Abstract
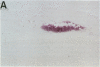
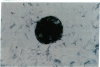
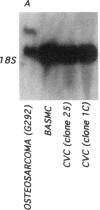
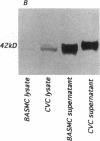
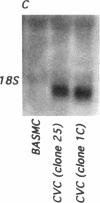
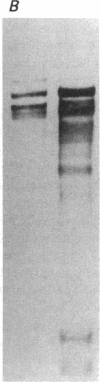
Previous studies in our laboratory demonstrated messenger RNA for bone morphogenetic protein-2a in human calcified plaque, suggesting that arterial calcification is a regulated process, similar to osteogenesis. To further test this hypothesis, we have isolated and cloned a subpopulation of cells from bovine aortic media that show osteoblastic potential. These novel cells are primarily distinguished from smooth muscle cells by expression of a surface marker preliminarily identified as a modified form of the ganglioside sialyl-lactosylceramide (GM3). Osteoblastic potential was indicated by high levels of alkaline phosphatase and collagen I, expression of osteopontin and osteonectin (SPARC), and production of bone-specific osteocalcin and hydroxyapatite. Cultures of these cells were stimulated to form increased numbers of calcium-mineral-producing nodules by the oxysterol 25-hydroxycholesterol as well as by transforming growth factor-beta 1, both known to be present in atherosclerotic lesions. The stimulation of calcifying vascular cells in the artery wall by these two factors suggests a possible mechanism for the colocalization of calcification with atherosclerosis in vivo.
Full text
PDF

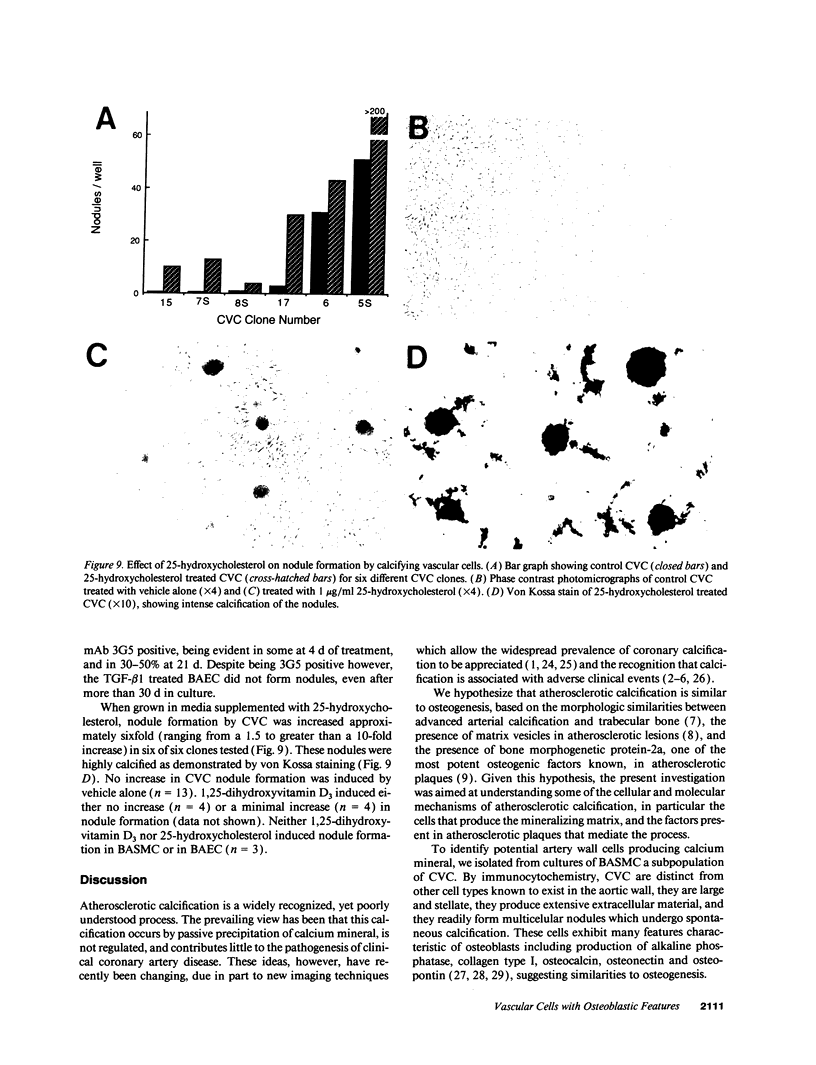


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agatston A. S., Janowitz W. R., Hildner F. J., Zusmer N. R., Viamonte M., Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar 15;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- Anderson H. C. Calcific diseases. A concept. Arch Pathol Lab Med. 1983 Jul;107(7):341–348. [PubMed] [Google Scholar]
- Andrianarivo A. G., Robinson J. A., Mann K. G., Tracy R. P. Growth on type I collagen promotes expression of the osteoblastic phenotype in human osteosarcoma MG-63 cells. J Cell Physiol. 1992 Nov;153(2):256–265. doi: 10.1002/jcp.1041530205. [DOI] [PubMed] [Google Scholar]
- BEADENKOPF W. G., DAOUD A. S., LOVE B. M. CALCIFICATION IN THE CORONARY ARTERIES AND ITS RELATIONSHIP TO ARTERIOSCLEROSIS AND MYOCARDIAL INFARCTION. Am J Roentgenol Radium Ther Nucl Med. 1964 Oct;92:865–871. [PubMed] [Google Scholar]
- Baroldi G., Silver M. D., Mariani F., Giuliano G. Correlation of morphological variables in the coronary atherosclerotic plaque with clinical patterns of ischemic heart disease. Am J Cardiovasc Pathol. 1988;2(2):159–172. [PubMed] [Google Scholar]
- Boström K., Watson K. E., Horn S., Wortham C., Herman I. M., Demer L. L. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993 Apr;91(4):1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton C. T., Lorich D. G., Kupcha R., Reilly T. M., Jones A. R., Woodbury R. A., 2nd The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992 Feb;(275):287–299. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L., Gutierrez R., Lopez-Alonso A., Gonzalez R., Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992 Feb;(275):280–286. [PubMed] [Google Scholar]
- Díaz-Flores L., Valladares F., Gutierrez R., Varela H. The role of the pericytes of the adventitial microcirculation in the arterial intimal thickening. Histol Histopathol. 1990 Apr;5(2):145–153. [PubMed] [Google Scholar]
- Fitzgerald P. J., Ports T. A., Yock P. G. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992 Jul;86(1):64–70. doi: 10.1161/01.cir.86.1.64. [DOI] [PubMed] [Google Scholar]
- Gadeau A. P., Campan M., Millet D., Candresse T., Desgranges C. Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb. 1993 Jan;13(1):120–125. doi: 10.1161/01.atv.13.1.120. [DOI] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Guenther H. L., Hofstetter W., Stutzer A., Mühlbauer R., Fleisch H. Evidence for heterogeneity of the osteoblastic phenotype determined with clonal rat bone cells established from transforming growth factor-beta-induced cell colonies grown anchorage independently in semisolid medium. Endocrinology. 1989 Oct;125(4):2092–2102. doi: 10.1210/endo-125-4-2092. [DOI] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki K., Termine J. D., Whitson S. W., Young M. F. Bone matrix mRNA expression in differentiating fetal bovine osteoblasts. J Bone Miner Res. 1992 Jul;7(7):743–754. doi: 10.1002/jbmr.5650070704. [DOI] [PubMed] [Google Scholar]
- Johansen J. S., Williamson M. K., Rice J. S., Price P. A. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res. 1992 May;7(5):501–512. doi: 10.1002/jbmr.5650070506. [DOI] [PubMed] [Google Scholar]
- Kragel A. H., Reddy S. G., Wittes J. T., Roberts W. C. Morphometric analysis of the composition of atherosclerotic plaques in the four major epicardial coronary arteries in acute myocardial infarction and in sudden coronary death. Circulation. 1989 Dec;80(6):1747–1756. doi: 10.1161/01.cir.80.6.1747. [DOI] [PubMed] [Google Scholar]
- Kragel A. H., Reddy S. G., Wittes J. T., Roberts W. C. Morphometric analysis of the composition of coronary arterial plaques in isolated unstable angina pectoris with pain at rest. Am J Cardiol. 1990 Sep 1;66(5):562–567. doi: 10.1016/0002-9149(90)90482-g. [DOI] [PubMed] [Google Scholar]
- Mahonen A., Pirskanen A., Keinänen R., Mäenpä P. H. Effect of 1,25(OH)2D3 on its receptor mRNA levels and osteocalcin synthesis in human osteosarcoma cells. Biochim Biophys Acta. 1990 Jan 30;1048(1):30–37. doi: 10.1016/0167-4781(90)90018-w. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Molloi S., Detrano R., Ersahin A., Roeck W., Morcos C. Quantification of coronary arterial calcium by dual energy digital subtraction fluoroscopy. Med Phys. 1991 Mar-Apr;18(2):295–298. doi: 10.1118/1.596674. [DOI] [PubMed] [Google Scholar]
- Nayak R. C., Berman A. B., George K. L., Eisenbarth G. S., King G. L. A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med. 1988 Mar 1;167(3):1003–1015. doi: 10.1084/jem.167.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhov A. N., Andreeva E. R., Krushinsky A. V., Novikov I. D., Tertov V. V., Nestaiko G. V., Khashimov Kh A., Repin V. S., Smirnov V. N. Intimal cells and atherosclerosis. Relationship between the number of intimal cells and major manifestations of atherosclerosis in the human aorta. Am J Pathol. 1986 Nov;125(2):402–415. [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Wolf O., Naumann A., Minne H. W., Mundy G. R., Ziegler R. Chemotactic response of osteoblastlike cells to transforming growth factor beta. J Bone Miner Res. 1990 Aug;5(8):825–830. doi: 10.1002/jbmr.5650050805. [DOI] [PubMed] [Google Scholar]
- Powers A. C., Rabizadeh A., Akeson R., Eisenbarth G. S. Characterization of monoclonal antibody 3G5 and utilization of this antibody to immobilize pancreatic islet cell gangliosides in a solid phase radioassay. Endocrinology. 1984 Apr;114(4):1338–1343. doi: 10.1210/endo-114-4-1338. [DOI] [PubMed] [Google Scholar]
- Ravindranath R. M., Graves M. C. Monoclonal IgM antibodies from cytomegalovirus-infected mice recognize the GlcNAc-containing receptor determinant of murine CMV as well as neutralizing anti-CMV IgG antibodies. Virology. 1992 May;188(1):143–151. doi: 10.1016/0042-6822(92)90743-9. [DOI] [PubMed] [Google Scholar]
- Rekhter M. D., Andreeva E. R., Andrianova I. V., Mironov A. A., Orekhov A. N. Stellate cells of aortic intima: I. Human and rabbit. Tissue Cell. 1992;24(5):689–696. doi: 10.1016/0040-8166(92)90039-a. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968 Dec;25(5):452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Flanders K. C., Roche N. S., Kondaiah P., Reddi A. H., Termine J. D., Sporn M. B., Roberts A. B. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987 Jul;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Allen T. D., Canfield A. E., Sloan P., Schor S. L. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990 Nov;97(Pt 3):449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Israel D. H., Kamean J. L., Bodian C. A., Ambrose J. A. Clinical, angiographic, and procedural determinants of major and minor coronary dissection during angioplasty. Am Heart J. 1993 Jul;126(1):39–47. doi: 10.1016/s0002-8703(07)80008-1. [DOI] [PubMed] [Google Scholar]
- Simons D. B., Schwartz R. S., Edwards W. D., Sheedy P. F., Breen J. F., Rumberger J. A. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol. 1992 Nov 1;20(5):1118–1126. doi: 10.1016/0735-1097(92)90367-v. [DOI] [PubMed] [Google Scholar]
- Sims D. E. Recent advances in pericyte biology--implications for health and disease. Can J Cardiol. 1991 Dec;7(10):431–443. [PubMed] [Google Scholar]
- Smith L. L., Johnson B. H. Biological activities of oxysterols. Free Radic Biol Med. 1989;7(3):285–332. doi: 10.1016/0891-5849(89)90136-6. [DOI] [PubMed] [Google Scholar]
- Strong D. D., Beachler A. L., Wergedal J. E., Linkhart T. A. Insulinlike growth factor II and transforming growth factor beta regulate collagen expression in human osteoblastlike cells in vitro. J Bone Miner Res. 1991 Jan;6(1):15–23. doi: 10.1002/jbmr.5650060105. [DOI] [PubMed] [Google Scholar]
- Tilton R. G. Capillary pericytes: perspectives and future trends. J Electron Microsc Tech. 1991 Nov;19(3):327–344. doi: 10.1002/jemt.1060190308. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]