Abstract
SIRT3 is the primary mitochondrial deacetylase that modulates mitochondrial metabolic and oxidative stress regulatory pathways. However, its role in response to nutrient excess remains unknown. Thus, we investigated SIRT3 regulation of the electron transfer chain and evaluated the role of SIRT3 in hepatic lipotoxic stress. SIRT3 depleted HepG2 cells shows diffuse disruption in mitochondrial electron transfer chain functioning, a concurrent reduction in the mitochondrial membrane potential, and excess basal reactive oxygen species levels. As this phenotype may predispose to increased lipotoxic hepatic susceptibility we evaluated the expression of SIRT3 in murine liver following chronic high-fat feeding. In this nutrient-excess model SIRT3 transcript and protein levels are downregulated in parallel with increased hepatic fat storage and oxidative stress. Palmitate was used to investigate lipotoxic susceptibility in SIRT3 knockout mouse primary hepatocytes and SIRT3 siRNA depleted HepG2 cells. Under SIRT3 deficient conditions palmitate enhances reactive oxygen species and increases hepatocyte cell death. Reconstitution of SIRT3 levels and/or treatment with N-acetylcysteine ameliorates these adverse effects. In conclusion SIRT3 functions to ameliorate hepatic lipotoxicity, although paradoxically, exposure to high-fat downregulates this adaptive program in the liver. This SIRT3-dependent lipotoxic susceptibility is possibly modulated, in part, by SIRT3 mediated control of electron transfer chain flux.
Keywords: SIRT3, Lipotoxicity, Electron Transfer Chain, Reactive Oxygen Species, Palmitate
Introduction
Lysine-residue acetylation as a mechanism of post-translation modification and regulation of proteins are evident from prokaryotes to eukaryotes, [1, 2] and proteomic screening has identified nutrient-flux dependent changes in the acetylation state of a numerous hepatic mitochondrial metabolic regulatory proteins [1, 3]. SIRT3 is enriched in the liver [4] and is a major mitochondrial NAD-dependent protein deacetylase [5]. Known targets activated by SIRT3 include complex I and II proteins in the electron transfer chain (ETC), [4, 6] and proteins that facilitate the generation of tricarboxylic acid (TCA) cycle intermediates [5, 7, 8]. Whether further components of the electron transfer chain (ETC) are functionally modified by SIRT3 is unknown. Additionally as the acetylation status of the mitochondrial proteome is modified by nutrient flux whether SIRT3 plays a functional role in response to hepatic nutrient stressors warrants investigation.
In this study we investigate the role of SIRT3 in the modulation of mitochondrial electron transfer and the consequences of hepatocyte SIRT3 deficiency to lipid-mediated toxicity. We show that following SIRT3 knockdown that complex II initiated respiration in mitochondria in-situ is not perturbed, however, complex I and complex IV – V substrate dependent oxygen consumption is significantly blunted compared to controls. In parallel, SIRT3 depletion results in a reduction in the mitochondrial membrane potential with increased reactive oxygen species (ROS) levels. N-acetylcysteine (NAC) administration reverses the increased ROS levels. As a functional correlation, hepatic tolerance to palmitate is diminished in parallel with palmitate mediated induction of ROS. This lipotoxic susceptibility is also reversed by the reconstitution of SIRT3 to knockout primary hepatocytes. Together these data show that SIRT3 is integral for global ETC functioning and its depletion results in diminished mitochondrial oxygen consumption, excess reactive oxygen levels and enhanced susceptibility to palmitate-mediated hepatocyte cell death.
Materials and Methods
Cell cultures and transfections
HepG2 human hepatocyte cell line was from American Type Cell Culture (ATCC, Manassas, VA) and was maintained in DMEM containing 25mM glucose and 10% fetal bovine serum (FBS). Primary mouse hepatocytes and mouse embryonic fibroblasts were isolated and cultured as described previously [9, 10]. For siRNA transfection, 106 HepG2 cells were electroporated with 100nmol of SIRT3 or control ON-TARGET plus SMARTpool siRNA (Thermoscientific) according to the manufacturer’s instruction (Amaxa). Unless specified, all the experiments were performed 64–68 hours after transfection. For plasmid transfection, mouse primary hepatocytes were transfected with pcDNA3.1(+) (Invitrogen) or pcDNA3.1 (+) containing full length human SIRT3 cDNA (hSIRT3, Addgene) at 2μg DNA/5μl lipofectamine 2000 reagent in a 6-well type I collagen-coated culture plate. The cells are harvested 48 hours after transfection for further experiments.
Cellular oxygen consumption assay
Steady state cell respiration in HepG2 cells and primary hepatocytes were measured in non-buffered DMEM containing 5.5 mM glucose for HepG2 cells or 10 mM glucose for hepatocytes with XF24 analyzer (Seahorse Bioscience) according to the manual. To examine mitochondrial complex activities, HepG2 cells were permeabilized with 10μg digitonin/106 cells, and incubated with the medium containing 250mM sucrose, 2 mM KH2PO4, 10mMMgCl2, 0.5 mM EGTA, 0.1% fatty acid free BSA, ADP 2mM, 20mM HEPES, pH7.1 in a non-CO2 incubator for 1 hour before experiments. The cells were then subjected to three to four baseline measurement, followed by injection of the following reagents: 10mM glutamate/5 mM malate for complex I activity, 0.1 μM rotenone/10 mM succinate for complex II activity, antimycin 20nM/0.5 mM TMPD/2 mM ascorbate for complex IV+V activities.
ATP production assay
Steady state cellular ATP levels were measured by using ATP bioluminescence assay kit CLS II in accordance with the protocol (Roche).
Determination of reactive oxygen species (ROS) production
Cellular ROS production was detected with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Invitrogen). Briefly, 106 cells were incubated with 5 μM CM-H2DCFDA in serum free medium at 37°C for 20min, after three wash with PBS, the cells were suspended with 100μl of PBS and transferred into a 96 well plate with black walls, measured with a Magellan microplate reader (Tecan) every 2 min for 1hour at 37°C. The excitation and emission wave length are 492nm, and 525nm respectively. In some experiments, the cells were pre-incubated with 10 mM NAC for 1 hour before staining with CM-H2DCFDA. In other experiments, 0.5 mM palmitate/0.5% fatty acid free BSA or 0.5% BSA were added to the cell suspension immediately before measurement. ROS production was calculated as the maximal slope (RFU/min) in each reaction. Mitochondrial ROS production was additionally measured using Mitosox (Invitrogen), following the same protocol for CM-H2DCFDA above. After incubation with 1 μM Mitosox for 20 min, 106 cells were analyzed on the plate reader using an excitation and emission wavelength of 492 nm and 595 nm, respectively.
Immunoblot analysis and immunoprecipitation
HepG2 cells or mouse liver tissues were extracted in RIPA lysis buffer and 20μg of total proteins were separated in 4–20% tris-glycine gel. After transferred into nitrocellulose membranes, the proteins were blotted with the following primary antibodies: rabbit polyclonal anti-human SIRT3 antibody (Cell Signaling), affinity-purified rabbit polyclonal anit-SIRT3 antibody was raised against the 15 amino acids of the mouse SIRT3 C-terminus [5], monoclonal anti-alpha tubulin antibody (Santa Cruz) and mitochondrial F1F0-ATPase subunit α (Invitrogen).
For immunoprecipitation, liver mitochondrial proteins from WT and SIRT3 KO mice were purified by differential centrifuge as described [11], and 1mg mitochondrial proteins were solubilized with 1% n-dodecyl-β-D-maltopyranoside and mitochondrial complex V components were precipitated with ATPase synthase immunocapture kit (MitoSciences), followed by immunoblotting using the following antibodies: affinity-purified rabbit polyclonal anti-SIRT3 antibody was raised against the 15 peptides of mouse SIRT3 C-terminus, rabbit polyclonal anti-acetylated lysine antibody was from Cell Signaling, and F1F0-ATPase subunit α as described above. To assess human SIRT3 levels the Sigma antibody was used. Immunoblots were visualized and analyzed using the Odyssey infrared imaging system (Li-Cor Biosciences).
Gene expression analysis
Following isolation and quantification of RNA from liver extracted from mice fed for 12 weeks on a caloric equivalent chow diet (% calories – carbohydrates - 70, protein – 20 and fat - 10) versus high fat (% calories – carbohydrates - 20, protein - 20 and fat - 60 (Research Diets Inc.)). The reverse transcription reaction was performed using Super-Script III reverse transcriptase kit (Invitrogen). Real time quantitative-PCR was performed using SYBR green PCR Master Mix (Applied Biosystems) and MJ Research DNA Engine Opticon 2 fluorescence detection system (BioRad). The murine QuantiTect SIRT3 primers were obtained from Qiagen. The mRNA concentration was calculated from the cycle threshold values using a standard curve and normalized to the expression levels of 18S ribosomal RNA.
Liver confocal microscopy
For nitrotyrosine staining frozen liver sections were fixed with methanol at −20°C for 15min and permealized with 0.1% Triton X-100 in PBS for 5min at room temperature. After three wash with PBS, the slides were blocked with 10% normal goat serum for 30min at room temperature, followed by incubation with anti-nitrotyrosine antibody (1:100, Upstate) at 4°C over night. The slides were then incubated with Alexa Fluor 594-conjugated goat antimouseIgG (1:250; Invitrogen) at room temperature for one hour. Nuclei were counterstained with 4′6′-diamidino-2 phenylindole (DAPI; Invitrogen). The images were collected using a Zeiss LSM 510 confocal microscope.
Lipotoxicity assay
To induce lipotoxicity, 0.5mM or 0.75 mM palmitate conjugated to 0.5% fatty acid free BSA or BSA alone was added to HepG2 cells or primary hepatocytes in the presence or absence of 10 mM NAC for 24 hours. Then the cells were harvested and stained with propidium iodide (PI) dye, then subjected to FACS analysis to detect cell viability. The lipotoxicity was calculated as the percentage of PI positive cells (dead cells) in a cell population.
Statistical Analysis
Differences between data groups were evaluated for significance using the Student t-test. P< 0.05 was considered statistically significant, and data are expressed as mean ± SEM.
Results
SIRT3 regulates mitochondrial respiration via modulation of multiple complexes in the electron transfer chain
To investigate modulation of the mitochondrial ETC by SIRT3, we employed siRNA to deplete SIRT3 in HepG2 cells (Fig. 1a) and transient transfection to restore SIRT3 levels into the SIRT3 knockout primary hepatocytes (Fig. 1b). Consistent with prior studies in SIRT3−/− mouse embryonic fibroblasts cellular oxygen consumption and ATP levels were significantly reduced compared to levels in scrambled siRNA transfected cells (Fig. 1c–d, p < 0.001). Interestingly, the reduction in oxygen consumption is also evident in SIRT3 knockout primary hepatocytes. The role of SIRT3 in this phenotype is confirmed by the restoration of oxygen consumption following the reintroduction of SIRT3 into primary SIRT3−/− hepatocytes (Fig. 1c). In parallel with these mitochondrial bioenergetic perturbations, the inner mitochondrial membrane potential was diminished following the knockdown of SIRT3 (Fig. 1e) with a parallel increase in ROS levels (Fig. 1f).
Figure 1. SIRT3 regulates mitochondrial functions in hepatic cells.
(A) Immunoblot analysis of SIRT3 expression levels in HepG2 cells transfected with control or SIRT3 siRNA for 72 hours. F0F1-ATPase subunit α was used as loading controls. (B) Immunoblot analysis shows the absence of SIRT3 in primary knockout (KO) hepatocytes and the capacity to reconstitute SIRT3 levels following transfection studies. (C) Intact cellular basal oxygen consumption rates (OCR) of control or SIRT3 siRNA-treated HepG2 cells, wild type (WT) or SIRT3 KO hepatocytes, and SIRT3 KO hepatocytes transfected with pcDNA3.1 or plasmid encoding hSIRT3 measured by Seahorse XF24 analyzer. (D) Steady-state cellular ATP levels in control or SIRT3 siRNA-treated HepG2 cells. Data represent mean ± SEM from four independent experiments. (E) Comparison of mitochondrial membrane potential in control or SIRT3 siRNA-treated HepG2 cells, as measured by TMRM. (F) Representative traces of ROS production as indicated by DCF fluorescence intensity in control or SIRT3 siRNA-treated HepG2 cells.
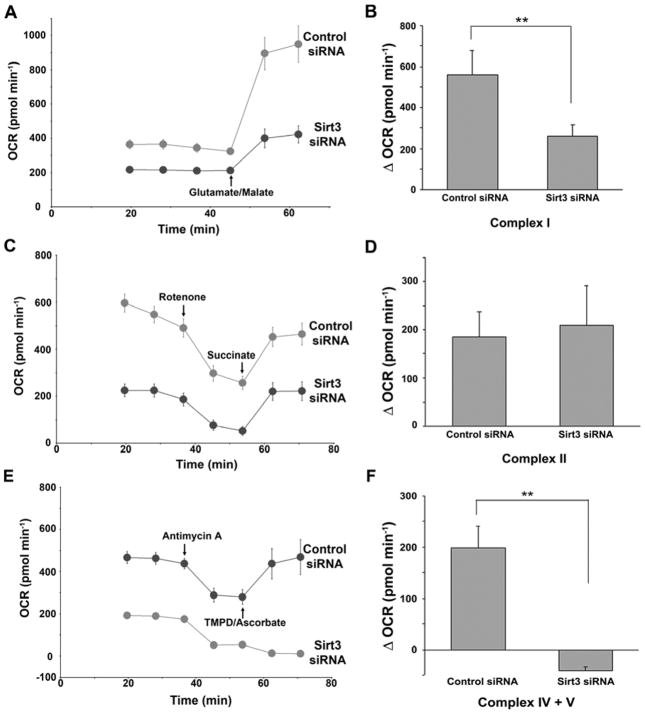
As protein components of complex I, II and V and cytochrome C of the electron transfer chain proteins have been shown to undergo lysine residue acetylation [1, 8, 12] we delineated individual electron transfer chain complex specific substrate capacity to facilitate oxygen consumption following RNAi knockdown of SIRT3. HepG2 cells were permeabilized and complex I, II and IV/V substrates were administered. As shown in Figure 2, the capacity to enhance oxygen consumption was significantly blunted following the supply of substrate feeding into complexes I, III and IV (Fig. 2a–b) and downstream of complex III (Fig. 2e–f) in the SIRT3 depleted cells compared to scramble siRNA controls (P< 0.001). Conversely, although basal respiration was lower prior to the administration of succinate to supply reducing equivalents to complex II in the SIRT3 knockdown cells, the relative enhancement of oxygen consumption by complex II was similar in the SIRT3 knockdown and control cells (Fig. 2c–d). It is interesting to note, that while global reduction in oxygen consumption in the absence of SIRT3 is ≈30% (Fig. 1c), and consistent with prior publications [4, 10], when interrogating the different complex substrates (Fig. 2), we find much larger differences in baseline oxygen consumption. We propose that as the knockdown of SIRT3 is known to result in lower ETC complex I and II activity [4, 6], the incubation of the permeabilized cells with 2mM ADP (see methods section) in the presence of endogenous metabolites and reducing equivalents may exaggerate the basal differences in oxygen consumption prior to the experimental introduction of the ETC complex specific substrates. Although this approach does appear to accentuate differences when interrogating the distinct ETC complexes, it should be recognized that these differential oxygen consumption rates do not reflect the composite oxygen consumption as shown in intact cells.
Figure 2. SIRT3 regulates mitochondrial electron transfer chain activities.
Left, representative traces of OCRs in control or SIRT3 siRNA-treated, digitonin-permealized HepG2 cells follow the addition of glutamate/malate (A), succinate (C), or TMPD/ascorbate (E). The quantification of OCR change in each group was shown in the right of respective figures. B, D, and F, Data represent average of 10 replicates from one plate ± SD, each experiment repeated at least three times. **, p<0.01.
As the acetylation status of complex V is modulated by the manipulation of SIRT3 [8, 12], we investigated whether SIRT3 interacts with, and deacetylates, subunits of complex V. This potential function of SIRT3 is supported by protein interaction studies and the differential acetylation levels of the complex V subunit α in wildtype versus SIRT3 knockout MEF cells (supplemental section Fig. S1a–c).
SIRT3 levels are downregulated in response to a high fat diet
Disrupted mitochondrial function has been shown to result in a predisposition or exacerbation of numerous pathologies including, for example, premature aging, insulin resistance and hepatic lipotoxicity [13–15]. As the liver is susceptible to fat-induced toxicity, [16] we reasoned that it would be important to evaluate the role of SIRT3 in mitochondrial homeostasis in response to this nutrient-excess mediated stress. We began by delineating whether SIRT3 is modulated in the liver in response to chronic fat feeding. Following 12 weeks of a diet with a 60% fat content the mouse liver showed evidence of increased lipid deposits (Fig. 3a) and evidence of redox stress (Fig. 3b). In parallel transcripts encoding for SIRT3 and steady-state protein levels were significantly downregulated in wild type mice compared to chow-fed controls (Fig 3c–d).
Figure 3. Effects of long term high fat diet on hepatic SIRT3 expression and oxidative stress.
(A) Representative images of oil red O staining of liver sections from mice fed with normal diet or high fat diet for four months. (B) Nitrotyrosine staining (red) of liver sections as described in A. The nuclei were counterstained with DAPI (blue). (C) Liver SIRT3 mRNA expression levels in four month chow diet or high fat diet-fed mice, as quantified by qPCR. Data represent mean ± SEM, n = 5 for chow diet, and n = 8 for high fat diet-fed mice. **, p<0.01. (D) Immunoblot analysis of SIRT3 protein expression levels in mouse liver total proteins as described above. β-tubulin was used as loading controls.
SIRT3 depletion increases ROS levels and enhances susceptibility to lipotoxicity
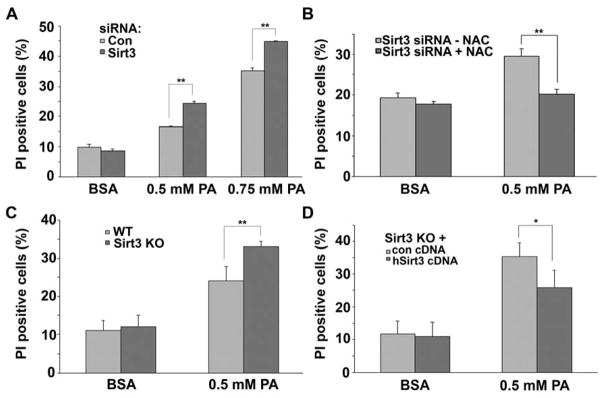
As a high-fat diet downregulates SIRT3 and the depletion of SIRT3 disrupts mitochondrial function, we investigated whether exposure of hepatocytes to the saturated fatty acid palmitate in the presence of normal or diminished levels of SIRT3 would modulate mitochondrial respiration and ROS levels. Interestingly, the acute exposure to palmitate does increase oxygen consumption compared to BSA vehicle administration in both scrambled and SIRT3 siRNA transfected HepG2 cells. Although, in parallel with basal respiration, the palmitate-mediated increase in oxygen consumption is lower following SIRT3 depletion (Fig. 4a). In parallel, ROS levels as measured by DCF fluorescence are disproportionally induced by palmitate when SIRT3 levels are diminished (Fig 4b) or in SIRT3 knockout primary hepatocytes (Fig 4c). The excess palmitate-induced ROS levels evident in SIRT3 depleted cells are rescued by the co-administration of the anti-oxidant NAC (Fig. 4d). As saturated fatty acids and excess ROS can predispose to lipid peroxidation and cellular injury, we evaluated the response of these cells to increasing concentrations of palmitate administration versus BSA administration. 0.5 mM palmitate increased cell death by ≈ 80% in the control siRNA cells and by 150% in the SIRT3 depleted HepG2 cells (Fig. 5a). 0.75 mM of palmitate further increased cell death in the control and SIRT3 knockdown cells although demonstrating a continued disadvantage of SIRT3 depletion (Fig. 5a). In parallel with the ROS findings, the co-administration of NAC reversed palmitate-induced cell death in the SIRT3 depleted HepG2 cells.
Figure 4. A. Palmitate modulates oxygen consumption and ROS levels in a SIRT3-dependent manner.
(A) Oxygen consumption of control or SIRT3 siRNA-treated HepG2 cells in response to 0.1 % BSA or 0.1 mM palmitate (PA). Data represent average of 5 replicates from one plate ± SD, each experiment repeated at least three times. (B) Mitochondrial superoxide production of control or SIRT3 siRNA-treated HepG2 cells in the absence or presence of 0.5 % BSA or 0.5 mM PA. Data mean ± SEM from 10 measurements and are representative of at least 3 independent experiments. (C) ROS production in WT and SIRT3 KO primary hepatocytes in the absence and presence of 0.5 mM PA. The data were expressed as the percentage of maximal slope compared to WT control cells. Data represent mean ± SEM from three independent experiments. (D) ROS production in control or SIRT3 siRNA-treated HepG2 cells in the absence or presence of 0.5 mM PA and/or 10 mM NAC. The data were expressed as the percentage of maximal slope compared to control siRNA-treated HepG2 cells. Data represent mean ± SEM from four independent experiments. *, p<0.05, **, p<0.01.
Figure 5. Oxidative stress partially mediates SIRT3-dependent lipotoxicity.
(A) Cell viability of control or SIRT3 siRNA-transfected HepG2 cells following treatment of BSA or different amounts of palmitate (PA). The dead cells are identified as propidium iodide (PI)-positive cells and quantified by FACS analysis. Data represent mean ± SEM of four independent experiments. (B) Cell viability of control or SIRT3 siRNA-treated HepG2 cells with or without pretreatment of 10mM NAC, followed by incubation with 0.5 mM PA for 24 hours. Data represent mean ± SEM from four independent experiments. (C) Cell viability of primary WT and SIRT3 KO hepatocytes following treatment of BSA or 0.5 mM palmitate (PA). (D) Cell viability of pcDNA3.1 or hSIRT3 cDNA-transfected SIRT3 KO hepatocytes treated with 0.5 mM PA. Data represent mean ± SEM of three independent primary cell studies for each group in C and D. A–D, **, p<0.01.
To confirm the role of SIRT3 in this adverse phenotype we explored palmitate-tolerance and the response to SIRT3 repletion in primary SIRT3−/− mouse hepatocytes compared to wild-type controls. As in the siRNA studies, basal cell viability was unchanged comparing wild-type and SIRT3−/− hepatocytes (Fig. 5c). 0.5 mM palmitate significantly increased cell death in the SIRT3−/− hepatocytes compared to the response in wildtype hepatocytes (Fig. 5c). The restoration of SIRT3 levels in the SIRT3−/− hepatocytes following transient transfection rescued this detrimental phenotype with the return of palmitate-exposed cell viability to similar levels of the wild-type controls (Fig. 5d).
Discussion
Similar to other sirtuins, the mitochondrial enriched SIRT3 is activated by caloric restriction and fasting [5]. The functional targets of SIRT3 support its regulatory role in mitochondrial bioenergetics [4, 6, 10] and SIRT3 levels are enriched in the liver [4], an important nutrient homeostasis organ. Thus, we reasoned that the delineation of the role of SIRT3 in nutrient-excess mediated hepatic pathology is important to further delineate nutrient-dependent SIRT3 functioning. The major findings presented in this manuscript identify: i) that novel electron transfer sites are regulated by SIRT3; ii) that SIRT3 is downregulated in the liver in response to a high-fat diet, and iii) that the depletion of SIRT3 exacerbated lipotoxicity via the modulation of ROS.
Mitochondrial metabolism and redox stress proteins have been shown to undergo deacetylation in response to fasting in the liver [1]. However, whether SIRT3 is the deacetylase that orchestrates these effects is predominantly unknown and the functional effect of known targets of SIRT3 deacetylation are only beginning to be explored (reviewed [17]). The known targets of SIRT3 deacetylation modulate substrate entry into the TCA cycle and regulate proximal subunits in the ETC. Recently proteomic analysis implicates ATP synthase alpha subunit of the ETC as a probably additional target of SIRT3 deacetylation [12]. Our functional in vivo oxygen consumption studies support that components of the ETC downstream of complex III are modulated and subsequently activated by SIRT3. In addition, we show that numerous subunits of complex V are deacetylated in wild-type compared to SIRT3 knockout mice liver tissue, and that one or more ATP synthase subunits directly interacts with SIRT3. Additionally, we demonstrate that ATP synthase subunit α shows a higher level of acetylation in SIRT3 deficient compared to wildtype MEF cells. Although these data support the ATPase subunit α as a substrate of SIRT3, further work will be required to fully delineate the extent of the functional interaction between these two proteins Interestingly, we show that complex II of the ETC is not directly modulated by SIRT3, which although compatible with the findings of Ahn et al [4], differs from the observations of Cimen et al [6]. This discrepancy is currently unexplained but warrants further investigation.
A primary method of post-translational modification and signaling in mitochondria appears to be via a change in protein acetylation, and SIRT3 is one of the primary protein deacetylases. SIRT3 is activated, at least in part, by changes in the ratio of NAD+/NADH and SIRT3 deacetylase activity may play a role in metabolic diseases such as obesity or diabetes since these conditions are associated with a diminished NAD+/NADH ratio and lower NADH oxidase activity [18, 19]. Interestingly, a recent study has shown that SIRT3 activates a pivotal mitochondrial fatty acid oxidation enzyme [20]. This observation would further suggest that SIRT3 may play an adaptive role in handling excess fat in the diet whereby it could facilitate the catabolism of these nutrients.
Chronic liver damage is also associated with a reduction in NAD levels [21]. In this regard, our study shows that chronic high-fat feeding increases hepatosteatosis and oxidative liver damage in parallel with the downregulation of SIRT3 levels. We therefore postulated that the mitochondrial dysfunction associated with SIRT3 downregulation or depletion may exacerbate injury in response to lipotoxic insults. The plausibility of this postulate is further supported in that lipotoxicity in the liver is associated with mitochondrial dysfunction [15] and saturated fats per se increase mitochondrial ROS levels [22]. In this study we demonstrate that the SIRT3 knockdown enhances ROS levels and that the ROS scavenger NAC, reverses this SIRT3 depletion effect. In parallel, SIRT3 depletion enhanced susceptibility to palmitate toxicity and this injury is reversed by both NAC and the reconstitution of SIRT3. Taken together, these results suggest that the downregulation of SIRT3 in the liver responding to excess fatty acids would be a maladaptive response and might play a role in hepatic pathophysiology in obesity.
Interestingly, SIRT3 deficiency has previously been shown to increase susceptibility to direct oxidative stress and in facilitating ROS-dependent oncogenic transformation [23, 24]. Mechanisms identified through which the reduction or absence of SIRT3 mediates these ROS-dependent effects include the disruption of anti-apoptotic programs [23] and via the attenuation of anti-oxidant defense programs [24]. Whether the direct disruption of ETC functioning with the diminution in SIRT3 is an additional mechanism enabling excess ROS generation, resulting in increased susceptibility to biological stressors, is suggested by the palmitate-mediated adverse effects in this study. However, as additional targets of SIRT3 deacetylation are being identified, it is increasingly being recognized that the number of targets that may be operational in the control of the mitochondrial and cellular redox state are probably multi-factorial [17].
Although the predominant focus of investigation into SIRT3 has been studied under conditions that mimic caloric restriction, the reduction in its function, under nutrient excess conditions, may play a similarly important biological role. Excess fat in the diet gives rise to both diabetes and obesity and both of these conditions can give rise to non-alcoholic fatty liver disease. Finally, we expand the targets of SIRT3 to include the modulation of distal ETC complexes and confirm that the reduction in SIRT3 levels results in excess ROS levels that is further accentuated in the presence of palmitate. An interesting question arising from this research is whether the retardation of ETC flux with low or absent SIRT3 levels is a significant contributor to the cytotoxic effects of excess ROS generation. The complexity of dissecting this possible pathophysiology from the myriad of additional yet, uncharacterized targets of SIRT3 is an important challenge in understanding the full scope of the functioning of SIRT3. The importance of the delineation of this biology is, however, underscored by the prevalence of nutrient excess in society, the increasing pathology associated with nutrient excesses and the potential that the regulation of SIRT3 may play a role in ameliorating these pathologies.
Supplementary Material
(A) WT mouse liver mitochondrial proteins were immunoprecipitated with complex V antibody or nonspecific mouse IgG and subjected to Western blot analysis with anti-SIRT3 antibody. F0F1-ATPase subunit α was used as input controls. (B) Increased lysine acetylation levels of liver mitochondrial complex V in SIRT3 KO mice. Liver mitochondrial proteins purified from WT and SIRT3 KO mice were immunoprecipitated with complex V immunocapture kit, followed by Western blot analysis with anti-acetylated lysine antibody. F0F1-ATPase subunit α was used as input controls. n=3 in both A and B. (C) Increased lysine acetylation levels of ATPase subunit α in SIRT3 KO MEF cells compared to WT controls. To validate the change in acetylation status of ATPase subunit α specifically, lysine modified proteins were immunoprecipitated from MEF cell lysates using the anti-acetylated lysine antibody. Western blot analysis using an antibody directed at the α subunit of ATPase shows increased acetylated protein levels in the KO versus WT MEF extracts. The input level of ATPase was the same in both samples.
Acknowledgments
This study was funded by the NHLBI Division of Intramural research. We acknowledge with appreciation the technical assistance obtain from the NHBLI Intramural flow cytometry and light microscopy core facilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–18. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, et al. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics. 2009;8:215–25. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–4. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–52. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–14. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–11. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A. 2006;103:10224–9. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 9.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–8. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao J, Lu Z, Joseph JJ, Carabenciov D, Dimond CC, Pang L, et al. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J Cell Biochem. 2010;110:238–47. doi: 10.1002/jcb.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2:287–95. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 12.Law IK, Liu L, Xu A, Lam KS, Vanhoutte PM, Che CM, et al. Identification and characterization of proteins interacting with SIRT1 and SIRT3: implications in the anti-aging and metabolic effects of sirtuins. Proteomics. 2009;9:2444–56. doi: 10.1002/pmic.200800738. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–92. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagel-Langenickel I, Bao J, Joseph JJ, Schwartz DR, Mantell BS, Xu X, et al. PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem. 2008;283:22464–72. doi: 10.1074/jbc.M800842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Lechon MJ, Donato MT, Martinez-Romero A, Jimenez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106–16. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Scott I, Webster BR, Sack MN. The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ Res. 2009;105:830–41. doi: 10.1161/CIRCRESAHA.109.204974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ido Y, Kilo C, Williamson JR. Cytosolic NADH/NAD+, free radicals, and vascular dysfunction in early diabetes mellitus. Diabetologia. 1997;40(Suppl 2):S115–S117. doi: 10.1007/s001250051422. [DOI] [PubMed] [Google Scholar]
- 19.Ritov VB, Menshikova EV, Azuma K, Wood RJ, Toledo FG, Goodpaster BH, et al. Deficiency of Electron Transport Chain in Human Skeletal Muscle Mitochondria in Type 2 Diabetes Mellitus and Obesity. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer JF, Bahr MJ, Boker KH, Manns MP, Tietge UJ. Plasma levels of PBEF/Nampt/visfatin are decreased in patients with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G196–G201. doi: 10.1152/ajpgi.00029.2008. [DOI] [PubMed] [Google Scholar]
- 22.Seifert EL, Estey C, Xuan JY, Harper ME. Electron Transport Chain-dependent and -independent Mechanisms of Mitochondrial H2O2 Emission during Long-chain Fatty Acid Oxidation. J Biol Chem. 2010;285:5748–58. doi: 10.1074/jbc.M109.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28:6384–401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) WT mouse liver mitochondrial proteins were immunoprecipitated with complex V antibody or nonspecific mouse IgG and subjected to Western blot analysis with anti-SIRT3 antibody. F0F1-ATPase subunit α was used as input controls. (B) Increased lysine acetylation levels of liver mitochondrial complex V in SIRT3 KO mice. Liver mitochondrial proteins purified from WT and SIRT3 KO mice were immunoprecipitated with complex V immunocapture kit, followed by Western blot analysis with anti-acetylated lysine antibody. F0F1-ATPase subunit α was used as input controls. n=3 in both A and B. (C) Increased lysine acetylation levels of ATPase subunit α in SIRT3 KO MEF cells compared to WT controls. To validate the change in acetylation status of ATPase subunit α specifically, lysine modified proteins were immunoprecipitated from MEF cell lysates using the anti-acetylated lysine antibody. Western blot analysis using an antibody directed at the α subunit of ATPase shows increased acetylated protein levels in the KO versus WT MEF extracts. The input level of ATPase was the same in both samples.