Abstract
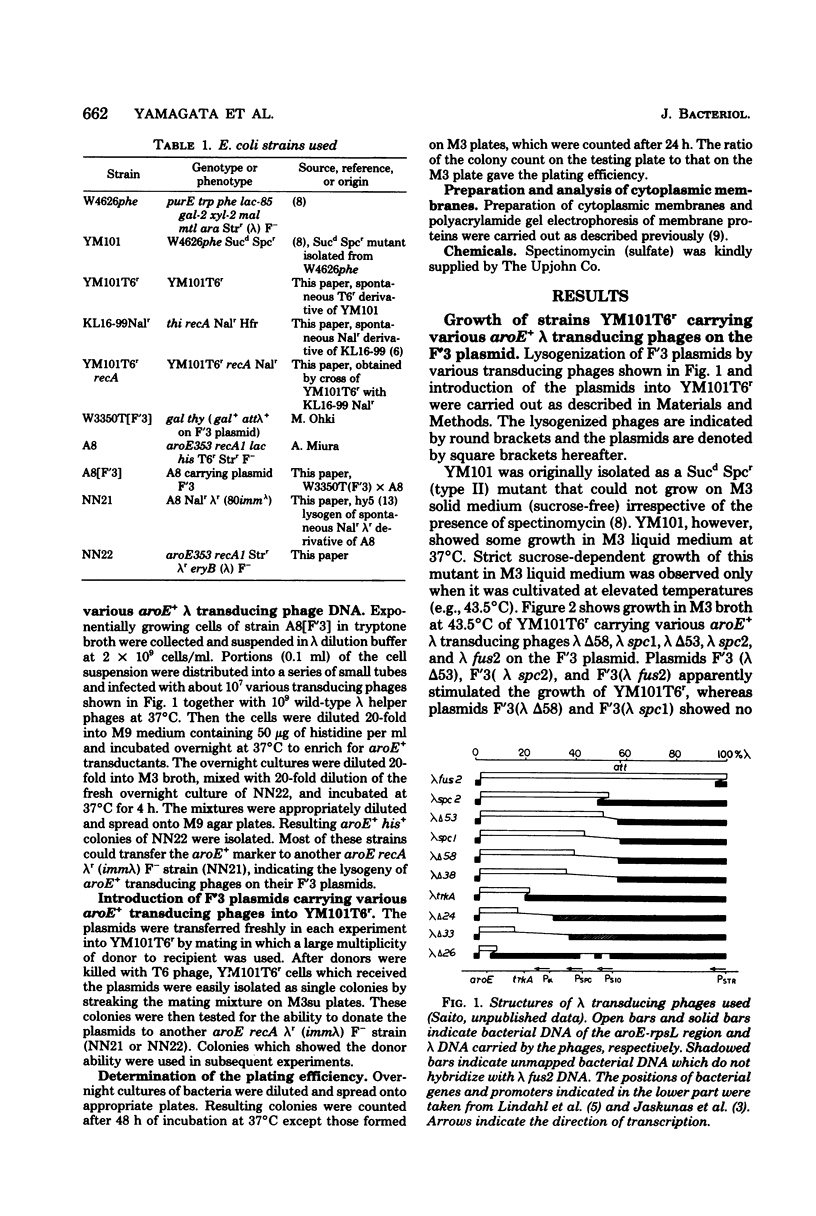
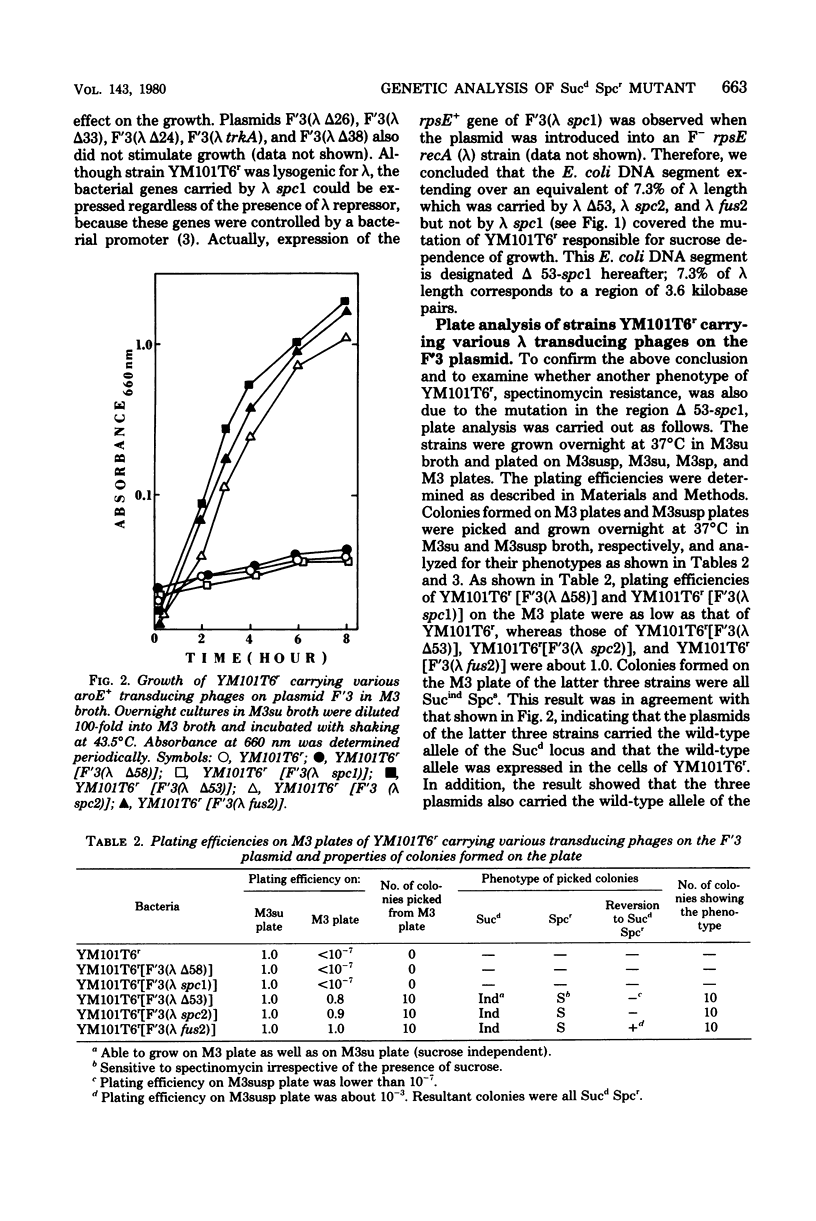
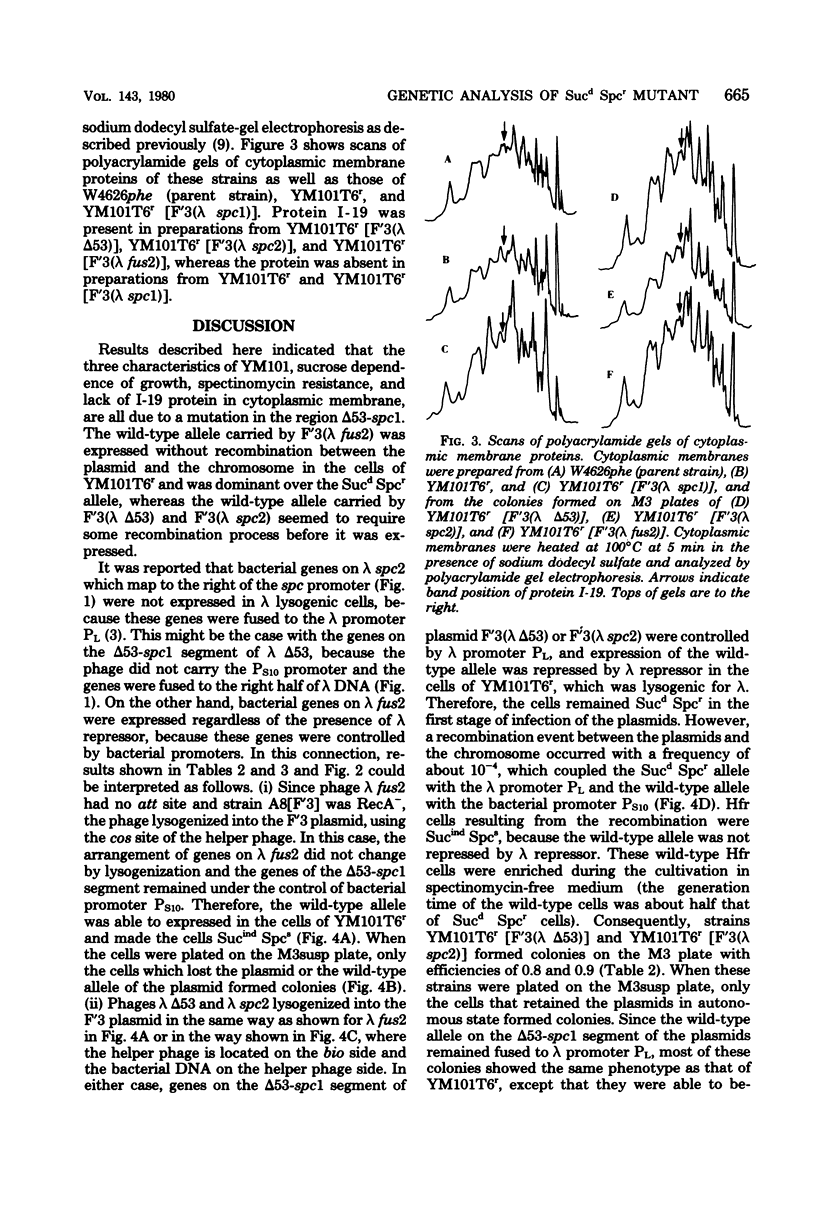
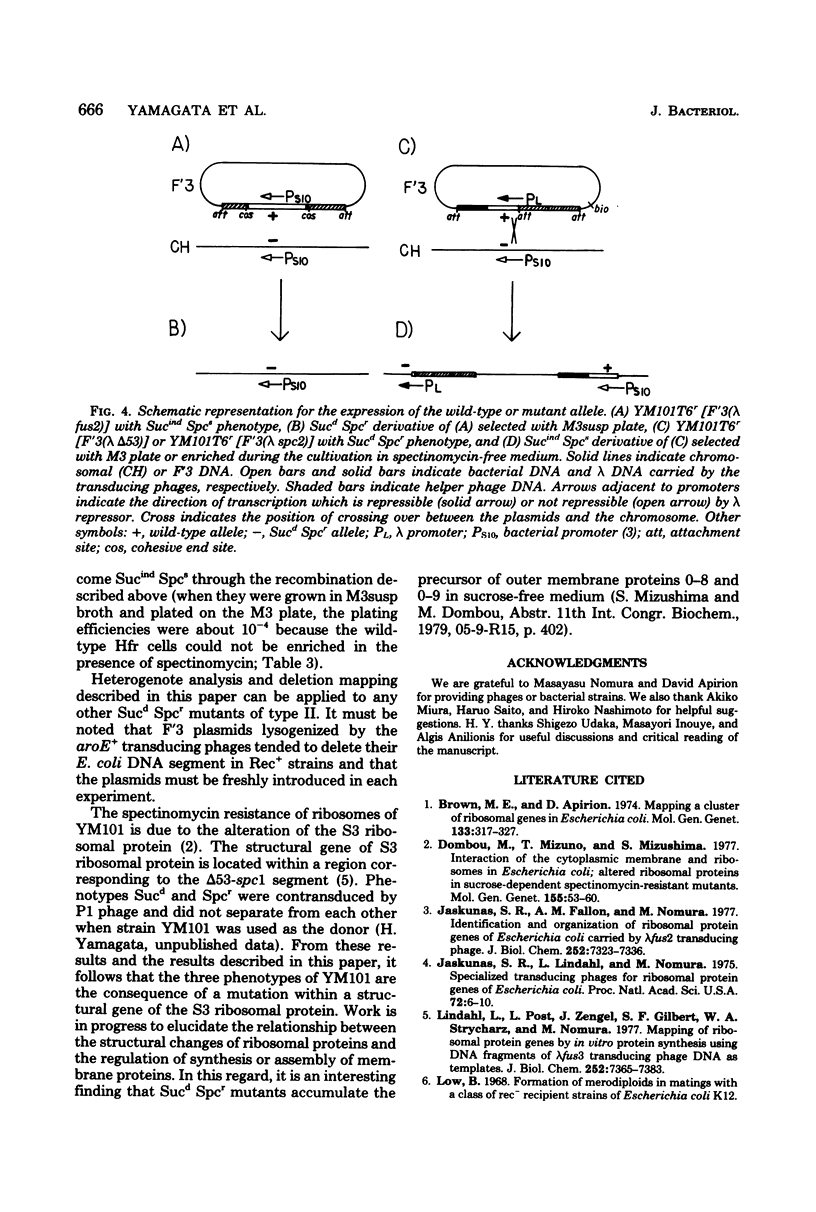
Lambda transducing phages carrying Escherichia coli deoxyribonucleic acid of various lengths from the aroE-rpsL region were lysogenized into the F'3 plasmid and were used for heterogenote analysis of YM101, a sucrose-dependent, spectinomycin-resistant mutant of E. coli. Three characteristics of the mutant strain, resistance to spectinomycin, sucrose dependence of growth, and lack of I-19 protein in the cytoplasmic membrane, were shown to be the result of a mutation in a region designated delta 53-spcl. This region extends over 3.6-kilobase pairs and is located within a cluster of ribosomal genes. The mutation is recessive to the wild-type allele.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. E., Apirion D. Mapping a cluster of ribosomal genes in Escherichia coli. Mol Gen Genet. 1974;133(4):317–327. doi: 10.1007/BF00332707. [DOI] [PubMed] [Google Scholar]
- Dombou M., Mizuno T., Mizushima S. Interaction of the cytoplasmic membrane and ribosomes in Escherichia coli; altered ribosomal proteins in sucrose-dependent spectinomycin-resistant mutants. Mol Gen Genet. 1977 Sep 21;155(1):53–60. doi: 10.1007/BF00268560. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Fallon A. M., Nomura M. Identification and organization of ribosomal protein genes of Escherichia coli carried by lambdafus2 transducing phage. J Biol Chem. 1977 Oct 25;252(20):7323–7336. [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Specialized transducing phages for ribosomal protein genes of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jan;72(1):6–10. doi: 10.1073/pnas.72.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Post L., Zengel J., Gilbert S. F., Strycharz W. A., Nomura M. Mapping of ribosomal protein genes by in vitro protein synthesis using DNA fragments of lambdafus3 transducing phage DNA as templates. J Biol Chem. 1977 Oct 25;252(20):7365–7383. [PubMed] [Google Scholar]
- Miura A., Tomizawa J. I. Studies on radiation-sensitive mutants of E. coli. 3. Participation of the rec system in induction of mutation by ultraviolet irradiation. Mol Gen Genet. 1968;103(1):1–10. doi: 10.1007/BF00271151. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Yamagata H. Sucrose-dependent spectinomycin-resistant mutants of Escherichia coli. J Bacteriol. 1976 Jan;125(1):142–148. doi: 10.1128/jb.125.1.142-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Yamada H., Yamagata H., Mizushima S. Coordinated alterations in ribosomes and cytoplasmic membrane in sucrose-dependent, spectinomycin-resistant mutants of Escherichia coli. J Bacteriol. 1976 Feb;125(2):524–530. doi: 10.1128/jb.125.2.524-530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Yamagata H., Mizushima S. Interaction of cytoplasmic membrane and ribosomes in Escherichia coli: spectinomycin-induced disappearance of membrane protein I-19. J Bacteriol. 1977 Jan;129(1):326–332. doi: 10.1128/jb.129.1.326-332.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto H., Nomura M. Structure and function of bacterial ribosomes. XI. Dependence of 50S ribosomal assembly on simultaneous assembly of 30S subunits. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1440–1447. doi: 10.1073/pnas.67.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer J., Thomas R., Radding C. M. Hybrids of bacteriophages lambda and phi 80: a study of nonvegetative functions. Virology. 1969 Apr;37(4):585–596. doi: 10.1016/0042-6822(69)90276-1. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]



