 1983;17:201–211. doi: 10.1016/0277-9536(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 1998. [Google Scholar]
- Knafl K, Deatrick JA, Gallo AM. The interplay of concepts, data, and methods in the development of the Family Management Style Framework. Journal of Family Nursing. 2008;14:412–428. doi: 10.1177/1074840708327138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koocher G, O’Malley J. The Damocles Syndrome: Psychosocial consequences of surviving childhood cancer. New York: McGraw-Hill; 1981. [Google Scholar]
- Kovacs M. The Child Depression Inventory. Psychopharmocological Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lavigne JV, Faier-Routman J. Psychological adjustment to pediatric physical disorders: A meta-analytic review. Journal of Pediatric Psychology. 1992;17:133–157. doi: 10.1093/jpepsy/17.2.133. [DOI] [PubMed] [Google Scholar]
- Loney JC. Anxiety and depressive symptoms in children presenting with first seizure. Pediatric Neurology. 2008;39:236–240. doi: 10.1016/j.pediatrneurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Mishel MH. Parents’ perception of uncertainty concerning their hospitalized child. Nursing Research. 1983;32:324–330. [PubMed] [Google Scholar]
- Mishel MH. Uncertainty in illness. Image: Journal of Nursing Scholarship. 1988;20:225–231. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Mishel MH. Uncertainty in acute illness. Annual Review of Nursing Research. 1997a;15:57–80. [PubMed] [Google Scholar]
- Mishel MH. Uncertainty in Illness scales manual. 1997b. Available upon request from the author. [Google Scholar]
- Mishel MH. Uncertainty in chronic illness. Annual Review of Nursing Research. 1999;17:269–294. [PubMed] [Google Scholar]
- Neville K. The relationships among uncertainty, social support, and psychological distress in adolescents recently diagnosed with cancer. Journal of Pediatric Oncology Nursing. 1998;15:37–46. doi: 10.1177/104345429801500106. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Fears TR, Wexler LH, McClure LL, Wilson DL, McCalla JL, et al. Experiences of cancer in children and adolescents. Cancer Nursing. 1996;19:54–59. doi: 10.1097/00002820-199602000-00007. [DOI] [PubMed] [Google Scholar]
- Pela OA, Reynolds CR. Cross-cultural application of the Revised Children’s Manifest Anxiety Scale: Normative and reliability data for Nigerian primary school children. Psychological Reports. 1982;51:1135–1138. doi: 10.2466/pr0.1982.51.3f.1135. [DOI] [PubMed] [Google Scholar]
- Perrin S, Last CG. Do childhood anxiety measures measure anxiety? Journal of Abnormal Clinical Psychology. 1992;20:567–578. doi: 10.1007/BF00911241. [DOI] [PubMed] [Google Scholar]
- Phipps S, Srivastava DK. Repressive adaptation in children with cancer. Health Psychology. 1997;16:521–528. doi: 10.1037//0278-6133.16.6.521. [DOI] [PubMed] [Google Scholar]
- Reynolds CR. Concurrent validity of What I Think and Feel: The Revised Children’s Manifest Anxiety Scale. Journal of Consulting and Clinical Psychology. 1980;48:774–775. doi: 10.1037//0022-006x.48.6.774. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I Think and Feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Santacroce SJ, Lee Y. Uncertainty, posttraumatic stress, and health behavior in young adult cancer survivors. Nursing Research. 2006;55:250–266. doi: 10.1097/00006199-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Seligman LD, Ollendick TH, Langley AK, Baldacci HB. The utility of measures of child and adolescent anxiety: A meta-analytic review of the Revised Children’s Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. Journal of Clinical Child and Adolescent Psychology. 2004;33:557–565. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- Stewart JL. Children living with chronic illness: An examination of their stressors, coping responses, and health outcomes. Annual Review of Nursing Research. 2003a;21:203–243. [PubMed] [Google Scholar]
- Stewart JL. “Getting used to it”: Children finding the ordinary and routine in the uncertain context of cancer. Qualitative Health Research. 2003b;13:394–407. doi: 10.1177/1049732302250336. [DOI] [PubMed] [Google Scholar]
- Stewart JL. Unpublished dissertation. University of North Carolina; Chapel Hll: 2003c. Test of a conceptual model of uncertainty in children with cancer. [Google Scholar]
- Stewart JL, Lynn MR, Mishel MH. Psychometric evaluation of a new instrument to measure uncertainty in children with cancer. Nursing Research. doi: 10.1097/NNR.0b013e3181d1a8d5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Mishel MH. Uncertainty in childhood illness: A synthesis of the parent and child literature. Scholarly Inquiry for Nursing Practice. 2000;14:299–319. [PubMed] [Google Scholar]
- Thompson RJ, Jr, Gustafson KE. Adaptation to chronic childhood illness. Washington, DC: American Psychological Association; 1996. [Google Scholar]
- Wallander JL, Thompson RJ., Jr . Psychosocial adjustment of children with chronic physical conditions. In: Roberts MC, editor. Handbook of pediatric psychology. 2. New York: Guilford Press; 1995. pp. 124–141. [Google Scholar]
- Weekes DP, Kagan SH. Adolescents completing cancer therapy: Meaning, perception, and coping. Oncology Nursing Forum. 1994;21:663–670. [PubMed] [Google Scholar]
- Weisz JR. Development of control-related beliefs, goals, and styles in childhood and adolescence: A clinical perspective. In: Rodin J, Schooler C, Schaie KW, editors. Self-directedness: Cause and effects throughout the life course. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 147–154. [Google Scholar]
- Weisz JR, McCabe MA, Dennig MD. Primary and secondary control among children undergoing medical procedures: Adjustment as a function of coping style. Journal of Consulting and Clinical Psychology. 1994;62:324–332. doi: 10.1037//0022-006x.62.2.324. [DOI] [PubMed] [Google Scholar]
- White MM, Chaney JM, Mullins LL, Wagner JL, Hommel KA, Andrews NR, et al. Children’s perceived illness uncertainty as a moderator in the parent-child distress relation in juvenile rheumatic diseases. Rehabilitation Psychology. 2005;50:224–231. [Google Scholar]
- Wood BL, Lim J, Miller BD, Cheah PA, Simmens S, Stern T, et al. Family emotional climate, depression, emotional triggering of asthma, and disease severity in pediatric asthma: Examination of pathways of effect. Journal of Pediatric Psychology. 2007;32:542–551. doi: 10.1093/jpepsy/jsl044. [DOI] [PubMed] [Google Scholar]