Abstract
In order to evaluate novel stroke therapies, it is essential to utilize a highly reproducible model of focal cerebral ischemia. Though a range of rodent stroke models has been employed in the literature, there are persistent issues regarding reproducibility of the ischemic zone, as there is considerable inter-animal and inter-laboratory variation. We have developed a highly reproducible model of stroke that involves direct electrocoagulation of the MCA in SCID (CB-17/lcr-scid/scidJcl) and CB-17 (CB-17/lcr-+/+Jcl) mice. Using a modification of the Tamura method, our results demonstrate reproducible cortical infarction with high survival in the chronic period (up to 180 days) in SCID and CB-17, but not in C57BL/6, mice. We believe that our preclinical model represents a step forward for testing future therapeutic methods potentially applicable to patients with stroke.
Keywords: stroke model, permanent cerebral ischemia, reproducibility
Introduction
A range of stroke models has been developed to simulate clinically relevant subtypes of stroke(Liu et al. 2009). Focal cerebral ischemia has been used to model ischemic stroke, and the Tamura(Tamura et al. 1981) and intraluminal filament models(Longa et al. 1989) have been employed most commonly. A key issue in the evaluation of data obtained in these models concerns reproducibility of the stroke-affected region and the survival rate of animals during the chronic period. Various modifications of the latter methods have been employed to address these issues(Chen et al. 1986; Hirakawa et al. 1994; Kuge et al. 1995).
Because murine models are preferable for screening new therapies, though vascular structures in mice can demonstrate inter-strain variability, we have assessed the reproducibility of the stroke-affected area in different strains by simple direct ligation of the MCA. We have found that ligation of the distal portion of the MCA in SCID or CB-17 mice induces reproducible and selective cortical infarction. In previous reports, we have outlined our stroke model in SCID(Nakagomi et al. 2009; Taguchi et al. 2004) and CB-17(Taguchi et al. 2007) mice. In this manuscript, we provide the detailed methods, allowing for easy replication of our work, and discuss the advantages and limitations of our model.
Materials and Methods
All procedures were performed under auspices of an approved protocol from the National Cardiovascular Center Animal Care and Use Committee.
Induction of Permanent Focal Cerebral Ischemia
Male SCID (CB-17/lcr-scid/scidJcl), CB-17 (CB-17/lcr-+/+Jcl) and C57BL/6 (C57BL/6NJcl) mice were purchased from Clea Japan (Tokyo, Japan). Animals were allowed access to food and tap water ad libitum. Powdered chow and sterilized water were also provided during the first 7 days after induction of stroke.
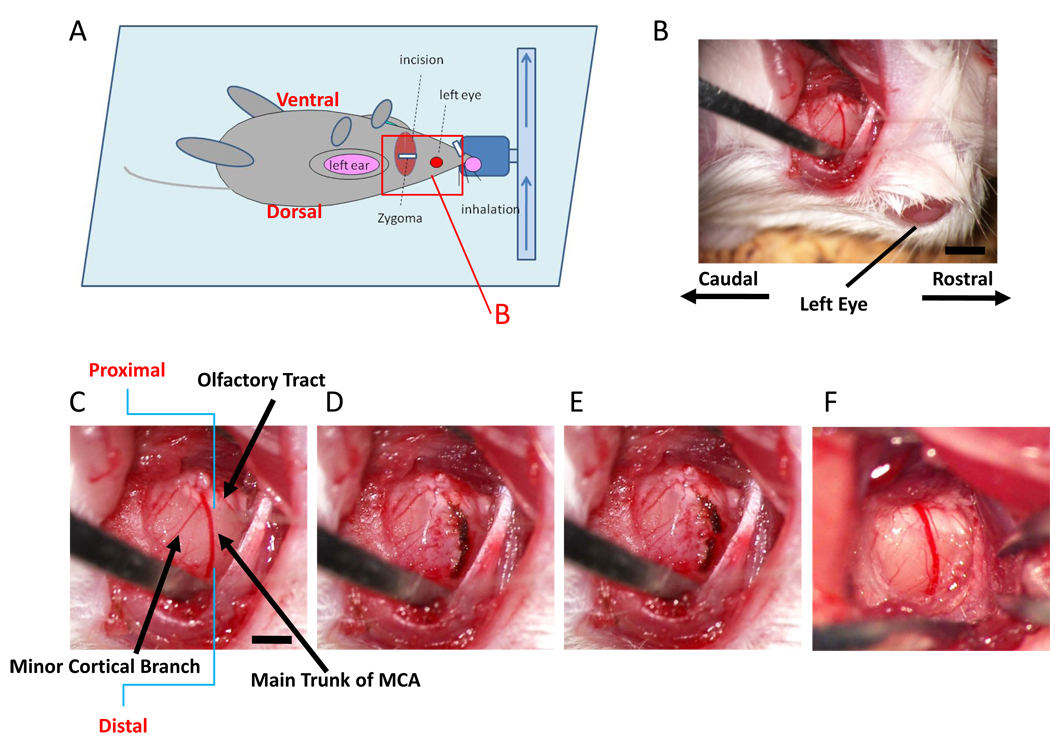
Cerebral infarction was studied in 8 week-old mice according to a modification of the Tamura method. General anesthesia was induced and maintained by inhalation of 3% and 1.5% halothane (7025 Rodent Ventilator, Ugo Basile, Italy), respectively. Mice were oriented as indicated, and a skin incision was made between left eyeball and left ear hole (Figure 1A). The left salivary gland and veins were carefully removed to visualize the zygoma. The left zygoma was dissected under an operating microscope (KOM300S, Konan Medical, Nishinomiya, Japan), and the jaw joint was detached to allow visualization of the MCA through the cranial bone. A hole (diameter, 1.5 mm) was made in the bone using a dental drill (C710, Senko Medical Instrument Manufacturing, Tokyo, Japan). Subsequently, the dura matter was carefully removed with caution not to damage the surface of the brain. Then, the MCA was isolated (Figure 1B, lower magnification; C, higher magnification), electrocauterized (Figure 1D) and disconnected distal to crossing the olfactory tract (distal M1 portion, Figure 1E). It is notable that very mild and gradual coagulation is required in order for electrocoagulation to avoid bleeding from the MCA. We used an electrocoagulator designed for ophthalmologic surgery (MERA MS-50, Senko Medical Instrument, Tokyo, Japan) and the bipolar forceps (MERA NF-14) at an output level of 1.0 Watt. Sequential, very brief coagulation with the top of the bipolar forceps is recommended, starting from the distal portion of MCA to the proximal portion. Repetition of this procedure, usually 2 to 4 times, stopped blood flow in the vessel. After that, the MCA was coagulated from the distal to the proximal portion. This method resulted in a very low risk of bleeding from the MCA.
Figure 1. Ligation of the distal portion of MCA.
(A) Schematic depiction showing orientation of the animal in our model for induction of stroke. The red rectangle represents the portion shown in panel B. (B–E) After dissecting the left zygoma, a hole was made and the dura was carefully removed (B; lower magnification, C; higher magnification). The main trunk of the MCA was electrocoagulated (D) and disconnected using the tip of 30G needle (E). This mouse had a minor cortical branch (C), but electrocoagulation and disconnection of main trunk resulted in disappearance of blood flow in the minor cortical branch. Panel (F) shows a representative picture of the vascular structure without a minor cortical branch around olfactory tract. Scale bar: 1 mm (B) and 0.5 mm (C).
Cerebral blood flow (CBF) in the MCA area was monitored as described(Taguchi et al. 2007). Briefly, an acrylate column (Neuroscience Co., Ltd., Osaka, Japan) was attached to the intact skull using stereotactic coordinates (1 mm anterior and 3 mm lateral to the bregma), and CBF was monitored using a linear probe (1 mm in diameter) by laser Doppler flowmetry (Neuroscience Co., Ltd.). Body temperature was maintained at 36.5–37°C using a heat lamp and animal blanket controller (ATB-1100, Nipponkoden,Tokyo, Japan) during the operation.
Evaluation of Stroke Volume
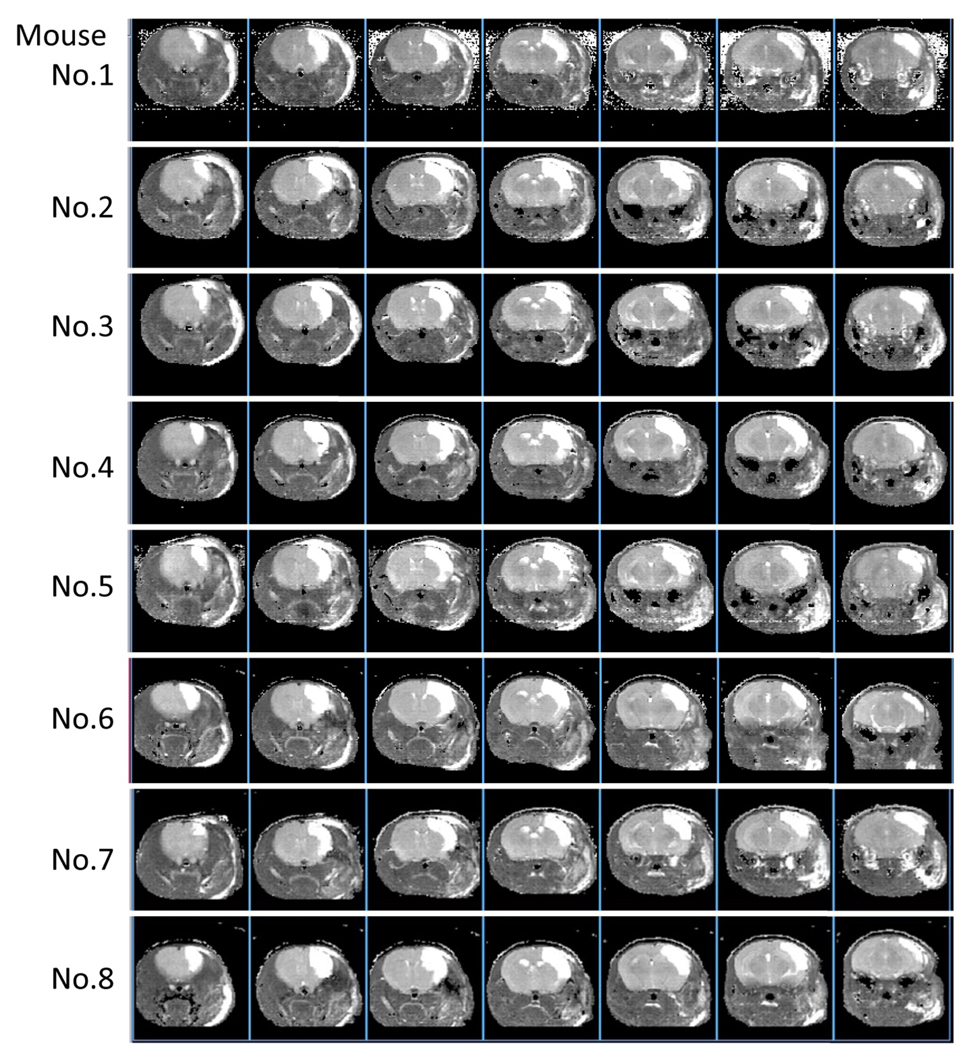
Twenty-four hours after induction of stroke, the area subject to ischemic damage was evaluated by 7T MRI (N=8; Inova 300 Imaging System; Varian, Inc., Palo Alto, CA. USA). The volume coil for RF excitation was actively decoupled and used in combination with a fixed tuned and matched receiver surface coil (Rapid Biomedical GmbH, Germany) that was placed over the skull. For MRI measurements, mice were placed in a stereotactic head holder and anesthetized with 2.0–2.5% halothane in a mixture of O2 and N2O gas delivered through a facemask. The fraction of inspired oxygen (FiO2) was adjusted to 30%. Rectal temperature was feedback-controlled at 37.5 ± 0.5°C using warm air. T2 images were obtained by a multislice spin echo sequence. Parameters included: TR = 3000 ms; TE = 10, 30, 50, 70 and 90 ms; and, NS = 1. Slice thickness was 1 mm, matrix size was 256 × 128, and images were zero-filled to 256 × 256. The field of view was 30 mm × 30 mm. MR image analysis employed the image browser (VNMR 6.1C and Solaris 7, Varian, Inc., Palo Alto, CA, USA). Lesion area in T2 images was calculated as the region with T2 above T2+3SD of each contralateral value. Ischemia-affected volumes from MR images were calculated by summing lesion areas in 7 slices and multiplying by slice thickness (1 mm). Percent ischemia-affected volume was calculated according to (ischemia affected volume) / [(contralateral cortex volume) × 2] × 100%.
Twenty-six (SCID; N=8) or 24 (CB-17; N=8 and C57BL/6; N=8) hours after stroke, viability of brain tissue was evaluated using 1% 2,3,5-triphenyltetrazolium (TTC; Sigma-Aldrich, St. Louis, MO, USA) for 20 min at 37°C. Tissues were then fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS; pH 7.4). Images of sections were captured using a microscopic digital camera system and % stroke volume was estimated with an image analysis program (BZ-2: Keyence, Osaka, Japan) which compared data in the stroke-affected hemisphere to that in the contralateral hemisphere. As with MRI images, % stroke volume was calculated by (stroke volume) / [(contralateral cortex volume) × 2] × 100 %.
Data Analysis
In all experiments, mean ± standard deviation is reported. JMP 7.01 (SAS Institute Inc, Co, North Carolina, USA) was used for statistical analysis.
Results
Stroke Model in SCID Mice
Cerebral infarction was induced by direct electrocoagulation of the distal portion of the MCA in SCID mice. In the current experiment, we used 8 mice and all animals displayed vascular structures with either one major MCA without other visible cortical branches (Figure 1E, N=5) or with just one significantly thinner cortical branch (Figure 1B, N=3) in the M1 distal portion of the MCA. The main trunk of the MCA was electrocauterized (Figure 1C) and disconnected (Figure 1D). Twenty-four hours after induction of permanent ischemia, brain damage was evaluated by T2-weighted MRI (all of the images are shown in Figure 2). Our results demonstrate that the mean % schemia-affected volume was 17.1 ± 1.4% and the variation coefficient was 7.8%.
Figure 2. MRI T2 images 24 hours after induction of stroke.
The current method results in highly reproducible cortical ischemic damage in all mice.
To assess viability of the cerebral cortex following ischemia, brain sections were stained with TTC 26 hours after stroke. Similar to MRI images, highly reproducible cerebral damage was observed between animals and the mean % stroke volume and variation coefficient were 14.9 ± 1.0 % and 6.6 %, respectively.
Before and after ligation of the MCA, the change in CBF in the cerebral cortex in the MCA area was evaluated using laser Doppler. Compared with before stroke, mean CBF decreased to 17.9 ± 5.0 and 19.7 ± 3.9 %, immediately and 5 minutes after induction of stroke, respectively. Comparing % viable ipsilateral cerebral volume and decreased CBF showed no significant correlation between size of the subsequent infarct and CBF immediately (R2=0.05, P=0.61) and 5 minutes (R2=0.08, P=0.48) after induction of ischemia.
To access the survival in the chronic period, 16 mice were induced cerebral infarction. The survival rate at 90 and 180 days after induction of stroke was 100 (16/16) and 94 (15/16) %, respectively.
Stroke Model in CB-17 Mice
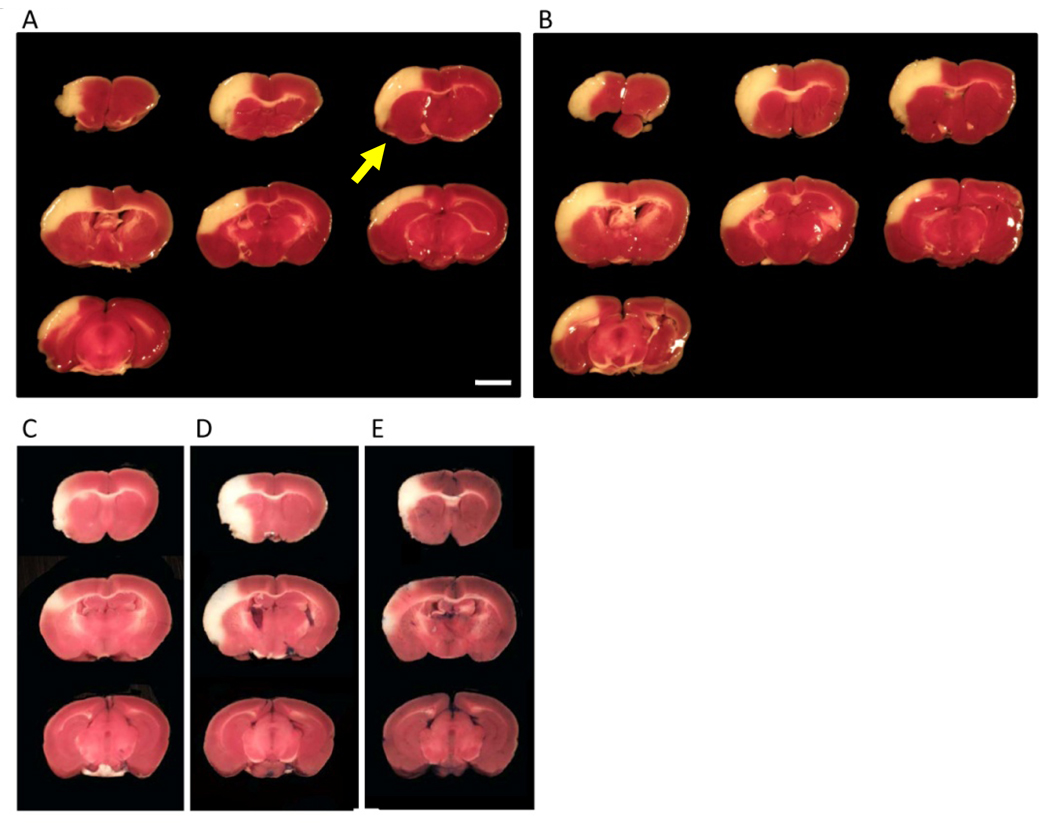
Similarly, cerebral ischemia was induced in CB-17 mice (N=8). Twenty-four hours after induction of cerebral ischemia, stroke area was evaluated by TTC staining (representative images are shown in Figure 3A,B). Mean stroke volume was 14.3 ± 0.9 %, and variation coefficient was 6.3 %. Compared with before stroke, mean CBF decreased to 18.1 ± 3.1 and 20.0 ± 4.2 %, immediately and 5 minutes after induction of stroke, respectively. There was no significant correlation between the size of infarction and CBF immediately (R2=0.001, P=0.94) and 5 minutes (R2=0.02, P=0.73) after induction of stroke. It is notable that a small TTC-negative area was observed around the immediate area of coagulation of the MCA (Figure 3). As a control, brain sections from sham-operated mice were also stained with TTC. In contrast, no defect in TTC staining was observed in the controls at the 24 hr point after induction of stroke (not shown).
Figure 3. TTC images obtained 24 after stroke.
(A,B) Representative images of TTC-stained tissue 24 hours after induction of stroke in CB-17 mice. Similar to the MRI images of SCID mice (Figure 2), reproducible cortical ischemia was observed by TTC staining, comparing the results in different animals. (C–E) Representative images of TTC staining obtained from different C57BL/6 mice are shown. In contrast to the reproducibility of SCID and CB-17 mice, lesion size and even location varied substantially in C57BL/6 mice comparing different animals. Scale bar: 2 mm (A). The arrow indicates the portion around the area subject to electric coagulation (A).
Survival at 180 days after induction of stroke was 100 (16/16) % (N=16).
Stroke Model in C57BL/6 Mice
As a control, we induced cerebral ischemia in C57BL/6 mice (N=8) by ligation of the M1 distal portion of the main trunk of the MCA. Only 4 mice showed decreased CBF to less than 25% compared with the baseline before ligation. Twenty-four hours after induction of permanent ischemia, stroke area in these 4 mice was evaluated by TTC staining (representative images are shown in Figure 3C–D). Mean stroke volume was 5.0 ± 3.2 %, and the variation coefficient was 63.4%.
Discussion
In this article, we have demonstrated that ligation of the distal portion of the MCA in SCID and CB-17 mice, using a modification of the Tamura method, induces highly reproducible and selective cortical infarction.
The advantages of this method include: a) the model produces very reproducible areas of cortical infarction, allowing for effective screening of various experimental treatments using smaller numbers of mice; b) because of its simplicity, the method is broadly accessible to a wide range of investigators; c) the long-term survival rate (up to 180 days) is high, allowing assessment of therapeutic and/or side effects of experimental treatments during the chronic period; d) as a model of focal permanent ischemia, the model mimics, in part, human cerebral infarction; e) CB-17 and SCID mice are easy to procure and require no special diet or pre-treatment regimen; f) use of SCID mice allows evaluation of cell-based therapies with donor cells from different species/strains; and, g) reproducible stroke area is observed during the chronic period(Taguchi et al. 2004; Taguchi et al. 2007) and the latter corresponds to the TTC-negative area observed 24 hours after induction of stroke.
Of course, there are also short-comings of our model: a) there are few transgenic/knockout mice in CB-17 strain, so animals must be bred into that background; b) craniotomy results in stress to the overall animal and trauma to local tissues; and, c) our model results in minimal motor dysfunction accompanying focal ischemia. We have demonstrated that the open field test a reliable and simple test of cortical function after stroke in this model(Taguchi et al. 2004; Taguchi et al. 2007). However, further investigation will be needed for a complete evaluation of cortical function.
According to our previous experiments, most SCID and CB-17 mice displayed the same vascular structures/organization. Though the great majority of mice showed highly reproducible stroke area, 1.1 % (4/362) of SCID and 4.9% (6/122) of CB-17 mice displayed an expanded area extending across the corpus callosum to the striatum. Expansion of the stroke-affected was rarely observed in mice with a thin cortical branch, but was frequently observed in mice with a major cortical branch. These observations suggest that evaluation of MCA branching in a range of mouse strains might provide a means of increasing the reproducibility of other stroke models. To reduce variability using our method of focal cerebral ischemia, we recommend excluding from the experimental protocol mice with major variations in MCA structure, such as changes in the width of the cortical branch artery (branched before olfactory tract) especially when this represents more than one-third that of the main trunk.
In our experiments, all mice showed significant reduction (less than 25%, compared with before ligation) of CBF by ligation of the MCA, and no significant correlation was observed between decreased CBF after the procedure and subsequent stroke volume. Furthermore, no significant correlation was observed between decreased CBF and cortical function in our previous work(Taguchi et al. 2004). These data indicate that determination of CBF in this model may not be especially important for initial screening of various treatments after stroke. If the investigator excludes measurement of CBF, all procedures can be finished within 15 minutes when the method is performed by experienced hands. The latter should facilitate screening multiple drugs or other types of interventions.
In conclusion, our focal permanent cerebral ischemia model using SCID and CB-17 mice provides a reproducible method for induction of cerebral infarction, results in high long-term survival, and employs a relatively simple procedure. Though we have not confirmed the level of inter-lab variation, especially outside of Japan, where SCID and CB-17 mice might display greater variability in cerebral vascular structure, our findings provide, we believe, a new strategy for evaluating potential future therapeutic methods for the benefit of patients with stroke.
Acknowledgments
This work was supported by a Research Grant for Cardiovascular Diseases (21A-7) from the Ministry of Health, Labour and Welfare.
Footnotes
Disclosure / Conflict of interest
The authors indicate no potential conflicts of interest.
References
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Hirakawa M, Tamura A, Nagashima H, Nakayama H, Sano K. Disturbance of retention of memory after focal cerebral ischemia in rats. Stroke. 1994;25:2471–2475. doi: 10.1161/01.str.25.12.2471. [DOI] [PubMed] [Google Scholar]
- Kuge Y, Minematsu K, Yamaguchi T, Miyake Y. Nylon monofilament for intraluminal middle cerebral artery occlusion in rats. Stroke. 1995;26:1655–1657. doi: 10.1161/01.str.26.9.1655. discussion 1658. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhen G, Meloni B, Campbell K, Winn H. Rodent stroke model guidlines for preclinical stroke trials (1st edtiton) J Exp Stroke Transl Med. 2009;2:2–27. doi: 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, Yoshikawa H, Stern DM, Matsuyama T, Taguchi A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–2195. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wen Z, Myojin K, Yoshihara T, Nakagomi T, Nakayama D, Tanaka H, Soma T, Stern DM, Naritomi H, Matsuyama T. Granulocyte colony-stimulating factor has a negative effect on stroke outcome in a murine model. Eur J Neurosci. 2007;26:126–133. doi: 10.1111/j.1460-9568.2007.05640.x. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]