Abstract
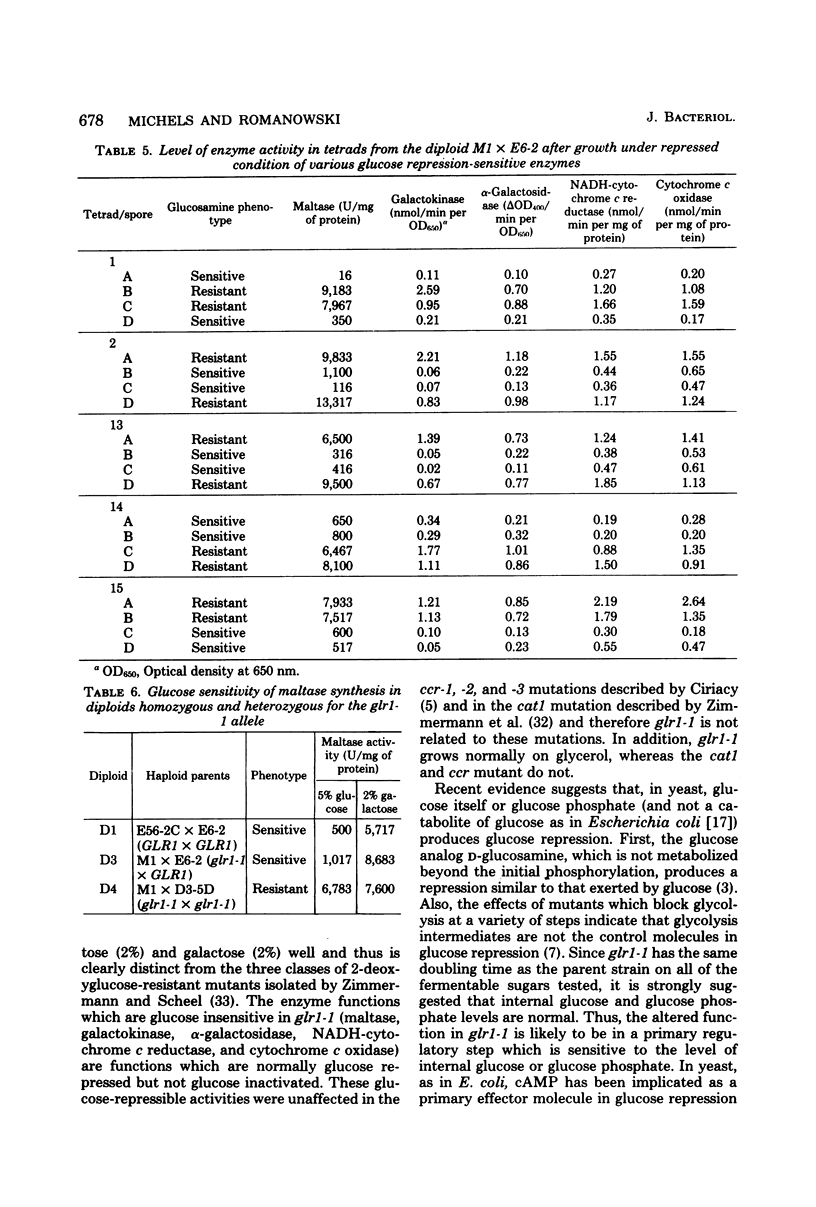
We describe the characterization of a mutation of the locus GLR1. This mutation allowed for (i) the glucose repression-insensitive synthesis ot the enzymes maltase, galactokinase, alpha-galactosidase, reduced nicotinamide adenine dinucleotide-cytochrome c reductase, and cytochrome c oxidase and (ii) growth on maltose in the presence of the gratuitous glucose repressor D-glucosamine. The glucosamine resistance cosegregated with the glucose-insensitive synthesis of the enzymes listed above. In addition, crosses between the glucosamine-resistant mutant and isogenic sensitive strains gave only tetrads containing two resistant and two sensitive spores. Thus, a single pleiotropic mutation is responsible for both phenotypes. We call the locus GLR1, for glucose regulation, and the glucose repression-insensitive mutation glr1-1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Induction of galactokinase in Saccharomyces cerevisiae: kinetics of induction and glucose effects. J Bacteriol. 1972 Aug;111(2):308–315. doi: 10.1128/jb.111.2.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B. G. Method for decryptification of -glucosidase in yeast with dimethyl sulfoxide. Anal Biochem. 1972 Jan;45(1):137–146. doi: 10.1016/0003-2697(72)90014-0. [DOI] [PubMed] [Google Scholar]
- Chapman C., Bartley W. The kinetics of enzyme changes in yeast under conditions that cause the loss of mitochondria. Biochem J. 1968 Apr;107(4):455–465. doi: 10.1042/bj1070455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriacy M. A yeast mutant with glucose-resistant formation of mitochondrial enzymes. Mol Gen Genet. 1978 Feb 27;159(3):329–335. doi: 10.1007/BF00268270. [DOI] [PubMed] [Google Scholar]
- Ciriacy M., Breitenbach I. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol. 1979 Jul;139(1):152–160. doi: 10.1128/jb.139.1.152-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K., Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphyorylation. Mol Gen Genet. 1977 Nov 4;156(1):99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- Fang M., Butow R. A. Nucleotide reversal of mitochondrial repression in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1579–1583. doi: 10.1016/0006-291x(70)90568-1. [DOI] [PubMed] [Google Scholar]
- Furst A., Michels C. A. An evaluation of D-glucosamine as a gratuitous catabolite repressor of Saccharomyces carlsbergensis. Mol Gen Genet. 1977 Oct 24;155(3):309–314. doi: 10.1007/BF00272810. [DOI] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Douglas H. C. Genetic co-regulation of galactose and melibiose utilization in Saccharomyces. J Bacteriol. 1976 Jan;125(1):33–41. doi: 10.1128/jb.125.1.33-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A., Eaton N. R. Purification and characterization of maltase and alpha-methyl glucosidase from yeast. Biochim Biophys Acta. 1967 Sep 12;146(1):173–180. doi: 10.1016/0005-2744(67)90084-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Montenecourt B. S., Kuo S. C., Lampen J. O. Saccharomyces mutants with invertase formation resistant to repression by hexoses. J Bacteriol. 1973 Apr;114(1):233–238. doi: 10.1128/jb.114.1.233-238.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamhart D. H., Ten Berge A. M., Van De Poll K. W. Isolation of a catabolite repression mutant of yeast as a revertant of a strain that is maltose negative in the respiratory-deficient state. J Bacteriol. 1975 Mar;121(3):747–752. doi: 10.1128/jb.121.3.747-752.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system: isolation of nuclear and cytoplasmic mutants of Saccharomyces cerevisiae with specific defects in mitochondrial functions. J Bacteriol. 1975 Jun;122(3):826–831. doi: 10.1128/jb.122.3.826-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk R., Ouwehand J., van den Bos T., Koningsberger V. V. Induction and catabolite repression of alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta. 1969 Jul 22;186(1):178–191. doi: 10.1016/0005-2787(69)90501-2. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. DNA-dependent in vitro synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1971;112(1):14–27. doi: 10.1007/BF00266928. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]
- van Rijn J., van Wijk R. Differential sensitivities of the two malate dehydrogenases and the maltose permease to the effect of glucose in Saccharomyces carlsbergensis. J Bacteriol. 1972 May;110(2):477–484. doi: 10.1128/jb.110.2.477-484.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poll K. W., Kerkenaar A., Schamhart D. H. Isolation of a regulatory mutant of fructose-1,6-diphosphatase in Saccharomyces carlsbergensis. J Bacteriol. 1974 Mar;117(3):965–970. doi: 10.1128/jb.117.3.965-970.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



