Abstract
Introduction
Current mesenchymal stromal cell (MSC) delivery methods require infusion/implantation through needles and/or catheters. Little investigation into the effect of delivery via catheter injection has been completed. We hypothesize that injection of rat and human MSCs through various clinically relevant-sized catheters and flow rates will not affect cell viability, characterization, or function.
Methods
Both rat and human MSCs were injected through 20-, 25-, and 30-gauge needles, as well through an SL-10 microcatheter at rates of 60, 120, 240, and 500 mL/h. MSC viability and apoptotic fraction was measured. MSCs were characterized 24 h after injection with flow cytometric immunophenotyping, and multilineage differentiation was completed.
Results
Catheter diameter or flow rate did not affect rat MSC viability. No clinically significant decrease in human MSC viability was observed immediately after injection; however, a delayed decrease in viability was observed at 24 h. No difference in the surface markers CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1 or the capacity for multilineage differentiation (adipogenesis, osteogenesis, and chondrogenesis) was observed for either rat or human MSCs.
Conclusion
The injection of human and rat MSCs through various clinically relevant catheters and flow rates did not have a clinically significant effect on viability immediately after injection, indicating compliance with recently published Food and Drug Administration guidelines (viability >70%). Further, no changes in cell characterization or function were observed via measurement of cell surface markers and the capacity for multilineage differentiation, respectively. These results ensure the biocompatibility of MSCs with commonly used delivery methods.
Introduction
Adult progenitor (stem) cells have shown promise as a novel therapy for a range of medical disorders and remain a focus of intense scientific investigation.1–3 Goals for regenerative medicine using progenitor cell therapy include treating myocardial infarction,4 liver disorders,5 diabetes,6 and kidney failure.7 Regenerative therapies directed at central nervous system disorders are also the subject of vigorous research. Traumatic brain injury and stroke are devastating events that result in the hospitalization of hundreds of thousands of patients annually.
Optimization of cell therapy requires delivery to the target area without significant loss of cellular function or viability. Current delivery methods require the infusion/implantation of progenitor cells through needles and/or catheters. A large body of preclinical research has investigated both in vivo and in vitro progenitor cell engraftment and viability; however, the effect of cell infusion on viability remains unknown. Tol et al. showed that infusion of the mononuclear cell fraction isolated from human bone marrow was not affected by needle diameter or infusion rate8; however, few studies have been completed to investigate the effect on adult tissue stem cells. Mesenchymal stromal cells (MSCs) are adult tissue stem cells that have shown potential therapeutic benefits in preclinical stroke and traumatic brain injury models.9 The mononuclear cell data may not necessarily apply to MSCs due to their much smaller size (5–6 vs. 12–15 μm) and the adherent properties of MSCs.
The translation to clinical trials requires investigation into optimal therapeutic dosage. To accurately calculate the number of live progenitor cells delivered to a target zone, the effect of catheter/needle infusion on viability needs to be understood. Exposing cells to certain flow regimens may damage or destroy the cell, eliminating any chance of therapeutic effect. One major cause of cell damage is shear stress. Virtually all types of physiological fluid flow are considered laminar, meaning that a defined velocity profile exists across the cross section of the fluid channel. Traditional models of laminar flow use “no-slip” boundary conditions, thus assuming that fluid velocity is zero at the wall of the channel.10 The velocity profile in laminar flow generally takes a parabolic shape, with a maximum velocity in the center of the channel. The difference in velocity along with the viscous nature of a fluid results in shear stress. The magnitude of shear stress is maximum at the walls, and is dependent upon the difference in pressure, length, and cross-sectional area of the volume under consideration.11 Any sudden change in geometry, such as the abrupt narrowing of a syringe as it tapers to its hub and needle, can also result in zones of high shear.
Shear stress has been found to be destructive to cells under certain circumstances. Significant investigation has been focused on shear-based damage to erythrocytes to reduce hemolysis in catheters and blood pumps.12,13 However, these results cannot be applied directly to MSCs due to differences in cell shape and size. Prior work concluded that cell damage is based upon both the amount of shear stress present as well as the residence time within zones of shear. Damage may also occur due to cell–surface interactions with the wall of the vessel. Complete destruction of the cell may not necessarily be the only negative outcome, as studies on erythrocytes have shown that excessive stretching or deformation of the cell membrane may result in loss of function.13
Recently, the Food and Drug Administration (FDA) has published guidelines requiring investigation into the biocompatibility of MSCs after injection/infusion through catheter delivery systems. Recommendations included investigation into various clinically relevant flow rates and catheter/sample temperatures.14 Additional FDA guidelines created for human somatic cell therapy Investigational New Drug Applications require cell viability of >70% after delivery. Further, if these viability requirements are not satisfied, additional testing to ensure the safety of increased dead cells/debris must be completed.15 Therefore, the translation of preliminary in vivo progenitor cell models requires stringent biocompatibility testing before clinical trials.
In the context of progenitor cell delivery for regenerative medicine, it is desirable to determine the conditions where the viability of the injected cells is not compromised by the properties of delivery (i.e., the rate of delivery or geometry of the needle). This study was designed to determine the viability, apoptotic fraction, and characterization of MSC cell populations after infusion through needles and catheters of different diameters at multiple flow rates.
Experimental Design
MSCs were harvested from the bone marrow of Sprague-Dawley rats and human organ donors and expanded in culture. After the third passage, the MSCs were suspended in multipotent adult progenitor cell (MAPC) media, a medium at a concentration of 106 MSCs/mL. The cell suspension was then injected through 20-, 25-, and 30-gauge needles (Table 1 displays needle internal diameter), as well as an SL-10 microcatheter (Boston Scientific, Natick, MA) at rates of 60, 120, 240, and 500 mL/h (n = 4 for rat MSCs and n = 3 for human MSCs). MSC viability was measured immediately after infusion through the catheters. Additionally, MSCs were plated and allowed to incubate overnight. After 24 h, the MSC apoptotic and dead cell fractions were measured using an Annexin stain. In addition, MSCs were injected through the various catheters at maximum flow rate (500 mL/h), plated, and allowed to incubate overnight (n = 3 for rat MSCs and n = 1 for human MSCs). Subsequently, MSCs were immunophenotyped using flow cytometry as previously described.16 To ensure that MSCs function after catheter infusion, the MSCs were cultured with the induction medium to promote adipogenesis, osteogenesis, and chondrogenesis (n = 3 for rat MSCs and n = 2 for human MSCs).
Table 1.
Internal Diameter (mm) and Length (cm) of Various Needles and SL-10 Catheter
| Internal diameter (mm) | Length (cm) | |
|---|---|---|
| 20-gauge needle | 0.584 | 2.54 |
| 25-gauge needle | 0.240 | 2.54 |
| 30-gauge needle | 0.140 | 2.54 |
| SL-10 catheter | 0.420 | 150 |
Materials and Methods
All protocols involving the use of animals were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Institutional Animal Care and Use Committee (protocol HSC-AWC-07-055). Additionally, all protocols involving human patients were in compliance with NIH guidelines and approved by the University of Texas Internal Review Board. Informed consent had been granted before the harvest of any human tissue.
Isolation, characterization, and labeling of rat mesenchymal stem cells
MSCs were isolated from the bone marrow of Sprague-Dawley rats and human organ donors, and then expanded in the multipotent adult progenitor cell (MAPC) medium as previously described.16 Flow cytometric immunophenotyping was used to ensure that the MSCs were CD11b−, CD45−, CD29+, CD49e+, CD73+, CD90+, CD105+, and Stro-1 + before use in any experiment. In addition, cellular diameter was measured using the Beckman Coulter counter, and both human and rat MSCs were found to have a diameter of 15–19 μm as previously described.16
Catheter infusion
Passage 3 rat MSCs and passage 3–5 human MSCs were detached from culture plates and resuspended in the MAPC medium at 1 × 106 MSCs/mL. The cell suspension was aspirated into a 10 mL syringe with a 1000 μL pipette tip (internal diameter 1.49 mm) and infused through 20-, 25-, 30-gauge needles, and an SL-10 microcatheter (Boston Scientific) at rates of 60, 120, 240, and 500 mL/h (n = 4 for rat MSCs and n = 3 for human MSCs). Table 1 outlines the needle and catheter specifications. After injection through the various catheters at varying flow rates, MSC viability was immediately measured. In addition, MSCs from each sample were plated and allowed to incubate overnight. After 24 h of incubation, MSC viability and apoptotic fraction were measured. Of note, unmanipulated control samples of the same passage as experimental groups were tested for viability on day 1. Further, an additional group of control samples were separated from culture plates and re-plated on day 1. These samples were then used on day 2 as viability/apoptosis control samples (exact procedure as completed for experimental samples except for catheter injection).
Immediate viability measurement
Cells were stained using a cell viability kit. The protocol used was that recommended by the manufacturer (BD Biosciences, San Jose, CA). Briefly, the MSCs were detached from the plates and resuspended in the MAPC medium at 1 × 106/mL. After infusion through respective needle or catheter, the cells were stained with propidium iodide (PI) and thiazole orange. Flow cytometry was used to analyze viability after 5 min of incubation in the dark at room temperature.
Apoptosis measurement
Cells were stained using fluorescein-isothiocyanate-conjugated Annexin V. The protocol used was recommended by the manufacturer (BD Biosciences). Briefly, after 24 h in an incubator, the adherent cells were detached from the plates. Next, cells were washed three times with phosphate-buffered saline and resuspended in binding buffer at 1 × 106/mL. Fluorescein-isothiocyanate-conjugated Annexin V and PI were added to the cell suspension and incubated at room temperature in the dark for 15 min. Finally, viability and the apoptotic fraction were calculated using flow cytometry.
Repeat MSC immunophenotyping
After injection through the various catheters at maximal flow rate (500 mL/h), MSCs were allowed to incubate overnight (n = 3 for rat MSCs and n = 1 for human MSCs). After 24 h, flow-cytometry-based immunophenotyping was repeated to measure the surface markers CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1 as previously described.15
MSC induction/differentiation protocol
Before differentiation, MSCs were injected through the various catheters at the maximal flow rate (500 mL/h) and plated in the MAPC medium at 5000 cells/cm2 in 12-well plates (n = 3 for rat MSCs and n = 2 for human MSCs). The MSCs were placed in the incubator for 24–48 h until confluent before the addition of the induction medium.
For adipogenic and osteogenic differentiation, the induction medium supplied by Chemicon (Temecule, CA) was used per the manufacturer's protocol. Briefly, MSC cultures were allowed to reach confluence as described above. Next, the supplied adipogenic or osteogenic medium was exchanged every 48 h. After 10 days in culture, MSC cultures were characterized by staining with Oil Red to assess adipogenesis and Alizarin red to assess osteogenesis.
For chondrogenic differentiation, the induction medium supplied by Invitrogen (Carlsbad, CA) was used per the manufacturer's protocol. Briefly, MSC cultures were allowed to reach confluence as previously described. Next, the chondrogenic base medium or supplement was exchanged every 48 h. After 14 days in culture, MSCs were characterized by staining with Alcian blue to assess chondrogenesis.
Statistical analysis
Data were analyzed using both two-factor analysis of variance and one-factor analysis of variance with Tukey–Kramer post hoc testing. A p-value ≤0.05 was considered statistically significant.
Results
Rat MSCs
Control samples were stained with PI on study day 1 (97.6% viability) and Annexin V on study day 2 (93.7% viability). Table 2 shows the average immediate postinjection viability and 24 h viability for all MSC study sample populations. Data analysis showed that varying the catheter size or flow rate did not have a significant effect upon the immediate postinjection viability or 24 h viability.
Table 2.
Mesenchymal Stromal Cell Postinfusion Viability and 24 h Viability (Values Represent Percentage of Total Cell Number)
| |
60 mL/h |
120 mL/h |
240 mL/h |
500 mL/h |
||||
|---|---|---|---|---|---|---|---|---|
| Postinfusion viability | 24 h viability | Postinfusion viability | 24 h viability | Postinfusion viability | 24 h viability | Postinfusion viability | 24 h viability | |
| 20-gauge | 96.8 | 93.6 | 97.4 | 95.2 | 97.7 | 94.8 | 97.8 | 94.9 |
| 25-gauge | 97.0 | 92.7 | 97.0 | 94.7 | 96.7 | 93.9 | 96.8 | 94.7 |
| 30-gauge | 97.9 | 95.2 | 98.0 | 94.3 | 97.3 | 94.1 | 96.5 | 94.3 |
| SL-10 | 97.5 | 93.9 | 97.8 | 94.0 | 97.6 | 93.8 | 98.4 | 94.1 |
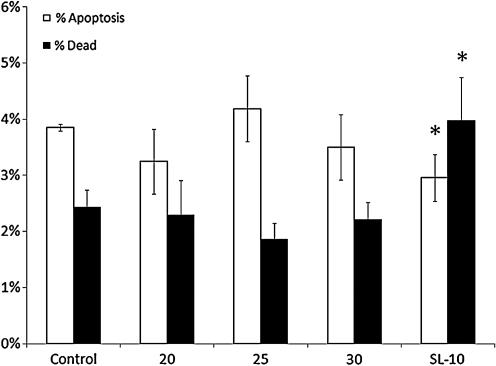
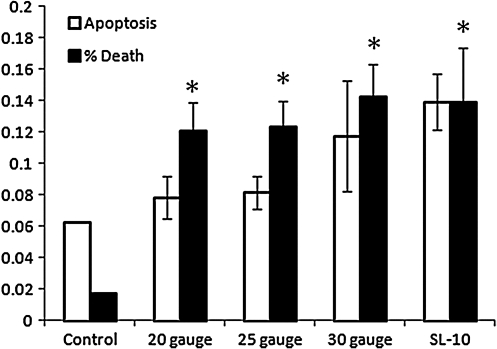
After 24 h of incubation, Annexin V stain showed control sample apoptotic and dead cell fractions of 3.85% and 2.42%, respectively. Data analysis showed a decrease in the apoptotic fraction (2.95%) (p = 0.0011) with a concurrent increase in dead cell fraction (3.98%) (p = 0.0001) for the SL-10 microcatheter only (60, 240, and 500 mL/h). Further analysis showed no difference in apoptotic or dead cell fractions for differing flow rates. Figure 1 shows the apoptotic and dead cell fractions at 24 h for the various catheters.
FIG. 1.
Apoptotic and dead cell fractions at 24 h for control sample and all catheters. Asterisk (*) indicates statistical significance compared to control sample (analysis of variance [ANOVA] with Tukey–Kramer post hoc p < 0.05).
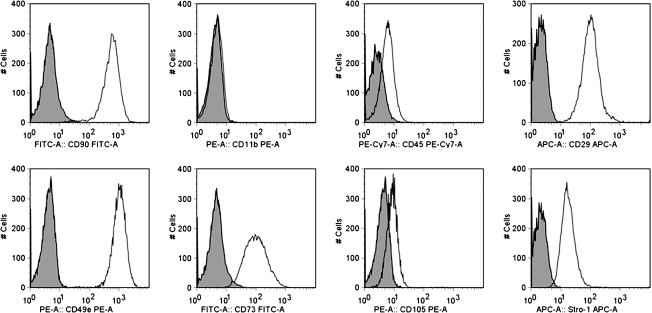
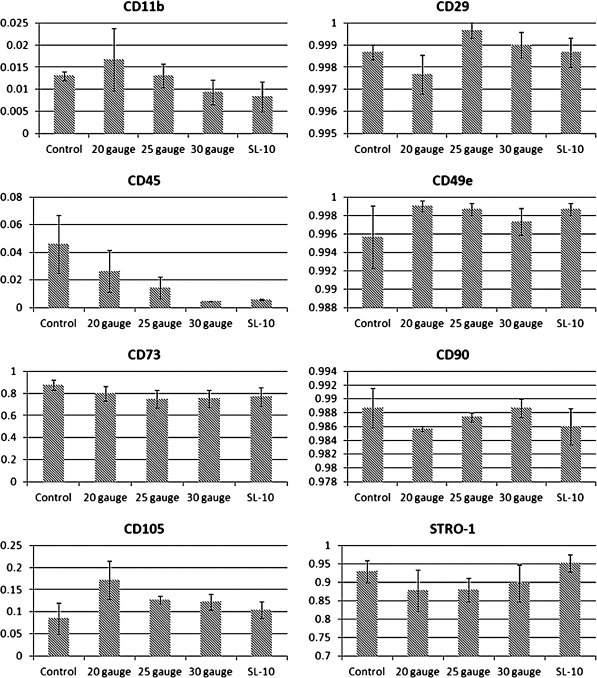
MSCs were injected through all catheters at a maximum flow rate (500 mL/h), plated, and allowed to incubate overnight (n = 3). After 24 h of incubation, immunophenotyping with flow cytometry was completed to measure the surface markers CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1. Figure 2 shows flow cytometry overlays completed on the MSC control, indicating that the cell population was CD11b−, CD45−, CD29+, CD49e+, CD73+, CD90+, CD105+, and Stro-1+ as previously described. Measurement of the above-mentioned surface markers on MSCs after infusion through all catheters at the maximum flow rate showed no difference in MSC characterization based upon flow cytometric immunophenotyping. Figure 3 displays the MSC characterization after infusion through each catheter.
FIG. 2.
Flow cytometry overlays of rat mesenchymal stromal cell (MSC) control samples showing the cell population to be CD11b−, CD45−, CD29 + , CD49e + , CD73 + , CD90 + , CD105 + , and Stro-1 + as previously described.
FIG. 3.
After injection of rat MSCs through various catheters at the maximum flow rate (500 mL/h) flow cytometric immunophenotyping was completed that showed no difference in the surface markers CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1.
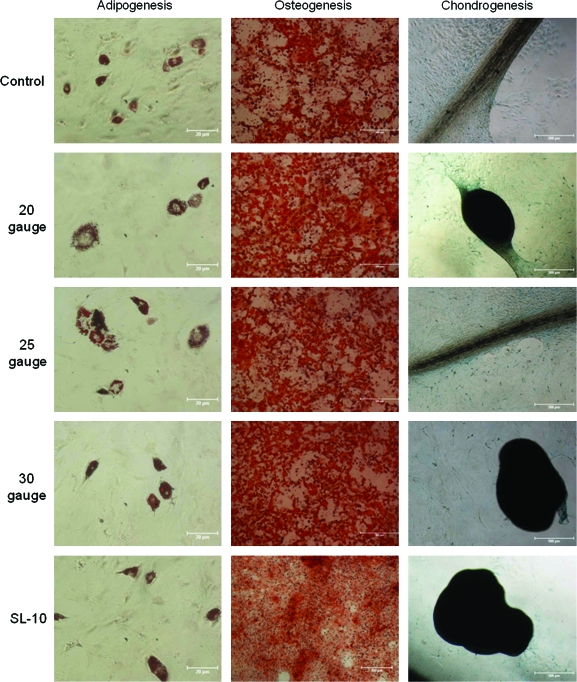
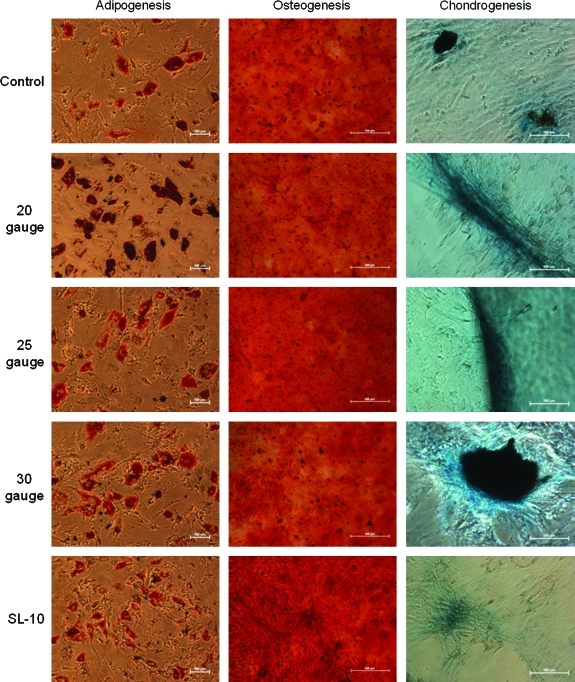
Additional samples of MSCs were plated after injection at maximal flow rate and were placed for 24–48 h until confluent (n = 3). Once confluent, adipogenic, osteogenic, and chondrogenic induction media were exchanged every 48 h as previously described. Figure 4 shows that all samples of MSCs completed multilineage differentiation with no observable difference based upon catheter injection observed.
FIG. 4.
After injection through various catheters at the maximum flow rate (500 mL/h), rat MSCs were placed in culture with subsequent addition of the induction medium. All MSC samples completed multilineage differentiation into adipocytes, osteocytes, and chondrocytes. Adipogenesis (10 × ). Osteogenesis and chondrogenesis (4 × ). Of note, scale bar lengths are 20 μm for the adipogenesis pictures and 500 μm for the osteogenesis and chondrogenesis pictures. Color images available online at www.liebertonline.com/ten.
Human MSCs
Control samples were stained with PI on day 1 (98.1% viability) and Annexin V on study day 2 (92.1% viability). Table 3 shows MSC viability immediately after injection. Although significant decreases in viability after injection through the 30-gauge needle and SL-10 microcatheter are observed (95.0% and 97.3%, respectively), these values fail to reach clinical significance as indicated by FDA guidelines. Table 4 shows human MSC viability 24 h after injection. A significant decrease in cell viability is observed for all catheters and flow rates with samples from the 30 gauge needle and SL-10 microcatheter dropping below the threshold set by the FDA (70%).
Table 3.
Human Mesenchymal Stromal Cell Viability (%) Immediately After Infusion Through Various Catheters at Clinically Relevant Flow Rates
| 60 mL/h | 120 mL/h | 240 mL/h | 500 mL/h | |
|---|---|---|---|---|
| 20-gauge | 98.1 ± 0.06 | 98.2 ± 0.09 | 98.0 ± 0.19 | 98.0 ± 0 |
| 25-gauge | 98.1 ± 0.06 | 97.6 ± 0.07a | 97.9 ± 0.07 | 98.2 ± 0.03 |
| 30-gauge | 98.0 ± 0.09 | 97.4 ± 0.06a | 97.6 ± 0.07 | 95.0 ± 0.25a |
| SL-10 | 94.8 ± 0.43a | 94.0 ± 0.09a | 97.5 ± 0.19 | 97.3 ± 0.09a |
Control samples of MSCs had a viability of 98.1 ± 0.14. Although a statistically significant decrease in cell viability is seen with the 30-gauge catheter and SL-10 microcatheter, the difference fails to reach clinical significance.
Indicates statistical significance compared to control sample (ANOVA with Tukey–Kramer post hoc p < 0.05).
MSC, mesenchymal stromal cell.
Table 4.
Human Mesenchymal Stromal Cell Viability (%) 24 h After Infusion Through Various Catheters at Clinically Relevant Flow Rates
| 60 mL/h | 120 mL/h | 240 mL/h | 500 mL/h | |
|---|---|---|---|---|
| 20-gauge | 81.2 ± 1.7 | 79.1 ± 3.8a | 78.3 ± 2.8a | 77.9 ± 3.0 |
| 25-gauge | 79.8 ± 1.3a | 77.5 ± 0.6a | 80.2 ± 0.3a | 80.5 ± 4.3a |
| 30-gauge | 77.6 ± 0.2a | 73.4 ± 0.9a | 78.9 ± 1.7a | 66.2 ± 2.7a |
| SL-10 | 66.7 ± 1.8a | 65.2 ± 2.6a | 78.0 ± 1.2a | 83.1 ± 1.4a |
Control samples of MSCs had a viability of 92.1 ± 0.10. A significant decrease in cell viability is seen with all catheters and flow rates.
Indicates statistical significance compared to control sample (analysis of variance with Tukey–Kramer post hoc p < 0.05).
After 24 h of incubation, Annexin V stain showed a control sample apoptotic fraction of 6.2% and a dead cell fraction of 1.75%. Figure 5 shows the apoptotic and dead cell fractions for the various catheters across all flow rates. Of interest, a trend toward increased apoptosis is observed with decreasing needle diameter and length; however, this fails to reach significance. Additionally, a significant increase (p = 0.005) in the dead cell fraction is seen with all catheters as expected in accordance with the aforementioned decrease in viability.
FIG. 5.
Human MSC apoptotic and dead cell fractions for various catheters across all flow rates. Of interest, a trend toward increased apoptosis is observed with decreasing needle diameter and length; however, this fails to reach significance. Additionally, a significant increase (p = 0.005) in the dead cell fraction is seen with all catheters as expected in accordance with the aforementioned decrease in viability. Asterisk (*) indicates statistical significance compared to control sample (ANOVA with Tukey–Kramer post hoc p < 0.05).
As previously detailed, human MSCs (n = 1) were injected through the various catheters at maximal flow rate (500 mL/h). Flow cytometric analysis showed that after injection through all catheters, the viable human MSC populations were CD45−, CD29 + , CD49e + , CD73 + , CD90 + , CD105 + , and Stro-1 + comparable to the control samples (data not shown).
Additional samples of human MSCs were plated after injection at maximal flow rate and were placed in the incubator for 24–48 h until confluent (n = 2). Once confluent, adipogenic, osteogenic, and chondrogenic induction media were exchanged every 48 h as previously described. Figure 6 shows that all samples of MSCs completed multilineage differentiation with no observable difference based upon catheter injection.
FIG. 6.
After injection through various catheters at the maximum flow rate (500 mL/h), human MSCs were placed in culture with subsequent addition of the induction medium. All MSC samples completed multilineage differentiation into adipocytes, osteocytes, and chondrocytes. Adipogenesis (10 × ), osteogenesis (4 × ), and chondrogenesis (20 × ). Of note, scale bar lengths are 100 μm for the adipogenesis and chondrogenesis pictures and 500 μm for the osteogenesis pictures. Color images available online at www.liebertonline.com/ten.
Discussion
Rat MSCs
Our study shows that clinically relevant cell delivery systems do not meaningfully affect rat MSC viability. No difference in rat MSC viability immediately after or 24 h after injection was observed. The small amount of variability in rat MSC viability (92.7% –98.0%) ensures adequate cell population viability regardless of catheter size or injection flow rate and compliance with current FDA recommendations.
A decrease in the apoptotic cell fraction and an increase in the dead cell fraction were observed at 24 h for the SL-10 catheter only (MSCs). The longer catheter length likely prolonged the cells' exposure to shearing forces, causing membrane disruption and immediate cell death during infusion. However, it is important to note that there was no recorded difference in overall cell viability even for these highly adherent cell populations. Therefore, the overall fraction of dead and dying (apoptotic) cells showed no difference. The increased mechanical shear likely accelerated the apoptotic pathways, resulting in the observed decrease in apoptotic cell fraction and increase in dead cell fraction at 24 h.
Flow cytometric immunophenotyping showed no significant difference in surface marker (CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1) expression for rat MSCs injected through any catheter or flow rate. In addition, all MSC samples completed multilineage differentiation as previously described.15 Our data show that injection of rat MSCs does not affect cell characterization (immunophenotyping) or cell function (capacity for multilineage differentiation).
Human MSCs
Our data showed no clinically significant decrease in human MSC viability immediately after injection through the various catheters at clinically relevant flow rates as defined by FDA guidelines (viability > 70%); however, a significant decrease in viability is observed for all catheters 24 h after infusion. Further, human MSC viability recorded for the 30-gauge needle and SL-10 microcatheter dropped below 70% (66.2% and 65.2%, respectively). It is important to note that control sample viability dropped from 98.1% on day 1 to 92.1% on day 2. Therefore, although the shearing forces due to catheter injection played a significant role in the observed decrease in human MSC viability, a portion of cell death was likely secondary to the additional cell passage 24 h after injection. Further, the majority of human MSC samples maintained viability > 70%. Despite the observed delayed decrease in MSC viability, the immediate viability remains well above 70%. Therefore, the catheters and flow rates tested still meet published FDA requirements.
Due to the significant effect of catheter injection on delayed (24 h) human MSC viability, investigation into cell characterization and function were imperative. In accordance with this, flow cytometric immunophenotyping showed no difference in surface marker (CD11b, CD45, CD29, CD49e, CD73, CD90, CD105, and Stro-1) expression and no observable difference in cell function as evident by capacity for multilineage differentiation.
Future Considerations
The translation of preclinical work to multicenter trials requires optimization of cell delivery. Although some in vitro and in vivo studies have evaluated optimal cell dosage, little work has been completed to evaluate the effect of cell infusion through needles and/or catheters on cell viability. The injection of rat and human MSCs through 20-, 25-, and 30-gauge needles and the SL-10 microcatheter did not have a clinically significant effect on progenitor cell viability (immediately after injection), characterization, or function, indicating compliance with published FDA guidelines.
Although our study investigated MSC viability, characterization, and cell function, future projects could provide additional benefit via investigation into MSC delivery biocompatibility. One potential area of research could be the evaluation of increased cell concentrations. Increasing cell concentration could increase shearing forces leading to increased cell death. We have found that increasing rat or human MSC concentration causes significant cell clumping that does not allow for reproducible experimentation; however, investigation into alternate progenitor cell lines such as neuronal stem cells or bone marrow mononuclear cells could be of benefit.
Overall, we have tested the injection of human and rat MSCs through various needles/catheters at flow rates that would be most consistent with intravenous delivery. Although a decrease in viability of human MSCs is observed at 24 h, we have ensured adequate immediate (postinjection) viability of rat and human MSCs, indicating the biocompatibility of MSC delivery via several clinically relevant devices.
Acknowledgment
Supported by grants from NIH T32 GM 08 79201, M01 RR 02558, Texas Higher Education Coordinating Board, and Children's Memorial Hermann Hospital Foundation.
Disclosure Statement
There are no known conflicts between the authors and the information presented in this paper.
References
- 1.Harting M.T. Sloan L.E. Jimenez F. Baumgartner J. Cox C.S., Jr. Subacute neural stem cell therapy for traumatic brain injury. J Surg Res. 2009;153:188. doi: 10.1016/j.jss.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood A. Lu D. Lu M. Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 53 doi: 10.1227/01.neu.0000079333.61863.aa. discussion 703, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Lu D. Sanberg P.R. Mahmood A. Li Y. Wang L. Sanchez-Ramos J. Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275. [PubMed] [Google Scholar]
- 4.Britten M.B. Abolmaali N.D. Assmus B. Lehmann R. Honold J. Schmitt J. Vogel T.J. Martin H. Schachinger J. Dimmeler S. Zeiher A.M. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 5.Herrera M.B. Bruno S. Buttiglieri S. Tetta C. Gatti S. Deregibus M.C. Bussolate B. Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- 6.Fellous T.G. Guppy N.J. Brittan M. Alison M.R. Cellular pathways to beta-cell replacement. Diabetes Metab Res Rev. 2007;23:87. doi: 10.1002/dmrr.692. [DOI] [PubMed] [Google Scholar]
- 7.Bussolati B. Hauser P.V. Carvalhosa R. Camussi G. Contribution of stem cells to kidney repair. Curr Stem Cell Res Ther. 2009;4:2. doi: 10.2174/157488809787169129. [DOI] [PubMed] [Google Scholar]
- 8.Tol M. Akar A.R. Durdu S. Ayyildiz E. Ilhan O. Comparison of different needle diameters and flow rates on bone marrow mononuclear stem cell viability: an ex vivo experimental study. Cytotherapy. 2008;10:98. doi: 10.1080/14653240701762356. [DOI] [PubMed] [Google Scholar]
- 9.Qu C. Mahmood A. Lu D. Goussev A. Xiong Y. Chopp M. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truskey G.A. Yuan F. Katz D.F. Upper Saddle River. NJ: Pearson education group; 2004. Transport Phenomena in Biological Systems. [Google Scholar]
- 11.Sharp M.K. Mohammad S.F. Scaling of hemolysis in needles and catheters. Ann Biomed Eng. 1998;26:788. doi: 10.1114/1.65. [DOI] [PubMed] [Google Scholar]
- 12.Leverett L.B. Hellums J.D. Alfrey C.P. Lynch E.C. Red blood cell damage by shear stress. Biophys J. 1972;12:257. doi: 10.1016/S0006-3495(72)86085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutera S.P. Flow-induced trauma to blood cells. Circ Res. 1977;41:2. doi: 10.1161/01.res.41.1.2. [DOI] [PubMed] [Google Scholar]
- 14.FDA. Draft Guidance for Industry: Somatic Cell Therapy for Cardiac Disease. Rockville, MD: 2009. [Google Scholar]
- 15.FDA. Guidance for FDA Reviewers and Sponsors: content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigation New Drug Applications (INDs) Rockville, MD: 2009. [Google Scholar]
- 16.Harting M. Jimenez F. Pati S. Baumgartner J. Cox C., Jr. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10:243. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]