Abstract
Purinergic signaling is a crucial component of disease whose pathophysiological basis is now well established. This review focuses on P2X7, a unique bifunctional purinoreceptor that either opens a non selective cation channel or forms a large, cytolytic pore depending on agonist application and leading to membrane blebbing and to cell death either by necrosis or apoptosis.
Activation of P2X7 receptor has been shown to stimulate the release of multiple proinflammatory cytokines by activated macrophages, with the IL-1b to be the most extensively studied among them. These findings were verified by the use of knockout P2X7 (-/-) mice.
Update information coming from all fields of research implicate this receptor at the very heart of diseases such as rheumatoid arthritis, multiple sclerosis, depression, Alzheimer disease, and to kidney damage, in renal fibrosis and experimental nephritis.
Clinical studies are currently underway with the newly developed selective antagonists for P2X7 receptor, the results of which are eagerly anticipated. These studies together with data from in-vivo experiments with the P2X7 knockout mice and in-vitro experiments will shed light in this exciting area.
Keywords: P2X7, NLRP3 inflammasome, IL-1b ATP, purinergic signaling, purinergic receptors, review
Whilst conducting experiments in the guinea-pig taenia coli, G. Burnstock, in the early 70s, observed the presence of a third, non adrenergic, non cholinergic neurotransmitter, and claimed it to be the extracellular Adenosine- 5- Triphosphate (ATP)1,2. Two decades later, the discovery of purinergic receptors3 shed light on the mechanism underlying this purinergic signaling and demonstrated that ATP exerted its multiple actions via specific purinoreceptors located in plasma membranes.
In 1994, classification and nomenclature of purinergic receptors, based on their pharmacological properties, revealed the existence of a distinct P1 adenosine receptor and P2 receptors, further divided into P2X ionotropic receptors and P2Y metabotropic G-coupled protein receptors4–7.
Purinergic signaling was finally accepted as a crucial component of disease and was found to mediate a vast array of biological processes such as neuronal transmission, signal transduction to the cardiovascular system, mediators of exocrine and endocrine functions, and involvement in immunity, inflammation and cancer8.
ATP and purinergic receptors
ATP is constantly generated intracellularly by mitochondrial oxidase phosphorylation and via cytolytic glycolysis and stored in the cytoplasm of cells such as platelets9 macrophages10, microglia11 and activated immune cells in concentrations that reach molar range (3M).
ATP cannot be transported across lipid bilayers by simple diffusion because of its size and charge and is currently thought to exit cells either through vesicular transport or channel-mediated release like the ATP-binding cassette transporters, gap junction hemichannels, connexin hemichannels (CX), and anion channels such as: cystic fibrosis transmembrane conductance regulator (CFTR), volume sensitive outwardly rectifying (VSOR) and maxi-anion channels12.
Dying13 or stressed cells secondary to hypoxia, ischemia, osmotic swelling and mechanical stimulation14,15 may release high concentrations of ATP into the pericellular space. The regulated release of ATP plays an essential role in autocrine and/or paracrine cell-to-cell signaling. As soon as ATP successfully crosses the plasma membrane borders, it is rapidly degraded by the ubiquitously extracellurly present ectonucleotidases16. In these compartments ATP resynthesising enzymes known as kinases, has been documented.
Purinergic receptors: classification, nomenclature, cloning
All cells express plasma membrane receptors for extracellular nucleotides called the purinergic receptors3. These receptors are classified as P1 and P2 receptors. P1- adenisine receptors activated by adenosine, include four cloned members; A1, A2B, A2A, and A317. P2 receptors are further subdivided into P2X receptors known as ionotropic purinergic receptors, that include seven members P2X7 and the P2Y metabotropic receptors activated by ATP and Adenosine Diphosphate (ADP). P2Y receptors have eight different forms6. The discovery of P1 adenosine receptor was first proposed by Burnstock in 1978 and later the distinction of P2 receptors in P2X and P2Y was based on their separate pharmacological properties7.
Structure and properties of P2X7 receptor
P2X7 receptor is a protein that results from the homomerization of three subunits18 and was first cloned from a rat brain library19, then from human monocytes20 and finally from mouse microglia cells21. P2X7 is mainly expressed in cells of the haemopoetic lineage such as: antigen- presenting immune cells and epithelia, monocytes/ macrophages, leukocytes, red cells, fibroblasts, dendritic cells, keratinocytes, astrocytes, microglia, lymphocytes, mast cells and Langerhans cells of the epidermis20,22,24.
P2X7 receptor constitutes from:
Two transmembrane protein domains with 472 amino acids.
A long extracellular loop, with 10 residues of cysteine, that may form disulphide bonds25. The disulphide bonds are thought to be ATP-binding sites26 positive charged residues of lysine, 2-6 posts of glykosylation.
Two intracellular termini, one amino N-terminus and one carboxyl, C-terminus19,27. The C-terminus in P2X7 is at least 120 amino acids longer than the rest of its family members28.
Ion current properties
Brief exposure to agonist ATP leads to the opening of cation channel that permits K+ efflux and Ca2+ and Na+ influx into the cells. This causes a major disturbance to the ionic gradient across the plasma membrane allowing calcium influx into the cell, thus triggering several intracellular signaling cascades.
Recently another agonist was found to activate P2X7 receptor , and this was cathelicidin (LL37) which is a potent antimicrobial peptide produced predominantly by neutrophils and epithelial cells. LL37 has been shown to activate the P2X7 receptor at much lower concentrations than ATP and promotes IL-1b processing and release without causing cytotoxicity29.
Prolonged activation of the agonist on P2X7 receptor results in the formation of a large aqueous pore permeable to molecules of a molecule mass up to 900 Da. There are also rapid membrane and mitochondrial morphological changes, cytoskeletal rearrangement, and eventual cell death30,19,31,32.
Current evidence implicates the C-terminul as a critical size for the formation of the large pore, since it interacts with 11 intracellular proteins, cytoskeletal and signal transduction proteins33. Deletion of the cytoplasmic tail did not affect ion channel properties but severely affected the ability to form a large pore and to induce activation of caspases34.
Pore formation is essential for P2X7 stimulated IL- 1b release35. The mechanism of pore formation by the P2X7 is a matter of debate. Some investigators believe that pore formation is due to an ATP-dependent increase in size of the P2X7 channel itself whilst others believe that the pore is a separate molecular structure activated by the P2X7 36, 37.
The P2X7 is non-desensitizing receptor. The pore stays open as long as it is bound by its ATP ligand. Removal of the nucleotide, by rinsing or apyrase-catalyzed hydrolysis38 causes pore closure, thus allowing reversible plasma membrane permeabilization.
The gene that encodes P2X7 protein is on chromosome 12q24 and is a highly polymorphic gene. More than 260 single nucleotide polymorphisms (SNPs) have been described in the human P2X7 gene, but only a few have been functionally characterized. Several studies39–41 have identified four loss-of-function single amino acid substitutions26,42,43. No convincing disease associations have been demonstrated for these SNPs.
Cabrini et al group has characterized the first gain-of-function polymorphism so far identified (H155Y)44. This raises the obvious question whether or not some of the actions that have been associated with P2X7 receptor may be due to the participation of more members of P2X family. Alternatively P2X7 receptors close connection to massive release of mature IL-1b by activated macrophages, may suggest these receptors acting as danger sensors, that make the critical decision of continuing inflammation to the next level or turn off inflammatory detrimental consequences thereby preventing it from becoming chronic. The responses of P2X7 to ATP and 3'-0-(4-benzoyl) benzol adenosine 5'-triphosphate (BzATP) are increased by reducing the concentration of extracellular divalent cations30,45–50 such as zinc and copper whereas, other P2X receptors are strongly potentiated or unaffected7.
IL-1beta and P2X7 receptor activation
IL-1b is a master proinflammatory cytokine that exerts a broad range of inflammatory processes regulating the host response to infections, activating macrophages and neutrophils and inducing Th1 and Th2 cellular responses51– 53. Given its detrimental role in inflammation, living organisms have developed a tight control mechanism in the release of bioactive IL-1b.
IL-1b is a leaderless protein thus it is not possible to exit the cell by using the classical Golgi route. IL-1b requires a proteolytic cleavage by the intracellular protease, caspase-154.
In recent years, increasing evidence exist about the key role that P2X7 plays in this process. The first observation was that Lipopolysaccharides (LPS) primed macrophages managed only to synthesize a 33kD precursor of IL-1b that remained in the cytosol55. The addition of ATP resulted in a massive release of the biologically active 17kD form of IL-1b to the pericellular space by macrophages and other cells56–60. Further experiments revealed that it was specifically P2X7-receptor that mediated this ATP-driven mature IL-1b release61–64. Overexpression of P2X7 receptors results in a massive release of mature IL-1b through secretory lysosomes within minutes and its absence prevents the secretion of IL-1b65.
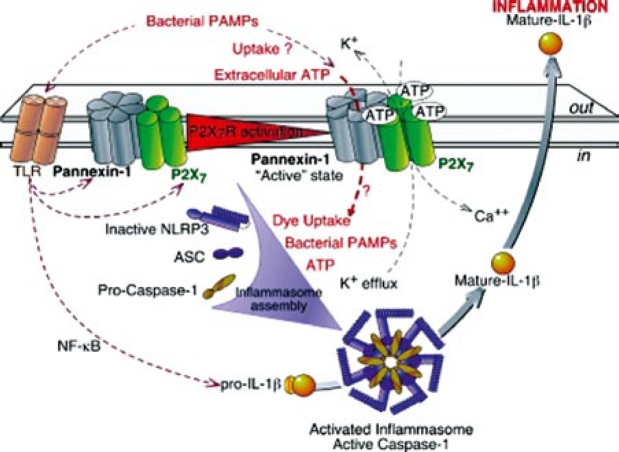
In summary the following are the major steps, currently known of the coplex IL-1b maturation and release pathway shown in Figure 1:
Figure 1. Pelegrin and Suprenant, Purinergic Signalling 2009 (with permission).
Toll-like receptors, like TLR4, become activated by pathogen associated molecular patterns (PAMPs) or lipopolysaccharide, bacterial endotoxin (LPS) and this activation leads to the production of pro-IL-1b and P2X7 and panx1 activation66–68.
When extracellular concentrations of ATP reach the micromolar range required for P2X7 activation, a non selective cation channel opens and Ca++ and Na+ influx as well as K+ efflux begins thereby decreasing intracellular K+ levels.
Panx1 is activated and allows a dye uptake pathway and bacterial PAMPs and extracellular ATP enter the cell and activate the nucleotide leukin rich polypeptide 3 (NLRP3) inflammasome - a reaction facilitated by the low intracellular K+ levels- (nucleotide binding domain and leucine rich repeat containing a pyrin domain) using the apoptosis associated speck like protein (ASC) containing caspase recruiting domain (CARD)69–72.
Once the inflammasome is assembled and activated, caspase1 is produced and enzymatically converts pro-IL- 1b to its mature form that finally exits the plasma membrane either via exocytosis or via microvesicles. Several theories propose that IL-1 is released by apoptotic cell death and shedding of microvesicles73, 74. Exocytosis of secretory lysosomes75–77 where proIL-1b is transported to endosomic vesicles together with caspase 1. In this protected compartment it is proteolytically cleaved and converted to the mature form.
The mechanism whereby this receptor is activated under physiological conditions is currently unknown78. The theory presumes the receptor acts as a danger sensor and ATP as a danger signal, as rather high ATP concentrations are required to activate P2X7-receptor. This may create a vicious cycle where the receptor decides to perpetuate or halt the inflammatory process.
P2X7-receptor and disease
There is an increasing body of evidence implicating P2X7 receptor in various pathological conditions, such as: rheumatoid arthritis and chronic obstructive pulmonary disease, where inflammation is the cornerstone of these disorders. Selective P2X7 antagonists are currently being investigated in clinical trials due to its close connection to IL-1b and TNF-a production79, 80.
In neurological disorders, studies in rodent models revealed a close connection of P2X7 to Alzheimer disease81, Parkinson's disease82, multiple sclerosis86, sensory neuropathies87, and neuropathic pain83–85. In cancer where apoptotic cell death is an important mechanism of disease, P2X7 with its direct effect in apoptosis plays a significant role as it was shown in skin cancers88,89 and uterine epithelial cancers compared to normal tissues. Perhaps P2X7 will be of future use as abiomarker to distinct normal from cancer uterine epithelial tissues90. Early apoptotic cell death to the retina in diabetes in rodent models has been linked to P2X7 activation in that part of the eye, suggesting apossible connection to diabetic microvascular injury91.
Reported data for P2X7 receptor and hypertension
A connection to aldosterone-mediated signaling to distal renal tubules was found;92
P2X7 receptor polymorphisms may be linked to hypertension. A family based quantitative genetic association study involving 248 families was performed to investigate a possible association between P2X7 genes (and P2X4, P2X6) and ambulatory blood pressure. Significant evidence of association between the single nucleotide polymorphism rs591874 in the first intron of the P2X7 gene and blood pressure was found. The strongest association was found for nocturnal diastolic blood pressure although an association was present for both systolic and diastolic blood pressures measured by an observer during the day and at night92.
Production of proinflammatory cytokines and promotion of apoptosis to endothelial cells may be linked to vascular remodeling in hypertension93. A possible link to hypotensive responses in inflammatory diseases via IL-1b release with nitric oxide synthesis may be postulated44.
P2X7 receptors are expressed in cells of the cardiovascular system and drugs affecting this signaling system may provide promising new therapies in hypertension and prevention of thrombotic events94.
Current findings for P2X7 associated with renal dysfuntion
The following findings are in favour of the purinergic signalling involvement in the pathogenesis of renal disease:
Under physiological conditions renal cells may release ATP that is found in tubular fluid and the final urine at a concentration that approximately reaches 200nM95, 98 as well as several ectonucleotidases at cell surfaces and in tubular fluid96,99.
When inflammation is present, several new events are taking place in the inflammatory milieu such as: ATP degrading enzymes are downregulated thus prolonging its action, and ionotropic P2X7 needs only milliseconds to act as it does not require a second intracellular messenger100. Large amounts of ATP are released by dying or stressed cells101,102, P2X7 activation threshold decreases thus facilitating ATP binding at lower concentrations103, 104. Proinflammatory cytokines and bacterial products upregulate P2X7 expression and increase its sensitivity to ATP105,106.
Once activated, P2X7 may become a self-activating source of ATP, as concentrations released at the cell surface of living cells reach 100200 µM108.
The combined published clinical data investigating the role of P2X7 in disease demonstrate that in pathological conditions, P2X7 activation may happen more frequently than predicted by studies derived from in vitro experiments.
Polycystic kidney disease and P2X7
Investigating nephrogenesis and renal cyst growth in a congenital mouse model of polycystic kidney disease, P2X7 was detected in metanephric mesenchyme, in the collecting ducts in the later stages of nephrogenesis and receptor's expression was obvious between cysts suggesting a non apoptotic role of this receptor in cyst enlargement107.
In a mouse model of autosomal recessive polycystic kidney disease (ARPKD) Hilman et al, activating and blocking P2X7 receptors demonstrated that the activation of this receptor reduced the number of the cysts96. P2X7 receptor's protein was upregulated in human fetal ARPKD model versus the normal fetal collecting duct.
Turner et al, investigated cyst lining cells from Han:SPRD (cy/+) rat model of polycystic kidney disease cyst lining cells, homozygotic and heterozygote rat kidneys. P2X7 receptor's mRNA was found to be increased in heterozygote (cy/+) but not in homozygote(cy/cy) rat kidneys. P2X7 receptors were clearly expressed in the above mentioned cyst lining cells of this model of renal cystic disease, and its expression was increased in the cystic tissue108.
Further investigation will determine whether the P2X7 receptors plays a role in cell turnover and tissue remodeling of the cysts108.
Renal fibrosis and P2X7
P2X7 receptors have been linked to inflammation by its association to the synthesis of IL-1b and release in activated macrophages and in rat brain astrocytes. P2X7 - receptors mRNA was detected in the kidney raising the possibility that it may be involved with TGF-β production109, a major cytokine involved in renal fibrogenesis. Solini clearly pointed out a role for P2X7 receptor activation on macrophage function and matrix formation in the models of glomerular disease used, and its association to TGF-β release110.
The molecular basis of renal fibrosis is yet to be clarified so in 2006, Goncalves et al.111 evaluated the role of this intriguing receptor to renal inflammation and fibrosis. They used a well established model of fibrosis the unilateral ureteral obstruction (UUO) that has been proven to produce a typical fibrotic picture, with infiltration of macrophages, increased extracellular matrix protein deposition and tubular atrophy112–115.
They used C57BI6 mice as a wild type, P2X7 (-/-) knockout mice and control mice. Goncalves, et al., found that in the animals lacking P2X7 receptor, tubulointerstitial injury following UUO, significantly attenuated injury compared to WT animals, as well as myofibroblasts. Collagen deposition and TGF-β expression were significantly reduced. The population of myofibroblasts was significantly reduced in the knockout mice, implying that P2X7 receptor presence is somehow involved in this epithelial to mesenchymal transition. This may involve IL-1b secretion,that has been found to promote fibroblast proliferation and collagen production116,117.
P2X7 expression in healthy and diseased kidney
Turner et al, published a paper in 2003 where they used polyclonal antibodies and immunohistochemistry to prove that there is little if any expression of P2X7 receptors in healthy kidneys. Indeed a very low level of P2X7 receptor immunoreactivity was detectable in a few glomeruli and this was the first study in native renal tissues rather than using cell cultures.
Later experiments provided evidence for P2X7 -receptor's expression in cultured mesangial cells in rodent models and showed that it can mediate ATP-induced cell death by apoptosis118,119. In cell cultures it has also been detected in mouse podocytes and medullary collecting duct cells.
Turner et al120,121 showed the distribution of P2X7 receptor in diseased renal tissue. They carried out their work in diabetic animals-rats with Streptozocin induced diabetes and hypertensive rats of transgenic (mRen2) models. This study proved expression of P2X7 receptors in the glomeruli of the two rodent models of disease and electron microscopy showed that the predominant expression was in podocytes, endothelial and mesangial cells. Altered P2X7 receptor expression and increased sensitivity to ATP-induced apoptosis have been reported in cultured fibroblasts exposed to high concentrations of extracellular glucose111.
Solini et al., also investigated the role of P2X7 receptor in fibroblasts derived from skin biopsies of diabetic patients122. They found that these fibroblasts had increased expression of P2X7 receptor, in addition to increased apoptosis, IL-6 secretion, shape changes and enhanced fibronectin, responses known to be mediated by the receptors. These finding unravel an interesting mechanism that alters the cellular and extracellular structural components of the arterial wall, and at the same time, generates a proinflammatory milieu.
Apoptosis is an important mechanism in the kidney, leading either to healing or to renal scarring, therefore regulation of this process could be important in normal tissue repair and remodeling after injury. The finding that P2X7 expression was increased in the glomeruli of the TGR2 hypertensive rodent model and also in the diabetic animals, compared to control animals, may indicate a role for the P2X7 receptor in glomerular repair by deleting damaged cells whilst simultaneously encouraging proliferation and repair.
Another study by Solini et al110 demonstrated in mesangial cell cultures that BzATP agonist of P2X7 receptors, both in normal and high glucose environment that extracellular matrix protein production and TGF- β production were increased, while the application of oxidized ATP had the opposite effect. This suggests a potential relationship between P2X7 receptor and the major profibrotic cytokine TGF-β. Harada et al used BzATP in cell cultures of mesangial cells where typical ladders of oligonucleosomal fragments of extracted DNA were yielded confirming the assay that P2X7 receptor activation in them induces apoptotic death in mesangial cells118.
P2X7 and experimental nephritis
In a mouse model of experimental nephritis, increased expression of P2X7 receptor protein levels were detected as well as increased apoprtosis in glomeruli. In the same study, increased protein levels of the receptor were detected in both tubular and glomerular cells derived from renal biopsies from patients with autoimmune related glomerulonephritis124.
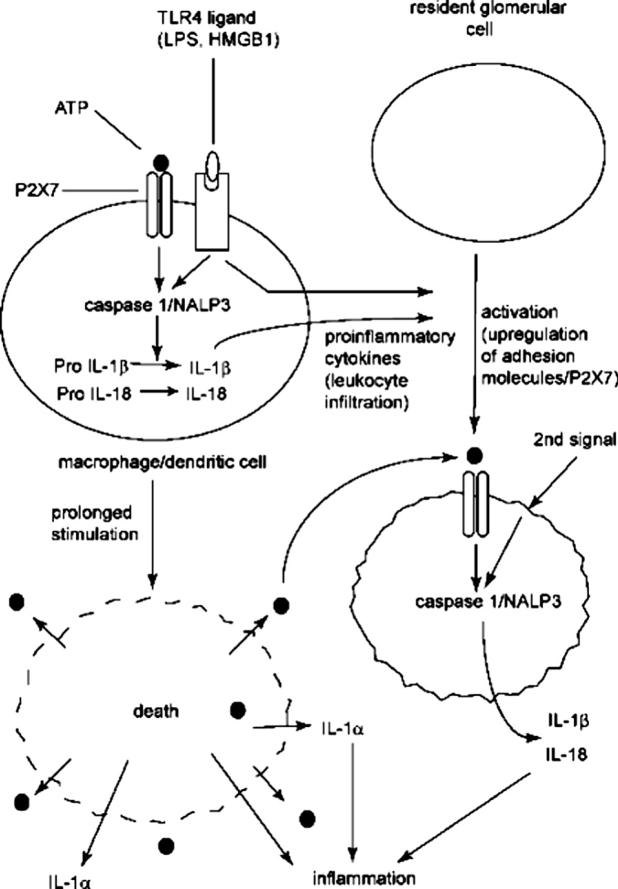
Furthermore, in a rat model of proliferative glomerulonephritis increased mRNA levels of P2X7 receptor were found to coincide with the onset of proteinuria and maximally increase mRNA levels of IL-1b on the 4th day after the injection of the nephrotoxic serum124. These findings suggest that P2X7 receptor could be an important factor in the pathogenesis of glomerulonephritis, either by the creation of apoptosis or by regulating the proinflammatory cytokines production (Figure 2).
Figure 2. A possible mechanism of perpetuating renal inflammation via the activation of P2X7. ATP binding P2X7 receptors in activated macrophages or dendritic cells activate P2X7. This may lead to: a) cell death in prolonged activation of P2X7 and exacerbation of renal inflammation or b) to the activation of resident glomerular cells and the infiltration of leukocytes by proinflammatory cytokines released under the effect of activated P2X7. c) P2X7 is further upregulated, leading to caspase 1 activation expression and NLRP3 assembly releasing 1L-1b. d) 1L-1b exacerbates renal inflammation.
More recently a novel selective antagonist (A438079) as well as the knockout P2X7(-/-) mice were used in an attempt to investigate the role of this receptor in a rodent model of experimental nephritis created with the injection of nephrotoxic serum125. Renal function, urinary MCP- 1 levels, macrophage infiltration and a dramatic increase in proteinuria were all observed in the mice lacking the P2X7 receptors comparing to wild type animals. The use of the antagonist prevented the antibody mediated glomerulonephritis in rats suggesting that in autoimmune renal injury perhaps the missing link to successful treatment involves P2X7 receptors125.
Conclusion
P2X7 receptor could be viewed as a danger sensor, a key point, where the decision whether inflammation will proceed further or not is taken.
Perhaps a new era is emerging where novel anti-analgesic and anti-inflammatory therapies would be developed specifically targeting at P2X7 receptors.
The field of P2X7- receptor might be so broad that it includes neurological disorders, such as depression and Alzheimer disease, lung disease, such as copd, rheumatoid arthritis, diabetic microvascular damage, including the area of diabetic nephropathy, renal fibrosis and experimental nephritis, and cardiovascular disease.
Many questions are yet to be answered for this intriguing receptor i.e. regarding the pore forming events and mutogenesis, mass spectroscopy, in vivo experiments with the novel selective antagonists and the knock in and knockout P2X7 mice, will further contribute to unraveling the multiple aspects of P2X7 receptor's involvement in disease.
References
- 1.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 2.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 4.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinoceptors: ontogeny and phylogeny. Drug Dev Res. 1996a;39:204–242. [Google Scholar]
- 6.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, et al. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- 8.Burnstock G. Pathophysiology and Therapeutic Potential of Purinergic Signaling. Pharmacological Reviews. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Belgi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface attached firefly luciferase. Am J Physiol Cell Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 11.Fillipini A, Taffs RE, Agui T, Sitkovsky MV. EctoATPase activity in cytolytic T lymphocytes.Protection of the cytolytic effects of extracellular ATP. J Biol Chem. 1997;265:334–340. [PubMed] [Google Scholar]
- 12.Sabirov RZ, Okada Y. ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn J Physiol. 2004;54:7–14. doi: 10.2170/jjphysiol.54.7. [DOI] [PubMed] [Google Scholar]
- 13.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol Cell Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 14.Lazarowski ER, Boudher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 15.Nieber K, Eschke D, Brand A. Brain hypoxia : effects of ATP and adenosine. Prog.Brain Res. 1999;120:287–297. doi: 10.1016/s0079-6123(08)63563-3. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 17.Schwiebert EM, Zsembery Akos, Geibel John P. Cellular mechanisms and physiology of nucleotide and nucleoside release from cells: current knowledge, novel assays to detect purinergic agonists, and future directions. Curr Top Membr. 2003;54:31–58. [Google Scholar]
- 18.Egan TM, Cox JA, Voigt MM. Molecular structure of P2X receptors. Curr.Top.Med.Chem. 2004;4:821–829. doi: 10.2174/1568026043451005. [DOI] [PubMed] [Google Scholar]
- 19.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 20.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 21.Chessel IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PP. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- 22.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–83. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 23.Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circulation Research. 1998;83:196–203. doi: 10.1161/01.res.83.2.196. [DOI] [PubMed] [Google Scholar]
- 24.Duan S, Nery JT. P2X7 receptors: properties and relevance to CNS function. Glia. 2006;15:738–746. doi: 10.1002/glia.20397. [DOI] [PubMed] [Google Scholar]
- 25.Freist W, Verhey JF, Stuhmer W, Gauss DH. ATP binding site of P2X channel proteins: structural similarities with class II aminoacyl-tRNA synthetases. FEBS Lett. 1998;434:61–6514. doi: 10.1016/s0014-5793(98)00958-2. [DOI] [PubMed] [Google Scholar]
- 26.Gu BJ, Sluyter R, SkarRatt KK, Shemon AN, Dao-Ung LP, Fuller SJJ. An Arg307 to Gln polymorphism within the ATPbinding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287–31295. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- 27.Buell GN, Talabot F, Gos A, Lorenz J, Lai E, Morris MA, Antonarakis SE. Gene structure and chromosomal localization of the human P2X7sub> receptor. Receptors Channels. 1998;5:347–354. [PubMed] [Google Scholar]
- 28.Denlinger LC, Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, et al. Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- 29.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 30.North A. Molecular physiology of P2X receptors. Physiol Rev. 2002:82–1013. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 31.Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, et al. Extracellular ATP causes ROCK 1-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14:2655–2664. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 33.Becker D, Woltersdorf R, Boldt W, Schmitz S, Braam U, Schmalzing G, Markwardt F. The P2X7 Carboxyl Tail Is a Regulatory Module of P2X7 Receptor Channel Activity. J Biol Chem. 2008;283:25725–25734. doi: 10.1074/jbc.M803855200. [DOI] [PubMed] [Google Scholar]
- 34.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 35.Sluyter R, Shemon AN, Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 release from human monocytes. J. Immunol. 2004;172:3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 36.Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG. Maitotoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. Am J Physiol. 1999;277:C766–C776. doi: 10.1152/ajpcell.1999.277.4.C766. [DOI] [PubMed] [Google Scholar]
- 37.Verhoef PA, Kertesy SB, Estacion M, Schilling WP, Dubyak GR. Maitotoxin induces biphasic interleukin-1 secretion and membrane blebbing in murine macrophages. Mol Pharmacol. 2004;66:909–920. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg TH, Silverstein SC. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987;262:3118–3122. [PubMed] [Google Scholar]
- 39.Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, et al. A Glu496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 40.Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, et al. An Ile568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- 41.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, SkarRatt KK. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2005;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 42.SkarRatt KK, Fuller SJ, Sluyter R, Dao-Ung LP, Gu BJ, Wiley JS. A 5' intronic splice site polymorphism leads to a null allele of the P2X7 gene in 12% of the Caucasian population. FEBS Lett. 2005;579:2675–2678. doi: 10.1016/j.febslet.2005.03.091. [DOI] [PubMed] [Google Scholar]
- 43.Li CM, Campbell SJ, Kumararatne DS, Bellamy R, Ruwende CSJ, McAdam KP, et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis. 2002;186:1458–1462. doi: 10.1086/344351. [DOI] [PubMed] [Google Scholar]
- 44.Chiao CW, Tostes RC, Webb RC. P2X7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1β release. J Pharmacol Exper Therap. 2008;326:864–870. doi: 10.1124/jpet.107.135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wildman SS, King BF, Burnstock G. Modulation of ATP responses at recombinant rP2X4 receptors by extracellular pH and Zinc. B J Pharmacol. 1999;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wildman SS. Modulatory activity of extracellular H+ and Zn+ on ATP responses at rP2X1 and rP2X3 receptors. B J Pharmacol. 1999;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, et al. Differential modulation by copper and zinc of P2X2 and P2X4 function. J Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]
- 48.Acua-Castillo C, Morales B, Huidobro-Toro JP. Zinc and copper modulate differentially P2X4 receptor. J Neurochem. 2000;74:1529–1537. doi: 10.1046/j.1471-4159.2000.0741529.x. [DOI] [PubMed] [Google Scholar]
- 49.Clyne JD, LaPointe LD, Hume RI. The role of histidine residues in modulation of the rat P2X2 purinoreceptor by zinc and pH. J Physiol. 2002;539:347–359. doi: 10.1113/jphysiol.2001.013244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorca RA, Coddou C, Gazita MC, Bull P, Arredondo C, Huidobro-Toro JP. Extracellular histidine residues identify common structural determinants in the copper/zinc P2X2 receptor modulation. J Neurochem. 2005;95:499–512. doi: 10.1111/j.1471-4159.2005.03387.x. [DOI] [PubMed] [Google Scholar]
- 51.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 52.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 53.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–S13. [PubMed] [Google Scholar]
- 54.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dinarello CA. Unraveling the NALP-3/IL-1 inflammasome:a big lesson from a small mutation. Immunity. 2004;20:243–244. doi: 10.1016/s1074-7613(04)00055-x. [DOI] [PubMed] [Google Scholar]
- 56.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 200;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 57.Hogquist KA, Unanue ER, Chaplin DD. Release of IL-1 from mononuclear phagocytes. J Immunol. 1991;147:2181–2186. [PubMed] [Google Scholar]
- 58.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 59.Griffiths RJ, Stam EJ, Downs JT, Otterness IG. ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol. 1995;154:2821–2828. [PubMed] [Google Scholar]
- 60.Perregaux DG, Gabel CA. Human monocyte stimulus-coupled IL-1 beta posttranslational processing: modulation via monovalent cations. Am J Physiol. 1998;275:1538–1547. doi: 10.1152/ajpcell.1998.275.6.C1538. [DOI] [PubMed] [Google Scholar]
- 61.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- 62.Ferrari D, Wesselborg S, Bauer MKA, Schulze-Osthoff K. Extracellular ATP Activates Transcription Factor NF-{kappa}B through the P2Z Purinoreceptor by Selectively Targeting NF-{kappa}B p65. p65. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 64.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key play in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 65.Gudipaty L, Munetz J, Verhoef PA, Dubyak GR. Essential role for Ca2+ in the regulation of IL-1b secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am J Physiol Cell Physiol. 2003;285:C286–C299. doi: 10.1152/ajpcell.00070.2003. [DOI] [PubMed] [Google Scholar]
- 66.Pelegrin P, Suprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxinand nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 68.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 69.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 70.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 71.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 72.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 73.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 74.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 release from microglia. J.Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 75.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc Natl Acad Sci. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol. 2001;28:340–350. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 78.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Botsios C. Safety of tumour necrosis factor and interleukin-1 blocking agents in rheumatic diseases. Autoimmunity Reviews. 2005;4:162–170. doi: 10.1016/j.autrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 80.McInnes IB, Snell NJ, Perrett JH, Parmar H, Wang MM, Astbury C. Results of a phase II clinical trial of a novel P2X7 receptor antagonist, AZD9056, in patients with active rheumatoid arthritis (CREATE study). ACR/ARHP Annual Scientific Meeting; presentation 2085 (Abstract) [Google Scholar]
- 81.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 82.Jun D, Kim K. ATP-mediated necrotic volume increase (NVI) in substantia nigra pars compacta dopaminergic neuron. Abstract Viewer/Itinerary Planner; Program 2004. 2004;222:18. Society for Neuroscience. [Google Scholar]
- 83.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fulgenzi A, Ticozzi P, Gabel CA, Dell AG, Quattrini A, Franzone JS, Ferrero ME. Periodate oxidized ATP. (oATP) reduces hyperalgesia in mice: involvement of P2X7 receptors and implications for therapy. Int J Immunopathol Pharmacol. 2008;2:61–71. doi: 10.1177/039463200802100108. [DOI] [PubMed] [Google Scholar]
- 85.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Witting A, Chen L, Cudaback E, Straiker A, Walter L, Rickman B, et al. Experimental autoimmune encephalomyelitis disrupts endocannabinoid-mediated neuroprotection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6362–6367. doi: 10.1073/pnas.0510418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sperlαgh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Progress in Neurobiology. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Fu W, McCormick T, Qi X, Luo L, Zhou L, Li X, et al. Activation of P2X(7)-mediated apoptosis Inhibits DMBA/TPA-induced formation of skin papillomas and cancer in mice. BMC Cancer. 2009;9:114. doi: 10.1186/1471-2407-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorodeski GI. P2X7-mediated chemoprevention of epithelial cancers. Expert Opin Ther Targets. 2009;11:1313–1332. doi: 10.1517/14728220903277249. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI. The P2X7 Receptor: A Novel Biomarker of Uterine Epithelial Cancers. Cancer Epidemiol Biomarkers Prev. 2006;10:1906–1913. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG, Kobayshi M. Enhancement of P2X7-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Investig Ophthalmol Vis Sci. 2004;45:1026–1032. doi: 10.1167/iovs.03-1062. [DOI] [PubMed] [Google Scholar]
- 92.Palomino-Doza J, Rahman TJ, Avery PJ, Mayosi BM, Farrall M, Watkins H, et al. Ambulatory Blood Pressure Is Associated With Polymorphic Variation in P2X Receptor Genes. Hypertension. 2008;52:980–985. doi: 10.1161/HYPERTENSIONAHA.108.113282. [DOI] [PubMed] [Google Scholar]
- 93.Gibbons GH. Autocrine-paracrine factors and vascular remodeling in hypertension. Current Opinion in Nephrology and HyHypertension. 1993;2:291–298. doi: 10.1097/00041552-199303000-00017. [DOI] [PubMed] [Google Scholar]
- 94.Ralevic V, Burnstock G. Involvement of purinergic signaling in cardiovascular diseases. Drug News and Perspectives. 2003;3:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- 95.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol. 2006;17:1841–1847. doi: 10.1681/ASN.2005111171. [DOI] [PubMed] [Google Scholar]
- 96.Vekaria RM, Shirley DG, Svigny J, Robert J. Unwin Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol. 2006;290:F550–F560. doi: 10.1152/ajprenal.00151.2005. [DOI] [PubMed] [Google Scholar]
- 97.Hillman KA, Johnson TM, Winyard PJ, Burnstock G, Unwin RJ, Woolf AS. P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol. 2002;10:34–42. doi: 10.1159/000049896. [DOI] [PubMed] [Google Scholar]
- 98.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol. 1999;10:218–229. doi: 10.1681/ASN.V102218. [DOI] [PubMed] [Google Scholar]
- 99.Belgi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface attached firefly luciferase. Am J Physiol Cell Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 100.Franke H, Gónther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, et al. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 101.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 102.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milius D, Grφger-Arndt H, Stanchev D, Lange-Dohna C, Rossner S, Sperlagh B, et al. Oxygen/glucose deprivation increases the integration of recombinant P2X7 receptors into the plasma membrane of HEK293 cells. Toxicology. 2007;238:60–69. doi: 10.1016/j.tox.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 104.Wirkner K, Kφfalvi A, Fischer W, Gónther A, Franke H, Grφger-Arndt H, et al. Supersensitivity of P2X receptors in cerebrocortical cell cultures after in vitro ischemia. J Neurochem. 2005;95:1421–1437. doi: 10.1111/j.1471-4159.2005.03465.x. [DOI] [PubMed] [Google Scholar]
- 105.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64:265–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- 106.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hillman K, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJ. The P2X7 ATP receptor modulates renal cyst development in vitro. Biochemical and Biophysical Research Communications. 2004;332:434–439. doi: 10.1016/j.bbrc.2004.07.148. [DOI] [PubMed] [Google Scholar]
- 108.Turner CM, Ramesh B, Srai SK, Burnstock G, Unwin RJ. Altered ATP-sensitive P2 receptor subtype expression in the Han: SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs. 2004;178:168–179. doi: 10.1159/000082247. [DOI] [PubMed] [Google Scholar]
- 109.Wang CM, Chang YY, Sun SH. Activation of P2X7 purinoceptor-stimulated TGF-βeta 1 mRNA expression involves PKC/MAPK signalling pathway in a rat brain-derived type-2 astrocyte cell line, RBA-2. Cell Signal. 2003;15:1129–1137. doi: 10.1016/s0898-6568(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 110.Solini A, Ricci C, Chiozzi P, Amadio L, Pricci F, Di Mario U, et al. Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int. 2005;67:875–885. doi: 10.1111/j.1523-1755.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- 111.Goncalves RG, Gabrich L, Rosrio A, Takiya CM, Ferreira MLL, Chiarini LB, et al. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 2006:70–1599. doi: 10.1038/sj.ki.5001804. [DOI] [PubMed] [Google Scholar]
- 112.Klahr S. Urinary tract obstruction. Semin Nephrol. 2001;21:133–145. doi: 10.1053/snep.2001.20942. [DOI] [PubMed] [Google Scholar]
- 113.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 114.Diamond JR. Macrophages and progressive renal disease in experimental hydronephrosis. Am J Kidney Dis. 1995;26:133–140. doi: 10.1016/0272-6386(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 115.Diamond JR, van Goor H, Ding G, Engelmyer E. Myofibroblasts in experimental hydronephrosis. Am J Pathol. 1995;146:121–129. [PMC free article] [PubMed] [Google Scholar]
- 116.Vesey DA, Cheung CW, Cuttle L, Endre ZA, Gob G, Johnson DW. Interleukin-1 induces human proximal tubule cell injury, -smooth muscle actin expression and fibronectin production. Kidney Int. 2002;62:31–40. doi: 10.1046/j.1523-1755.2002.00401.x. [DOI] [PubMed] [Google Scholar]
- 117.Vesey DA, Cheung CW, Cuttle L, Endre ZA, Gob G, Johnson DW. Interleukin-1beta stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-beta-dependent mechanism. J Lab Clin Med. 2002;140:342–350. doi: 10.1067/mlc.2002.128468. [DOI] [PubMed] [Google Scholar]
- 118.Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57:949–958. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 119.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brne B, et al. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol. 1998;275:F962–F969. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- 120.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. C Cell Tissues Organs. 2003;175:105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 121.Vovend O, Turner CM, Chan CM, Loesch A, DellAnna GC, Srai KS, et al. Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–166. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 122.Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia. 2000;43:1248–1256. doi: 10.1007/s001250051520. [DOI] [PubMed] [Google Scholar]
- 123.Solini A, Chiozzi P, Morelli A, Adinolfi E, Rizzo R, Baricordi OR, et al. Enhanced P2X7 Activity in Human Fibroblasts From Diabetic Patients.Arteriosclerosis. Thrombosis and Vascular Biology. 2004;24:1240. doi: 10.1161/01.ATV.0000133193.11078.c0. [DOI] [PubMed] [Google Scholar]
- 124.Turner CM, Tam FWK, Lai PC, Tarzi RM, Burnstock G, Pusey CD, et al. Increased expression o f the pro-apoptotic ATPsensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant. 2007;22:386–395. doi: 10.1093/ndt/gfl589. [DOI] [PubMed] [Google Scholar]
- 125.Taylor SRJ, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, et al. P2X7 Deficiency Attenuates Renal Injury in Experimental Glomerulonephritis. J Am Soc Nephrol. 2009;20:1275–1281. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]