Abstract
Objective
Many investigators now routinely classify children with fragile X syndrome (FXS) according to whether or not they also meet diagnostic criteria for autism. To determine whether this classification is appropriate, we examined the profiles of autistic behaviors shown by boys and girls with FXS.
Method
Individuals with FXS, aged 5 to 25 years, were assessed on two established measures of autism - the Social Communication Questionnaire (SCQ) and the Autism Diagnostic Observation Schedule (ADOS).
Results
We found that 35.1% of boys and 4.3% of girls with FXS scored in the “autism” category on both instruments. Analysis of the symptom profile indicated that both boys and girls with FXS showed lower rates of impairment on communication and reciprocal social interaction items than the reference autism samples on the measures. Furthermore, a regression model showed that IQ was significantly negatively associated with the SCQ total score in both boys and girls with FXS, when controlling for age, medication use, and FMRP levels.
Conclusions
These data suggest that there are significant differences in the profile of social and communicative symptomatology in FXS compared to individuals diagnosed with idiopathic autism. Given these differences, the implementation of standard autism interventions for individuals with FXS may not be optimal. Maintaining the conceptual distinction between FXS (an established biological disease) and idiopathic autism (a phenomenologically-defined behavioral disorder) may also facilitate the development of more targeted and thus, effective interventions for individuals with FXS in the future.
Keywords: fragile X syndrome, IQ, autism, autistic behaviors, FMRP
Introduction
Many children with fragile X syndrome (FXS), the most common known cause of inherited intellectual disability, show behaviors (e.g., gaze aversion, stereotyped behaviors, repetitive vocalizations) that, on the surface, appear very similar to children diagnosed with idiopathic autism 1-3. Because of these apparent similarities, several investigators now routinely classify children with FXS according to whether or not they also have autism 4-13. This classification is usually made using DSM-IV diagnostic criteria 14 and/or on the basis of scores obtained on standardized measures of autism, such as the Autism Diagnostic Interview-Revised (ADI-R) 15, or the Autism Diagnostic Observation Schedule (ADOS) 16.
For example, Rogers and colleagues 17 reported that 33% of 2 to 4 year old children with FXS met diagnostic criteria for autism on the ADOS; Kaufman and colleagues 11 reported that 25% of 3 to 8 year old boys with FXS met criteria for autism on the ADI-R and DSM-IV; and Clifford and colleagues 18 reported that 18% of boys and 10% of girls with FXS aged 5 to 80 years met diagnostic criteria on the ADI-R and ADOS. These investigators then proceeded to divide their sample into two groups: those diagnosed with autism and those without autism, and conducted subsequent analyses on those two groups.
Several investigators, however, have noted that inconsistencies in diagnostic rates can occur when different instruments are employed. For example, Harris and colleagues 19 assessed autistic symptoms in a sample of 63 boys with FXS aged 2 to 19 years on the ADOS, ADI-R and DSM-IV. Only 24% of the boys met the diagnostic criteria for autism on all three measures, while an additional 44% fulfilled the criteria on one or two of the three measures. In the study by Clifford and colleagues, 67% of boys and 23% of girls were classified on the “autism spectrum,” but these rates varied significantly according to whether one or two diagnostic instruments were employed. These studies highlight the difficulties applying dichotomous, phenomenologically defined diagnoses to symptoms and behaviors that may exist as continuous variables in the population being studied.
The distinction between a condition that can be diagnosed on a genetic basis (e.g., FXS), and a disorder like autism, that can only be diagnosed behaviorally, may be important in understanding these discrepancies. FXS is caused by mutations to the FMR1 gene at locus q27.3 on the X chromosome, typically resulting in an expansion of CGG trinucleotide repeats in the promoter region of the gene 20. Once the sequence expands to approximately 200 repeats or more, production of the protein product of the gene, the Fragile X Mental Retardation Protein (FMRP), is severely reduced. Reduced FMRP in the developing brain leads to aberrant regulation of a specific subset of neuronal proteins and, consequently, manifestation of cognitive and behavioral abnormalities. While FXS occurs in both genders, girls with FXS are less affected by the disorder because their second X chromosome does not have the mutation. FXS is thus an established disease with a well-defined genetic basis.
In contrast, the cause of autism in most affected individuals is largely unknown 21 so the diagnosis necessarily depends on behavioral criteria – the so-called “triad of impairments” in communication, reciprocal social interaction and the presence of restricted and repetitive stereotyped behaviors 14. Refinements in the assessment of these three behavioral criteria over the last two decades may have contributed to the rise in the prevalence rates of autism (from 6 per 10,000 to 60 per 10,000 children over the previous two decades, to as high as 1 in 150 children according to recent estimates) 22, 23. This “moving diagnostic target” has hindered the ability of investigators to replicate research results 23, 24 as well as hamper policy decisions 25. Despite this problem, a number of investigators have persisted in diagnosing autism in children with FXS, partly because the additional diagnosis may allow the child access to early intervention services designed for children with autism 8, 19, 26 particularly given that there are virtually no educational programs designed specifically for children with FXS. It is also hoped that comparing children with FXS with and without autism may also shed light on common etiological mechanisms in the two disorders 6.
Although several investigators have concluded that autism and FXS are “related”, others have questioned this notion 27-29. The debate has centered around several issues. First, some investigators have argued that there are distinct qualitative differences in the behaviors of children diagnosed with FXS and those diagnosed with “idiopathic” autism. For example, Fisch 29 noted that the core symptoms of autism (impairments in communication and reciprocal social interaction) usually manifest as abnormal speech and “embarrassed” social behavior in children with FXS; a behavior pattern different from the severe social withdrawal observed in the “prototypical” autistic child originally described by Leo Kanner 30. Similarly, Sudhalter and colleagues 3 have reported that individuals with FXS show significantly less deviant language than individuals diagnosed with autism. Finally, in their observational studies of individuals with FXS and individuals with idiopathic autism, Cohen and his collaborators 2, 31 found that children with FXS showed less gaze avoidance when interacting with their mothers than when interacting with strangers, whereas children with autism showed similar levels of gaze avoidance to both familiar and unfamiliar people. However, given that FXS is a disease where affected individuals share the same risk factor, it could be argued that children with FXS appear different from children with autism simply because the diagnostic criteria of autism allows for more variance across behavioral dimensions. It is also important to remember that autism groups are selected based on high levels of autistic symptomatology whereas individuals with FXS are not. Thus, it is not surprising that FXS groups have lower mean scores on autistic symptoms. Qualitative differences and the general profile of social, communicative, and stereotyped behavior symptoms may therefore be more important than overall mean scores on autism measures.
A second issue concerns the possibility that autistic behaviors in FXS may be associated with the low levels of intellectual ability usually found in FXS 27, 29. Several studies, for example, have reported that children diagnosed with autism and FXS have significantly lower IQ scores than individuals with FXS without autism 7, 10, 11, 17, 32. This finding is corroborated by the fact that autism or autistic behavior has been identified in other genetic disorders that are known to be associated with low levels of intellectual ability. These disorders include (but are not limited to) PKU 33, Down syndrome 34, Smith-Lemli-Opitz syndrome 35, Angelman syndrome 36, CHARGE syndrome 37, 38, and Williams syndrome 39. However, individuals diagnosed with these syndromes do not appear have the same number and severity of autistic symptoms as those seen in individuals with FXS 26.
In an attempt to move the debate forward, in the present study, we measured autistic behaviors and IQ in a large group of boys and girls with FXS. Given that autism is an extremely heterogeneous behaviorally-defined disorder, we compared our sample of children with FXS to the available reference samples of children diagnosed with idiopathic autism on two well-established instruments that measure autistic symptoms: the Social Communication Questionnaire (SCQ) 40 and the ADOS 41. By doing so, we sought to determine whether autistic symptoms in FXS would differ from individuals with “established” (or idiopathic) autism. We had three aims: first, to examine the prevalence of autistic behaviors in individuals with FXS on these well-known measures of autism; second, to examine the profile of autistic behaviors in FXS by comparing rates of individual symptoms shown by individuals with FXS to the reference autism samples provided on these measures; and third, to determine whether IQ and autistic behaviors were associated in FXS while controlling for age, medication use, and FMRP levels.
Method
Subjects
Participants were 120 children, adolescents and young adults (47 girls, 73 boys) aged 5 to 25 years. The mean ages of the boys and girls were 13.24 (SD = 3.27) years and 13.47 (SD = 4.6) years respectively. Sixty (50%) participants had taken part in a previous study investigating the association between autistic, self-injurious and compulsive behaviors in FXS 42. Participants had been diagnosed with the full mutation associated with FXS (> 200 CGG repeats and evidence of aberrant FMR1 methylation) using PCR and Southern Blot DNA analysis (Kimball Genetics). Eleven (15.1%) boys and four (8.5%) girls showed evidence of mosaicism. All mothers of the participants were carriers of the FMR1 premutation (55 < CGG repeats < 200 with no evidence of aberrant FMR1 methylation). Participants were recruited from across the United States through the National Fragile X Foundation, flyers distributed to special interest groups, local contacts, and our research website. Written informed consent was obtained from the parents of all participants. Fifty-two (71.2%) boys with FXS were taking psychoactive medications: these included stimulants (49.3%), antidepressants, (31.5%), antipsychotics (12.3%), antihypertensives (17.8%), anticonvulsants (12.33%) and anxiolytics (2.7%). Seventeen (36.2%) girls with FXS were taking psychoactive medications: these included stimulants (17%), antidepressants (21.3%) and antipsychotics (4.3%).
Measures
The Autism Diagnostic Observation Schedule (ADOS) (Lord, Rutter, DiLavore, & Risi, 2002) is a semi-structured observational measure of autistic behavior administered directly to the participant by a trained researcher or clinician. The assessment lasts approximately 30-60 minutes, during which the researcher or clinician engages the child in a number of activities designed to elicit symptoms of autism. Participants receive one of four modules, assigned on the basis of the participant's expressive language age. Items contributing to three domains - “communication”, “reciprocal social interaction” and “repetitive behavior and stereotyped patterns” – are rated by the clinician according to standardized criteria. A subset of algorithm items from the communication and reciprocal social interaction domains are used to generate a total score. A total score of 12 and above for Modules 1 and 2 and a total score of 10 and above for Modules 3 and 4 are indicative of an autism diagnosis. However, given that each module contains slightly different items (and different numbers of items), the total score cannot be compared across modules. Modules were administered to participants in the present study as follows: Module 1 (9 boys, 0 girls), Module 2 (9 boys, 2 girls), Module 3 (9 boys, 12 girls) and Module 4 (10 boys, 19 girls). The psychologist in this study (AAL) was certified in ADOS administration according to the standards established by the University of Michigan where the scale was developed. Our psychologist was trained to consensus reliability of 80% and above on all items and followed the standardized protocol for rating items contained in the manual. In order to compare individuals with FXS to individuals with autism on this measure, mean scores for each of the items were compared to those listed in Appendix B of the ADOS manual. These mean item scores were obtained on a sample of individuals diagnosed with autism (N = 78, mean age = 8.78 years, SD = 3.86 years).
The Social Communication Questionnaire (SCQ) 43 (also known as the ADI screener and formerly known as the Autism Screening Questionnaire) is a 40-item checklist of autistic behaviors that parallels the content of the ADI-R i.e. the SCQ asks the same questions as the ADI-R, but in a different format (i.e., questionnaire). Raters check either “yes” or “no” to items in 3 domains: “communication” (e.g., stereotyped utterances, inappropriate questions, pronoun reversal), “reciprocal social interaction” (e.g., inappropriate facial expressions, eye gaze, social smiling), and “restricted, repetitive and stereotyped patterns of behavior” (e.g., verbal rituals, compulsions, unusual preoccupations). For some behaviors where developmental delay is likely to affect ratings, the instructions on the SCQ require caregivers to focus on the period during which the child was between 4 and 5 years old. (This is also done in the ADI-R.) A score of 1 is given for the presence of an abnormal behavior and a score of 0 is given for its absence. A total score is obtained by summing the items assigned a score of 1, with scores of 15 or more being considered to be indicative of an autism diagnosis. In order to compare individuals with FXS to individuals with autism on this measure, the percentage of individuals scoring on each item was compared to the percentages listed in Table 5 of the SCQ manual. These individuals had received a prior diagnosis of autism spectrum disorder (N = 160, mean age = 16.74 years, SD = 7.22 years).
Fragile X Mental Retardation Protein (FMRP)
Blood drawing kits and consent forms were mailed directly to each family in order to obtain FMRP levels for the child with FXS. Blood draws were performed by a local physician and samples were mailed directly to Kimball Genetics (Denver, CO) using overnight mail. FMRP immunostaining, an indirect alkaline phosphatase technique, was used 44. Slides were analyzed under the microscope, distinguishing lymphocytes from other blood cell types by morphology. For each slide, 200 lymphocytes were scored, and the percentage of lymphocytes expressing FMRP was determined. Scoring was performed in blinded fashion with respect to DNA results.
IQ
All participants were administered an age-appropriate IQ test: the Wechsler Intelligence Scale for Children – Third Edition (WISC-III) 45 (children aged 5 to 16 years), or the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) 46 (participants aged 17 years and over). Each test yields a standardized IQ score.
Procedures
Two researchers arrived at the family home and administered a battery of assessments to the participant over 1 to 1.5 days. IQ assessments were usually administered in the morning by one of the researchers in a quiet location in the house and the ADOS was usually administered in the afternoon by the other trained researcher in the same quiet location. The SCQ was completed by the participant's main caregiver in addition to a number of other questionnaires. Due to scheduling constraints and study design issues, only 37 (50.7%) boys and 33 (70.2%) girls were administered the ADOS assessment. There were no differences in either age, IQ or FMRP between those who received an ADOS assessment and those who did not receive an ADOS assessment. There were also no differences in SCQ total score between those who received an ADOS and those who did not receive an ADOS assessment. A $100 honorarium was paid to each family upon completion of their participation.
Data Analysis
Data for boys and girls were analyzed separately. For each item on the SCQ, items endorsed in participants with FXS were compared to the reference percentages contained in the SCQ manual 43 using odds ratio statistics with a 99% confidence interval. For algorithm items on the ADOS, mean scores shown by participants with FXS were compared to mean item scores listed for the autism sample contained in the ADOS manual 41 using 2-sample t-tests with a 99% confidence interval (www.dimensionresearch.com/resources/calculators/ttest.html). Given that most algorithm items in the ADOS appear in more than one module, means were pooled across modules where appropriate.
All other statistical analyses were conducted using SPSS (SPSS, Inc). To examine the association between IQ and autistic behaviors in FXS, a multiple regression model was estimated with SCQ total score as the dependent variable and IQ, age, FMRP level and use of psychoactive medication as the independent variables.
Results
As expected with an X-linked genetic disorder, IQ scores were significantly higher in girls with FXS (M = 76.77, SD = 22.76, range = 40 to 116) than in boys with FXS (M = 45.66, SD = 7.94, range = 40 to 74) [t(118) = 10.78, p < 0.001] and FMRP values were significantly higher in girls (M = 53.02, SD = 18.59, range = 14.5 to 90) than in boys (M = 13.91, SD = 14.42, range = 1.5 to 74), [t(118) = 12.67, p < 0.001)]. Total scores on the SCQ were significantly higher in boys (M = 17.14, SD = 6.74, range = 3 to 39) than in girls (M = 7.83, SD = 6.90, range = 0 to 28), [t(118) = 7.38, p < 0.001)].
Preliminary correlation analyses were conducted separately for boys and girls to examine the association between age, IQ, FMRP, and medication use in each group. These analyses indicated that there was a significant association between IQ and FMRP levels in boys with FXS [r(73) = .27, p = 0.03]. There were no other significant associations between these variables.
Prevalence and Phenomenology
Of the 120 participants who were assessed on the SCQ, 9 of 47 (18.8%) girls with FXS and 44 of 73 (58.7%) boys with FXS scored in the “autism” category (i.e., obtained a score of 15 or greater). Of the 70 participants who were administered the ADOS, 7 of 33 (21.2%) girls with FXS and 19 of 37 (51.4%) boys with FXS scored in the autism category (i.e., obtained a total score of 12 or greater for those who were administered modules 1 and 2 and a total score of 10 or greater for those who were administered modules 3 and 4). Of the 70 children who were assessed on both instruments, only 3 of 33 (4.3%) girls and 13 of 37 (35.1%) boys scored in the autism category on both instruments. Agreement between the measures on diagnostic category was therefore poor (Cohen's kappa = .33 and .13 for girls and boys respectively).
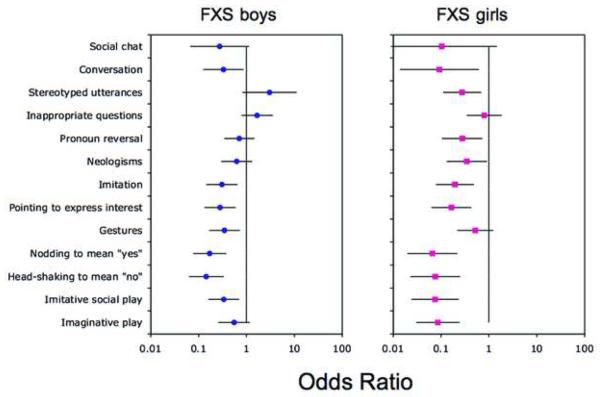
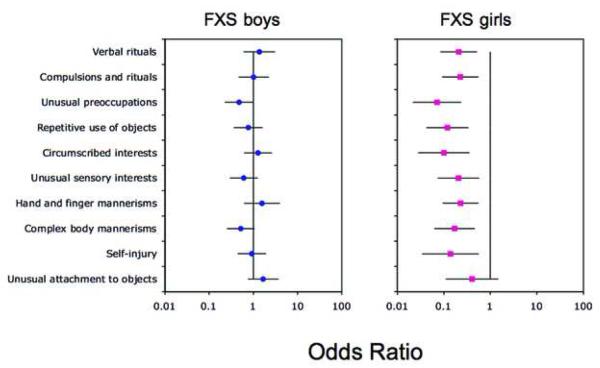
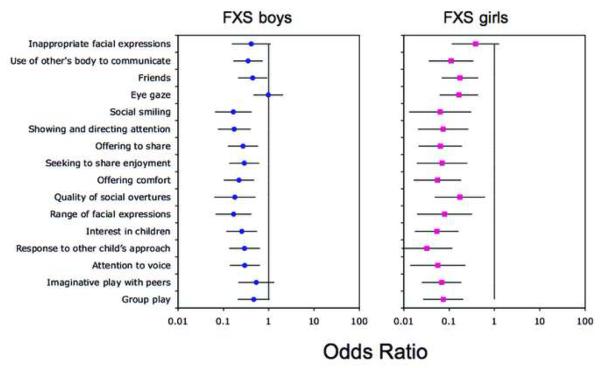
Table 1 shows the percentages of boys and girls with FXS who scored on each of the SCQ items. In boys with FXS, percentages ranged from 5.5% (“social chat”) to 93.2% (“stereotyped utterances”). In girls with FXS, percentages ranged from 0% (“social chat”) to 51.1% (“gestures”). Odds ratio analyses were performed to compare the percentage of boys and girls with FXS on items in each domain of the SCQ to those given in the SCQ manual. The results of these analyses for each domain are shown in Figures 1 to 3.
Table 1.
Percentage of boys and girls with fragile X syndrome scoring on each item of the Social Communication Questionnaire.
| Domain | Item | % Girls with FXS (N=47) |
% Boys with FXS (N=73) |
|---|---|---|---|
| Communication | Social Chat | 0.00 | 5.48 |
| Conversation | 4.26 | 13.70 | |
| Stereotyped utterances | 55.32 | 93.15 | |
| Inappropriate questions | 51.06 | 68.49 | |
| Pronoun reversal | 23.40 | 43.84 | |
| Neologisms | 23.40 | 35.62 | |
| Imitation | 31.91 | 42.47 | |
| Pointing to express interest | 25.53 | 36.99 | |
| Gestures | 51.06 | 41.10 | |
| Nodding to mean “yes” | 12.77 | 27.40 | |
| Head-shaking to mean “no” | 12.77 | 21.92 | |
| Imitative social play | 14.89 | 43.84 | |
| Imaginative play | 19.15 | 60.27 | |
|
| |||
| Social Interaction | Inappropriate facial expressions | 12.77 | 13.70 |
| Use of other's body to communicate | 14.89 | 35.62 | |
| Friends | 29.79 | 52.05 | |
| Eye gaze | 23.40 | 64.38 | |
| Social smiling | 6.38 | 15.07 | |
| Showing and directing attention | 10.64 | 21.92 | |
| Offering to share | 17.02 | 46.58 | |
| Seeking to share enjoyment | 10.64 | 32.88 | |
| Offering comfort | 12.77 | 36.99 | |
| Quality of social overtures | 10.64 | 10.96 | |
| Range of facial expressions | 8.51 | 16.44 | |
| Interest in children | 17.02 | 49.32 | |
| Response to other child's approach | 10.64 | 52.05 | |
| Attention to voice | 8.51 | 32.88 | |
| Imaginative play with peers | 29.79 | 76.71 | |
| Group play | 23.40 | 65.75 | |
|
| |||
| Repetitive behavior | Verbal rituals | 31.91 | 75.34 |
| Compulsions and rituals | 34.04 | 69.86 | |
| Unusual preoccupations | 12.77 | 49.32 | |
| Repetitive use of objects | 19.15 | 60.27 | |
| Circumscribed interests | 10.64 | 60.27 | |
| Unusual sensory interests | 19.15 | 41.10 | |
| Hand and finger mannerisms | 42.55 | 83.56 | |
| Complex body mannerisms | 21.28 | 45.21 | |
| Self-injury | 8.51 | 38.36 | |
| Unusual attachment to objects | 10.64 | 32.88 | |
Figure 1.
Social Communication Questionnaire (SCQ) Communication domain. Note: Odds ratios comparing the percentage of boys and girls with fragile X syndrome (FXS) on items in the communication domain of the SCQ to those given in the SCQ manual. A 99% confidence interval for each odds ratio is also shown.
Figure 3.
Social Communication Questionnaire (SCQ) repetitive behavior domain. Note: Odds ratios comparing the percentage of boys and girls with fragile X syndrome (FXS) on items in the repetitive behavior domain of the SCQ to those given in the SCQ manual. A 99% confidence interval for each odds ratio is also shown.
An odds ratio significantly less than 1 would indicate that the item was significantly less likely to be endorsed in individuals with FXS than in the reference group. An odds ratio significantly greater than 1 would indicate that the item was significantly more likely to be endorsed in individuals with FXS than in the reference group. Figure 1 shows that for boys with FXS, 7 of the 13 items in the communication domain of the SCQ were significantly less likely to be endorsed in comparison to the SCQ reference sample. These items were: “conversation”, “imitation”, “pointing to express interest”, “gestures”, “nodding to mean yes”, “head-shaking to mean no” and “imitative play”. Figure 2 shows that 12 of the 16 items in the social interaction domain of the SCQ were significantly less likely to be endorsed in boys with FXS than in the SCQ reference sample. These items were: “use of other's body to communicate”, “friends”, “social smiling”, showing and directing attention”, “offering to share enjoyment”, “offering comfort”, “quality of social overtures”, “range of facial expressions”, “interest in other children”, “response to others child's approach” and “attention to voice”. Figure 3 shows that, by contrast, there were no differences between the two samples on the items in the repetitive behavior domain. In girls with FXS, Figures 1 to 3 show that 10 of the 13 communication domain items, 15 of the 16 social interaction domain items, and 9 of the 10 repetitive behavior domain items of the SCQ were significantly less likely to be endorsed than in the SCQ reference sample. There were no items on the SCQ in either boys or girls with FXS that were more likely to be endorsed than the reference sample.
Figure 2.
Social Communication Questionnaire (SCQ) social interaction domain. Note: Odds ratios comparing the percentage of boys and girls with fragile X syndrome (FXS) on items in the social interaction domain of the SCQ to those given in the SCQ manual. A 99% confidence interval for each odds ratio is also shown.
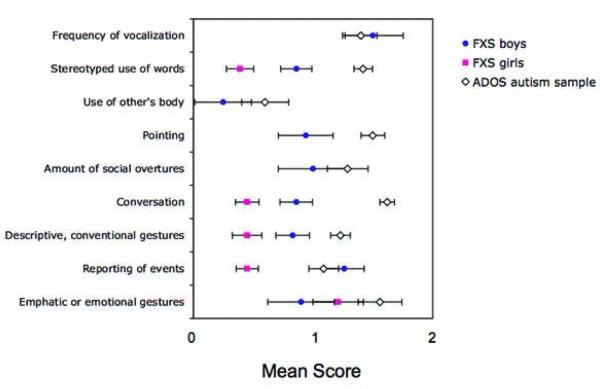
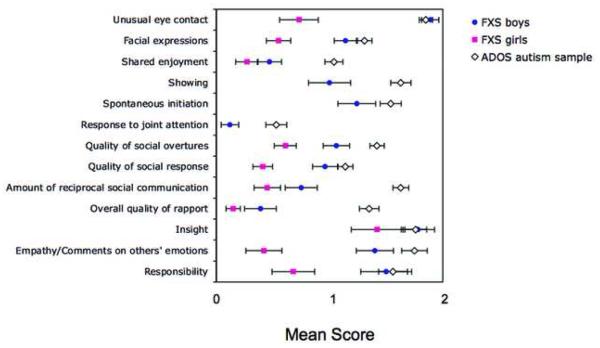
Figures 4 and 5 show the mean scores obtained on each of the items listed on the ADOS scoring algorithm. (In girls with FXS, means could not be calculated for some ADOS items because only two girls with FXS received Module 2 and no girls received Module 1). In boys with FXS, mean scores ranged from .12 (“response to joint attention”) to 1.78 (“insight”). In girls with FXS, mean scores ranged from .15 (“overall quality of rapport”) to 1.42 (“insight”).
Figure 4.
Autism Diagnostic Observation Schedule (ADOS) communication domain. Note: Mean item scores obtained on the communication domain of the ADOS for boys and girls with fragile X syndrome (FXS) compared to the ADOS reference sample.
Figure 5.
Autism Diagnostic Observation Schedule (ADOS) social interaction domain. Note: Mean item scores obtained on the social interaction domain of the ADOS for boys and girls with fragile X syndrome (FXS) compared to the ADOS reference sample.
Statistical analysis of the ADOS items indicated that boys with FXS received significantly lower scores than the ADOS reference autism sample on 4 of 9 items in the communication domain of the ADOS. These items were: “stereotyped use of words”, “pointing” “conversation”, and “descriptive, conventional gestures”. Boys with FXS also received significantly lower scores on 8 of 13 items in the social interaction domain of the ADOS. These items were: “shared enjoyment”, “showing”, “spontaneous initiation”, “response to joint attention”, “quality of social overtures”, “amount of reciprocal social communication”, “overall quality of rapport”, and “empathy on other's emotions”. Girls with FXS received significantly lower scores than the autism reference sample on 4 of 5 communication domain items. These items were: “stereotyped use of words”, “conversation”, “descriptive gestures” and “reporting of events”. Girls with FXS received significantly lower scores on 9 of 10 reciprocal social interaction domain items.
Association between IQ and autistic behavior
To examine the association between autistic behavior and IQ, a multiple regression model, estimated separately for boys and girls, was conducted with SCQ total score entered as the dependent variable and IQ, age, FMRP and use of psychoactive medication as the independent variables in each analysis. Both models showed that there was a significant effect of IQ on SCQ total score in boys [b = −.21 (SE = .09), B = −.28, p = .03] and in girls [b = −.13 (SE = .04), B = −.43, p = .002]. These data indicated that lower IQ scores were associated with significantly higher autistic symptoms, when controlling for age, FMRP and medication use in both boys and girls with FXS. The effect of age on SCQ total score when controlling for IQ, FMRP levels and medication use was not significant nor was the effect of FMRP on SCQ total score when controlling for age, IQ and medication use in either boys or girls with FXS. In girls with FXS, there was a significant effect of medication use on SCQ total score [b = 4.45 (SE = 1.89), B = .31, p = .02], indicating that medication use was associated with higher autistic symptoms when controlling for age, FMRP and IQ levels. The r-square values for the overall models were 28% and 35% for boys and girls with FXS respectively, with IQ accounting for 8% and 18% of the variance in each case.
To understand the magnitude of the apparent IQ effect, imagine two boys with FXS whose IQ's differed by 30 points (the range in the sample of boys with FXS was 40 to 74 points). The regression model predicts that the difference in their SCQ scores would be approximately 6 points (i.e., 30 × .21). Similarly, for two girls with FXS whose IQ's differed by 30 points (the range in the sample of girls with FXS was 40 to 116 points), the difference in their SCQ scores would be approximately 4 points (i.e., 30 × .13). Given that the mean scores on the measure were approximately 17 points for boys and 8 points for girls, a change of 4 to 6 points on the SCQ could result in the classification of some individuals moving from “non-autistic” to “autistic” or vice versa, depending on IQ.
Discussion
The first aim of this study was to examine the prevalence of autistic behaviors in individuals with FXS. To accomplish this objective, we administered two well-known measures of autistic behaviors to a large sample of individuals with FXS. Both of these instruments are widely used in the autism literature - the SCQ is a checklist that parallels ADI-R content, while the ADOS is an observational measure of autistic behaviors that is often considered the “gold-standard” in autism research. Results showed that 35.1% of boys and 4.3% of girls scored in the autism category on both instruments, similar to the prevalence rates of autism found in previous studies of individuals with FXS 11, 18, 19. However, agreement on diagnostic category was poor. The low diagnostic agreement would be expected given that one measure involved parent report (the SCQ) and the other measure involved direct observations of the child under controlled conditions by an expert rater (the ADOS). In boys with FXS, 51.4% scored in the autism category on the ADOS, and 58.7% scored in the autism category on the SCQ, yet only 35.1% scored in the autism category on both instruments. These findings once again highlight the difficulty of applying dichotomous, phenomenologically defined diagnoses (i.e., using cut-off points) to symptoms and behaviors that may exist as continuous variables.
Another explanation for the discrepancy between the prevalence rate of autism observed on the two instruments concerns differences in the content of the items employed in each instrument. For some behaviors (e.g., eye gaze, facial expressions, quality of social overtures, repetitive speech), the content of the items could be considered comparable across instruments (e.g., “stereotyped utterances” on the SCQ vs “stereotyped use of words” on the ADOS). For other items, however, the content between the two instruments appears to be quite different. For example, the SCQ item “inappropriate questions” has no comparable item on the ADOS, while the ADOS items “insight”, “empathy on others' emotions”, “quality of rapport”, and “response to joint attention have no comparable items on the SCQ. Another major difference between the two scales concerns the fact that the repetitive behaviors domain is not included in the scoring algorithm of the ADOS 41. Interestingly, in their longitudinal investigation of autism in FXS, Hernandez and colleagues 10 reported that the ADOS appeared to generate a high number of “false-positive” cases of autism in their sample of boys with FXS (i.e., 21 of 22 boys with FXS were classified in the autism category on the ADOS while only 12 of these children were classified as autistic using a combination of ADI-R and DSM-IV criteria). The discrepancy between the measures in the present study (and in the Hernandez et al study) could therefore result from the different numbers of items and domains being included in the scoring algorithms of the two measures. Future studies could employ alternate measures of autistic symptoms and/or social behaviors and language function to further assess differences between FXS and autism groups.
The second aim of the study was to determine whether the behaviors shown by individuals with FXS are similar to those diagnosed in individuals with idiopathic autism. Analysis of the items on the two measures indicated that boys with FXS showed particular forms of behaviors (e.g., stereotyped behaviors, repetitive vocalizations and eye gaze avoidance) that appeared at similar rates to those diagnosed with autism. However, impairments in a large number of social behaviors (e.g., social smiling, range of social expressions, quality of social overtures, joint attention) as well as impairments in communicative behaviors (gestures, pointing, imitation), occurred at significantly lower rates than that of individuals typically diagnosed with autism. While girls with FXS also showed a few behaviors that were similar to the autism samples (e.g., attachment to objects, inappropriate questions, gestures, and inappropriate facial expressions), the majority of items on the measures occurred at significantly lower rates than the reference samples. Taken together, these data support previous observations that while individuals with FXS exhibit high levels of social avoidance and repetitive behaviors and language, their reciprocal social interaction skills and communication skills may be qualitatively (and quantitatively) less impaired than in some samples of individuals diagnosed with idiopathic autism 29, 31.
A third aim of the study was to determine whether the autistic behaviors of individuals with FXS were associated with IQ levels. A multiple regression analysis indicated that, when controlling for age and FMRP levels, IQ was significantly negatively associated with autistic behaviors in both boys and girls with FXS. Assuming that the negative association between IQ and autistic behavior does not result from item overlap in the measures of autism and IQ, there are several possible explanations for the observed association. First, decreased levels of IQ, or some variable that is correlated with IQ, could trigger or amplify autistic behaviors. Second, autistic symptoms, or some variable that is correlated with autistic symptoms, could impair performance on intelligence testing. Third, IQ and autistic behaviors could result from the effects of an unidentified “third” variable with simultaneous causal effects on both IQ and on autistic symptoms.
Can we conclude from these analyses that the impairment in IQ in children with FXS actually causes autistic symptoms? This would not be a valid conclusion. Although FMRP levels were not predictive of autistic behaviors, this does not preclude the possibility that there is some other unmeasured variable, such as generalized brain dysfunction, that leads to the impaired intellectual development as well as the autistic symptoms in individuals with FXS. In the regression model, the amount of variance in autistic symptoms explained by IQ, age, FMRP and psychoactive medication use was 51% in girls and 28% in boys with FXS. While the low percentage of variance explained in boys with FXS was most likely due to severe range restriction in IQ scores and FMRP levels in this group, there are clearly other important factors, such as the family environment or other biological factors that could be involved in the appearance of autistic behaviors in FXS 47.
We found that there was no effect of FMRP levels on autistic behaviors when controlling for age, IQ, and psychoactive medication use. Bailey and colleagues 4 also found no association between FMRP levels and autistic behaviors, as assessed on the Childhood Autism Rating Scale (CARS). By contrast, in a longitudinal study of 83 young children with FXS (mean initial age = 4.5 years), Hatton and colleagues 8 found that FMRP levels and autistic behavior on the CARS were significantly negatively associated. However, intellectual ability levels were not included in their analysis.
Several studies have shown that the caudate nucleus, a region of the brain thought to be involved in learning and memory, is significantly larger in children with FXS compared to typically developing controls 48, 49. Intriguingly, studies have also shown that the caudate nucleus and amygdala are significantly larger in children with autism, suggesting that there may be common underlying brain pathology in the two disorders 50, 51. However, in a recent brain imaging study, Hazlett and colleagues 52 reported that children with FXS had a significantly larger caudate nucleus and a smaller amygdala than children with autism. These authors suggested that this “double dissociation” in brain pathology points to differing pathogenic mechanisms underlying FXS and autism.
There are a number of limitations associated with this study. First, only a proportion of the study sample (70 out of 120 participants) received the ADOS assessment. Thus, agreement between the measures on diagnostic category was limited to the 70 participants who had received both instruments. Second, for the 33 girls who received the ADOS assessment, the majority (31 out of 33 participants) had received either Module 3 or 4 because they had fluent expressive language skills. Consequently, data were not available in that group for items on the ADOS that appear only in Modules 1 and 2. A third limitation concerns the potential differences between the current sample of children with FXS and the reference samples. For example, the mean age of the children in the present study was approximately 13 years, while the mean age of the reference samples on the ADOS and SCQ were approximately 9 and 17 years respectively. While we did not find age to be associated to autistic behaviors in this study, there is some evidence from longitudinal studies that autistic behaviors may decrease with age 8. To circumvent this limitation, we could have included an age- and gender-matched autism control group in our study. This would facilitate minimizing differences between subject groups (e.g., gender and age) and would also ensure that the ADOS assessments were performed in a similar manner in the two groups, if possible, conducted blind to subject diagnosis. Future studies could therefore compare FXS and idiopathic autism groups recruited at the same study site and assessed by the same study team. However, had we recruited our own sample of children with autism at Stanford, they would likely have had some characteristics that differed from the autism reference groups contained in the manuals of the measures (and, incidentally, also from other autism control groups recruited by investigators at other institutions). We therefore felt that comparing our sample of individuals with FXS to individuals with “established” autism (defined by the particular autism measure) was a sound strategy for this initial analysis.
The findings of this study may have important implications for interventions. Currently, children with FXS who show autistic-like behaviors may be referred to intervention services designed for children with autism. However, the data in this study suggests that continuing to implement interventions designed for individuals with autism may not be optimal since individuals with FXS do not necessarily show the same profile or severity of behaviors associated with idiopathic autism. While some subjects with FXS generated summary scores that reached criteria for autism, the scores on many individual symptoms were relatively mild. It is possible that interventions designed to address the specific phenotypic features of FXS (e.g., with an emphasis on repetitive-stereotypic motor behavior and language), rather than the broad symptoms of autism, may therefore be more beneficial to individuals with FXS. Future studies are needed to test this premise. A number of investigators, for example, have begun to evaluate medications targeted to the specific underlying pathology in FXS i.e., the downstream effects of reduced FMRP (for reviews see 53, 54). Interventions have also been conducted to address specific cognitive and behavioral symptoms shown by individuals with FXS55, 56. If these FXS-specific interventions are found to be more successful than standard autism interventions, these treatments should be prioritized in comparison to those developed (and relatively non-specifically) for children with autism. Service agencies would also need to recognize FXS as its own entity, with services being made available to all individuals with FXS irrespective of whether or not they have also been assigned a diagnosis of autism. Until more FXS-specific interventions are developed however, it is possible that individuals with FXS who have an additional diagnosis of “autism” may receive more appropriate school services relative to an “other health impaired” diagnosis.
While we could not rule out other factors that might have contribute to the appearance of autistic behaviors in FXS, it seems likely that degree of intellectual ability is at least a proxy for some of those factors. Given this result, and the finding that the profile of behaviors associated with FXS appears to differ from individuals with idiopathic autism, the practice of diagnosing children with FXS as autistic may become increasingly obsolete in the future. In his book “The Concept of Mind”, the philosopher Gilbert Ryle 57 identified several types of “category mistake” that pervade scientific discourse. A category mistake is an instance in which items are suggested to be components or equal members of the same category despite the fact that they exist at different levels of explanation (e.g., “brain” and “mind”). It is possible that the grouping together of FXS (a biological disease) with autism (a phenomenologically-defined behavioral disorder) may be another such type of category mistake. We acknowledge however, that the issue of whether to give an autism diagnosis to individuals with a known genetic syndrome (e.g., FXS, Rett's disorder, Down syndrome and others) is a complex one. We believe that further study of this issue may facilitate the implementation of more specific and effective treatments for individuals with FXS in the future.
Acknowledgments
This research was supported by NIMH grants MH50047 and MH01142 and the Canel Fragile X Family Fund.
The authors wish to thank Dr. David Burns of Stanford University for his comments on an earlier draft of the manuscript. The authors would also like to thank the families of children with fragile X syndrome for their participation in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. J Am Acad Child Adolesc Psychiatry. 2009 Mar;48(3):320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Parent-child dyadic gaze patterns in fragile X males and in non-fragile X males with autistic disorder. J Child Psychol Psychiatry. 1989 Nov;30(6):845–856. doi: 10.1111/j.1469-7610.1989.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 3.Sudhalter V, Cohen IL, Silverman W, Wolf-Schein EG. Conversational analyses of males with fragile X, Down syndrome, and autism: comparison of the emergence of deviant language. Am J Ment Retard. 1990 Jan;94(4):431–441. [PubMed] [Google Scholar]
- 4.Bailey DB, Jr., Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. J Autism Dev Disord. 2001 Apr;31(2):165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- 5.Brown WT, Jenkins EC, Friedman E, et al. Autism is associated with the fragile-X syndrome. J Autism Dev Disord. 1982 Sep;12(3):303–308. doi: 10.1007/BF01531375. [DOI] [PubMed] [Google Scholar]
- 6.Dissanayake C, Bui Q, Bulhak-Paterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. J Child Psychol Psychiatry. 2009 Mar;50(3):290–299. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 7.Hagerman RJ, Jackson AW, 3rd, Levitas A, Rimland B, Braden M. An analysis of autism in fifty males with the fragile X syndrome. Am J Med Genet. 1986 Jan-Feb;23(1-2):359–374. doi: 10.1002/ajmg.1320230128. [DOI] [PubMed] [Google Scholar]
- 8.Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006 Sep 1;140A(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 9.Philofsky A, Hepburn SL, Hayes A, Hagerman R, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. Am J Ment Retard. 2004 May;109(3):208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: a longitudinal evaluation. Am J Med Genet A. 2009 Jun;149A(6):1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann WE, Cortell R, Kau AS, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004 Sep 1;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 12.Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in Fragile X syndrome: differential contribution of adaptive socialization and social withdrawal. Am J Med Genet A. 2006 Sep 1;140A(17):1814–1826. doi: 10.1002/ajmg.a.31405. [DOI] [PubMed] [Google Scholar]
- 13.Kau AS, Tierney E, Bukelis I, et al. Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am J Med Genet A. 2004 Apr 1;126A(1):9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- 14.American_Psychiatric_Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 15.Rutter M, LeCouteur C, Lord C. Autism Diagnostic Interview-Revised. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- 16.Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989 Jun;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 17.Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001 Dec;22(6):409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007 Apr;37(4):738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 19.Harris SW, Hessl D, Goodlin-Jones B, et al. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008 Nov;113(6):427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 21.Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006 Oct;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 22.Wing L, Potter D. The epidemiology of autistic spectrum disorders: is the prevalence rising? Ment Retard Dev Disabil Res Rev. 2002;8(3):151–161. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]
- 23.Holburn CS. Detrimental effects of overestimating the occurrence of autism. Intellect Dev Disabil. 2008 Jun;46(3):243–246. doi: 10.1352/2008.46:243-246. [DOI] [PubMed] [Google Scholar]
- 24.Reiss AL. Childhood developmental disorders: an academic and clinical convergence point for psychiatry, neurology, psychology and pediatrics. J Child Psychol Psychiatry. 2009 Jan;50(1-2):87–98. doi: 10.1111/j.1469-7610.2008.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skellern C, Schluter P, McDowell M. From complexity to category: responding to diagnostic uncertainties of autistic spectrum disorders. J Paediatr Child Health. 2005 Aug;41(8):407–412. doi: 10.1111/j.1440-1754.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 26.Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009 Oct;53(10):852–873. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- 27.Einfeld S, Molony H, Hall W. Autism is not associated with the fragile X syndrome. Am J Med Genet. 1989 Oct;34(2):187–193. doi: 10.1002/ajmg.1320340211. [DOI] [PubMed] [Google Scholar]
- 28.Fisch GS. Is autism associated with the fragile X syndrome? Am J Med Genet. 1992 Apr 15;43(1-2):47–55. doi: 10.1002/ajmg.1320430107. May 1. [DOI] [PubMed] [Google Scholar]
- 29.Fisch GS. What is associated with the fragile X syndrome? Am J Med Genet. 1993 Jul 15;48(2):112–121. doi: 10.1002/ajmg.1320480210. [DOI] [PubMed] [Google Scholar]
- 30.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 31.Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. Am J Med Genet. 1991 Feb-Mar;38(2-3):498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- 32.Bailey DB, Jr., Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. J Autism Dev Disord. 1998 Dec;28(6):499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- 33.Baieli S, Pavone L, Meli C, Fiumara A, Coleman M. Autism and phenylketonuria. J Autism Dev Disord. 2003 Apr;33(2):201–204. doi: 10.1023/a:1022999712639. [DOI] [PubMed] [Google Scholar]
- 34.Starr EM, Berument SK, Tomlins M, Papanikolaou K, Rutter M. Brief report: autism in individuals with Down syndrome. J Autism Dev Disord. 2005 Oct;35(5):665–673. doi: 10.1007/s10803-005-0010-0. [DOI] [PubMed] [Google Scholar]
- 35.Sikora DM, Pettit-Kekel K, Penfield J, Merkens LS, Steiner RD. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2006 Jul 15;140(14):1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- 36.Peters SU, Beaudet AL, Madduri N, Bacino CA. Autism in Angelman syndrome: implications for autism research. Clin Genet. 2004 Dec;66(6):530–536. doi: 10.1111/j.1399-0004.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 37.Johansson M, Rastam M, Billstedt E, et al. Autism spectrum disorders and underlying brain pathology in CHARGE association. Dev Med Child Neurol. 2006 Jan;48(1):40–50. doi: 10.1017/S0012162206000090. [DOI] [PubMed] [Google Scholar]
- 38.Hartshorne TS, Grialou TL, Parker KR. Autistic-like behavior in CHARGE syndrome. Am J Med Genet A. 2005 Mar 15;133A(3):257–261. doi: 10.1002/ajmg.a.30545. [DOI] [PubMed] [Google Scholar]
- 39.Klein-Tasman BP, Mervis CB, Lord C, Phillips KD. Socio-communicative deficits in young children with Williams syndrome: performance on the Autism Diagnostic Observation Schedule. Child Neuropsychol. 2007 Sep;13(5):444–467. doi: 10.1080/09297040601033680. [DOI] [PubMed] [Google Scholar]
- 40.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999 Nov;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 41.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Manual. Western Psychological Services; Los Angeles: 2002. [Google Scholar]
- 42.Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. Am J Ment Retard. 2008 Jan;113(1):44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Rutter M, Bailey A, Lord C. The Social Communication Questionaire. Manual. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- 44.Willemsen R, Smits A, Mohkamsing S, et al. Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Hum Genet. 1997 Mar;99(3):308–311. doi: 10.1007/s004390050363. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler D. Wechsler Intelligence Scale for Children - Third Edition. Manual. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 46.Wechsler D. Wechsler Adult Intelligence Scale- Third Edition. Manual. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 47.Hessl D, Dyer-Friedman J, Glaser B, et al. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 2001 Nov;108(5):E88. doi: 10.1542/peds.108.5.e88. [DOI] [PubMed] [Google Scholar]
- 48.Eliez S, Blasey CM, Freund LS, Hastie T, Reiss AL. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain. 2001 Aug;124(Pt 8):1610–1618. doi: 10.1093/brain/124.8.1610. [DOI] [PubMed] [Google Scholar]
- 49.Gothelf D, Furfaro JA, Hoeft F, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008 Jan;63(1):40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007 Aug 1;62(3):262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 51.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009 May;66(5):509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazlett HC, Poe MD, Lightbody AA, et al. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. Journal of Neurodevelopmental Disorders. 2009;1(1):81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall SS. Treatments for fragile X syndrome: a closer look at the data. Dev Disabil Res Rev. 2009;15(4):353–360. doi: 10.1002/ddrr.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reiss AL, Hall SS. Fragile X syndrome: assessment and treatment implications. Child Adolesc Psychiatr Clin N Am. 2007 Jul;16(3):663–675. doi: 10.1016/j.chc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Hall SS, Maynes NP, Reiss AL. Using percentile schedules to increase eye contact in children with Fragile X syndrome. J Appl Behav Anal. 2009 Spring;42(1):171–176. doi: 10.1901/jaba.2009.42-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall SS, Debernardis GM, Reiss AL. The acquisition of stimulus equivalence in individuals with fragile X syndrome. J Intellect Disabil Res. 2006 Sep;50(Pt 9):643–651. doi: 10.1111/j.1365-2788.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 57.Ryle G. The concept of mind. The University of Chicago Press; Chicago: 1949. [Google Scholar]