Abstract
Tissue fibrosis is believed to be a manifestation of dysregulated repair following injury, in association with impaired reepithelialization, and aberrant myofibroblast activation and proliferation. Numerous pathways have been linked to the pathogenesis of fibrotic lung disease, including the death receptor Fas, which contributes to apoptosis of lung epithelial cells. A redox imbalance also has been implicated in disease pathogenesis, although mechanistic details whereby oxidative changes intersect with profibrotic signaling pathways remain elusive. Oxidation of cysteines in proteins, such as S-glutathionylation (PSSG), is known to act as a regulatory event that affects protein function. This manuscript will discuss evidence that S-glutathionylation regulates death receptor induced apoptosis, and the potential implications for cysteine oxidations in the pathogenesis of in fibrotic lung disease.
Keywords: S-glutathionylation, glutaredoxin, Fas, apoptosis, fibrosis, lung
A role for oxidants in pulmonary injury and fibrosis
A number of inhaled agents that include hyperoxia, mineral dust, cigarette smoke, radiation, etc. cause acute injury, and subsequent interstitial fibrotic reactions.1 These agents are all well known to cause oxidative stress, highlighting the possibility that changes in the oxidative environment may contribute to chronic remodeling of the lung and tissue fibrosis.1 Oxidants, such as superoxide, hydrogen peroxide (H2O2), and nitric oxide are generated by NADPH oxidases and nitric oxide synthases, respectively, and can give rise to other more damaging species, such as the hydroxyl radical, hypochlorous acid, and nitrogen dioxide. Although it is well established that the aforementioned environmental agents, and inflammatory cells are responsible for the excessive production of these oxidant species, the concept that mild oxidants, produced by structural cells of the lung, can function as signaling molecules has emerged more recently.2
Protection from oxidative damage, or oxidations is achieved by an extensive battery of classical antioxidant enzymes (catalase, glutathione peroxidase, superoxide dismutases), complemented with nonenzymatic factors (urate, vitamins, glutathione), metal-binding proteins (metallothioneine, others), and enzymes involved in glutathione homeostasis. In addition, other enzyme systems that also regulate the redox homeostasis were relatively recently identified and include peroxiredoxins, sulfiredoxins, thioredoxins, and glutaredoxins (Grxs).1,2 It is of relevance to note that a number of these enzymes are regulated by a redox-sensitive transcription factor, Nrf2. Intriguingly, mice lacking Nrf2 are more sensitive to the development of bleomycin-induced fibrosis.3 Similarly, mice that lack the antioxidant enzyme, extracellular superoxide dismutase also had enhanced collagen deposition in their lungs after exposure to asbestos,4 while on the other hand, administration of the glutathione precursor and antioxidant, N-Acetyl-L-cysteine protects against bleomycin-induced fibrosis,5 highlighting the causal involvement of oxidative imbalances in the process of fibrogenesis. It was recently demonstrated that the profibrotic cytokine transforming growth factor-beta (TGF-β), induces activation of the nonphagocytic oxidase-4 (NOX4), and that the resulting production of H2O2 was important in causing differentiation of myofibroblasts, extracellular matrix production, and contractility. Importantly, genetic or pharmacologic inhibition of NOX4-attenuated collagen deposition in two independent models of fibrosis, highlighting the functional importance of NOX4-derived H2O2 in fibrogenesis.6 These experimental findings are corroborated by an increase in multiple parameters of oxidative stress in samples from patients with idiopathic pulmonary fibrosis (IPF).7 Furthermore, other studies have demonstrated that antioxidant enzymes are lowered in fibrotic lesions of IPF lungs, while elevations are present in areas of epithelial regeneration (reviewed in Ref. 1), suggesting that the lowered antioxidant complement within the fibrotic areas many contribute to the remodeling process. Levels of GSH are decreased in epithelial lining fluid from patients with IPF. It is of significance to note that antioxidant therapies have been suggested for patients with IPF, and a recent clinical trial of the IFI-GENIA study group that involved administration of the GSH precursor N-Acetyl-L-cysteine has shown some clinical efficacy in terms of preserving vital capacity and single-breath carbon monoxide diffusing capacity at 6 and 12 months.8
S-glutathionylation and glutaredoxin: a newly recognized module in redox signaling
In contrast to their role in causing injury, a role of oxidants as signaling molecules and response modifiers also has emerged and the importance of H2O2 herein is becoming apparent. Indeed, stimulation of growth factor receptors, toll-like receptors (TLRs), and cytokine receptors, leads to the activation of signaling cascades that are regulated by H2O2 as a result of activation of nonphagocytic oxidases, or dual oxidase (DUOX).9 The expression of multiple isoforms of these enzymes in tissues provides evidence that the deliberate production of low levels of oxidants is a feature of many cells.10 H2O2 exerts its regulatory role in signal transduction by causing precisely regulated and targeted oxidation of thiol sulfhydryl groups in a low pKa, thiolate state. It is important to highlight that numerous classes of proteins contain free-reactive cysteine residues that are highly conserved across species. These cysteine residues have the potential to become reversibly oxidized by H2O2, leading to the formation of sulfenic acid (SOH) residues, disulfides (S-S), or S-glutathionylated residues.2 Thus, cysteines are currently believed to serve as molecular switches, capable of processing different redox-based signals into distinct functional responses.
The main function of glutathione is to maintain the reduced state of cellular protein thiol groups, accomplished in part through the function of glutathione peroxidases. Under conditions of oxidative stress, glutathione can form mixed S-S with protein thiol groups, causing reversible S-glutathionylation (also known as S-glutathiolation, mixed S-S, or PSSG).11 S-glutathionylation of thiols can confer protection against further irreversible oxidations. However, if the targeted cysteine is a functionally critical amino acid, S-glutathionylation has the potential to modify protein function. For instance, S-glutathionylation of the p50 subunit of NF-κB12 has been linked to repression of DNA-binding activity of this transcription factor. The activities of protein kinase C,13 IKKβ,14 among others are also inhibited via by S-glutathionylation, while on the other hand S-glutathionylation of SERCA,15 Ras16 enhances activity of these proteins. These contrasting results demonstrate that the outcome of S-glutathionylation depends on the protein that is targeted. Since S-glutathionylation has been established to alter protein function, it is considered a posttranslational modification through which oxidants can transduce signals and serve as second-messenger molecules. The relevance of S-glutathionylation to cell biology is further bolstered by the existence of mammalian Grxs, also known as thiol transferases, which under physiological settings catalyze the specific reversal of the glutathionylated moiety to the sulfhydryl group, thereby rapidly restoring protein function17,18 (Fig. 1). Two mammalian Grx enzymes have been characterized to date; Grx1 is a cytosolic protein with a Cys-Pro-Tyr-Cys active site, whereas Grx2 contains a Cys-Pro-Phe-Cys active site and is directed to the mitochondria, and can also occur in the nucleus.2 The Grx-catalyzed reversible reduction of S-glutathionylated proteins to free sulfhydryl groups occurs through a monothiol mechanism that only depends on the N-terminal Cys22 (Cys23 in mouse Grx1), which displays an unusually low pKa (3.5).19 Cys22 will become S-glutathionylated itself in this reaction, and the reduced state of Grx will subsequently be restored using GSH coupled to GSSG reductase.19 Although Grx-catalyzed deglutathionylation is prominent, it is important to note that in certain settings Grx can also catalyze PSSG, via a mechanism that involves the glutathione thiyl radical.20
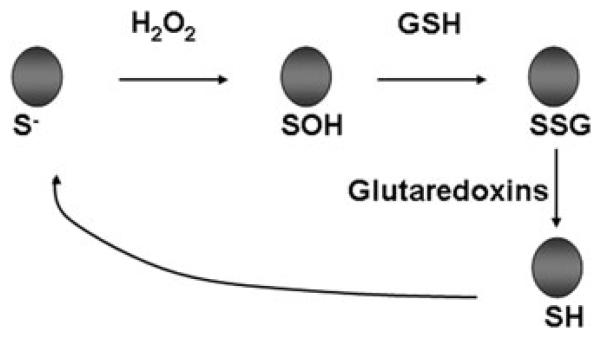
Figure 1.
Schematic representation of one of the mechanisms whereby hydrogen peroxide (H2O2) causes protein S-glutathionylation (PSSG), and reversal of PSSG catalyzed by glutaredoxins. SH = reduced sulfhydryl group. SOH represents unstable sulfenic acid intermediate. S. denotes reactive cysteine. For illustrative purposes, this is a simplified schematic and additional reactions that lead to PSSG have been identified. As is highlighted in the text, glutaredoxins catalyze reversible reactions, but under physiological conditions where high concentrations of reduced GSH exist, the predominant reaction is deglutathionylation.
Grx are among a few enzymes known to date to specifically act on an oxidative modification in an amino acid, and therefore provide an important opportunity to unravel the functional significance of a specific form of oxidative protein modification (S-glutathionylation) in cell and tissue (patho)biology. Studies on Grx enzymes in the lung are scant. Hyperoxia was found not to affect Grx expression in the lung,21 and mice systemically lacking Glrx1 did not display altered susceptibility to hyperoxia-induced acute lung injury, compared to WT mice.22 Grx expression was mainly detected in alveolar macrophages, with some expression in bronchiolar epithelium. Of relevance to the topic of this perspective is the finding that Grx expression was decreased or not detectable in fibroblast foci and other fibrotic areas of patients with IPF.23 Our laboratory has demonstrated that multiple stimuli affect expression of Grx1 but not Grx2 in primary lung epithelial cells. Increases in Grx activity and Grx1 mRNA were observed in mice with allergic airways disease. Intriguingly, the profibrotic cytokine transforming growth factor-beta 1 (TGF-β1) resulted in decreases in Grx activity and decreases in Grx1 mRNA levels in primary lung epithelial cells, in association with marked increases in PSSG content.24
The role of Fas-induced apoptosis in lung disease
Fas (CD95, Apo-1) is a member of the tumor necrosis factor receptor superfamily of death receptors that shares a conserved 80 amino acid death domain in their cytoplasmic tail critical in apoptosis signaling.25 The Fas pathway becomes activated after ligation of Fas by Fas ligand (FasL)-expressing effector cells, or by soluble FasL. Upon ligation of Fas, the sequential association of FADD, pro forms of caspases 8 and 10 occurs leading to the formation of a death-inducing signaling complex (DISC) with resulting oligomerization, processing, and activation of caspase-8, and execution of apoptosis via direct or indirect programs.26 In addition to the well-documented presence and role of Fas in cells of the immune system, Fas is also expressed on resident pulmonary cells, including airway epithelium,27 although its role in the pathophysiology of pulmonary disease is just emerging. In order to address the relevance of Fas in pulmonary disease, a number of approaches have been undertaken that involve the use of agonistic, Fas-activating antibodies, neutralization strategies, and the use of mice that systemically lack functional Fas or FasL. A key observation that linked Fas to apoptosis and subsequent development of pulmonary fibrosis was made in a study where mice were exposed to agonistic Fas antibody (which mimics cross-linking between FasL and Fas) and demonstrated apoptosis of bronchial and alveolar epithelial cells, and subsequent development of pulmonary fibrosis.28 Conversely, bleomycin-induced fibrosis could be prevented using soluble anti-Fas, or anti-Fas ligand antibodies, and did not occur in mice that lack functional Fas (lpr) or FasL (gld),29 corroborating the essential role of Fas in the development of fibrosis in the mouse.
Molecular links between Fas-induced epithelial apoptosis and the development of fibrosis
Although the mechanistic links between apoptosis and fibrosis in the lung remain to be fully unraveled, the current paradigm is that apoptosis of epithelial cells represents a key event in the subsequent development of fibroproliferative disease, and the cardinal role of caspases in bleomycin or TGF-β1-dependent apoptosis has been demonstrated.30,31 Administration of agonistic Fas antibody increases TGF-β1 expression in the lung,28 which is mechanistically relevant because of the well-known profibrotic role of TGF-β. In this regard, TGF-β1 can synergize with Fas to induce apoptosis of epithelial cells32 through mechanisms that are unclear. In patients with IPF, apoptosis has been reported in bronchiolar and alveolar epithelial cells,33 in association with upregulation of Fas expression in these cells.29 Similarly, in the bleomycin model of fibrosis excessive apoptosis has been detected in bronchiolar and alveolar epithelial cells, in association with enhanced Fas expression.34 In the lung, FasL expression has been detected on multiple cell types, including epithelial cells, macrophages, lymphocytes, neutrophils, and myofibroblasts.34,35 Recent studies have demonstrated that expression of FasL was increased in lung myofibroblasts of patients with IPF, and in lungs of mice with bleomycin-induced fibrosis. Importantly, FasL-expressing myofibroblasts themselves were resistant to Fas-induced apoptosis, and were demonstrated to be critical cytotoxic effectors that cause apoptosis in Fas-expressing lung epithelial cells.35 Broncho alveolar lavage fluids from patients with IPF were capable of inducing apoptosis in small airway epithelial cells, and causal roles for TGF-β and Fas in the induction of apoptosis were apparent.32 Myofibroblasts that were isolated from lungs of mice with bleomycin-induced fibrosis were also found to be immuno-privileged based upon findings that they could live in an allogeneic environment and were resistant to killing by FasL bearing naïve or activated T-lymphocytes.36 Collectively, these findings raise the intriguing possibility that myofibroblast-dependent killing of Fas bearing epithelial cells may underlie the lack of adequate reepithelialization, resulting in chronic wound healing that culminates in fibrosis (Fig. 2).
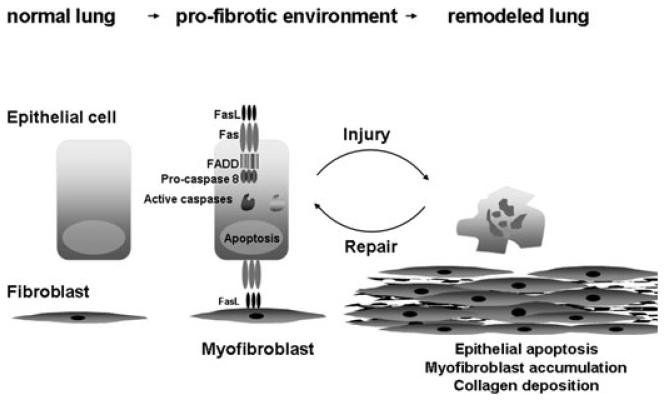
Figure 2.
Hypothetical schematic whereby Fas-induced apoptosis of epithelial cells can lead to fibrosis. Myofibroblast-dependent killing of Fas bearing epithelial cells may contribute to impaired reepithelialization, resulting in chronic wound healing that culminates in fibrosis.
Redox regulation of Fas-induced apoptosis
Because of the documented importance of both Fas-induced apoptosis and redox changes in fibrotic lung disease, the question can be raised whether these processes are functionally linked? Indeed numerous studies have demonstrated the importance of redox regulation of Fas-induced apoptosis. S-nitrosylation of caspases has been demonstrated to prevent their activation, and FasL stimulated denitrosylation of caspases which was catalyzed by thioredoxins was required for caspase activation.37,38 Our laboratory recently demonstrated that subsequent to ligation, Fas becomes S-glutathionylated on cysteine 294 in the death domain. S-glutathionylation of Fas occurs following caspases eight- and/or three-mediated degradation of Grx1, and promotes its recruitment into lipid rafts and assembly of the DISC. Importantly, overexpression of Grx1 attenuates Fas-induced apoptosis, while ablation of Grx1 increases the sensitivity of cells to undergo Fas-induced apoptosis. Thus, S-glutathionylation of Fas constitutes a feed forward signaling mechanism through which the apoptotic strength is regulated.39
Future directions
The ability to reveal reversible cysteine oxidation events in diverse protein targets enables investigators to unravel with precision how redox events control biological outcomes. While this area of investigation has classically been considered as “oxidative stress,” given the importance of reversible cysteine oxidations in biological functions and cell homeostasis, this research area perhaps is more appropriately considered as “redox biology.” This perspective has centered on S-glutathionylation and Grx as one of the modules that affect redox biology and Fas-induced apoptosis. However, many additional studies are needed to unravel the hierarchy whereby redox events control this as well as other biological outcomes, and should be aimed at the differentiation of redox events important in homeostasis versus those oxidations that control pathophysiology, and those that mediate overt damage. A reaction scheme that delineates the roles of diverse oxidation events along the spectrum from cell biology to pathology has been published.40 Additional studies also are needed to document the functional significance of reversible cysteine oxidations to the pathogenesis of diseases. This will require identification of proteins modified through reversible cysteine oxidation in tissues, and demonstration of the functional importance of such event in vivo. Those challenging endeavors will require a trans disciplinary approach that integrates redox biochemistry, transgenics, and clinical studies and are much needed not only to advance our knowledge, but also to develop therapeutic strategies aimed at restoring the balance of functional cysteine oxidations.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kinnula VL, et al. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen-Heininger YM, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho HY, et al. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 4.Fattman CL, et al. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic. Biol. Med. 2006;40:601–607. doi: 10.1016/j.freeradbiomed.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am. J. Respir. Crit. Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 6.Hecker L, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwano K, et al. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur. Respir. J. 2003;21:232–240. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 8.Demedts M, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N.Engl.J.Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 9.Sundaresan M, et al. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 10.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 11.Shackelford RE, et al. Cellular and molecular targets of protein S-glutathiolation. Antioxid. Redox Signal. 2005;7:940–950. doi: 10.1089/ars.2005.7.940. [DOI] [PubMed] [Google Scholar]
- 12.Pineda-Molina E, et al. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 13.Ward NE, et al. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry. 2000;39:10319–10329. doi: 10.1021/bi000781g. [DOI] [PubMed] [Google Scholar]
- 14.Reynaert NL, et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc.Natl.Acad.Sci.USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi T, et al. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 16.Adachi T, et al. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 17.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, et al. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry. 1998;37:17145–17156. doi: 10.1021/bi9806504. [DOI] [PubMed] [Google Scholar]
- 20.Starke DW, Chock PB, Mieyal JJ. Glutathionethiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J. Biol. Chem. 2003;278:14607–14613. doi: 10.1074/jbc.M210434200. [DOI] [PubMed] [Google Scholar]
- 21.Barrios R, et al. Oxygen-induced pulmonary injury in gamma-glutamyl transpeptidase-deficient mice. Lung. 2001;179:319–330. doi: 10.1007/s004080000071. [DOI] [PubMed] [Google Scholar]
- 22.Ho YS, et al. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic. Biol. Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltoniemi M, et al. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum. Pathol. 2004;35:1000–1007. doi: 10.1016/j.humpath.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Reynaert NL, Wouters EF, Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am. J. Respir. Cell Mol Biol. 2007;36:147–151. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peter ME, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 26.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 27.Hamann KJ, et al. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am. J. Respir. Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- 28.Hagimoto N, et al. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am. J. Respir. Cell Mol Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 29.Kuwano K, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J. Clin. Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CG, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwano K, et al. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 32.Hagimoto N, et al. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J. Immunol. 2002;168:6470–6478. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 33.Kuwano K, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am.J.Respir.Crit.CareMed. 1996;154:477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 34.Hagimoto N, et al. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Cell Mol Biol. 1997;16:91–101. doi: 10.1165/ajrcmb.16.1.8998084. [DOI] [PubMed] [Google Scholar]
- 35.Golan-Gerstl R, et al. Epithelial cell apoptosis by fas ligand-positive myofibroblasts in lung fibrosis. Am. J. Respir. Cell Mol Biol. 2007;36:270–275. doi: 10.1165/rcmb.2006-0133OC. [DOI] [PubMed] [Google Scholar]
- 36.Wallach-Dayan SB, Golan-Gerstl R, Breuer R. Evasion of myofibroblasts from immune surveillance: a mechanism for tissue fibrosis. Proc. Natl. Acad. Sci. USA. 2007;104:20460–20465. doi: 10.1073/pnas.0705582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benhar M, et al. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mannick JB, et al. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 39.Anathy V, et al. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J. Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forrester MT, Stamler JS. A classification scheme for redox-based modifications of proteins. Am. J. Respir. Cell Mol Biol. 2007;36:135–137. doi: 10.1165/rcmb.2006-001ED. [DOI] [PubMed] [Google Scholar]