Abstract
Debates on 6 controversial topics were held during the Fourth International Workshop on Seizure Prediction (IWSP4) convened in Kansas City (July 4–7, 2009). The topics were 1) Ictogenesis: focus vs. network? 2) Spikes and seizures: step-relatives or siblings? 3) Ictogenesis: a result of hyposynchrony? 4) Can focal seizures be caused by excessive inhibition? 5) Do high-frequency oscillations (HFOs) provide relevant independent information? and 6) Phase synchronization – is it worthwhile as measured? This manuscript, written by the IWSP4 organizing committee and the debaters, summarizes the arguments presented during the debates.
Keywords: seizure focus, seizure onset area, network, interictal activity, spikes, ictogenesis, inhibition, excitation, hyposynchrony, hypersynchrony, high frequency activity, high frequency oscillations, phase synchronization
Introduction
Automated seizure detection and prediction are extremely active areas of research. The ability to make substantive progress in these areas depends to some extent on our ability to understand and address existing controversies in epilepsy. These controversies run the gamut of inquiry into this brain disorder. While some of these controversial issues are relatively new others have remained unresolved for years, even decades.
The Fourth International Workshop on Seizure Prediction (IWSP4; June 4–7, 2009 Kansas City, MO) brought together investigators from very diverse technical and scientific backgrounds to, amongst other goals, assess the state of seizure detection and prediction and identify impediments to progress in this important field. The IWSP4 Organizing Committee for the workshop (IO, MF, SA and HZ) preselected six controversial topics due to their relation to the workshop s central objectives, and invited debaters they thought would do justice to the particular topics. These debates were not designed to resolve the selected topics and no winner was selected. The format was primarily designed to highlight an open topic and clarify opposing perspectives. In fact, not all of the debaters even fully supported the positions they were asked to argue for.
This paper, written by the organizing committee and the debaters, seeks to concisely summarize the arguments and counter-arguments presented. Many of the arguments presented were taken from the literature, although some included new and/or previously unpublished work. The debater listed first spoke first and the debaters were each allowed 5 minutes to present evidence to support their position. This exchange was followed by a 10 minute open forum for audience participation and a 1 minute rebuttal/closing statement for each debater.
Debate 1. Ictogenesis: focus vs. network?
“Ictogenesis: Focus” – Gregory Bergey and Christophe Jouny, Ph.D. (Johns Hopkins University, Baltimore, MD, US)
Partial epileptic seizures, whether simple or complex, by definition, begin from focal regions in the brain (e.g. hippocampus, neocortex) [1]. Certainly partial seizures, particularly as they spread regionally, involve neural networks and indeed many of the clinical manifestations experienced by the patient are a reflection of this propagation. The fact that partial epileptic seizure evolution and propagation involve neural networks, however, should not result in neglecting the fact that these are seizures that originate from focal regions of the brain, often with identifiable focal pathology (e.g. cavernoma, dysplasia). Taking the position of focal ictogenesis does not discount the important role of networks, nor does it require one to embrace the concept of an “epileptic neuron.” A detailed discussion of cellular, membrane, or synaptic changes that contribute to epileptogenesis is beyond the scope of this summary.
Most partial seizures, with the notable exception of the childhood syndromes of centrotemporal and occipital epilepsy are of symptomatic or cryptogenic origin. The various pathologies can be associated with clear focal changes such as the disorganization of cortical dysplasia or the changes (cell loss, sprouting) of mesial temporal sclerosis or post-traumatic epilepsy. The surrounding cerebral regions, which may well be involved in epileptic seizure propagation, are often pathologically normal. While some pathology such as balloon cell dysplasia may be involved in actual ictogenesis, other lesional pathology such as cavernomas may produce seizures by effects on adjacent cerebral cortex.
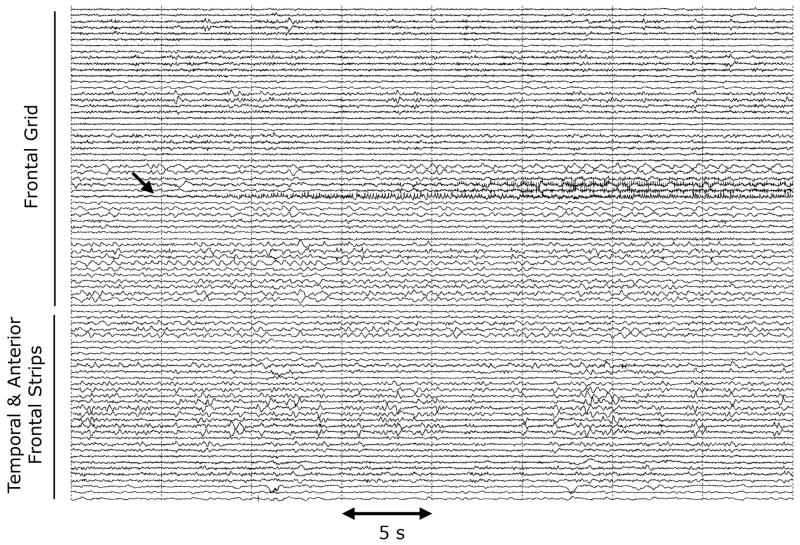
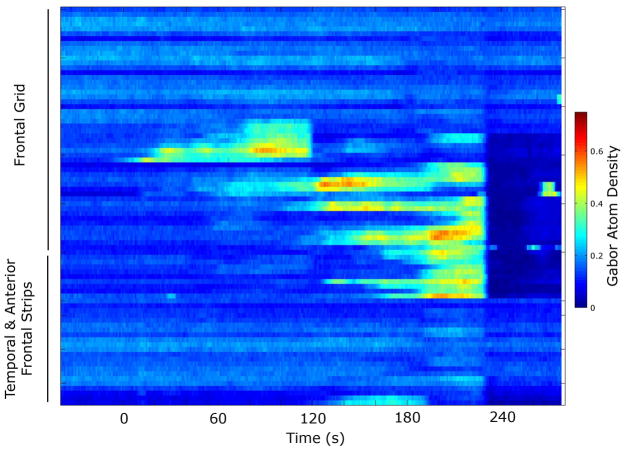
The ictal EEG will often reveal a focal seizure onset (Figure 1), recognizing that in some instances, (e.g. neocortical onset), intracranial recordings may be necessary to reveal the seizure onset and that some seizures (e.g. nonlesional neocortical) may have regional seizure onset patterns. Analysis of partial seizure dynamics from multichannel intracranial recordings reveals that there are changes in signal complexity that are also focal. The Gabor atom density (GAD) composite measure of signal complexity based on the matching pursuit method of time-frequency analysis reveals increased focal changes that occur in partial seizures, changes that correlate both temporally and spatially with the region of seizure onset [2,3]. Later regional seizure propagation is accompanied by increases in regional complexity in these areas (Figure 2).
Figure 1.
Intracranial EEG (ICEEG) of a partial seizure onset in a patient with a calcified frontal vascular malformation. The initial ~35 sec of the focal onset of the seizure is shown.
Figure 2.
Propagation map for the entire focal seizure illustrated in Figure 1. Each channel represents the composite GAD measure of complexity. Blue represents low GAD values and red represents high GAD values. This illustrates the focal onset from the frontal region but also during this 5 minute analysis, the patterns of propagation distinct from the focus are appreciated. Time 0 is the approximate seizure onset.
The most compelling evidence for the focal concept of ictogenesis is that patients with lesional partial epilepsy who undergo resective surgery are often seizure free [4]. While there is some debate as to how much surrounding tissue needs to be resected to optimize surgical outcome, it is clear that it is not necessary to resect areas remote from the lesion even if these areas are involved in seizure propagation. In summary, while the neuronal synchrony that characterizes partial seizure onset is a manifestation of local network involvement, partial seizures originate from focal brain regions prior to regional or generalized propagation.
“Network” – Klaus Lehnertz (University of Bonn, Bonn, Germany)
Seizures are inherently network phenomena that are intimately related to pathologic synchronization of neuronal networks in various brain regions [5]. Clinical observations together with invasive electroencephalography and functional neuroimaging now provide increasing evidence for the existence of specific cortical and subcortical networks in the genesis and manifestation of not only primary generalized but also focal onset seizures [6–8]. These epileptic networks are complex systems within the brain and are characterized by altered interactions between and within networks of networks of neurons and altered properties of neurons themselves. Epileptic networks exhibit a high spatial and temporal variability and involve interconnected areas distributed in distant cerebral structures. Studies on the predictability of seizures with EEG analysis techniques that aim at characterizing the strength of interactions between different brain regions identified seizure precursors in more remote and, in some cases, even contralateral brain areas [9]. Similar findings could also be achieved with functional magnetic resonance imaging and optical imaging of cerebral blood volume and deoxygenated hemoglobin. EEG analysis techniques that had been developed recently to characterize directed interactions between different brain regions identified complex driver-responder relationships between the epileptogenic zone and distant cerebral structures [10]. Studies on interaction mechanisms underlying propagation and termination of seizures indicate that network randomization, accompanied by an increasing synchronization of neuronal activity, may be considered as an emergent self-regulatory mechanism for seizure termination [11]. These findings underline the importance of brain tissue outside the epileptogenic zone but within an epileptic network in generating, maintaining, and terminating clinical seizures.
The unequivocal identification of the epileptic focus or epileptogenic zone [12] is regarded as a prerequisite for successful surgical treatment. With the concept of an epileptic network an epileptic focus may be interpreted as a strongly connected network node (or hub) whose successful removal may be interpreted as a so called grateful degradation of network activity. With the rapid developments in the quantitative analysis of complex networks, based largely on graph theory [13,14], further insights into the network mechanisms underlying ictogenesis can be expected in the near future.
Debate 2. Spikes and seizures: step-relatives or siblings?
“Spikes and seizures: Distant Step-relatives” – Jean Gotman (Montreal Neurological Institute, Montreal, Canada)
The EEG of most patients with epilepsy is characterized by two important changes compared to the EEG of healthy individuals: the ictal discharge occurs at the time of a seizure and inter-ictal, usually brief, discharges occur between seizures, most often without any clinical accompaniment. Whereas the seizure is of course the clinical and EEG event of most interest to characterize the seizure disorder and to localize the source of its generation, the interi-ctal discharges (the spikes) are commonly used for the same purposes. The reason for this are principally that spikes are much more frequent than seizures and are therefore much more easily recorded, but also that seizure recordings (at least with scalp EEG) are often obscured by EMG artifact, in contrast to spikes.
The question then naturally arises as to the relationships between spikes and seizures. Although it has long been established that there is a clear relationship between the two, we would like to argue here that the relationship may not be as tight as thought by many and we will emphasizes two aspects of the relationship that point to their difference.
In focal epilepsy, there is a frequent discrepancy between the localization of spikes and the localization of seizure onset. For instance one often sees patients with bitemporal independent spikes but seizures originating from a single temporal lobe. One often sees an important difference in extent: a spike may be quite focal and the seizure onset diffuse or the patient may show a widespread spike and wave discharge but a focal seizure onset, often frontal. The discrepancy is often more striking in intracerebral recordings than in scalp EEG; when independent spiking can be frequent in multiple regions even when the seizure onset is consistently focal.
Whereas antiepileptic drugs (AEDs) are effective in reducing seizures in most patients, even if they may not stop them, they have little effect on spikes. It is often thought that reducing AEDs results in increased spiking but several studies have shown that this is not the case [15–17]. Of course reducing AEDs results in increased seizure frequency, an important difference with spikes. The same studies have shown that seizures are followed by increased spiking, sometimes for a few hours and sometimes for several days. It is this increase which is often interpreted as resulting from decreased medication.
The concept of a seizure as a spike that did not stop and turned into a seizure is not compatible with the above observations. One should rather consider that spikes and seizures are different and relatively independent manifestations of epileptogenic brain tissue. In some situations, the coupling between them is tight and in others it is looser. Unfortunately, we have not defined the situations in which one is more likely than the other.
“Spikes and seizures: Siblings” – Ivan Osorio (University of Kansas, Kansas City, KS, US)
Debates are prompted by and grounded in opinions that make for entertaining exchange of arguments and counter-arguments. Moreover, even if based on reproducible observations, the arguments rarely constitute evidence as they have not passed what qualifies in science as a “severe test” [18]. Thus, the value of debates lies not in the expectation of answering complex questions, but in stimulating testable hypothesis.
The arguments that spikes and seizures are not “siblings” but “step-relatives” may be further strengthened by more persuasive experimental observations [19] than those furnished by Gotman: 1. Non-NMDA glutamatergic mechanisms underlie the generation of “interictal” spikes while seizures result from the activation of NMDA and non-NMDA glutamatergic and of GABAA receptors; 2. Spikes originate in the hippocampal subfield CA3, spread to the entorhinal cortex via CA1 and subiculum to return to the CA3 via the perforant path/dentate gyrus, whereas seizures initiate in the entorhinal cortex and propagate to hippocampus; 3. The interictal spike rate does not change before seizure onset in human and animal temporal lobe epilepsy; 4. In “in-vitro” experiments, CA3-driven spikes outlast seizures and 5. Severing the Schaffer collaterals connecting CA3 - CA1, abolishes spikes but reestablishes seizures in entorhinal cortex. Interpretation of these observations as confirmation that seizures and spikes are different disregards the evidence that the brain is organized as a densely interconnected network of nonlinear noisy “oscillators”, whose behavior is likely to be misconstrued when analyzed with common statistics.
The position that seizures and spikes are different lacks proper bases being the by-product of the “focus” theory, which ignores network concepts and their emergent properties and presupposes spatial congruency (if seizures & spikes are the same they should originate from the same site), a feature “alien” to network dynamics. The claim that spikes and seizures are independent manifestations of epileptogenic tissue, because spike frequency does not consistently increase before seizure onset, adopts the erroneous presumption that temporal correlations (event x always precedes event y) is “proof” that x causes y. This “logic” would lead to the interpretation that day, not the earth s rotation, “causes” night, because day always precedes night. Moreover, in non-linear systems a phenomenon may depend sensitively on another, yet be statistically independent of it, a property known as mixing (the so-called “butterfly phenomenon” in chaos theory).
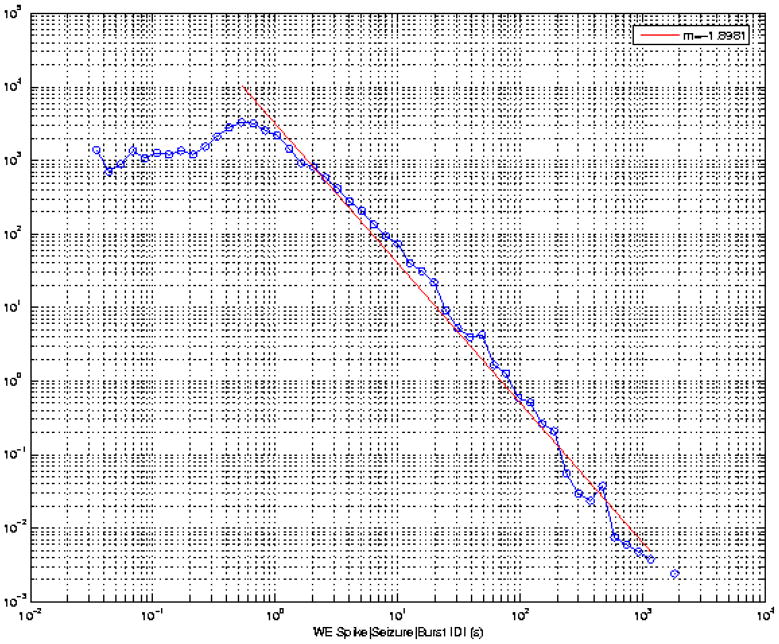
That spikes and seizure are “siblings” is supported by: 1. Single spikes disrupt visual processing in humans [20], a criterion that qualifies them as clinical events at least in certain epilepsies; 2. The probability distribution functions of spikes and seizure intervals have been shown to follow the same power law (Figure 3), an indication that they are governed by similar dynamics.
Figure 3.
Doubly logarithmic plots (y-axis: number of intervals; x-axis: durations in sec.) of times elapsed between the onset of events (defined as a single spike, bursts of spikes or seizures) recorded directly from the cortex of 8 rats treated intravenously with 3 mercapto-propionic acid. Onset times were marked visually. Inter-event (single spikes, bursts of spikes and seizures) time intervals are governed by the same power law suggesting they share common dynamics.
The claims that spikes and seizures are different lack probative value, as they are buttressed by incomplete observations, interpreted in an improper context; absolute distinctions have been made without adequate knowledge. The hypothesis that spikes and seizures are scale-free manifestations of epileptic networks and that their classification as interictal vs. ictal is baseless [21] is testable.
Debate 3. Ictogenesis: a result of hyposynchrony?
“Yes” – Theoden Netoff (University of Minnesota, Minneapolis, MN, US)
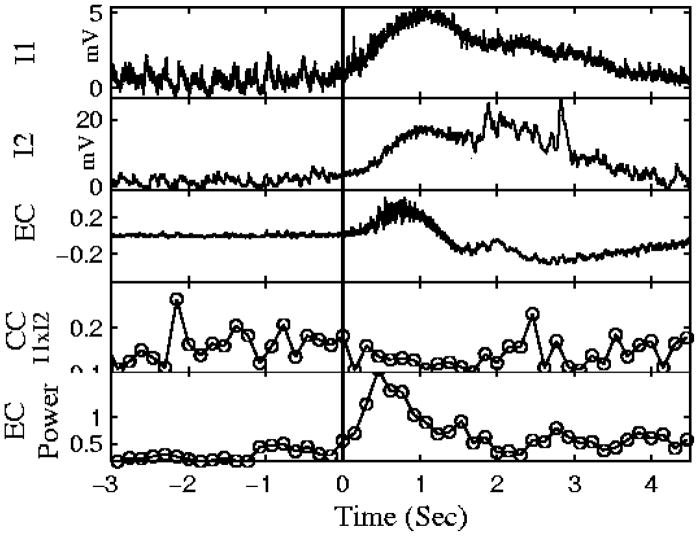
It has long been presumed that seizures are a result of “hyper-synchronous neuronal activity”. We tested this theory by measuring the correlation between synaptic inputs of two neurons during inter-ictal (between seizures) and ictal (seizure) activity in a brain slice model of epilepsy. These experiments revealed that correlation is in fact strong during the inter-ictal period. However, during the seizure, we see two different kinds of correlation as can be seen in Figure 4; there is strong correlation in the slow frequencies due to the summation of synaptic inputs during the seizure, however, correlation between the precise timing of the synaptic inputs decreases [22]. This finding has since been confirmed in intracranial EEG recordings [23], and during the seizure [24–26]. A stimulus that increases synchrony during a seizure has been shown to truncate the seizure [27]. We hypothesize that in this brain slice model of epilepsy that the seizure onset is caused by failure of the inhibitory population resulting in runaway excitation [28]. If this is the case, how can a network of excitatory coupled neurons desynchronize?
Figure 4.
Simultaneous recording of two intracellular (I1 & I2, top two signals) and extracellular recording (EC, third trace from top) during 4-AP induced seizures in slice. Crosscorrelation (CC) measured in short time bins during the seizure show that while both neurons are strongly depolarized during the seizure, the high frequency activity is not correlated. Towards the end of the seizure, correlation begins to increase again. Figure modified from [22].
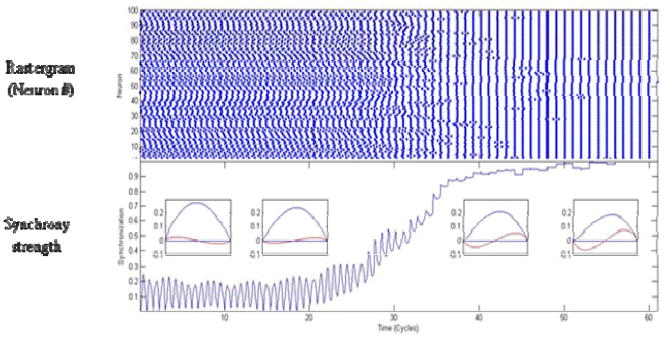
Synchrony within a network of neurons can be predicted from the phase response curves (PRCs) of the neurons. The PRC is a measure of how a synaptic input will affect the interspike interval of a neuron, depending on the phase that the synaptic input was applied [29]. The PRC of a neuron changes with firing frequency. We hypothesize that at the onset of the seizure, the firing rate is so high that the neurons desynchronize, as the firing rates slow, the PRCs change in shape and the network can synchronize, resulting in the post-ictal bursts, or the change from tonic phase of the seizure to the clonic phase. We demonstrate through a simulation shown in Figure 5. The simulation starts with a PRC that might be measured from a neuron firing at a high rate and then changes in shape to reflect the change in dynamics as the firing rate slows. When the peak of the PRC is in first half of the phase (at high firing rates), the neurons will prefer to fire in antiphase, as it shifts to the second half (at slower firing rates), the network prefers to synchronize [30]. This simulation illustrates that it is plausible that at the peak of a seizure where neurons are firing very rapidly that they actively desynchronize and as the firing rate slows they synchronize.
Figure 5.
Excitatory network to desynchronize and then synchronize as network activity decreases. Network of 100 excitatory cells all-to-all coupled. Top graph, rastergram of spike times. Middle panel, spectrogram of network activity, light colors indicate strong synchrony. Bottom panel, a network synchrony measure, zero is desynchronized, 1 is perfect synchrony. Phase response curves are being changed over simulation, insets show PRC and H functions (red sinusoid) at 4 time points. The change in PRC is designed to reflect that seen from decreasing firing rate. Network switches from splay to stable synchrony on 15th cycle.
“No” – Hitten Zaveri (Yale University, New Haven, CT, US)
The argument presented here is based on a consideration of 1) background EEG activity, 2) transition from background activity to seizure, 3) seizure, and 4) measurement of synchrony of brain activity.
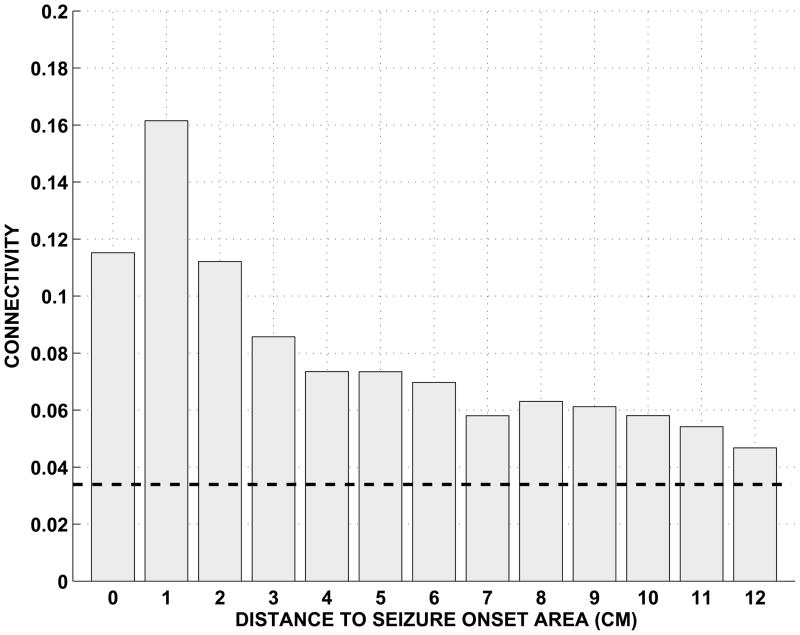
First, a number of studies employing a range of methods have found background intracranial EEG (icEEG) activity to be hypersynchronous and there has been no compelling evidence to the contrary [31–33]. See, for example, Figure 6 which documents the presence of excess background icEEG connectivity in the immediate vicinity of the seizure onset area.
Figure 6.
Intracranial EEG beta frequency band connectivity averaged for 6 unselected patients for integer distances from the seizure onset area [33]. The seizure onset contacts are located at distance 0. Average estimates greater than the indicated threshold are significantly non-zero (p < 10−5). Non-zero connectivity exists in the seizure onset area and several cm away from it, and an inverse relationship is apparent between average beta band connectivity and distance from the seizure onset area. Reproduced, with permission, from [33].
Second, studies have been equivocal on the role of synchrony in the transition from background to seizure [34,35]. Furthermore, our recent studies have revealed counter-intuitive observations of a decrease in univariate correlates of brain excitation with antiepileptic drugs (AEDs) during icEEG monitoring [36,37,17] which suggest a lack of support for the hypothesis that out-of-control excitation leads to seizure, and may instead suggest a role for hypersynchrony during the transition from background to seizure [36]. A change in synchrony, if any, during this transition remains to be better understood.
Third, the textbook definition of seizure equates it to a hypersynchronous discharge of neurons; indeed hypersynchrony is considered a sine qua non for seizure. Recent studies of seizure, including those documented in the opposing perspective, have observed hyposynchrony during seizure. It should be emphasized, however, that the results of these studies are not determinate as both hyposynchrony and hypersynchrony have been observed during seizure [37].
Fourth, the measurement of synchrony of brain activity is not without difficulty. The measures being employed are, primarily, linear, assume stationarity and statistical significance has historically been poorly evaluated [38]. If relationships being studied are non-linear or the time-series is non-stationary synchrony will be underestimated by these methods. Furthermore, measurements are typically performed at a single scale and ignore the possibility that synchrony can be different at different scales, for example decreased at one scale and increased at another, or that synchrony can be different in different systems for example thalamo-cortical and cortico-cortical systems, and importantly because of these reasons hyposynchrony and hypersynchrony are not necessarily mutually exclusive. Additionally, the propagation of synchronous discharges can change them, and current approaches will likely underestimate the synchrony of propagated discharges.
In conclusion, we argue that because seizure, the state where hyposynchrony is now being reported, is the most difficult of the three indicated periods to study for the reasons listed above, it is possible that synchrony in this state is being relatively underreported by current methods. In addition, given the hypersynchrony of background activity, equivocal findings in the transition from background to seizure and equivocal findings during seizure it remains possible that seizure consists of hypersynchronous activity as has been historically believed and is the result of hypersynchrony.
Debate 4. Can focal seizures be caused by excessive inhibition?
“Yes” – Walter Freeman (University of California, Berkeley, CA, US)
In my opening comment of the debate I stated: “This is a lousy question.” My reason for saying so is that seizures have many causes in many combinations. Complex systems have multiple pathways. Each event causes many effects, and each effect has many causes. Even worse, because of recursive paths in networks, each cause can be its own effect. Worse still is the butterfly effect of nonlinearities [39]. Any one of many local causes hidden in remote nodes of networks can have global effects, but as in a flock of butterflies, finding which one is the cause is usually impossible [40].
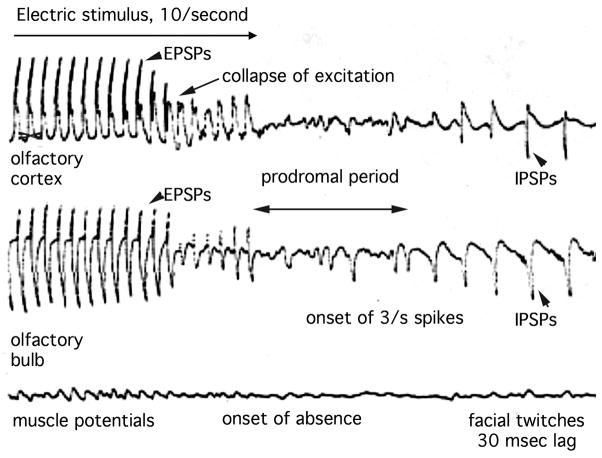
The question arises from inappropriate resort to linear thinking. One assumes that every seizure must have a time and place when it begins. Linear cause-and-effect is neatly demonstrated in a form of experimental epilepsy. I can place a pair of electrodes on the lateral olfactory tract and reliably cause a complex partial seizure by a few seconds of titanic electrical stimulation [41]. The seizure obviously begins at my focus of stimulation in time and space. It rapidly goes global as a 3/sec spike-and-wave train lasting 30–60 sec from the olfactory bulb and cortex (Figure 7). The animal (here cat, but also rat and rabbit) shows classic freezing, absence, twitching of lips, jaw and eyelids, salivation, occasional micturition. On repeated induction it kindles into tonic-clonic seizure.
Figure 7.
At high temporal resolution it can be seen that the seizure begins with the failure of excitation. The presynaptic compound action potential was unchanged, ergo the failure was due to depletion of glutamate in the presynaptic terminals from excessive utilization rate. It then becomes obvious that the seizure spikes are IPSPs, not EPSPs, because they have the same spatial distribution with overlap of the location of the active synapses on the pyramidal cells, but with opposite polarity.
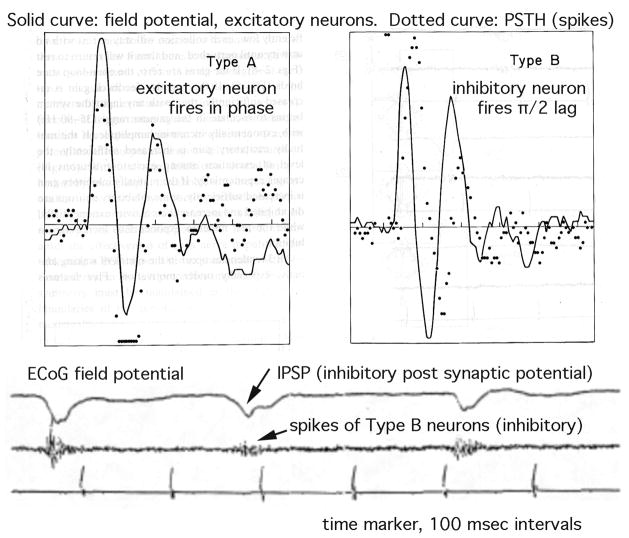
Obviously by excess excitation I cause the seizure. But wait. The excitatory post-stimulus potential (EPSP) abruptly vanishes well before the seizure spikes begin (Figure 7), so the cause is deficient excitation, with probable cause by transmitter depletion (the pre-synaptic action potentials are still observed). But wait again. The seizure spike is inhibitory. It is an IPSP from excitatory neurons, caused by intense firing by other neurons. But they are not the pyramidal cells that are generating the IPSP. The runaway activity is by inhibitory interneurons (Figure 8). The spike is caused by excess inhibition of the excitatory cells. Yet that shuts off the interneurons, opening the way for disinhibition of excitatory cells. But disinhibited inhibitory cells overtake them when they initiate the next IPSP. Clearly then the cause is deficient inhibition.
Figure 8.
In prepyriform cortex the IPSP of the excitatory neurons (A) is accompanied by vigorous discharge of interneurons, as identified by the 90° phase lag (B).
These statements are all valid inferences; taken together, they make the case that linear causal thinking is much too local to penetrate the mysteries of focal seizures to explain them and predict them. A model that can simulate the nonlinear feedback dynamics is needed.
Specifically, I challenge my opponent, and all others who record epileptic spikes. Can you prove that the spikes you observe are compound EPSPs from excitatory neurons? I believe that in most instances they cannot, because the proof must be based on the following evidence. The current sources and sinks of the spikes must be mapped to determine whether the epileptiform spikes are envelopes of action potentials or dendritic potentials [42,43]. If dendritic, the orientation of the fibers and the locations of synapses must be shown. Vigorous bursts of action potentials must be shown to drive PSPs. The type of the firing neurons (excitatory or inhibitory) must be demonstrated. To do that, a dynamic model of the system must be constructed that simulates the spike trains by deficient excitatory firing and excess inhibitory firing. I have done all this, and have shown that a GABA blocker reduces the susceptibility of animals to experimental seizure induction [44]. And my colleagues and I have published a successful algorithm for seizure prediction [45,46], which is based on the theory that excess inhibition causes seizure spikes but not the seizure. I believe that my program offers complex models that go beyond linear thinking into the realm of self-organization and deal effectively with circular causality.
“No” – John Jefferys (University of Birmingham, Birmingham, United Kingdom)
The meaningful interpretation of this question is whether or not clinically-relevant focal seizures are caused by excessive inhibition. Experimental seizures certainly can result from aberrant inhibition, although even here aberrant does not necessarily mean excessive.
Blocking GABAA receptors, or HCO3− production, blocks seizure-like events produced by: low-[Mg2+]o [47], 4-aminopyridine [48,49], and brief repetitive stimulation of hippocampal CA1 [50]. In each case seizure-like activity arguably is caused by excessive GABAA-receptor activation, but the physiological consequence is a shift from inhibition to excitation, not an increase in inhibition, because [Cl−]i accumulation allows HCO3− efflux dominate, while [K+]o accumulates.
GABAA-dependent depolarizing potentials appear in human epileptic tissue in vitro [51–53], apparently due to reversion of cation-chloride cotransporters to early developmental states. These brief interictal depolarizations may play a role in epileptic activity but there is no evidence they cause seizures, and again they represent aberrant rather than excessive inhibition.
Inhibition can play a critical role in oscillations [54]. There is one example of such inhibitory pacing (and “rebound” excitation) in epilepsy: Autosomal Dominant Frontal Lobe Epilepsy [54]. GABAA-receptor depression inhibits seizures in mouse models. This one example could justify the proposition (rather more skewed than the original “ictogenesis: excessive excitation or excessive inhibition?”). However even here, the root cause of the condition is mutation of nicotinic cholinergic receptors: the inhibitory network may well function normally in responding to an abnormal cholinergic input. Indeed the mouse models need low dose nicotine before they generate seizures. I am not aware of any reports of adverse effects of GABA promoters such as tiagabine in clinical ADNFLE, although to borrow a phrase from the UK s BSE enquiry, absence of evidence is not evidence of absence.
Ripples (100–250 Hz) and fast ripples (>250 Hz) have been implicated in focal epilepsies [55]. Unit recordings reveal phase locking of inhibitory neurons to ripples, implicating recurrent inhibition in their generation [56]. Ripples are associated with physiological function as well as epileptic activity. Fast ripples appear more specific for epileptic tissue than ripples, and are not associated with phase-locked firing of inhibitory neurons [56]. Whether they have a role in fast ripples without phase-locking is not clear. Even in the case of ripples it is not obvious whether inhibition is any more excessive than excitation: where inhibition sets rhythms or alters dynamics it may be the timing of the inhibition that matters more than whether or not it is excessive.
Clearly there are many ways in which inhibition can play a role in epileptic seizures. To get a sense of whether they do, we should look at the broader picture. Strengthening GABAergic inhibition is a common mechanism for AEDs [57]. Exceptions are carbonic anhydrase inhibitor, acetozolamide, and potentially NKCC1 transporter inhibitor, bumetanide, but neither controls excessive inhibition, instead they normalize aberrant inhibition. Drugs that depress GABAergic function are convulsants rather than AEDs, with one or two exceptions in experimental models outlined above. Epilepsy-related GABAA mutations cause loss, not gain, of function [58].
In conclusion, while there are interesting examples of abnormal inhibition causing seizures in experimental and theoretical models, the conventional, and robust, evidence is that strengthening GABA-ergic inhibition decreases clinical focal seizures, while impairing inhibition can cause seizures (as in some kinds of symptomatic seizures and genetic epilepsies).
Responses to Statements
Freeman
Readers should take note that Jefferys and I never engaged each other in our debate but presented cases that were completely skew, he arguing from neurochemistry, I from neurodynamics. We hold different parts of the elephant. Considering that many epilepsies are still idiopathic and inadequately treated, the most fertile ground for future research may be found in effort to combine these disparate disciplines. The requirements for training of new researchers in such a transdisciplinary program are daunting, but the impasse of our debate gives reason to try.
Jefferys
I fully agree with Freeman that we took different approaches to address the propositions we had been posed. The challenge he raises in his commentary should, and I am sure will, be addressed; it certainly is something that interests and intrigues me and my research group. In the mean time, I think there are some instances of evidence for the epileptic spikes being largely due to compound EPSPs, but I am certain that they are never pure EPSPs. The current source densities in [59] look like EPSPs, at least to start with. The polarity and conduction delays of the intra-ictal spikes in [60] also are consistent with a major role for excitatory synaptic activity.
However, these and similar studies do not provide clear answers to Dr Freeman’s challenge and I agree absolutely with him that the kind of transdisciplinary approach he advocates will go a long way towards answering these fundamentally important questions.
Debate 5. Do HFOs provide relevant independent information?
“Yes” – Gregory Worrell (Mayo Clinic, Rochester, MN, US)
Neuronal oscillations in human brain span a wide range of spatial and temporal scales that extend far beyond traditional clinical intracranial electroencephalography (iEEG). The iEEG records extracellular local field potentials (LFP) that include high frequency oscillations not captured on scalp recordings (Figure 9). The classification of LFP oscillations into physiological versus pathological oscillations is a fundamental challenge [61,62]. Despite the lack of mechanistic specificity for neuronal oscillations, i.e. LFP oscillation in a particular frequency range may be generated by different mechanism, clinical electrophysiology has a successful tradition of associating neural activity with pathological brain function [63]. Similar to interictal epileptiform spikes, studies in animals and humans have suggested that some interictal HFO may be electrophysiological biomarkers of epileptic brain [61,62,64–66] (Figure 10) and may be involved in epileptogenesis [63,64,68] and seizure generation [67,68]. Currently, there are no conclusive studies showing that HFO are precursor events in human partial epilepsy, but in vitro [69] and in some patients [67] HFO are increased prior to seizure. These results suggest that HFOs within the seizure onset zone may be useful for identifying periods of increased predisposition to clinical seizures [61,62,67,70].
Figure 9.
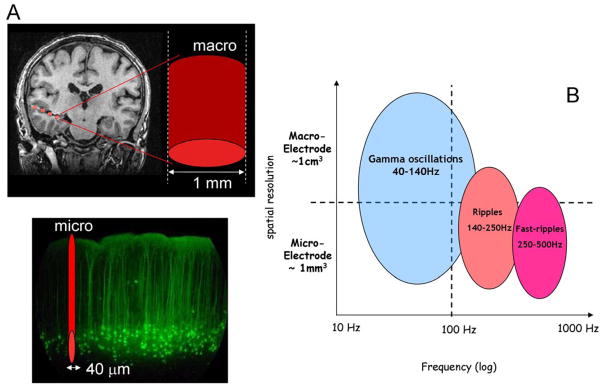
Macro and micro recordings of HFOs in the human brain. (A) Conventional intracranial depth macroelectrodes have a contact area around 1 mm2, which presumably record the activity of ~10 mm square of brain tissue. Microelectrodes are capable of probing the fine structure of cerebral cortex have and have a typical size of 30–40 μm in diameter. (B) The spatial scale of the expression of physiological HFO in the gamma (40 – 120 Hz) and ripple frequency (140 – 250 Hz) range and pathological fast ripple (250 – 500 Hz) HFO is frequency dependent (Adapted from [64]).
Figure 10.
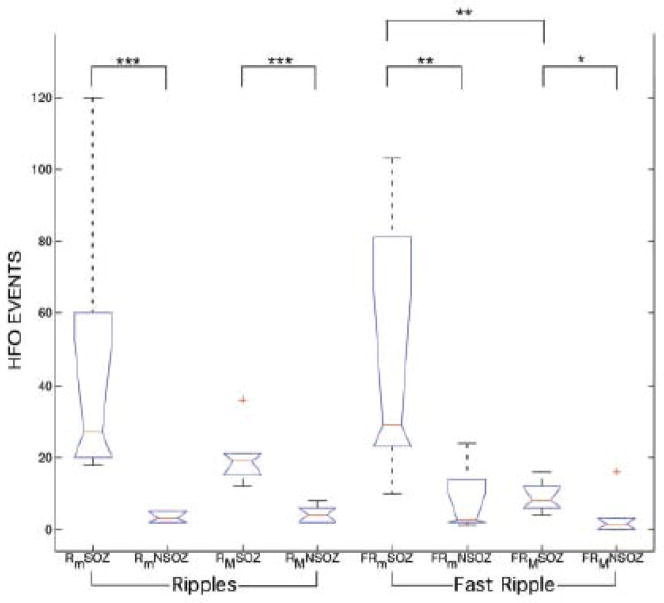
Analysis of variance applied to HFO (ripple/FR), electrode (micro/macroelectrode) and brain region (seizure onset/non-seizure onset). The number of microwire ripple (Rm) and fast ripple (FRm) oscillations are increased in the seizure onset zone (SOZ) compared to non-SOZ. The number of macroelectrode ripple (RM) and fast ripple (FRM) oscillations were increased in the SOZ. The microwire electrodes detect significantly more fast-ripple HFO compared to the clinical macroelectrodes (Adapted from [70]).
“No” – Michel Le Van Quyen (LENA-CNRS, Paris, France)
High-frequency oscillations (HFOs) are an electrical signature of focal epilepsy but are also instrumental in a wide range of physiological functions. Studies from both animals and humans describe gamma frequency oscillations (gamma: 25–80 Hz) that are involved in sensory binding [71], ripple frequency oscillations (ripple: 80–200 Hz) that may be important for memory consolidation [72,73], and ultra-fast oscillations (~600 Hz) associated with somatosensory evoked response [74]. This raises the fundamental issue of the differences between the physiological and pathological HFOs [62]. Are these different only in terms of their dominant frequency? The type of cellular substrate or network involved? The scale that they can be recorded? While epileptiform spikes and seizures are highly specific signatures for epileptic brain, it has proven more difficult to clearly define pathological HFO because normal physiological oscillations occur in the same frequency range [61,62]. In fact, while there is evidence for distinctly pathological LFP events, e.g. interictal epileptiform spikes [63] and fast ripple HFO [64], there is emerging evidence that gamma and ripple frequency HFO may exist on a continuum of activity and spanning both pathological and physiological activity [61,62]. In addition, the studies in humans are limited to patients with medically resistant partial epilepsy and the specificity of these findings remains unclear. Whether HFO recorded in epileptic brain are generated by unique pathological mechanism(s) or represent an aberration of normal physiological oscillations is also not clear, and there are currently no established quantitative criteria for distinguishing physiological from pathological HFO.
Debate 6. Phase synchronization – is it worthwhile as measured?
“No” – Steven J. Schiff (Pennsylvania State University, University Park, PA, US)
The brain creates mind as a function of the coupling and interaction between its neurons and their ensembles. The way we infer such coupled interactions is by testing for correlation (time) or coherency (frequency) to infer synchronization.
Our interest in phase synchronization in recent years was heightened by the finding that for certain nonlinear systems, that phase relationships might detect coupling when the amplitudes of signals might not provide an adequate signal (see [75] and references therein). Nevertheless, the application of phase synchronization to biological signals, especially EEG, is fraught with difficulty. The dangers of the pitfalls of detecting phase in EEG (reviewed by Schiff [76]) were well discussed by Nunez [77] and Fein et al [78]. The difference between the theoretical advantages of phase in systems composed with well defined phase oscillations and brain signals lies in the complexity of the brain dipole generators, the filtering and conductive properties of the coverings of the brain, and the inherent limitations of sampling. As shown by Guevara et al [79], magnetic encephalography (MEG) has clear advantages over any type of EEG in terms of signal processing for phase coherence, but MEG and EEG do not measure the same signals, and EEG is much more widely available. One can improve on the recording of EEG by using a common reference, but the theory here would require coverage of the entire head with finely spaced electrodes in order to have an adequate common reference – an impossibility. We can use bipolar recordings, but dipoles within the brain will produce very different signals on bipolar scalp surface recordings depending on the orientation of those dipoles. We can use Laplacian derivations to improve the estimate of the current actually exiting the brain or skull beneath electrodes, but Laplacians are typically measured with less accuracy than is reasonable, the calculations tend to only apply to divergence of potential within the plane of the electrodes themselves, and they act as spatial filters underestimating longer-range correlations.
Lastly is the nature of detecting weak synchronization, and detecting the lack of synchronization, in any system. If systems are uncoupled but share frequencies, one will always detect a finite estimate of correlation or coherency between the signals. A statistical model is thus required, with a null hypothesis, to gauge the significance of any estimate of synchrony in measurements. In [80], the statistical models required to detect a lack of phase synchronization in uncoupled systems were explored. Such models were then used on real and simulated data where the actual degree of coupling was known. The results were quite unexpected: phase correlation calculations might or might not be as effective for linear stochastic systems, nonlinear maps, nonlinear differential equations, and nonlinear experimental electrical circuits, in comparison with alternative methods (linear correlation, mutual information, and continuity). How much uncertainty and noise is present in such signals has a substantial effect on our ability to select the optimal synchrony detection tools [81].
So phase information can be extracted from brain signals, and elegant computational methods applied to detect synchronization and infer coupling. But such results must be presented with sufficient methods as required to probe the integrity of the results. This would involve recording with high densities of electrodes, and with the application of one or more reference free type of montage. There must be more than one method applied to detect synchrony – employing amplitude, phase, and frequency information. Statistical null hypotheses must be employed for each method of synchrony detection used. And in the end, congruent results advocating for the presence of true coupling must be evaluated in light of the complexities of the above in mind.
“Yes” – Florian Mormann (California Institute of Technology, Pasadena, CA, US)
Measures of phase synchronization like the mean phase coherence [82] can be quite useful to quantify phase relationships between EEG signals as long as certain caveats are observed. Two important caveats are the influence of the recording reference [77,79] and the question which frequency band the extracted oscillatory phase relates to.
In cases where an influence of a remote (e.g. scalp) reference is suspected, it is possible to identify and eliminate the influence of the reference from the recorded signal as long as the reference is sufficiently independent from the sources of the actual signal [83]. Another useful strategy is to record several independent references in a way that allows post hoc selection of different references. Any findings suspected to be influenced by the recording reference should be replicated with a different reference to prove that they are indeed reference-independent. Note that the problem of a common reference can affect not only phase synchronization measures, but any bivariate measure used to characterize relationship between two signals.
The second caveat relates to the question of which frequencies are represented by the phase variables of a synchrony measure. If the phases are extracted from broad band signals, e.g. via Hilbert transform [84], it is important to realize that a phase variable generally reflects the dominant frequency in the spectral composition of a signal [85,86]. If the dominant frequency changes over time, a broadband phase variable will follow this change and instantaneously reflect this dominant frequency (Figure 11). A phase variable extracted from a narrow-band signal, e.g. after filtering or through use of the wavelet transform, on the other hand will reflect a constant frequency band even if this band does not contain any substantial power in the spectrum of the signal. Depending on the problem under investigation, broad-band phase definitions may be favorable over narrow-band definitions or vice versa.
Figure 11.
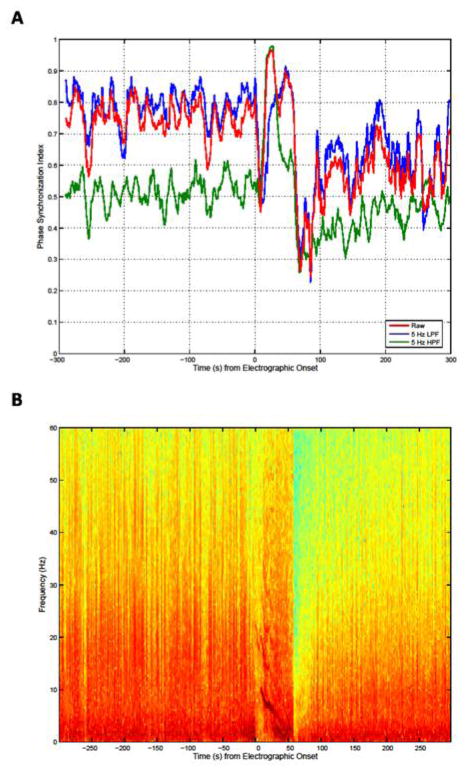
(A) Phase synchronization between two intracranial channels (bipolar montage) during a seizure recording. The seizure started at 0 s and lasted for approximately 60 s. Displayed is the time course of the mean phase coherence calculated from the raw broad band signals (red), from low-pass filtered signals below 5 Hz (blue), and from high-pass filtered signals. Note that the broad-band synchronization index mostly follows the low-pass-filtered index except during seizure onset when it briefly follows the high-pass-filtered index. (B) The time-frequency plot with the color-coded spectral power from one of the two channels shows that the dominant frequency is mostly below 5 Hz except during seizure onset when the dominant frequency is briefly found around 10 Hz. (Analysis and Figure provided by Mark Frei.)
As with any measure used to characterize biological signals, it is crucial to understand what a measure for phase synchronization represents and what types of signals are being processed.
Summary
IWSP4 was the fourth in a series of international scientific congresses held every 18–24 months to bring together the interdisciplinary international seizure prediction group to discuss progress on seizure prediction, seizure generation and the control of seizures. The debates described here allowed the IWSP4 Organizing Committee to highlight controversial topics in epilepsy which are of relevance to the workshop. The debates allowed a succinct presentation of positions through arguments and counter-arguments, and an open discussion around these positions. A survey of workshop participants after the conclusion of the workshop indicated that the debates were extremely successful for the purpose intended. Workshop participants found the debates very useful for clarifying issues. While the debaters did not necessarily present original results, the debates allowed the debaters to propose arguments from evidence presented in different studies to support their position. A clarification of positions in this manner helps to define where conflicts lie and clarify opposing perspectives, both of which contribute toward advancement in the field.
Acknowledgments
This work was supported by funding from the Epilepsy Therapy and Development Program (GW), National Institutes of Health R01-NS 06939 (GW), and the European Union-FP7 Project EPILEPSIAE (Evolving Platform for Improving Living Expectation of Patients Suffering from IctAl Events, Grant No 211713 (MLVQ)).
Financial Support. Funding for IWSP4 was received from the following foundations, government agencies, industries, university and hospital partners and individuals: Alliance for Epilepsy Research, UCB, Cyberonics, Deutsche Gesellschaft für Epileptologie, NeuroVista, American Epilepsy Society, CURE, University of Kansas Medical Center, Children’s Mercy Hospitals and Clinics, Honeywell - Kansas City Plant, Ad-Tech, Cardinal Health, Medtronic, DIXI, Boulevard Brewing Co., and Mary Shaw Branton, Don Alexander, and Frank and Helen Wewers. Funding was also made possible in part by grant number R13NS065535 from the National Institute of Neurological Disorders and Stroke (NINDS), Office of Rare Disease Research (ORDR) and National Institute of Child Health and Human Development (NICHD). The views expressed in written conference materials or publications and by speakers and moderators do not necessarily represent the official views of the NINDS, ORDR, NICHD or National Institutes of Health (NIH) and do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engel J., Jr Report of the ILAE Classification Core Group. Epilepsia. 2006;47:1558–1568. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 2.Jouny CC, Bergey GK, Franaszczuk PJ. Partial seizures are associated with early increases in signal complexity. Clin Neurophysiol. doi: 10.1016/j.clinph.2009.09.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franaszczuk PJ, Bergey GK, Durka PJ, Eisenberg HM. Time-Frequency analysis using the matching pursuit algorithm applied to seizures originating from the mesial temporal lobe. Electroenceph clin Neurophysiol. 1998;106:513–21. doi: 10.1016/s0013-4694(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 4.Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, Tetto A, Vitelli E, Vitezic D, Wiebe S. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Avoli M, Antuono MD, Louvel J, Köhling R, Biagini G, Pumain R, Arcangelo GD, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 6.Bartolomei F, Wendling F, Bellanger JJ, Regis J, Chauvel P. Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clin Neurophysiol. 2001;112:1746–60. doi: 10.1016/s1388-2457(01)00591-0. [DOI] [PubMed] [Google Scholar]
- 7.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–27. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- 8.Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49(Suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–33. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- 10.Lehnertz K, Bialonski S, Horstmann MT, Krug D, Rothkegel A, Staniek M, Wagner T. Synchronization phenomena in human epileptic brain networks. J Neurosci Methods. 2009;183:42–8. doi: 10.1016/j.jneumeth.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Schindler KA, Bialonski S, Horstmann MT, Elger CE, Lehnertz K. Evolving functional network properties and synchronizability during human epileptic seizures. Chaos. 2008;18:033119. doi: 10.1063/1.2966112. [DOI] [PubMed] [Google Scholar]
- 12.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 13.Arenas A, Diaz-Guilera A, Kurths J, Moreno Y, Zhou C. Synchronization in complex networks. Phys Rep. 2008;469:93–153. [Google Scholar]
- 14.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 15.Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- 16.Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroenceph Clin Neurophysiol. 1989;72:7–15. doi: 10.1016/0013-4694(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–92. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 18.Mayo DG. Error and the Growth of Experimental Knowledge. The University of Chicago Press; Chicago, Illinois: 1996. [Google Scholar]
- 19.Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr. 2006;6:203–7. doi: 10.1111/j.1535-7511.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time. I. General considerations. Electroencephalogr Clin Neurophysiol. 1988;69:319–37. doi: 10.1016/0013-4694(88)90004-1. [DOI] [PubMed] [Google Scholar]
- 21.Osorio I, Frei MG, Sornette D, Milton J. Pharmaco-resistant seizures: self-triggering capacity, scale-free properties and predictability? Eur J Neurosci. 2009;30:1554–8. doi: 10.1111/j.1460-9568.2009.06923.x. [DOI] [PubMed] [Google Scholar]
- 22.Netoff TI, Schiff SJ. Decreased neuronal synchronization during experimental seizures. J Neurosci. 2002;22:7297–307. doi: 10.1523/JNEUROSCI.22-16-07297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler K, Leung H, Elger CE, Lehnertz K. Assessing seizure dynamics by analysing the correlation structure of multichannel intracranial EEG. Brain. 2007;130:65–77. doi: 10.1093/brain/awl304. [DOI] [PubMed] [Google Scholar]
- 24.Garcia Dominguez L, Wennberg RA, Gaetz W, Cheyne D, Snead OC, 3rd, Perez Velazquez JL. Enhanced synchrony in epileptiform activity? local versus distant phase synchronization in generalized seizures. J Neurosci. 2005;25:8077–84. doi: 10.1523/JNEUROSCI.1046-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez Velazquez JL, Garcia Dominguez L, Wennberg R. Complex phase synchronization in epileptic seizures: Evidence for a devil’s staircase. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;75:011922. doi: 10.1103/PhysRevE.75.011922. [DOI] [PubMed] [Google Scholar]
- 26.García Dominguez L, Wennberg R, Pérez Velázquez JL, Guevara R. Enhanced measured synchronization of unsynchronized sources: inspecting the physiological significance of synchronization analysis of whole brain electrophysiological recordings. International Journal of Physical Sciences. 2007;2(11):305–17. [Google Scholar]
- 27.Schindler K, Elger CE, Lehnertz K. Increasing synchronization may promote seizure termination: Evidence from status epilepticus. Clin Neurophysiol. 2007;118:1955–68. doi: 10.1016/j.clinph.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–54. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci USA. 1998;95:1259–64. doi: 10.1073/pnas.95.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutkin BS, Ermentrout GB, Reyes AD. Phase-response curves give the responses of neurons to transient inputs. J Neurophysiol. 2005;94:1623–35. doi: 10.1152/jn.00359.2004. [DOI] [PubMed] [Google Scholar]
- 31.Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, McKhann G, Jr, Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage. 2007;35(1):140–8. doi: 10.1016/j.neuroimage.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega GJ, Menendez de la Prida L, Sola RG, Pastor J. Synchronization clusters of interictal activity in the lateral temporal cortex of epileptic patients: intraoperative electrocorticographic analysis. Epilepsia. 2008;49(2):269–80. doi: 10.1111/j.1528-1167.2007.01266.x. [DOI] [PubMed] [Google Scholar]
- 33.Zaveri HP, Pincus SM, Goncharova II, Duckrow RB, Spencer DD, Spencer SS. Localization-related epilepsy exhibits significant connectivity away from the seizure-onset area. NeuroReport. 2009;20(9):891–5. doi: 10.1097/WNR.0b013e32832c78e0. [DOI] [PubMed] [Google Scholar]
- 34.Bartolomei F, Wendling F, Regis J, Gavaret M, Guye M, Chauvel P. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Research. 2004;61(1–3):89–104. doi: 10.1016/j.eplepsyres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Kramer MA, Kolaczyk ED, Kirsch HE. Emergent network topology at seizure onset in humans. Epilepsy Research. 2008;79:173–86. doi: 10.1016/j.eplepsyres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Zaveri HP, Pincus SM, Goncharova II, Novotny EJ, Duckrow RB, Spencer DD, Spencer SS. Background EEG spectral change with anti-epileptic drug taper during intracranial monitoring. 2010 In press. [Google Scholar]
- 37.Zaveri HP, Pincus SM, Goncharova II, Novotny EJ, Duckrow RB, Spencer DD, Spencer SS. A reduction in energy accompanies anti-epileptic drug taper during intracranial monitoring. Epilepsy Research. 2009;86(2–3):153–62. doi: 10.1016/j.eplepsyres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Zaveri HP, Williams WJ, Sackellares JC, Beydoun A, Duckrow RB, Spencer SS. Measuring the coherence of intracranial electroencephalograms. Clinical Neurophysiology. 1999;110:1717–25. doi: 10.1016/s1388-2457(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 39.Freeman WJ. An Exploration of Mesoscopic Brain Dynamics. London: Springer; 2000. Neurodynamics. [Google Scholar]
- 40.Freeman WJ. Nonlinear dynamics of paleocortex manifested in the olfactory EEG. Biological Cybernetics. 1979;35:21–37. doi: 10.1007/BF01845841. [DOI] [PubMed] [Google Scholar]
- 41.Freeman WJ. Petit mal seizure spikes in olfactory bulb and cortex caused by runaway inhibition after exhaustion of excitation. Brain Research Reviews. 1986;11:259–84. doi: 10.1016/0165-0173(86)90015-9. [DOI] [PubMed] [Google Scholar]
- 42.Freeman WJ. Examination of the Neurophysiological Basis of Adaptive Behavior through the EEG. New York: Academic Press; 1975. Mass Action in the Nervous System. [Google Scholar]
- 43.Freeman WJ, Kozma R. Freeman’s mass action. Scholarpedia. 2010;5(1):8040. [Google Scholar]
- 44.Freeman WJ. Valium, histamine, and neural networks. Biological Psychiatry. 1993;34:1–2. doi: 10.1016/0006-3223(93)90249-d. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz YG, Guang L, Freeman WJ, Moreira EG. 1st Intern Conf Cogn Neurodyn. Ch 128. Shanghai, P.R. China: Springer; 2007. A new Approach to detect stable phase structure in high density EEG signals; pp. 741–5. [Google Scholar]
- 46.Ramon C, Holmes MD, Freeman WJ, McElroy R, Rezmanian E. Comparative analysis of EEG and phase synchronization of EEG to localize epileptic sites from high density scalp EEG interictal recordings. Abstract, Engineering in Medicine and Biology EMBS, Bimag2008; Japan. 2008. [DOI] [PubMed] [Google Scholar]
- 47.Köhling R, Vreugdenhil M, Bracci E, Jefferys JGR. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci. 2000;20:6820–9. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traub RD, Colling SB, Jefferys JGR. Cellular mechanisms of 4-aminopyridine-induced synchronized after-discharges in the rat hippocampal slice. J Physiol. 1995;489:127–40. doi: 10.1113/jphysiol.1995.sp021036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Antuono M, Louvel J, Kohling R, Mattia D, Bernasconi A, Olivier A, et al. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain. 2004;127:1626–40. doi: 10.1093/brain/awh181. [DOI] [PubMed] [Google Scholar]
- 50.Bracci E, Vreugdenhil M, Hack SP, Jefferys JGR. On the synchronising mechanisms of tetanically-induced hippocampal oscillations. J Neurosci. 1999;19:8104–13. doi: 10.1523/JNEUROSCI.19-18-08104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Köhling R, Lücke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, et al. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121:1073–87. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- 52.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 53.Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–73. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann EO, Mody I. The multifaceted role of inhibition in epilepsy: seizure-genesis through excessive GABAergic inhibition in autosomal dominant nocturnal frontal lobe epilepsy. Curr Opin Neurology. 2008;21:155–60. doi: 10.1097/WCO.0b013e3282f52f5f. [DOI] [PubMed] [Google Scholar]
- 55.Staba RJ, Frighetto L, Behnke EJ, Mathern G, Fields T, Bragin A, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–8. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 56.Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–64. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 58.Kang JQ, Macdonald RL. Making sense of nonsense GABAA receptor mutations associated with genetic epilepsies. Trends in Molecular Medicine. 2009;15:430–8. doi: 10.1016/j.molmed.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bragin A, Csicsvári J, Penttonen M, Buzsáki G. Epileptic afterdischarge in the hippocampal-entorhinal system: current source density and unit studies. Neuroscience. 1997;76:1187–203. doi: 10.1016/s0306-4522(96)00446-0. [DOI] [PubMed] [Google Scholar]
- 60.Finnerty GT, Jefferys JG. Investigation of the neuronal aggregate generating seizures in the rat tetanus toxin model of epilepsy. J Neurophys. 2002;88:2919–27. doi: 10.1152/jn.00211.2002. [DOI] [PubMed] [Google Scholar]
- 61.Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 62.Le Van Quyen M, Khalilov I, Ben-Ari Y. The dark side of high-frequency oscillations in the developing brain. Trends Neurosci. 2006;29:419–427. doi: 10.1016/j.tins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Gloor P. Contributions of electroencephalography and electrocorticography to the neurosurgical treatment of the epilepsies. Adv Neurol. 1975;8:59–105. [PubMed] [Google Scholar]
- 64.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- 66.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144–S52. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 67.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 68.Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 69.Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci. 2003;23:7873–80. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singer W. Neuronal synchrony: A versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 72.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-Frequency Network Oscillation in Hippocampus. Science. 1992;256:1025–27. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 73.Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28:6104–10. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curio G. Ain’t no rhythm fast enough: EEG bands beyond beta. J Clin Neurophysiol. 2000;17:339–40. doi: 10.1097/00004691-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Pikovsky A, Rosenblum M, Kurths J. Phase synchronization in regular and chaoric systems. Int J Bifurcations and Chaos. 2000;10:2291–305. [Google Scholar]
- 76.Schiff SJ. Dangerous Phase. NeuroInformatics. 2005;3:315–7. doi: 10.1385/NI:03:04:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nunez PL. Electric Fields of the Brain: The Neurophysics of EEG. New York: Oxford U. Press; 1981. [Google Scholar]
- 78.Fein G, Raz J, Brown FF, Merrin EL. Common reference coherence data are confounded by power and phase effects. Electroencephalogr Clin Neurophysiol. 1988;69:581–4. doi: 10.1016/0013-4694(88)90171-x. [DOI] [PubMed] [Google Scholar]
- 79.Guevara R, Velazquez JL, Nenadovic V, Wennberg R, Senjanovic G, Dominguez LG. Phase synchronization measurements using electroencephalographic recordings: what can we really say about neuronal synchrony? Neuroinformatics. 2005;3(4):301–14. doi: 10.1385/NI:3:4:301. [DOI] [PubMed] [Google Scholar]
- 80.Netoff TI, Carroll TL, Pecora LM, Schiff SJ. Detecting Coupling in the Presence of Noise and Nonlinearity. In: Schelter Björn, Winterhalder Matthias, Timmer Jens., editors. Handbook of Time Series Analysis. Wiley-VCH Verlag GmbH & Co; KGaA: 2006. pp. 265–82. [Google Scholar]
- 81.Netoff TI, Pecora LM, Schiff SJ. Analytical coupling detection in the presence of noise and nonlinearity. Physical Review E. 2004;69:017201. doi: 10.1103/PhysRevE.69.017201. [DOI] [PubMed] [Google Scholar]
- 82.Mormann F, Lehnertz K, David P, Elger CE. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D: Nonlinear Phenomena. 2000;144:358–369. [Google Scholar]
- 83.Hu S, Stead M, Worrell GA. Automatic identification and removal of scalp reference signal for intracranial EEGs based on independent component analysis. IEEE Trans Biomed Eng. 2007;54:1560–1572. doi: 10.1109/TBME.2007.892929. [DOI] [PubMed] [Google Scholar]
- 84.Gabor D. Theory of communication. IEEE Proc. 1946;93:429–57. [Google Scholar]
- 85.Boashash B. Time-Frequency Signal Analysis: Methods and Applications. Melbourne. Australia: Longman Cheshire; 1992. [Google Scholar]
- 86.Osterhage H, Mormann F, Staniek M, Lehnertz K. Measuring synchronization in the epileptic brain: A comparison of different approaches. Int J Bifurcat Chaos. 2007;17:3539–44. [Google Scholar]