Abstract
Although the importance of fluid flow for proper vascular development and function in vivo is well recognized, microvascular formation in response to flow has not been well evaluated in a three-dimensional (3D) environment in vitro. In this study, we developed a novel 3D in vitro perfusion system that allows direct investigation of the effects of shear stress on the development of microvasculature in vitro. This system utilizes a 3D collagen gel for suspension of vascular cells and mesenchymal stem cells, through which flow is directly perfused. We characterized the flow conditions and demonstrate the impact of flow on the development of microvasculature using a coculture of endothelial cells and mesenchymal stem cells. With the unique ability to apply bulk flow through the collagen gels, and to estimate shear stress within the constructs, this perfusion system provides a flexible platform for developing a controllable biomimetic environment that can be adapted for a variety of investigations of microvascularization.
Introduction
For decades, researchers have studied endothelial capillary morphogenesis (the so-called in vitro angiogenesis) on two-dimensional (2D) coated plates or on the surfaces of or inside three-dimensional (3D) gels.1–4 Folkman and Haudenschild demonstrated the first in vitro angiogenesis by capillary endothelial cells cultured in the tumor-conditioned medium.4 Other studies have demonstrated the invasion of endothelial cells cultured on top of a gelatinous matrix into the collagen gel,5 and lumen formation by endothelial cells in fibrin and collagen gels.6–8 Recently, a more complex multi-cellular approach revealed that human umbilical vein endothelial cells organize into cords within 24 h when cocultured with 10T1/2 cells (a mural precursor cell line) in collagen gels.9 In addition, the important role of mural cells, such as mesenchymal stem cells, has been shown for microvessel maturation and stabilization.10 Although microvessels grown in collagen gels can be implanted in vivo and can form stable vasculature in animals, such microvessels typically regress if maintained in vitro, perhaps due to a lack of flow through the newly formed vessels. Despite the critical role of blood flow and shear stress in the maintenance of the vasculature in vivo, no system has yet been described that allows the direct perfusion of gel-based vascular cells in vitro.
Although the importance of fluid flow and associated shear stress has long been postulated in vasculogenesis, only recently has it been proven that fluid shear stress is specifically required for remodeling of immature vessels during early embryonic development.11 Although vasculogenesis is a characteristic of early stages of embryonic development, vasculogenesis also occurs at later stages of development as well as in adulthood. Various in vivo studies also suggest that changes in shear stress play a role in stimulating arteriogenesis,12,13 and in maintaining blood vessels.14 Moreover, it is known that laminar flow induces quiescence of monolayer endothelium both in vivo and in vitro. Flow and associated shear stress modulate endothelial cell structure and function by inducing changes in proliferation, differentiation, intracellular kinases, and gene expression.15–17
Previously, it has been shown that interstitial flow can induce blood and lymphatic capillary morphogenesis in vitro.18,19 However, in these studies, a very low flow rate (average velocity 4.5 μm/s) was used, which is more than two orders of magnitude lower than the blood velocity found in arterioles.20 To date, the impact of flow on in vitro microvasculature stability and quiescence remains largely unknown,21 and the formation of branched, continuous, stable, and perfused endothelial cell networks in vitro still remains a challenge.22 Although several groups have studied the impact of mural cells on stabilization of microvasculature,22,23 no one has yet looked at the interaction of flow signals with microvascular formation and stabilization, in the presence or absence of mural cells. Moreover, although intravital microscopy techniques have allowed the observation of vascular formation in vivo, animal systems are difficult to control and study in detail, particularly with respect to paracrine signals between endothelial and mural cells.
In this study, we introduce a novel in vitro perfusion system that allows direct investigation of the effects of shear stress on the development of microvasculature in vitro. Assembled within a sterile perfusion system designed to deliver various flow rates, gel constructs that were seeded with endothelial and mesenchymal stem cells were subjected to physiological flow. The effects of coculture and flow on microvasculature formation and mesenchymal stem cell differentiation and interaction with endothelial cells in 3D in vitro cultures were investigated. This novel flow system provides a unique opportunity to better understand the role of fluid flow on microvascular development and vessel remodeling in vitro, with or without the presence of mesenchymal stem cells or other mural cells. We demonstrate that flow facilitates the formation of microvessels and impacts the growth factors that are elaborated by the cells. This system will allow more detailed characterization of the impact of bulk fluid flow on microvessel formation and stabilization in vitro, which is a process that is always accompanied by blood flow in vivo.
Materials and Methods
Cell culture
Rat aortic endothelial cells (RAECs) were purchased from VEC Technologies. Cells were cultured in 0.2%-gelatin-coated (Sigma) polystyrene tissue culture flasks in MCDB-131 complete medium (VEC Technologies) supplemented with 10% fetal bovine serum, antibiotics, and growth factors. The fresh culture medium was exchanged every 3–4 days. RAECs were passaged when they reached 80% confluency using 0.25% trypsin–ethylenediaminetetraacetic acid (Gibco BRL). The identity of the cells was confirmed by characteristic cobblestone morphology, and acetylated low-density lipoprotein uptake (Dil-Ac-LDL; Biomedical Technologies). RAECs of passage 6–10 were used in this study.
Rat mesenchymal stem cells (RMSCs) were purchased from Cell Applications. RMSCs were cultured in RMSC growth medium (Cell Applications) to maintain the multipotent phenotype of the cells. Phenotype of the RMSCs was examined and confirmed by fluorescence-activated cell sorting analysis. CD54, CD29 (Abcam), and CD90 (Santa Cruz) were used as positive markers for mesenchymal stem cell identity, and leukocyte markers CD45 (Abcam) and CD14 (Santa Cruz) as negative markers for mesenchymal cells.
Preparation of collagen gel and application of flow
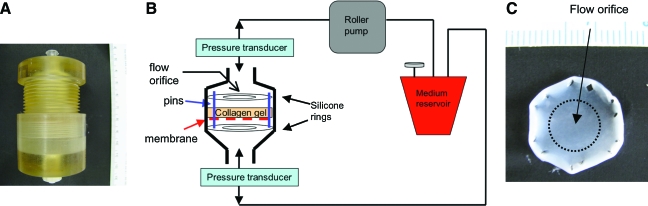
Collagen gel solutions were prepared by diluting rat-tail type I collagen (BD Sciences) in 0.02 N acetic acid and mixing it with 5× Dulbecco's modified Eagle's medium (Gibco), and 10× reconstitution buffer in a 6.5:2:1 ratio. Prepared collagen gel solutions were mixed with cell suspensions in a 15:1 ratio, yielding final concentrations of 3 × 106 cells/mL and 2.5 mg/mL of collagen. In the coculture experiments, a 1:1 ratio of RAEC:RMSC was utilized, while maintaining the total cell density in the gel at 3 × 106 cells/mL. The cell–collagen solution was pipetted onto a porous polyethylene terephthalate (PET) membrane (Becton Dickinson) in a custom-designed flow bioreactor (polysulfone, McMaster, Fig. 1A, B), to support the collagen gel solution in the system during initial polymerization. To reduce the resistance to flow created by the PET membrane, 20 extra holes were manually punched on the membrane using a 30G needle before the addition of collagen gel. Stainless steel pins were placed through the membrane and into the silicone ring below, and around the edge of the well to prevent collagen gel compaction during culture (Fig. 1B, C). Pins functioned to constrain the collagen gel from retracting from the periphery of the culture well. After 2 h of incubation at 37°C and 5% CO2 to allow gel polymerization, another silicone ring was placed on top of the gel to secure the gel in place and to force the fluid only through the gel by preventing fluid channeling around it. This created an effective flow orifice through the central region of the gel (Fig. 1C). The culture medium was perfused through the gel using a digital peristaltic pump (Masterflex, Cole-Parmer) at a flow rate of 5 mL/min for 3–7 days. A fluid reservoir provided the culture medium for circulation and a port for gas exchange with 5% CO2/95% air (Fig. 1B). Static controls were identically prepared in the tissue culture wells and cultured in 10 mL of the medium. The culture medium was exchanged every 3 days.
FIG. 1.
Flow bioreactor. (A) Polysulfone housing. (B) Schematic illustration of a flow system that directs flow exclusively through collagen gel tissue. Gas exchange with 5% CO2/95% air occurs via a syringe filter port attached to the medium reservoir. (C) Example of the resulting collagen gel after 7 days of culture, with the circular area marking the center region of the gel that is exposed to fluid flow. The holes on the edges resulted from the pins and constrained the gel to prevent inward radial contraction. Color images available online at www.liebertonline.com/ten.
Pressure measurement and permeability of collagen gels
Pressure transducers (Edwards Lifesciences) were connected to both the inlet and the outlet of the flow bioreactor to monitor the pressure drop in the bioreactor system at various flow rates (Fig. 1B). The pressure drop across the polysulfone chamber with and without a collagen gel was measured to determine the resistance of the perfusion flow bioreactor and the collagen gel constructs. The pressure signals were amplified and were acquired using the PowerLab data acquisition system (ADinstruments) with Chart5 software in a personal computer.
The hydraulic permeabilities of cell-seeded collagen gels were determined from measured flow rates and pressure drops across the gels. Darcy's law (Eq. 1) was used to calculate the permeability KD.
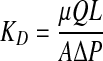 |
(1) |
where μ is the viscosity of the perfusing fluid (culture medium measured as 0.8 Cp using a viscometer at 37°C), Q is volumetric flow rate (cm3/s), L is the thickness of the sample (cm), A is the cross-sectional area (cm2), and ΔP is the pressure gradient. The resistance (R) was calculated using Equation 2, which is based on an Ohm's law analog for fluid flow, where U is an average fluid velocity.
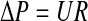 |
(2) |
The average shear stress applied over the cell surface embedded in collagen gel tissue was calculated by the modified Brinkman equation (Eq. 3), as described elsewhere by Wang and Tarbell.24
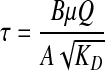 |
(3) |
where τ is the average shear stress on over the cell surface (dyn/cm2), and B is the Brinkman constant for flow around cylinders or spheres (B = 4/π for cylinders, and 3/π for spheres). The continuous collagen fiber matrix between the cells was assumed to be a homogeneous porous medium and the cells were assumed to be rigid circular spheres.
Histology and microscopy
For histological evaluation, samples were fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, and sectioned at 5 μm. To examine the 3D morphology of vascular structures and interactions between the two cell types in the collagen gels, tissues were fixed in 4% paraformaldehyde and stained with Rhodamine-phalloidin for F-actin and 4′-6-diamidino-2-phenylindole (DAPI) for nuclei (both from Molecular Probes). The whole-mount tissues were observed using a confocal laser scanning microscope (Leica). Capillary lumen formation was examined from paraffin sections of the tissue samples stained with hematoxylin and eosin (H&E).
Cell proliferation
To assess cell proliferation, paraffin-embedded sections were deparaffinized, and rehydrated according to standard protocols. Tissue sections were stained using a proliferating cell nuclear antigen (PCNA) staining kit (Zymed Laboratories).25 PCNA-positive cells were detected using a conjugated biotinylated PCNA monoclonal antibody and streptavidin peroxidase along with 3,3′-diaminobenzidine (DAB) as the chromagen to stain PCNA-positive nuclei a dark brown. The percentage of proliferating cells relative to the total number of cells was obtained from counting positive cells from images taken from four slides per sample (n = 3).
Enzyme-linked immunosorbent assay (ELISA) for transforming growth factor-beta1, vascular endothelial growth factor, and platelet-derived growth factor-BB
ELISA kits (R&D Systems) were used to quantify the levels of transforming growth factor-beta1 (TGF-β1), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF)-BB proteins produced by RAEC-only, RMSC-only, and RAEC-RMSC cocultures after 3 days of culture. The circulating culture medium was collected and assayed according to the manufacturer's instructions. The absorbance of each sample was read at 450 nm within 10 min. The baseline levels of growth factors in serum were subtracted from the measurements to obtain factors produced only by the cells in culture.
Western blot analysis
Construct homogenates were diluted (1:4) in Laemmili buffer (Bio-Rad) containing 5% mercaptoethanol and 2% sodium dodecyl sulfate and were boiled for 10 min to denature proteins. The proteins were separated on poly(vinylidene fluoride) (PDVF) gels by 1× Tris–glycine–sodium dodecyl sulfate running buffer (Boston Bioproducts) at a constant voltage of 100 V for 2 h at room temperature followed by electrophoretic transfer. For immunoblotting, the primary antibodies used were polyclonal rabbit anti-NOS3 (1:100; Santa Cruz), polyclonal rabbit anti-prostaglandin I synthase (1:100; Abcam), polyclonal rabbit anti-CD31 (1:100; Santa Cruz), monoclonal mouse anti-thrombomodulin (1:500; Abcam), α-smooth muscle actin (1:200; Dako), calponin (1:200; Dako), and monoclonal mouse anti-β actin (1:2000; Sigma). After multiple washes, blots were incubated with secondary goat anti-rabbit IgG HRP or goat anti-mouse IgG HRP antibodies (1:2000; Santa Cruz), and were developed using chemiluminescence (Supersignal, Pierce). Fresh rat aortic tissue homogenates and low passage of cultured RAEC lysates were used as positive controls.
Statistical analysis
Results are presented as mean ± standard deviation. Statistical differences were determined by a two-way analysis of variance with a post hoc Tukey's least significant difference among different cell types and flow conditions. Statistical significance was accepted for p < 0.05.
Results
Permeability and pressure measurement
The hydraulic permeability of collagen gels seeded with 3 × 106 RAEC/mL was assessed by measuring perfusion pressures and flow rates, and then determined from Equation 1. The gels were cultured for 3 days before testing. The permeability of RAEC-seeded collagen gels was 0.74 ± 0.32 × 10−8 cm2 and 1.18 ± 0.24 × 10−8 cm2 at flow rates of 1.3 and 5 mL/min, respectively, as shown in Table 1. The reported permeability values of either acellular or cellular collagen gels in the literature range from 10−7 to 10−11 cm2 depending on the experimental conditions.24,26,27 The resistance created by the porous membrane with extra holes, along with the other components of the polysulfone housing and tubing (see Fig. 1B), was determined by the pressure drop in the system at flow rates of 1.3, 5, and 10 mL/min without the gel (Table 1). With extra holes punched in the porous membrane, the pressure difference across the housing and membrane was very low, measuring 0.01 mmHg at 5 mL/min. This corresponds to a negligible estimated resistance of 0.001 dyn-s/cm3. As estimated from Equation 3, flow rates of 1.3–10 mL/min correspond to shear stresses of approximately 1.9–11.96 dyn/cm2 within the gel. The flow rate of 5 mL/min was used for our experiments, which resulted in shear stress of approximately 5.5 dyn/cm2. This is similar to what is estimated to be present in the microcirculation.28 This estimate of shear stress is derived assuming bulk flow around cylinders or spheres, which may not be a completely accurate reflection of the characteristics of flow in an amorphous collagen gel.
Table 1.
Characterization of the Perfusion Flow System at Flow Rates of 1.3, 5, and 10 mL/min
| Flow rate (mL/min) | Pressure (mmHg) | Permeability (× 10−8 cm2) | Resistance (dyn-s/cm3) | Shear stress (dyn/cm2) | |
|---|---|---|---|---|---|
| No collagen, membrane w/holes | 1.3 | 0.02 ± 0.19 | 0.76 ± 1.66 | 0.001 ± 0.01 | |
| 5 | 0.01 ± 0.09 | 1.49 ± 1.31 | 0.001 ± 0.0001 | ||
| 10 | 0.1 ± 0.2 | 1.61 ± 0.82 | 0.001 ± 0.001 | ||
| Collagen gels seeded w/3 × 106 RAECs/mL | 1.3 | 0.92 ± 0.37 | 0.74 ± 0.32 | 0.06 ± 0.02 | 1.9 ± 0.4 |
| 5 | 1.95 ± 0.37 | 1.18 ± 0.24 | 0.03 ± 0.007 | 5.51 ± 0.54 | |
| 10 | 4.57 ± 0.73 | 0.99 ± 0.17 | 0.04 ± 0.007 | 11.96 ± 0.99 |
Collagen gels were constructed with using 3 × 106 RAECs/mL and were tested after 3 days of culture. Pressure difference with or without the collagen gel (just the PET membrane) were measured, and the permeability and resistance of the flow system were calculated. Shear stress applied to the cells within the collagen gel construct was estimated using Equation 3.
RAEC, rat aortic endothelial cell.
Cell morphology and network formation
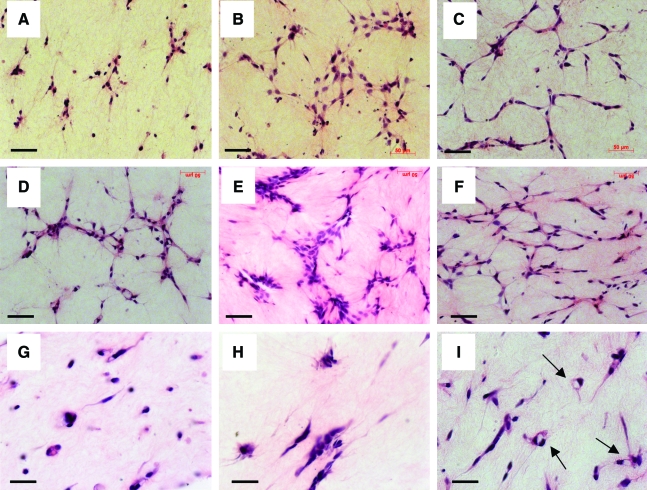
Engineered collagen gel tissues were created using RAEC-only, RMSC-only, or coculture of RAEC-RMSC under both static and flow conditions. Figure 2 shows H&E staining of longitudinal sections of collagen gels under static culture conditions. Cells were more spread out in RAEC-RMSC cocultures than in RAEC-only or RMSC-only gels at day 3 (Fig. 2C vs. Fig. 2A or B). The cells were more spread out in all the conditions by day 7 (Fig. 2D–F) than by day 3 (Fig. 2A–C). The cross section of the gels, however, revealed vacuole/lumen formation only in RAEC-RMSC cocultured gels, but not in RAEC-only or RMSC-only cultures, after 7 days (Fig. 2I vs. Fig. 2G or H).
FIG. 2.
Structure of engineered tissues under static conditions. Representative longitudinal hematoxylin and eosin micrographs: (A) RAEC-only, (B) RMSC-only, and (C) RAEC-RMSC coculture after 3 days. More spreading and networking of cells were observed after 7 days of culture in (D) RAEC-only, (E) RMSC-only, and (F) RAEC-RMSC coculture. Cross-sectional hematoxylin and eosin micrographs of (G) RAEC-only, (H) RMSC-only, and (I) RAEC-RMSC coculture after 7 days demonstrating vascular lumens only in the coculture indicated by the arrows in panel (I). Scale bar = 50 μm. RAEC, rat aortic endothelial cell; RMSC, rat mesenchymal stem cell. Color images available online at www.liebertonline.com/ten.
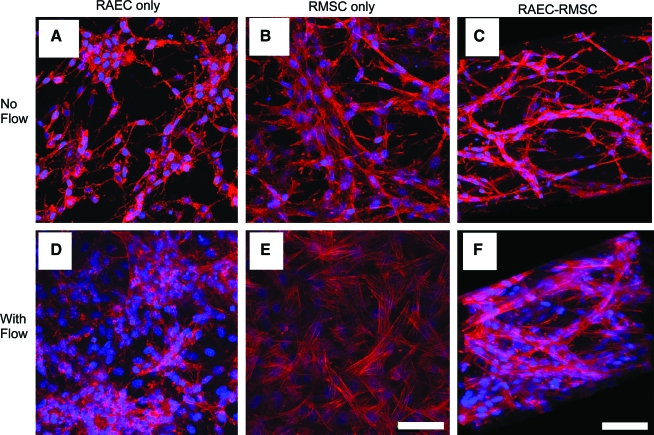
Representative fluorescence microscopic images of F-actin (red) and nuclei (blue) in RAEC-only, RMSC-only, and RAEC-RMSC 3-day cocultures showed significant morphological differences in the cocultured gels compared with gels with a single cell type under static conditions (Fig. 3). When RAECs were cultured statically without RMSC, cells were randomly oriented and were less spread out (Fig. 3A), which was also evidenced by H&E images (Fig. 2A). In RMSC-only collagen gels (Fig. 3B), cells were more spread out than in RAEC-only, yet not forming any noticeable networks. In contrast, when RAECs and RMSCs were cocultured, distinctive cell networks were observed, as they formed capillary-like structure as shown by a 3D reconstruction of the confocal image (Fig. 3C).
FIG. 3.
Structure of engineered tissues compared under static and flow conditions. Top panels show confocal microscopy images of rhodamine-phalloidin (red) and cell nuclei (blue) in (A) RAEC-only, (B) RMSC-only, and (C) RAEC-RMSC coculture gels for 3 days in static culture. Bottom panels show collagen gels cultured under flow at 5 mL/min for 3 days. (D) RAEC-only, (E) RMSC-only, and (F) RAEC-RMSC coculture. (C, F) Three-dimensional reconstruction of confocal microscopy images, showing spatial distribution of the microvessels formed in the absence and presence of flow. Scale bar = 50 μm. The direction of the flow is from top to bottom in the plane of the image. Color images available online at www.liebertonline.com/ten.
When flow was applied at 5 mL/min, cell networks became more evident compared to static cultures. RAECs became more widely spread than statically cultured cells (Fig. 3D vs. 3A). In RMSC-only gels, an increase in cell spreading and stress-fiber formation in response to flow was observed (Fig. 3E vs. Fig. 3B). Moreover, the impact of flow was striking in RAEC-RMSC cocultures, resulting in branched and denser vascular structures, which appeared to have wider diameters and more RMSC recruitment than vessels formed in the absence of flow (Fig. 3F vs. Fig. 3C). It is clear from these images that RAEC and RMSC become more conducive to microvessel formation in this 3D collagen gel system when they are cocultured under both static and flow conditions. It also appears that the application of flow affected the morphology of RAEC and RMSC in 3D monocultures. The effect of flow, however, was synergized in the cocultures of RAEC and RMSC as the cells formed 3D networks and what appeared to be a microvasculature.
Cell proliferation
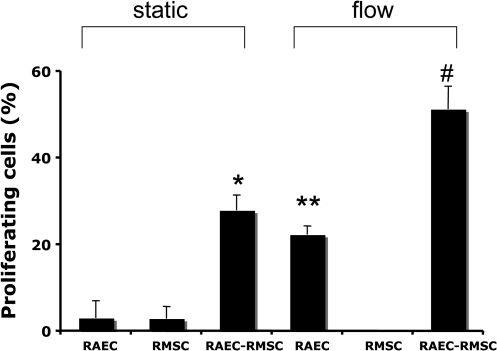
To assess the effects of coculture and flow on the proliferation of cells, collagen gels were cultured for 3 days under both static and flow conditions (5 mL/min). When RAECs or RMSCs were statically cultured alone for 3 days, very little proliferation occurred as assessed by PCNA staining (3.08% ± 3.98% in RAEC-only gels; 2.92% ± 2.81% in RMSC-only gels; Fig. 4). In RAEC-RMSC cocultures, however, a nearly 10-fold increase in the number of proliferating cells was observed compared to either RAEC- or RMSC-only gels at day 3 (27.94% ± 3.54%, *p < 0.001 compared with RAEC-only and RMSC-only cultures). When flow was applied for 3 days, there was a substantial increase of proliferating RAEC in RAEC-only gels, compared to statically cultured RAEC-only gels (22.36% ± 1.99% vs. 3.08% ± 3.98%, **p < 0.001 compared to RAEC-only static at day 3). Almost no proliferation was observed in RMSC-only cultures with flow, similar to the static culture. However, in RAEC-RMSC cocultured gels, the percentage of proliferating cells with flow was again significantly higher, nearly doubling from 27.94% ± 3.54% to 51.31% ± 5.30% compared to static coculture (#p < 0.001). Results after 7 days of culture showed nonsignificant increase in cell proliferation, though the same trends were exhibited in all the conditions as day 3 (data not shown). These results indicate that RAECs are intrinsically more proliferative and responsive to fluid flow than RMSCs in 3D collagen gels. This also provides evidence that paracrine signaling between RAEC and RMSC, as well as shear stress, may be important for stimulating cell proliferation in coculture.
FIG. 4.
The percentage of proliferating cells under static versus flow conditions after 3 days of culture (n = 3, four slides per samples). *p < 0.01 compared with RAEC-only and RMSC-only static, **p < 0.001 compared with RAEC-only static, and #p < 0.001 compared with RAEC-RMSC co-culture static.
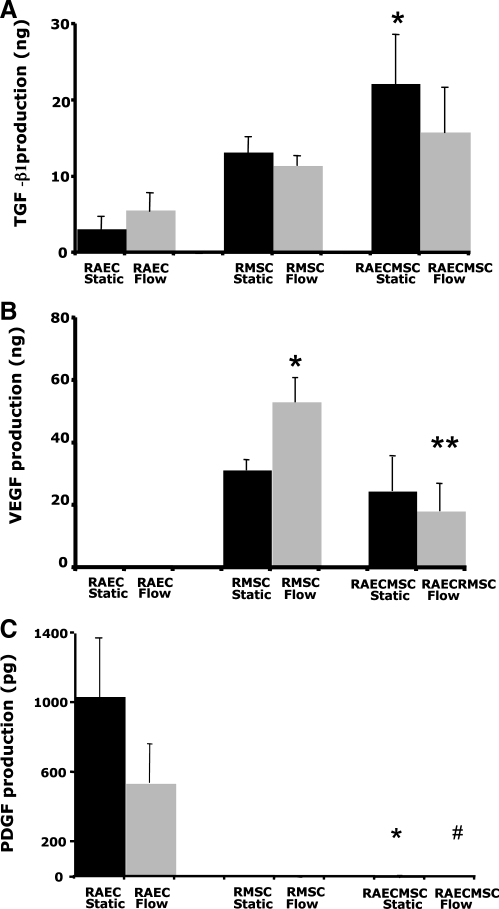
Secretion of angiogenic growth factors
To determine the impact of both flow and coculture on the paracrine signaling molecules that are known to be important for angiogenesis, the total production of growth factor by the cells in culture supernatants was examined. These measurements were corrected for baseline levels of growth factors in serum, and hence represent only the factors produced by the cells in culture. ELISA showed little production of TGF-β1 from RAEC-only gels in static culture (Fig. 5A). There was a nonsignificant increase in TGF-β1 production by RAEC with flow compared to static conditions. RMSC produced more TGF-β1 than RAEC, but no difference was observed between static versus flow conditions. On the other hand, when RAEC and RMSC were cocultured, there was a significant increase of TGF-β1 production compared to collagen gels with RAEC-only under static conditions (22.22 ± 6.48 ng vs. 3.21 ± 1.43 ng, respectively, *p < 0.05). No difference in TFG-β1 production was observed between static and flow in the coculture. This result shows that the coculture of RAEC-RMSC induces production of TGF-β1 by the cells, whereas the application of flow at the level utilized does not influence TGF-β1 production.
FIG. 5.
Total amount of growth factors produced by the cells in the collagen gel culture medium after 3 days of static or flow culture. The production of growth factors was normalized by the volume of the culture medium used for each condition. (A) TGF-beta 1, *p < 0.05 vs. RAEC-only static, (B) VEGF, *p < 0.05 vs. RMSC-only static, **p < 0.05 vs. RMSC-only flow, and (C) PDGF-BB, *p < 0.05 vs. RAEC-only static, #p < 0.05 vs. RAEC-RMSC co-culture static. Results are mean ± standard error of the mean, n = 4 per group. TGF-beta1, transforming growth factor-beta1; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor.
Unlike RAEC-only gels, which produced almost no measurable VEGF under either static or flow conditions, RMSC-only gels produced significant amounts of VEGF under both static and flow conditions (Fig. 5B). VEGF production was significantly increased with the application of flow to RMSC-only collagen gels compared to the statically cultured RMSC-only gels (53.81 ± 8.56 ng vs. 31.39 ± 3.27 ng, *p < 0.05). VEGF production, however, was decreased both with and without the flow when RAEC and RMSC were cocultured. The amount significantly decreased from 53.81 ± 8.56 to 18.82 ± 9.01 ng in the coculture compared to RMSC-only gels with flow (**p < 0.05), although this may have been due to 50% smaller number of RMSC in the cocultured gels (1:1 ratio of RAEC to RMSC). Our results suggest that RMSCs secrete angiogenic factors that can be used to recruit endothelial cells, and that flow regulates VEGF production by RMSC in collagen gels. However, there is little evidence that coculture per se influences total VEGF production in this system.
PDGF-BB, on the other hand, was produced mostly by RAEC and not by RMSC under both static and flow conditions (Fig. 5C). However, PDGF-BB was significantly reduced in RAEC-RMSC coculture in both static (*p < 0.05) and flow conditions (#p < 0.05), indicating that the cells may have consumed this growth factor, or that production was decreased in the coculture setting. PDGF-BB production was also decreased by fluid flow, despite that total RAEC number in flow cultures is substantially higher due to proliferation compared to static conditions. These results indicate that RAEC-derived PDGF-BB is modulated by fluid flow, and may function to recruit mural cells to form networks in the coculture.
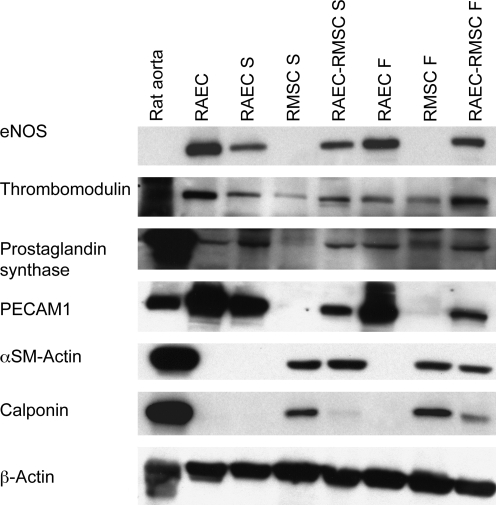
Protein analysis
Control RAEC grown in tissue culture flasks expressed endothelial cell markers such as endothelial nitric oxide synthase (eNOS), thrombomodulin, prostaglandin synthase, and platelet endothelial cell adhesion molecule-1 (PECAM-1), but not α-smooth muscle actin and calponin, since these latter two proteins are smooth muscle cell markers (Fig. 6). Immunoblotting of RAEC-only collagen gels after 3 days also revealed expression of eNOS, thrombomodulin, prostaglandin synthase, and PECAM-1 under both static and flow conditions. There was an upregulation of eNOS with the application of flow, although the expression level of thrombomodulin, prostaglandin synthase, and PECAM-1 remained similar with flow. The upregulation of eNOS with flow in RAEC-only collagen gels is consistent with the animal studies showing upregulation of eNOS expression by shear stress.29 No change in thrombomodulin and prostaglandin synthase is at variance with some previous reports, which suggest an upregulation of thromobomodulin30 and prostacyclin31,32 by shear stress. However, the magnitude of shear in the gels may not have been sufficient to generate changes in expression of these latter molecules.
FIG. 6.
Western blot analysis of collagen gels after 3 days of static or flow culture. Starting from the left, samples are rat aorta homogenates, cultured rat endothelial cells (RAEC), RAEC-only static (RAEC S), RMSC-only static (RMSC S), RAEC-RMSC static (RAEC-RMSC S), RAEC-only flow (RAEC F), RMSC-only flow (RMSC F), and RAEC-RMSC flow (RAEC-RMSC F). Endothelial cell markers endothelial nitric oxide synthase (eNOS), thrombomodulin, prostaglandin synthase, and PECAM-1, and smooth muscle cell markers α-smooth muscle actin and calponin were tested. PECAM -1, platelet endothelial cell adhesion molecule-1.
In RMSC-only gels, no expression of eNOS, prostaglandin synthase, or PECAM-1 was observed under static conditions, whereas there may be some upregulation of thrombomodulin and prostaglandin synthase expression with RMSC-only gels exposed to flow. This is consistent with previous studies that reported antithrombogenic properties of mesenchymal stem cells when exposed to shear.33 In addition, calponin expression by RMSC-only cultures was upregulated by flow, indicating that flow may have also induced differentiation of RMSC into a smooth-muscle-like phenotype. Hence, it may be that RMSC exposed to shear in collagen gels express markers associated with endothelium as well as with vascular smooth muscle.34
In RAEC-RMSC cocultured gels, we observed an upregulation of eNOS, thrombomodulin, and calponin expression in response to flow compared to the statically cocultured gels. PECAM-1 and α-smooth muscle actin expression appeared to be relatively unchanged by flow conditions.
Discussion
We have developed a novel perfusion system that allows the culture of endothelial cells and mesenchymal stem cells in a porous gel and in the presence of a bulk flow field. In contrast to traditional tissue culture systems where cells are grown on 2D solid surfaces, this custom-designed 3D in vitro culture system allows us to evaluate vascular endothelial cell function that cannot be assessed on 2D surfaces, such as tube formation and persistence, while allowing precise control of experimental variables such as flow and pressure. To the best of our knowledge, this is the first demonstration of perfusion of a 3D gel-based system to study the impact of flow on vascular formation in vitro.
Although our current study focuses on using cardiovascular cells, our perfusion system may provide a new tool for studying flow-induced effects on other cell types such as bone-forming cells and cancer cells, since the fluid flow is a potent regulator of bone cell behavior35 and tumor cells.36 Thus, until complete mineralization of the tissue occurs, which may take weeks, flow can continue in our system and the effects of flow on bone-forming cell differentiation and remodeling can be investigated. The Brinkman equation has been used by a number of groups to describe flow around and through several types of tissues,24,37 including fluid flow in the porous pericellular matrix surrounding the osteocytic process in a trabecula.38 This suggests that this equation should also be applicable in various coculture scenarios that are possible with this system.
One of the unique parts to this system is the ability to apply flow through the gel while using boundary constraints to prevent gel compaction during culture. Collagen gels can compact down to 30% of their original size without constraints, which would impact the fluid flow around and through the compacted gel. Collagen compaction also causes disorganization of the tissue structure and limits our ability to monitor structural changes in the gel that are caused exclusively by cells.39,40 By constraining the gel to a constant horizontal dimension, we maintain a constant cross-sectional area for application of flow, and provide a high degree of reproducibility in the system.
The flow system is designed such that flow rate can be easily changed, ranging from a fraction of a milliliter per minute to 10 mL per minute or higher. These flow rates produce estimated shear stresses over the surface of the cells embedded in collagen gel ranging from 1.9 to 11.96 dyn/cm2, which are within physiological ranges and do not damage the gel matrix. The flow rate of 5 mL/min, which was used in these experiments, corresponds to an estimated shear stress of approximately 5 dyn/cm2. This level of shear is similar to previous reports that examined the behavior of microvascular endothelial cell seeded on top of collagen gels and exposed to shear stress on gel surfaces of approximately 3 dyn/cm2.41
Our results demonstrate that cell spreading, stress fiber formation, cell density, and apparent microvascular formation by RAEC and RMSC in collagen gels are affected by both coculture and fluid shear stress. Enhanced cell–cell interactions with an increase in cell spreading, network, and lumen formation were observed in static cocultures of RAEC and RMSC. Fluid flow further enhanced both RAEC and RMSC spreading and network formation in monocultures, and more importantly resulted in formation of thicker and wider microvascular-appearing networks in RAEC-RMSC coculture. This suggests likely synergistic effects of coculture and fluid flow, which induce an increase in cellular responses and may facilitate in vitro microvascularization.
Elaboration of TGF-β1, VEGF, and PDGF-BB by cells is regulated by coculture and by flow in some cases. A significant increase in TGF-β1 production was observed in RAEC-RMSC coculture compared to the monoculture. TGF-β1 plays a role in trans-differentiation of endothelial cells into pericytes, and in driving smooth muscle cell differentiation from mesenchymal stem cells.42,43 The activated form of TGF-β1 is produced by coculture of endothelial cells and pericytes,44 although it is not clear whether cocultures simply activate latent material or whether TGF-β1 is secreted in an active form upon cocultivation. Our findings suggest that when RAEC are cocultured with RMSC, this causes an aggregate increase in the production of TGF-β1, which may induce RMSCs to differentiate into a smooth muscle cell–like phenotype, though significant SMC differentiation was not supported by our immunoblotting results. In contrast, fluid shear stress did not induce higher TGF-β1 production, indicating that shear stress that was applied in our study may be below the threshold for generation of active TGF-β1 as TGF-β1 normally secreted by endothelial cells is latent, unless stimulated.45 This is consistent with a previous report that showed failure to induce active TGF-β1 with shear stresses below 5 dyn/cm2.46
There was a lack of significant production of VEGF by RAEC in 3D collagen gels under both static and flow conditions, whereas a significant increase in VEGF production was induced in RMSC-only gels by flow. VEGF is an angiogenic molecule that has been shown to affect endothelial cell behavior, morphology, and survival, and to drive both angiogenesis and vasculogenesis.47 Thus, it appears that flow and shear stress induce VEGF production by RMSC, and may be a mechanism for inducing angiogenesis in vitro. PDGF-BB, which is a known potent mitogen and migratory factor for mural cells and is involved in recruitment of the cells to the developing vessel wall,48,49 was produced by RAEC-only cultures, but not by RMSC. In addition, a significant decrease in the PDGF-BB production in RAEC-RMSC cocultured gels compared to RAEC-alone suggests that RMSC recruitment may downregulate PDGF-BB production by RAEC. PDGF-BB exerts some effects on the smooth muscle differentiation state of mesenchymal stem cells, at least those derived from adult humans.50 Selective inhibition of this growth factor production by RAECs to evaluate its role in microvessel formation in this system awaits further investigation.
In RAEC-only gels, flow resulted in greater expression of eNOS, whereas upregulation of endothelial cell markers such as thrombomodulin and prostaglandin synthase was observed in RMSC-only gels under flow. Previous studies have demonstrated some antithrombogenic properties of mesenchymal stem cells that are exposed to shear,33 as well as an important role for fluid flow in the differentiation of mesenchymal precursor cells into endothelial cells.51 Therefore, we speculate that flow may impact the differentiation of (possibly a subset of ) mesenchymal stem cells into endothelial cells. Cocultures of RAEC-RMSC also had greater expression of eNOS and thrombomodulin in response to flow.
In summary, we have developed a novel 3D in vitro flow culture system to elucidate the effects of fluid flow on the development of microvascular in vitro. Coculture of endothelial cell and mesenchymal stem cells in a 3D collagen gel, and exposure of the gel to bulk fluid flow, resulted not only in changes in cellular architecture, cell proliferation, and growth factor signaling, but also in cell function and microvessel formation. Our novel system allows us to form and maintain microvessels for extended periods under controlled flow rates, shear stress, and highly defined conditions. This system demonstrates the feasibility of using engineered tissues to investigate the impact of flow on tissue vascularization and stabilization in vitro, and provides a flexible platform for studying paracrine interactions between vascular cells and other cell types such as smooth muscle cells and cardiac cells for applications in cardiovascular biology. In addition, other hydrogels such as fibrin, alginate, and matrigel may be used in this system, thereby increasing the flexibility and utility of this in vitro model.
Acknowledgments
The authors are grateful to Sevanne Halajian for her help in measuring permeability of collagen gels and pressure in the flow system. This work was funded by the National Institutes of Health (R01 HL-076485, R01-EB008366).
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Davis G.E. Bayless K.J. Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268:252. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- 2.Egginton S. Gerritsen M. Lumen formation: in vivo versus in vitro observations. Microcirculation. 2003;10:45. doi: 10.1038/sj.mn.7800174. [DOI] [PubMed] [Google Scholar]
- 3.Vailhe B. Lecomte M. Wiernsperger N. Tranqui L. The formation of tubular structures by endothelial cells is under the control of fibrinolysis and mechanical factors. Angiogenesis. 1998;2:331. doi: 10.1023/a:1009238717101. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 5.Meyer G.T. Matthias L.J. Noack L. Vadas M.A. Gamble J.R. Lumen formation during angiogenesis in vitro involves phagocytic activity, formation and secretion of vacuoles, cell death, and capillary tube remodelling by different populations of endothelial cells. Anat Rec. 1997;249:327. doi: 10.1002/(SICI)1097-0185(199711)249:3<327::AID-AR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Davis G.E. Camarillo C.W. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 7.Davis G.E. Black S.M. Bayless K.J. Capillary morphogenesis during human endothelial cell invasion of three-dimensional collagen matrices. In Vitro Cell Dev Biol Anim. 2000;36:513. doi: 10.1290/1071-2690(2000)036<0513:CMDHEC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Bayless K.J. Salazar R. Davis G.E. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koike N. Fukumura D. Gralla O. Au P. Schechner J.S. Jain R.K. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 10.Au P. Tam J. Fukumura D. Jain R.K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucitti J.L. Jones E.A. Huang C. Chen J. Fraser S.E. Dickinson M.E. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Royen N. Piek J.J. Schaper W. Bode C. Buschmann I. Arteriogenesis: mechanisms and modulation of collateral artery development. J Nucl Cardiol. 2001;8:687. doi: 10.1067/mnc.2001.118924. [DOI] [PubMed] [Google Scholar]
- 13.Schaper W. Therapeutic arteriogenesis has arrived. Circulation. 2001;104:1994. [PubMed] [Google Scholar]
- 14.Meeson A. Palmer M. Calfon M. Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 15.Chien S. Li S. Shyy Y.J. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 16.Li Y.S. Haga J.H. Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Lee E.J. Vunjak-Novakovic G. Wang Y. Niklason L.E. A biocompatible endothelial cell delivery system for in vitro tissue engineering. Cell Transplant. 2009;18:731. doi: 10.3727/096368909X470919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helm C.L. Zisch A. Swartz M.A. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol Bioeng. 2007;96:167. doi: 10.1002/bit.21185. [DOI] [PubMed] [Google Scholar]
- 19.Ng C.P. Helm C.L. Swartz M.A. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res. 2004;68:258. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Fung Y.C. Biomechanics. New York: Springer; 1984. [Google Scholar]
- 21.Tang D.G. Conti C.J. Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: an update. Semin Thromb Hemost. 2004;30:109. doi: 10.1055/s-2004-822975. [DOI] [PubMed] [Google Scholar]
- 22.Jain R.K. Au P. Tam J. Duda D.G. Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 23.Hirschi K.K. D'Amore P.A. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997;79:419. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 24.Wang S. Tarbell J.M. Effect of fluid flow on smooth muscle cells in a 3-dimensional collagen gel model. Arterioscler Thromb Vasc Biol. 2000;20:2220. doi: 10.1161/01.atv.20.10.2220. [DOI] [PubMed] [Google Scholar]
- 25.Waseem N.H. Lane D.P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96(Pt 1):121. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- 26.Ramanujan S. Pluen A. McKee T.D. Brown E.B. Boucher Y. Jain R.K. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J. 2002;83:1650. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng C.P. Swartz M.A. Fibroblast alignment under interstitial fluid flow using a novel 3-D tissue culture model. Am J Physiol Heart Circ Physiol. 2003;284:H1771. doi: 10.1152/ajpheart.01008.2002. [DOI] [PubMed] [Google Scholar]
- 28.Turitto V.T. Blood viscosity, mass transport, and thrombogenesis. Prog Hemost Thromb. 1982;6:139. [PubMed] [Google Scholar]
- 29.Nadaud S. Philippe M. Arnal J.F. Michel J.B. Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- 30.Takada Y. Shinkai F. Kondo S. Yamamoto S. Tsuboi H. Korenaga R. Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun. 1994;205:1345. doi: 10.1006/bbrc.1994.2813. [DOI] [PubMed] [Google Scholar]
- 31.McCormick S.M. Whitson P.A. Wu K.K. McIntire L.V. Shear stress differentially regulates PGHS-1 and PGHS-2 protein levels in human endothelial cells. Ann Biomed Eng. 2000;28:824. doi: 10.1114/1.1289472. [DOI] [PubMed] [Google Scholar]
- 32.Hanada T. Hashimoto M. Nosaka S. Sasaki T. Nakayama K. Masumura S. Yamauchi M. Tamura K. Shear stress enhances prostacyclin release from endocardial endothelial cells. Life Sci. 2000;66:215. doi: 10.1016/s0024-3205(99)00583-4. [DOI] [PubMed] [Google Scholar]
- 33.Hashi C.K. Zhu Y. Yang G.Y. Young W.L. Hsiao B.S. Wang K. Chu B. Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104:11915. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baenziger N.L. Becherer P.R. Majerus P.W. Characterization of prostacyclin synthesis in cultured human arterial smooth muscle cells, venous endothelial cells and skin fibroblasts. Cell. 1979;16:967. doi: 10.1016/0092-8674(79)90111-9. [DOI] [PubMed] [Google Scholar]
- 35.Fritton S.P. Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig D.H. Basson M.D. Biological impact of mechanical stimuli on tumor metastasis. Cell Cycle. 2009;8:828. doi: 10.4161/cc.8.6.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ethier C. Flow through mixed fibrous porous materials. AIChE J. 1991;37:1227. [Google Scholar]
- 38.Weinbaum S. Cowin S.C. Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 39.Costa K.D. Lee E.J. Holmes J.W. Creating alignment and anisotropy in engineered heart tissue: role of boundary conditions in a model three-dimensional culture system. Tissue Eng. 2003;9:567. doi: 10.1089/107632703768247278. [DOI] [PubMed] [Google Scholar]
- 40.Lee E.J. Holmes J.W. Costa K.D. Remodeling of engineered tissue anisotropy in response to altered loading conditions. Ann Biomed Eng. 2008;36:1322. doi: 10.1007/s10439-008-9509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueda A. Koga M. Ikeda M. Kudo S. Tanishita K. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am J Physiol Heart Circ Physiol. 2004;287:H994. doi: 10.1152/ajpheart.00400.2003. [DOI] [PubMed] [Google Scholar]
- 42.Ramsauer M. D'Amore P.A. Contextual role for angiopoietins and TGFbeta1 in blood vessel stabilization. J Cell Sci. 2007;120:1810. doi: 10.1242/jcs.003533. [DOI] [PubMed] [Google Scholar]
- 43.Hirschi K.K. Rohovsky S.A. D'Amore P.A. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonelli-Orlidge A. Saunders K.B. Smith S.R. D'Amore P.A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci USA. 1989;86:4544. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato Y. Rifkin D.B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno M. Cooke J.P. Dzau V.J. Gibbons G.H. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrara N. Gerber H.P. LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 48.Terranova V.P. DiFlorio R. Lyall R.M. Hic S. Friesel R. Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985;101:2330. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westermark B. Siegbahn A. Heldin C.H. Claesson-Welsh L. B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proc Natl Acad Sci USA. 1990;87:128. doi: 10.1073/pnas.87.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Z. Niklason L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H. Riha G.M. Yan S. Li M. Chai H. Yang H. Yao Q. Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]