Abstract
Background
Cannabinoid type 1 (CB1) receptors are involved in the regulation of gastrointestinal (GI) motility and secretion. Our aim was to characterize the roles of the CB1 receptor on GI motility and secretion in vitro and in vivo by using different classes of CB1 receptor antagonists.
Methods
Immunohistochemistry was used to examine the localization of CB1 receptor in the mouse ileum and colon. Organ bath experiments on mouse ileum and in vivo motility testing comprising upper GI transit, colonic expulsion, and whole gut transit were performed to characterize the effects of the inverse agonist/antagonist AM251 and the neutral antagonist AM4113. As a marker of secretory function we measured short circuit current in vitro using Ussing chambers and stool fluid content in vivo in mouse colon. We also assessed colonic epithelial permeability in vitro using FITC-labelled inulin.
Key Results
In vivo, the inverse agonist AM251 increased upper GI transit and whole gut transit, but it had no effect on colonic expulsion. By contrast, the neutral antagonist AM4113 increased upper GI transit, but unexpectedly reduced both colonic expulsion and whole gut transit at high, but not lower doses.
Conclusions & Inferences
CB1 receptors regulate small intestinal and colonic motility, but not GI secretion under physiological conditions. CB1 inverse agonists and CB1 neutral antagonists have different effects on intestinal motility. The ability of the neutral antagonist not to affect whole gut transit may be important for the future development of CB1 receptor antagonists as therapeutic agents.
Keywords: cannabinoid-1 receptor, inverse agonist/antagonist, intestinal motility, ion transport
INTRODUCTION
Pharmacological activation of cannabinoid receptors reduces motility in vitro and in vivo1,2. This has been extensively documented for the CB1 receptor, whereas the role of the CB2 receptor in regulation of GI motility is less well understood and remains to be fully resolved3-9. Cannabinoid receptors are part of the endocannabinoid system (ECS), which consists of endogenous ligands, as well as the biosynthetic and degradative enzymes for these ligands.10 The ECS is involved in the regulation of GI motility.3,11 Both in vitro and in vivo studies suggest that treatment with a CB1 receptor inverse agonist/antagonist under physiological conditions results in the opposite effects observed to that of treatment with a CB1 receptor agonist; increased contractility or motility of the gut.6,12-15 These effects of CB1 receptor inverse agonists/antagonists were shown in animals under normal conditions and in models of diarrhea or ileus.5,16-18 Interestingly, data from clinical trials of CB1 receptor inverse agonists/antagonists suggest that these findings hold true in humans as well, since nausea, vomiting and diarrhea were amongst the major dose-related side-effects observed in patients treated with rimonabant and taranabant19,20.
Our knowledge of the involvement of the CB1 receptor in GI physiology is largely based on data using CB1 receptor selective inverse agonists/antagonists like rimonabant (SR141716A), AM251, AM281 and LY32013521 or from conventional receptor knockout mice. Besides competitive antagonism at the CB1 receptor with the endogenous endocannabinoids, these compounds display inverse agonist activity, shifting a constitutively active CB1 receptor from the “on” state to the inactive “off” state.22,23 It is not possible in vivo to discriminate between inverse agonism at the receptor or the blockade of endogenously released endocannabinoids acting at a constitutively active receptor. We sought to understand which of the GI actions of the CB1 receptor antagonists are due to their inverse agonist activity by utilizing a novel CB1 receptor specific antagonist with no inverse agonist actions.
AM4113 is a novel CB1 receptor antagonist without inverse agonist activity and is a so called neutral antagonist.24 It is a pyrazole analog structurally related to AM251 and rimonabant. The aim of this study was to compare effects of the inverse agonist/antagonist AM251 and the neutral antagonist AM4113 on GI motility and secretion in vitro and in vivo. Using the neutral antagonist in this context is valuable in order to further characterize the physiological involvement of the ECS in the regulation of GI functions and to establish how the CB1 receptor is involved in these physiological processes. AM4113, like the CB1 receptor inverse agonists/antagonists, reduces food intake and body weight.24,25 However, we have shown that unlike AM251, AM4113 does not exacerbate emesis and others have shown that it does not have the same potential to cause nausea.24-26
METHODS
Animals
6-7 week old male C57BL/6N mice (20-26 g) and male CB1-/- mice (18-24 g) on a CB57BL/6N background as well as their wildtype littermates were used in the studies. The C57BL/6 mice were obtained from Charles River and the CB1-/- mice 27 were bred from heterozygous mice at the University of Calgary mouse breeding facility. Mice were housed at a constant temperature (22°C) and a photoperiod (12:12-h light-dark cycle) in sawdust-containing plastic cages with access to standard Laboratory Chow and tap water ad libitum. The mice were used after 1 week of habituation. Animal use for these studies was approved by the University of Calgary Animal Care Committee and the experiments were performed in accordance with institutional animal ethics committee guidelines that follow the guidelines established by the Canadian Council on Animal Care.
Immunohistochemistry
For preparation of whole mounts, segments of mouse ileum and colon (n=8) were opened along the mesenteric border, stretched, and pinned mucosal-side-up on a Sylgard-coated petri dish. For preparation of cross-sections, the lumen of the ileum and colon (n=16) was rinsed with PBS and stapled without stretching to cardboard. Tissues were fixed overnight with Zamboni's fixative (2% paraformaldehyde, 15% picric acid; pH 7.4) at 4°C and then rinsed for 3 × 10 min in phosphate buffered saline (PBS; pH 7.4).
Whole mounts were prepared by dissection with forceps under a dissecting microscope. Cross-sections of intact intestine were cyroprotected in PBS containing 20% sucrose at 4°C for several hours or overnight. Specimens were embedded in optimum cutting temperature compound (OCT; Tissue-Tek, Sakura Finetechnical Co. Ltd., Tokyo, Japan) and cryostat-sectioned at 14μm prior to thaw mounting onto poly-D-lysine coated slides. Whole mounts or sections were washed for 3 × 10 min in PBS containing Triton X-100 (0.1%) and incubated with goat anti-CB1 C-terminal antibody (K. Mackie; 1:500)28,29 for 48 h at 4°C. The tissue was then washed for 3 × 10 min in PBS and incubated with the secondary antibody donkey anti-goat conjugated to CY3 (Jackson ImmunoResearch; 1:100) for 60 min at room temperature, followed by washing (3 × 10 min in PBS) and mounting in bicarbonate-buffered glycerol (pH 8.6). Tissues were examined using a Zeiss Axioplan microscope. Photographs were taken using a digital imaging system consisting of a digital camera (Sensys Photometrics, Tucson, AZ, USA) and image analysis software (V for Windows; Digital Optics, Auckland, New Zealand).
Isolated smooth muscle strips
Male mice were killed by cervical dislocation. Segments of the distal ileum were removed and submerged in ice-cold oxygenated Krebs solution (mM): NaCl 115, KCl 8.0, KH2PO4 2.0, NaHCO3 25, MgCl2 2.4, CaCl2 1.3, and glucose 10. Luminal contents were gently flushed. Up to four segments of full-thickness strips, 1.5 cm each, were used from each animal. All experiments lasted less than 3 h and each preparation was used for a single experiment only.
The preparations were slipped through two platinum electrodes, which were 1 cm apart and placed in separate organ baths (25 mL; 37°C; oxygenated with 95% O2 / 5% CO2). Using a silk thread one end of the preparations was attached to the bottom of the organ bath, while the other end was connected to an isometric force transducer (Harvard Apparatus, model 50-7905, Kent, UK). 0.5 g tension was applied and the preparations were allowed to equilibrate for 30 min. Changes in tension were amplified by a transducer amplifier (Harvard Apparatus, model 50-7970, Kent, UK), relayed to a bioelectric amplifier (Hewlett-Packard, model 8811A, Mississauga, Ontario, Canada).
The data was converted using a CED 1401 PLUS analogue to digital converter (Cambridge Electronic Design, Cambridge, UK.) and recorded using the Spike 3 software (Cambridge Electronic Design).
AM4113 (1-100nM), AM251 (1-100nM) and rimonabant (SR141716A, 1-100nM) were added to the organ bath and effects on spontaneous activity and on muscular contractions induced by bethanechol (10μM) were recorded.
Electrical field stimulation (EFS) was used according to two different regimens. One was to assess the effects of the antagonists at 4 different frequencies (2, 4, 8 and 16 Hz for 10 sec, 60 V, and 0.5 msec pulse duration) and the other was used continuously at a frequency of 8Hz (60 V, stimulus duration 0.5 msec, train duration 5 sec, with 100 sec interval) to assess the effects of the antagonists on WIN55,212-2 inhibition of contractility. EFS was applied by a Grass Stimulator (S88 stimulator, Grass, Quincy, MA, USA). Under these conditions, EFS resulted in stable contractions mediated via cholinergic and neurogenic mechanisms, as contractions were absent in presence of atropine (1μM) or tetrodotoxin (100nM). Drugs (AM4113: 1-100nM; AM251: 1-100nM; rimonabant 1-100nM) were added cumulatively into the organ bath and effects on the EFS-induced contractions were recorded. Each concentration was allowed to incubate for 20 min. Before adding drugs, the mean of 2 successive contractions at each frequency was defined as the control response. Contractions produced by each drug concentration were reported as the percentage of control response. In additional control experiments, the effects of the vehicle were also tested.
Upper gastrointestinal transit
After an overnight fast (water ad libitum) upper GI transit experiments were performed as described in detail by others 30,31. Briefly, 20 min after intraperitoneal (i.p.) administration of drugs (or vehicle) 0.2 ml of 5% Evans blue suspension in 5% gum arabic was given by gastric gavage. 15 min later animals were killed by cervical dislocation and the intestine was immediately removed. The distance travelled by the colored marker was measured in cm and expressed as a percentage of the total length of the small intestine from pylorus to cecum.
Colonic expulsion
Distal colonic expulsion was measured using the method of Broccardo et al.32. Briefly, 20, 120 and 220 min after i.p. administration of drugs (or vehicle), a plastic bead (2.5 mm) was inserted 3 cm into the distal colon of each mouse using a silicone pusher. The time to expulsion of the bead was determined for each animal at each of the three time points and then the mean of these individual trials was taken. A higher mean expulsion time indicates a greater inhibition of colonic expulsion.
Fecal water content
Fecal water content was assessed in mice following a modification of a method described by Izzo et al.12 48 h prior to the experiment mice were housed in individual cages with water ad libitum and they were preconditioned with overnight-fasting and vehicle injections. On the day of the experiment, animals were given drugs i.p. and immediately transferred to an empty cage (devoid of bedding). The stool-pellets discharged at 20, 120 and 220 min were collected and weighed immediately (wet weight). After drying (overnight at 50°C) the dry weight was determined. The ratio of wet to dry weight was calculated and used as a marker of stool fluid content. Control mice received vehicle treatment only.
Whole gut transit time
Mice were housed in individual cages 72 h prior to the experiment. On the day of the experiment, they were acclimated to an empty cage (devoid of bedding) for 1 h prior to drug treatment. Twenty min after i.p. administration of drugs (or vehicle) 0.2 ml of 5% Evans blue suspension in 5% gum arabic was given by gastric gavage. The time to the first blue bowel movement was measured in min and constituted the whole gut transit time.
Ion transport
Whole thickness segments of mouse colon, taken from the mid-distal region of the colon, were mounted in modified Ussing chambers (0.38 cm2 opening). Both sides were bathed in a modified Krebs solution (mM): 115 NaCl, 2.0 KH2PO4, 2.4 MgCl2, 25.0 NaHCO3, 8.0 KCl, 1.3 CaCl2 containing 10 mM glucose (serosal side) or 10 mM mannitol (luminal side). Two tissue segments were used per mouse; one was used as a vehicle control, the other exposed to either AM251 or AM4113 (1μM). Segments receiving vehicle or drug were alternated to eliminate possible differences in ion transport responses between the mid and distal regions of the colon. Tissues were studied under short-circuited conditions in which the voltage was clamped to 0 mV using a WPI EVC-4000 voltage clamp (World Precision Instruments, Sarasota, FL, USA). Tissues were unclamped at the beginning and end of each experiment to record open PD values for the calculation of tissue conductance (Gt). After baseline short-circuit current (ISC) was established (15-30 min), either drug or an equal volume of vehicle (100% ethanol) was added to the serosal side of the tissue. The final concentration of ethanol in the bathing solution never exceeded 0.1% (v/v). 10 min following drug addition, tissues were exposed to electrical field stimulation at 1, 2.5, 5 and 10 Hz (pulse duration: 500 μs, stimulus duration: 5 sec, stimulus strength: 50 V) to assess neurally evoked secretory responses. ISC was allowed to return to baseline following each stimulation. Tissues were then exposed to tetrodotoxin (1μM, serosal addition) in order to reduce neural contributions to subsequent ion transport responses. Carbachol was added 10 min later (60 μM, serosal addition) and, finally, following a return to a steady baseline ISC, forskolin was added (10 μM, serosal addition). For each challenge, the peak change in ISC (ΔISC) was measured.
FITC-inulin flux
In some experiments, tissues mounted in Ussing chambers were exposed mucosally to FITC-labelled inulin, 1 mg/ml, to assess paracellular permeability. Aliquots of serosal buffer were sampled every 30 min and assayed for fluorescein fluorescence (wavelength 521nm) in a VICTOR3 fluorescence plate reader (Perkin-Elmer, Waltham, MA). Data were expressed as a percentage of baseline fluorescence obtained at time zero.
Statistics
Student's t-test was used to compare single treatment means with control means and ANOVA followed by Dunnet's post-hoc test was used for analysis of multiple treatment means (SigmaStat, Jandel Scientific, San Rafael, CA, USA). P values < 0.05 were considered significant. Data represent the mean ± SEM of experiments in n mice.
Drugs
AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazolone-3-carboxamide) was obtained from Tocris (Tocris, Ellisville, USA). AM4113 was synthesized in the laboratory of Alex Makriyannis at the Center for Drug Discovery, Northeastern University, USA. AM4113 is a pyrazole-3-carboxamide analog of AM251 with a very similar molecular weight. AM251 and AM4113 were dissolved in a solution of dimethyl sulfoxide [DMSO] 2% and Tween 80 1% in saline (0.9%). For ion transport studies, drugs were dissolved in 100% ethanol, as described above. Evans blue was obtained from Sigma-Aldrich (Oakville, ON, Canada). None of the vehicles used had effects on the observed parameters in vitro or in vivo.
RESULTS
Immunohistochemistry
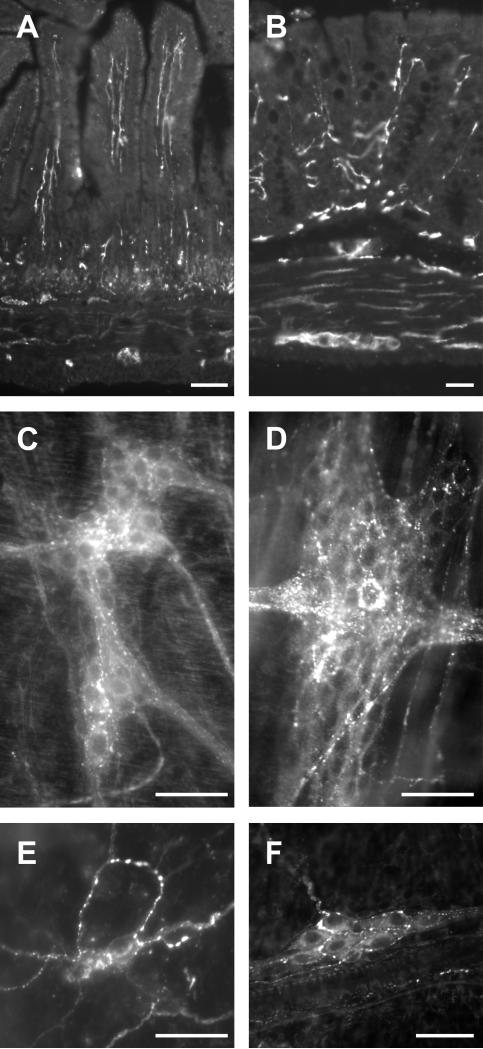
In order to establish the localization of CB1 receptor in the mouse GI tract we performed immunohistochemistry using an antibody that we confirmed does not react with CB1 receptors in CB1 receptor-knockout mice (data not shown). Sections of the mouse ileum and mouse colon were examined and revealed intense CB1 immunoreactivity in neural elements throughout the wall of the gut. CB1 immunoreactivity was localized to enteric neurons and nerve fibres of both the myenteric and submucosal plexus and nerve fibres in the mucosa. No immunoreactivity was detected on the epithelium (Fig. 1).
Figure 1.
Representative micrographs of CB1 immunoreactivity in cross-sections and whole mount preparations of ileum and colon from C57BL/6N male mice. Cross-sections of full thickness ileum (A) and colon (B). Whole mount preparations of the myenteric plexus of the ileum (C), colon (D) and submucosal plexus of the ileum (E) and colon (F). Note the dense innervation of the plexuses and extensive innervation of the mucosa. The epithelium was not labelled. Scale bars: 50μm.
Influence of CB1 receptor antagonists on ileal contractility in vitro
None of the antagonists tested, AM251 (1-100nM), rimonabant (1-100nM) or AM4113 (1-100nM) had an effect on the basal tone or spontaneous activity of mouse ileal smooth muscle or on contractions induced by bethanechol (10μM, data not shown; n=6). Electrical field stimulation was used to assess the effect of these compounds on nerve-mediated contractility of the ileum. These contractions were completely blocked by tetrodotoxin (100nM) and virtually abolished by atropine (1μM) (data not shown).
Electrical field stimulation of the ileum caused twitch contractions that were stable over 3 h. AM251 at doses up to 100nM had no significant effect on these contractions (Fig. 2A). This contrasts with the effects of rimonabant which concentration-dependently increased contractions (1-100nM) at stimulation frequencies of 4-16Hz (Fig. 2B). The neutral CB1 receptor antagonist AM4113 at concentrations up to 100nM had no effect on ileal contractility (Fig. 2C). We confirmed CB1 antagonism by assessing their ability to reverse the inhibitory effects of the non-selective cannabinoid agonist WIN55,212-2 on EFS-induced contractility (measured at 8Hz). WIN55,212-2 (100nM) significantly reduced contractility, this action was reversed in the presence of Rimonabant, AM251 and AM4113 (all 100nM) (Fig. 2D).
Figure 2.
Concentration-dependent effects of (A) AM251, (B) rimonabant, (C) AM4113 (all 1nM-100nM) on electrically-evoked contractions of the mouse ileum and (D) the effects of AM251, rimonabant and AM4113 prior to the addition of WIN55,212-2 on electrically-evoked contractions. Note the significant inhibitory effect of WIN55,212-2 is blocked by the presence of all the CB1 receptor antagonists. ** p < 0.01; *** p < 0.001; n = 6-9 each point.
Upper gastrointestinal transit
Rimonabant has already been shown to dose-dependently enhance upper GI transit in mice 33, consistent with the in vitro findings described above. We compared AM251 and AM4113 and found that both these compounds also enhanced upper GI transit, despite not enhancing contractility. AM251 (0.5 - 1 mg/kg) and AM4113 (0.1 - 1 mg/kg) significantly increased upper GI transit in a dose-dependent manner (Fig. 3A & 3B). AM4113 (0.1 mg/kg) and AM251 (0.5 mg/kg) at doses that did not significantly increase upper GI transit, reversed the WIN55,212-2 (1 mg/kg) induced inhibition of upper GI transit indicating receptor blockade at these concentrations (Fig. 3C). Neither AM251 (1 mg/kg) nor AM4113 (2 mg/kg) at doses that were effective in enhancing upper GI transit in wild type mice had a significant effect on upper GI transit in CB1-/- mice (Fig. 3D). This rules out a non-specific action at a site other than the CB1 receptor.
Figure 3.
(A) AM251 (0.5-1 mg/kg, n=8-12/group) and (B) AM4113 (0.1-1 mg/kg, n=5-6/group) increase upper GI transit of a marker dye in vivo. (C) AM4113 (0.1 mg/kg) and AM251 (0.5 mg/kg) completely block the inhibitory effects of the CB1 agonist WIN55,212-2 (1 mg/kg, n=4-9/group). Note, the effects of AM4113 (2 mg/kg) and AM251 (1 mg/kg) are absent in the CB1-/- mouse (D, n=4-8/group), which has an enhanced upper GI transit compared to wild type control mice. * p<0.05; ** p < 0.01; *** p < 0.001.
Colonic expulsion and fecal water content
AM251 (1-2 mg/kg) did not significantly alter the rate of colonic bead expulsion (Fig. 4A). Additionally, a high dose of AM251 (5mg/kg) also did not delay colonic expulsion (287 ± 64 sec; n=7). AM4113 (1-2 mg/kg) dose-dependently slowed expulsion of beads from the colon (Fig. 4B). AM4113 (1 mg/kg) and AM251 (2 mg/kg) at a doses that did not significantly alter colonic expulsion, completely blocked the effect of WIN55,212-2 (3 mg/kg) (Fig. 4C).
Figure 4.
(A) AM251 (1-2 mg/kg, n=4-12/group) and (B) AM4113 (1-2 mg/kg, n=6-16/group) have no significant effect on colonic expulsion time in vivo. (C) AM4113 (1 mg/kg) and AM251 (2 mg/kg) completely block the inhibitory effect of the CB1 agonist WIN55,212-2 (3 mg/kg, n=4-10/group). (D) Fecal water content is not altered by AM251 (1 mg/kg) or AM4113 (1 mg/kg) n=6-7/group. * p<0.05; ** p < 0.01; *** p < 0.001.
We assessed the fecal water content in animals treated with AM251 and AM4113. Neither the inverse agonist/antagonist AM251 (1 mg/kg), nor the neutral CB1 antagonist AM4113 (1 mg/kg), altered fecal water content (Fig. 4D).
Whole-gut transit
Since we observed an increase in small intestinal motility induced by AM251 and AM4113 in vivo, despite seeing no effect on contractility in some cases, we wished to assess the passage of food through the entire gut, as this is influenced by gastric, small and large intestinal motility, as well as secretion. Consistent with the actions of rimonabant, the inverse agonist/antagonist AM251 (1 and 5 mg/kg) speeds up transit by about 50% (Fig. 5). In contrast, the neutral antagonist AM4113 (5 mg/kg) did not alter whole gut transit time in vivo, and a lower dose (1 mg/kg) slowed whole gut transit (Fig. 5).
Figure 5.
Effect of the inverse agonist/antagonist AM251 and the neutral antagonist AM4113 on whole gut transit time. (A) shows the whole gut transit time when the antagonists are given 1 mg/kg (n=5/group), and (B) when the antagonists are given 5 mg/kg (n=8-9/group). * p<0.05; ** p < 0.01.
Effects of AM251 and AM4113 on colonic epithelial ion transport and permeability
Colonic tissue responded to EFS with frequency-dependent increases in ISC that were not affected by either AM251 or AM4113 (Fig. 6A & 6B). Similarly, no significant differences were observed between drug- and vehicle-treated groups with respect to both carbachol- and forskolin-induced ISC responses (Fig. 6C). It should also be noted that, on their own, neither AM251 nor AM4113 produced a change in ISC when added to the Ussing chambers (data not shown).
Figure 6.
Neither AM251 (A) nor AM4113 (B) affected Isc responses to electrical field stimulation or (C) serosal forskolin (FSK, 10 μM) or carbachol (CCh, 60 μM). (V – vehicle; n = 7 mice in A, n = 6 mice in B, n = 9-13 mice in C.
Fluorescein fluorescence in the serosal chamber increased over time, but neither drug appeared to affect the epithelial paracellular movement of FITC-labelled inulin in the colon (data not shown). Tissue conductance (Gt) was not significantly affected by either AM compound (data not shown).
DISCUSSION
CB1 receptors were first identified in 1988 34 and since then, there has been a wealth of evidence supporting their involvement in GI motility. CB1 receptor activation by specific CB1 receptor agonists was shown to reduce cholinergic contraction in various species in vitro 35-39. In vivo CB1 agonists attenuate upper GI transit and slow down colonic expulsion3,12,15,17,40,41. Since these actions are blocked by CB1 antagonists and are absent in CB1-/- mice, the CB1 receptor has been confirmed as the major CB receptor mediating these effects. In contrast to these consistent pharmacological reports, the involvement of the ECS and specifically the CB1 receptor in the regulation of motility under physiological and pathophysiological conditions is less well defined. The regulation of GI motility by the ECS is suggested by (i) levels of endocannabinoids in the gut that can activate receptors 40 (ii) by the ability of antagonists/inverse agonists to alter motility,23 (iii) altered motility in CB1 receptor gene-deficient mice 3,15 and (iv) by the activity of inhibitors of endocannabinoid degradation.42,43 There are some contradictory results in these studies, but overall the evidence is supportive of the involvement of the ECS and CB1 receptors in the control of GI motility. In order to strengthen this evidence further and to develop potential novel therapeutic approaches for the treatment of GI related diseases, such as irritable bowel syndrome, we investigated an alternative approach to studies of the role of the CB1 receptor in GI function by utilizing a CB1 receptor specific neutral antagonist.
Neutral antagonists do not alter receptor signalling when there is constitutive receptor activity. These contrast with the actions of CB1 antagonists/inverse agonists which can work in two ways: (i) they can act as antagonists and block the actions of endogenously released endocannabinoids or (ii) they can have an independent inverse agonist activity on constitutively active receptors. In tissues or whole animals it is not possible to distinguish these two possibilities as the extent of constitutive receptor activity is not known and likely varies considerably in different cells. Recently, a neutral CB1 receptor antagonist, AM4113, was developed that allows us to separate antagonism of the receptor without inverse agonism.24-26 We compared this compound with the well known inverse agonists/antagonists AM251 and rimonabant, in order to clarify the physiological involvement of the ECS in GI function.
We first investigated these compounds in vitro alone and found that despite AM251 and rimonabant being structurally related, they possessed markedly different actions. Rimonabant enhanced electrically stimulated contractility in a concentration-dependent manner, with its greatest effect at a stimulation frequency of 4Hz. In contrast, the inverse agonist/antagonist AM251 had no effect on contractility over the same concentration range, and neither did AM4113. Since all the compounds were completely effective at reversing the actions of WIN55,212-2, it suggests that their ability to act as antagonists is separable from other actions. Rimonabant has previously been found to enhance contractility in the guinea pig ileum,13,37 but interestingly it did not alter acetylcholine release.13 In the mouse colon, rimonabant enhanced tonic and phasic activity at high concentrations 44 whereas it did not alter nonadrenergic, noncholinergic contractions 45 and neither did it alter spontaneous contractile activity in the ileum 46, as we have also shown in this study. Finally, rimonabant was not shown to alter contractility in the human small or large intestine.47,48 The other compounds had not previously been examined, but on the basis of their effects it seems neither inverse agonism nor antagonism of endogenous tonically released endocannabinoids can completely account for the enhanced contractility observed with rimonabant in vitro. Since neither basal activity nor bethanechol induced contractions were altered by any of the compounds, we believe the site of action of rimonabant is on the enteric nervous system and not on the smooth muscle, as others have suggested.35,37-39,45
We next extended our findings to examine the effects of AM251 and AM4113 in vivo on upper GI transit. Both compounds increased upper GI transit in a dose-dependent manner as has previously been shown for rimonabant in both rats and mice 49,50, and for AM251 in rats.6 We confirmed that both compounds completely blocked the effects of the CB1 agonist WIN 55,212-2 and that effects of the antagonists were not present in CB1-/- mice, confirming that these are CB1 receptor mediated actions. As the effect of the neutral antagonist AM4113 cannot be explained by inverse agonist activity and it mirrors what is observed in the CB1-/- mice, these results strongly support the idea that under physiological conditions in vivo endogenous cannabinoid tone is importantly involved in the regulation of upper GI transit. Upper GI transit evaluated by the method used is a measure of gastric emptying and small intestinal transit, and interestingly rimonabant was shown to increase gastric emptying in mice.51
By contrast, AM251 had no effect on colonic bead expulsion, but AM4113 slowed bead expulsion at the highest dose tested (2mg/kg). Again, both compounds completely reversed the effects of the CB1 receptor agonist WIN 55,212-2 at doses that had no effect on bead expulsion. These data suggest that endocannabinoids act centrally or peripherally to modulate colonic function, either through slowing of motility or increasing the threshold for sensation of a colonic bead (or both). CB1 receptors have been implicated in the regulation of visceral sensation,2,11 but in rats rimonabant alone did not alter visceral sensitivity.52 It remains to be determined the exact site of action of AM4113 and whether this effect on bead expulsion is central or peripheral.
Since normal colonic function requires adequate lubrication, but excess secretion results in diarrhea, we also examined stool water content in animals treated with both AM251 and AM4113. In neither case was this altered, in contrast to rats treated with rimonabant, which have highly fluid stools.12 CB1 receptor expression in the submucosal plexus of the guinea pig 29 has been previously reported, we now confirm CB1 receptor expressions in mouse submucosal plexus. CB1 receptors have also been described on the colonic epithelium in the human,53 but this was not the case in the mouse, possibly indicating an important species difference that may be of functional significance.
Finally, we examined whole gut transit time to gain an impression of all elements of motility in a freely moving animal. Whereas AM251 enhanced whole gut transit, the neutral antagonist AM4113 did not, suggesting that inverse agonism is the feature of these compounds that leads to reduced whole gut transit time, which may translate into the symptoms of diarrhea in humans.19,20,54 This finding was not expected, because AM4113 enhanced transit along the small intestine, but is very exciting since it may allow this class of drug to be developed further for a range of indications without significant GI side-effects. Exactly why AM4113 did not speed up whole gut transit is not known, but may relate to pharmacodynamic or pharmacokinetic properties or to some aspect of lack of inverse agonism. In this regard, it is noteworthy that the inverse agonists rimonabant and taranabant both induce diarrhea and GI disturbances in humans.19,20,54 Whether reducing weight and cardiometabolic risk factors can also be achieved with a neutral CB1 receptor antagonist in humans and whether the side-effect profile is more favorable remains to be established, but our data suggest, that a neutral antagonist may have a more favorable GI side-effect profile. Moreover, AM4113 has been examined in animal studies and found to reduce weight and food intake whilst have less nauseogenic potential, a feature that is favorable considering future human use.24-26
Interestingly, neither antagonist had an effect on fecal stool water content, suggesting that the increased whole gut transit in animals treated with AM251 is a motor and not a secretomotor effect. Nevertheless, we were interested whether the CB1 antagonists altered other parameters of ion transport or barrier function, to address whether there may be a physiological regulation of these functions. Using various stimuli, including electrical field stimulation, carbachol and forskolin, which stimulate secretion indirectly via the enteric nervous system and directly at the epithelium, as shown previously,55 we did not observe any effects of the CB1 receptor antagonists on ion transport, tissue conductance or mucosal permeability. This lack of effect on secretion and barrier function suggests that the physiological role of the ECS may be limited to activation of CB1 receptors that regulate GI motility and is not a general involvement of CB1 receptors which are found throughout the enteric nervous system.
Our findings do not contradict previous reports in the guinea pig ileum, where pharmacological activation of CB1 receptors using the potent CB receptor agonist WIN55,212-2 resulted in a reduction of secretion, which was not observable in the presence of the CB1 antagonist rimonabant.29 Comparable data were also reported for rat ileal secretion.56 Both studies identified that CB1 receptors reduce neuronally induced secretion, but not secretion induced by direct activation of the epithelium.29,56 Experiments with the antagonist alone were not reported in these studies. This pharmacological effect is also observable in larger mammals like pigs, where the CB1 agonist HU 210 reduces secretion induced by the proinflammatory peptide kallidin 57. Therefore secretion in the gut can be pharmacologically reduced by drugs activating CB1 receptors and our study adds that secretion seems not to be under physiological control by the ECS. This is supported by another study in mice, where cannabinoid drugs are involved in pathophysiological but not in physiological states.43 The role of CB1 receptors on neurons of the submucosal plexus remains to be fully established. One possibility is that the CB1 receptors are only involved in the regulation of secretion under pathophysiological conditions, 43 a hypothesis that needs to be addressed in the future.
In conclusion, using a novel neutral CB1 receptor antagonist we have confirmed and extended findings that the ECS is involved in the regulation of intestinal motility under physiological conditions. We found no evidence that it is involved in the control of secretomotor or barrier functions under normal physiological conditions, despite the presence of CB1 receptors on enteric nerves throughout the wall of the gut. We also demonstrated markedly variable effects of CB1 receptor antagonists on contractility assessed in vitro and found no evidence that the ECS regulates electrically-evoked cholinergic contractility in the mouse ileum under these conditions. We made the interesting finding that whole gut transit time was not reduced by the neutral CB1 antagonist AM4113, suggesting that inverse agonism, but not CB1 receptor antagonism, is the feature that underlies the reduced whole gut transit seen when these compounds are administered to humans and animals.19,20 This unexpected and interesting finding is of importance for the future development of CB1 receptor antagonists as pharmacological agents for the treatment of a variety of metabolic and other disorders, as it may help to reduce their unwanted GI side-effects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Winnie Ho for performing the genotyping of the CB1 receptor gene deficient mouse colony. This work was supported by grants from the Canadian Institutes of Health Research (to KAS) and the Crohn's and Colitis Foundation of Canada (CCFC, to MS and KAS). Martin Storr was also supported by the University of Calgary Research Grant Committee. Keith Sharkey is an Alberta Heritage Foundation for Medical Research Medical Scientist and holds the CCFC Chair in IBD Research at University of Calgary. Wallace MacNaughton is a CCFC IBD Research Scientist. Christina Hirota holds a post-doctoral fellowship from the Canadian Association of Gastroenterology, CIHR and Abbott. Ken Mackie is supported by National Institutes of Health (NIH) grants DA11322 and DA21696. Alex Makriyannis is supported by NIH grants DA09158, DA7215, DA3801 and DA023142
Footnotes
Competing Interests
The authors declare they have no competing interests.
REFERENCES
- 1.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- 2.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- 3.Izzo AA, Mascolo N, Capasso F. The gastrointestinal pharmacology of cannabinoids. Curr Opin Pharmacol. 2001;1:597–603. doi: 10.1016/s1471-4892(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 4.Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Current Opinion in Pharmacology. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Li YY, Li YN, Ni JB, Chen CJ, Lv S, Wu RH, Yuece B, Storr M. Involvement of cannabinoid-1 and cannabinoid-2 receptors in septic ileus. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01419.x. in press. [DOI] [PubMed] [Google Scholar]
- 6.Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, Patel KD, Pittman QJ, Sharkey KA. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boesmans W, Ameloot K, van den Abbeel V, Tack J, Vanden BP. Cannabinoid receptor 1 signalling dampens activity and mitochondrial transport in networks of enteric neurones. Neurogastroenterol Motil. 2009;21:958–e77. doi: 10.1111/j.1365-2982.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- 9.Marquez L, Suarez J, Iglesias M, Bermudez-Silva FJ, Rodriguez de FF, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE. 2009;4:e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Storr M, Sharkey KA. The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol. 2007;7:575–582. doi: 10.1016/j.coph.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Izzo AA, Mascolo N, Borrelli F, Capasso F. Defaecation, intestinal fluid accumulation and motility in rodents: implications of cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:65–70. doi: 10.1007/pl00005325. [DOI] [PubMed] [Google Scholar]
- 13.Pertwee RG, Fernando SR, Nash JE, Coutts AA. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br J Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carai MA, Colombo G, Gessa GL, Yalamanchili R, Basavarajppa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. Br J Pharmacol. 2006;148:1043–1050. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuece B, Sibaev A, Broedl U, Marsicano G, Goeke B, Lutz B, Allescher HD, Storr M. Cannabinoid type 1 receptor modulates intestinal propulsion by an attenuation of intestinal motor responses within the myenteric part of the peristaltic reflex. Neurogastroenterol Motil. 2007;19:744–753. doi: 10.1111/j.1365-2982.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 16.Mascolo N, Izzo AA, Ligresti A, Costagliola A, Pinto L, Cascio MG, Maffia P, Cecio A, Capasso F, Di Marzo V. The endocannabinoid system and the molecular basis of paralytic ileus in mice. FASEB J. 2002;16:1973–1975. doi: 10.1096/fj.02-0338fje. [DOI] [PubMed] [Google Scholar]
- 17.Izzo AA, Pinto L, Borrelli F, Capasso R, Mascolo N, Capasso F. Central and peripheral cannabinoid modulation of gastrointestinal transit in physiological states or during the diarrhoea induced by croton oil. Br J Pharmacol. 2000;129:1627–1632. doi: 10.1038/sj.bjp.0703265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Filippis D, Iuvone T, D'Amico A, Esposito G, Steardo L, Herman AG, Pelckmans PA, De Winter BY, De Man JG. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil. 2008;20:919–927. doi: 10.1111/j.1365-2982.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- 19.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 20.Addy C, Rothenberg P, Li S, Majumdar A, Agrawal N, Li H, Zhong L, Yuan J, Maes A, Dunbar S, Cote J, Rosko K, Van Dyck K, De Lepeleire I, de Hoon J, Van Hecken A, Depre M, Knops A, Gottesdiener K, Stoch A, Wagner J. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, in healthy male volunteers. J Clin Pharmacol. 2008;48:734–744. doi: 10.1177/0091270008317591. [DOI] [PubMed] [Google Scholar]
- 21.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 22.Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG. Agonist-inverse agonist characterisation at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KA. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- 25.Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 28.Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol Gastrointest Liver Physiol. 2004;286:G863–G871. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- 30.Shook JE, Dewey WL, Burks TF. The central and peripheral effects of delta-9-tetrahydrocannabinol on gastrointestinal transit in mice. NIDA Res Monogr. 1986;67:222–227. [PubMed] [Google Scholar]
- 31.Seerden TC, De Winter BY, Van Den Bossche RM, Herman AG, Pelckmans PA, De Man JG. Regional differences in gastrointestinal motility disturbances during acute necrotising pancreatitis. Neurogastroenterol Motil. 2005;17:671–679. doi: 10.1111/j.1365-2982.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 32.Broccardo M, Improta G, Tabacco A. Central effect of SNC 80, a selective and systemically active delta-opioid receptor agonist, on gastrointestinal propulsion in the mouse. Eur J Pharmacol. 1998;342:247–251. doi: 10.1016/s0014-2999(97)01470-2. [DOI] [PubMed] [Google Scholar]
- 33.Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, Esposito G, Mascolo N, Di Marzo V, Capasso F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterisation of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 35.Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB1) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol. 2006;148:191–199. doi: 10.1038/sj.bjp.0706710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Redondo F, Lees GM, Pertwee RG. Effects of cannabinoid receptor ligands on electrophysiological properties of myenteric neurons of the guinea-pig ileum. Br J Pharmacol. 1997;122:330–334. doi: 10.1038/sj.bjp.0701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izzo AA, Mascolo N, Borrelli F, Capasso F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptors. Br J Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pertwee RG, Stevenson LA, Elrick DB, Mechoulam R, Corbett AB. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br J Pharmacol. 1992;105:980–984. doi: 10.1111/j.1476-5381.1992.tb09088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storr M, Gaffal E, Saur D, Schusdziarra V, Allescher HD. Effect of cannabinoids on neural transmission in rat gastric fundus. Can J Physiol Pharmacol. 2002;80:67–76. doi: 10.1139/y02-005. [DOI] [PubMed] [Google Scholar]
- 40.Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, Mascolo N, Di Marzo V, Capasso F. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- 41.Sibaev A, Yuece B, Kemmer M, Van NL, Broedl UC, Allescher HD, Goeke B, Timmermans JP, Storr M. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2008;296:G119–G128. doi: 10.1152/ajpgi.90274.2008. [DOI] [PubMed] [Google Scholar]
- 42.Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, Mascolo N, Petrosino S, Monory K, Valenti M, Di Marzo V, Izzo AA. Fatty Acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Izzo AA, Capasso F, Costagliola A, Bisogno T, Marsicano G, Ligresti A, Matias I, Capasso R, Pinto L, Borrelli F, Cecio A, Lutz B, Mascolo N, Di Marzo V. An endogenous cannabinoid tone attenuates cholera toxin-induced fluid accumulation in mice. Gastroenterology. 2003;125:765–774. doi: 10.1016/s0016-5085(03)00892-8. [DOI] [PubMed] [Google Scholar]
- 44.Mancinelli R, Fabrizi A, Del Monaco S, Azzena GB, Variu R, Colombo GC, Gessa GL. Inhibition of peristaltic activity by cannabinoids in the isolated distal colon of mouse. Life Sci. 2001;69:101–111. doi: 10.1016/s0024-3205(01)01110-9. [DOI] [PubMed] [Google Scholar]
- 45.Mule F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res. 2007;56:185–192. doi: 10.1016/j.phrs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Baldassano S, Serio R, Mule F. Cannabinoid CB(1) receptor activation modulates spontaneous contractile activity in mouse ileal longitudinal muscle. Eur J Pharmacol. 2008;582:132–138. doi: 10.1016/j.ejphar.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Croci T, Manara L, Aureggi G, Guagnini F, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Mukenge S, Ferla G. In vitro functional evidence of neuronal CB1 receptors in human ileum. Br J Pharmacol. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manara L, Croci T, Guagnini F, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Mukenge S, Ferla G. Functional assessment of neuronal cannabinoid receptors in the muscular layers of human ileum and colon. Dig Liver Dis. 2002;34:262–269. doi: 10.1016/s1590-8658(02)80146-3. [DOI] [PubMed] [Google Scholar]
- 49.Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- 50.Colombo G, Agabio R, Lobina C, Reali R, Gessa GL. Cannabinoid modulation of intestional propulsion in mice. Eur J Pharmacol. 1998;344:67–69. doi: 10.1016/s0014-2999(97)01555-0. [DOI] [PubMed] [Google Scholar]
- 51.Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, Romano B, Orlando P, Capasso F, Izzo AA. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol. 2008;153:1272–1280. doi: 10.1038/sj.bjp.0707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil. 2006;18:949–956. doi: 10.1111/j.1365-2982.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 53.Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 54.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 55.Fichna J, Schicho R, Andrews C, Bashashati M, Klompus M, McKay DM, Sharkey KA, Zjawiony JK, Janecka A, Storr M. Salvinorin A inhibits colonic transit and neurogenic ion transport in mice by activating ê-opioid and cannabinoid receptors. Neurogastroenterol Mot. 2009;21:1326–e128. doi: 10.1111/j.1365-2982.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 56.Tyler K, Hillard CJ, Greenwood-Van Meerveld B. Inhibition of small intestinal secretion by cannabinoids is CB1 receptor-mediated in rats. Eur J Pharmacol. 2000;409:207–211. doi: 10.1016/s0014-2999(00)00843-8. [DOI] [PubMed] [Google Scholar]
- 57.Green BT, Calvin A, O'Grady SM, Brown DR. Kinin-induced anion-dependent secretion in porcine ileum: characterization and involvement of opioid- and cannabinoid-sensitive enteric neural circuits. J Pharmacol Exp Ther. 2003;305:733–739. doi: 10.1124/jpet.102.047829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.