Abstract
Taste, which is almost always accompanied by other oral sensations, serves to identify potential nutrients and toxins. The present study was designed to determine the influence of sensory modality (chemesthetic vs. gustatory) and physiological significance (potentially nutritive vs. potentially harmful) on insular response to oral stimulation. Sixteen subjects underwent functional magnetic resonance imaging scanning while receiving 2 potentially nutritive solutions (sucrose and NaCl), 2 potentially harmful solutions (quinine and capsaicin, a chemesthetic stimulus), and a tasteless control solution. We identified a region of anterior ventral insula that responded to oral stimulation irrespective of modality or physiological significance. However, when subjects tasted a potentially nutritive stimulus, the connectivity between the insula and a feeding network including the hypothalamus, ventral pallidum, and striatum was greater than when tasting a potentially harmful stimulus. No differential connectivity was observed as a function of modality (gustatory vs. chemesthetic). These results support the existence of an integrated supramodal flavor system in the anterior ventral insula that preferentially communicates with the circuits guiding feeding when the flavor is potentially nutritive.
Keywords: capsaicin, chemesthesis, gustation, insula, physiological significance
Introduction
When we “taste” we also touch the food or drink in our mouths and sense its odor via retronasal olfaction. The term “flavor” describes this multimodal experience. Taste and oral somatosensation are particularly intimately related. Taste receptors lie side-by-side in the oral cavity with thermoreceptors, mechanoreceptors, and nociceptors; everything that is tasted induces tactile, thermal, and sometimes also chemesthetic sensations (e.g., burning, stinging, or coolness) (Green 2003; Simon et al. 2008). In addition, some taste stimuli can themselves evoke somatosensory sensations. These stimuli are referred to as chemesthetic—chemical compounds that activate somatosensory receptors involved in pain, touch, and/or thermal perception (Green et al. 1990; Lim and Green 2007). For example, in moderate-to-high concentrations, salts and acids can provoke chemesthetic sensations of burning, stinging, or pricking (Green and Gelhard 1989; Green and Lawless 1991). Consequently, even presumably “pure taste” stimuli can have an oral somatosensory component.
Accordingly, there is considerable evidence that taste and oral somatosensory inputs are integrated throughout the central nervous system. The taste signal is carried from taste receptor cells in the oral cavity (Chandrashekar et al. 2006) by cranial nerves VII, IX, and X to the nucleus of the solitary tract in the brainstem where taste inputs are joined by oral somatosensory projections from the spinal trigeminal nucleus (Beckstead et al. 1980). The precise locations of the trigeminal projections vary across species, but there is evidence (including in humans) of overlap with gustatory areas (Whitehead and Frank 1983; Whitehead 1990) and of tracts that run within the nucleus of the solitary tract that may facilitate crossmodal integration (Travers 1988). Somatosensory input also reaches the nucleus of the solitary tract via the glossopharyngeal nerve, which contains taste-sensitive, as well as mechano- and thermo-sensitive neurons (Bradley et al. 1992). Overlapping representation of gustatory and somatosensory information also occurs in the thalamus (Pritchard et al. 1989) and at the cortical level; (Pritchard et al. 1986; Cerf-Ducastel et al. 2001; de Araujo and Rolls 2004). For example, the primary gustatory cortex contains nearly as many somatosensory-specific as taste-specific neurons in addition to bimodal neurons responding to both somatosensory and taste stimulation (Yamomoto et al. 1985; Smith-Swintosky et al. 1991, 1992, 1996; Kadohisa et al. 2004).
In humans, 2 functional neuroimaging studies have shown overlapping insular responses to taste and oral somatosensory stimulation. In the first study, it was shown that both gustatory and “somato-gustatory” stimuli (strong pungent HCl and astringent aluminum potassium sulfate, both of which have a taste) activated the insula, but only the bimodal stimuli produced simultaneous and symmetrical activation of the pre- and postcentral opercula (i.e., the Rolandic operculum) (Cerf-Ducastel et al. 2001). In the second study, de Araujo and Rolls (2004) used carboxymethyl cellulose, a thickening agent, to compare responses to a tasteless and odorless viscous stimulus with responses to 1.0 M sucrose. As predicted, sucrose activated the anterior insular taste cortex. However, they also found that response to carboxymethyl cellulose in this region was proportional to the log of its viscosity. A similar effect was also observed in a mid-insular region that did not respond to sucrose. Thus, both studies found overlapping representation of taste and oral somatosensation in the anterior insula. More recently, taste neurons within this region were shown to respond to variations in physiological parameters such as gastrointestinal hormones or blood glucose levels (de Araujo et al. 2006), suggesting that the anterior insular cortex represents an integrative circuit dedicated to evaluating the biological significance of all intraoral stimuli (de Araujo and Simon 2009).
On the other hand, the 2 prior functional magnetic resonance imaging (fMRI) studies of oral somatosensation differ with respect to the regions found to be uniquely activated by the somatosensory component. The study by Cerf-Ducastel et al. (2001) suggests a preferential role for the pre- and postcentral opercula in lingual somatosensory perception, whereas the study by de Araujo and Rolls (2004) highlights the mid-insula. One problem with the interpretation that the Rolandic operculum is uniquely specialized for lingual somatosensation is that electrophysiological and neuroanatomical studies in primates have demonstrated gustatory inputs to the Rolandic operculum (Ogawa et al. 1985; Pritchard et al. 1986). Because stronger intensity ratings were given to the somato-gustatory stimuli, greater activation in the Rolandic operculum by a strong somato-gustatory stimulus is not unambiguous evidence of preferential activation by somatosensory stimuli. Similarly, interpretation of a specific role for the mid-insula in oral somatosensation is limited by the fact that study only employed a single taste stimulus. For example, it may be that the mid-insula would have responded to a bitter taste. In fact, Small et al. (2003) found that quinine produces preferential activation of posterior regions of the insula in comparison with sucrose.
Given the controversy surrounding the representation of taste and somatosensory oral stimuli, the goal of the current study was to compare brain responses to capsaicin, a chemesthetic stimulus, with the response to 3 taste stimuli, sucrose, NaCl, and quinine, which were matched in perceived intensity to capsaicin. Capsaicin is the best-known chemesthetic stimulus that, as the pungent chemical in chili pepper, is very widely consumed. Capsaicin stimulates the Transient Receptor Potential cation channel, Subfamily V, Member 1 receptor (Caterina et al. 1997; Zanotto et al. 2007; Mandadi and Roufogalis 2008) which is a widely accepted marker for nociceptive (pain) neurons (Koltzenburg 2004), but psychophysical studies have also shown that capsaicin can both evoke and desensitize bitter taste (Green and Hayes 2003; Green and Schullery 2003). Further work by Lim and Green (2007) showed that for some subjects bitter taste and weak burning sensations caused by dilute capsaicin solutions can be difficult to distinguish. These findings are indicative of a surprisingly close perceptual relationship between bitter taste and burning sensations. We reasoned that if there is a common site in the insula for all oral stimuli, then we should see overlapping responses not only for quinine (bitter) and capsaicin (burn) but also for capsaicin, sucrose (sweet), and NaCl (salty). In contrast, if oral somatosensation is represented by a distinct region of insula or operculum (e.g., mid-insula or Rolandic operculum), then we should identify specific responses to capsaicin. Yet another possibility was that quinine and capsaicin would produce overlapping responses that were distinct from NaCl and sucrose. Such a finding would be consistent with the fact that burning and bitter sensations signal potentially harmful substances, whereas sweet and salty signal potentially nutritive substances. This physiological significance can be difficult to dissociate from pleasantness, which in many cases (such as sucrose) are closely linked. Importantly, however, NaCl represents a molecule that is important to consume to ensure electrolyte balance and yet is considered unpleasant when consumed on its own in water. This hedonic dichotomy presents an opportunity to investigate the ways in which 2 physiologically important stimuli of differing valences are encoded in the brain.
Materials and methods
Subject selection
This research protocol was approved by the Yale University Human Investigation Committee. Forty-three subjects were recruited by advertisements posted around Yale University and the greater New Haven area. Only right-handed subjects between the age of 18 and 45 were included. Subjects were excluded if they had any nonremovable metal on their body, were currently or recently taking major medications such as antidepressants, were claustrophobic, had a history of food allergies, had diabetes, or had any history of psychiatric disorder or drug abuse. Subjects also completed an intake and training session. Data from this session were used to exclude 22 subjects. Reasons for exclusion included the perception of capsaicin as bitter, burn ratings persisting beyond 5 rinse trials (Figure 1a; see below), or discomfort with the scanning environment. Prior to the experiment, subjects were given a description of the paradigm and provided written informed consent. All subjects participated in an intake session and an fMRI training session in a mock scanner with 21 participating in the fMRI session. After excluding subjects with excessive head movement in the scanner, 16 subjects (11 female; age 25.3 ± 5.8) were used for final fMRI analysis.
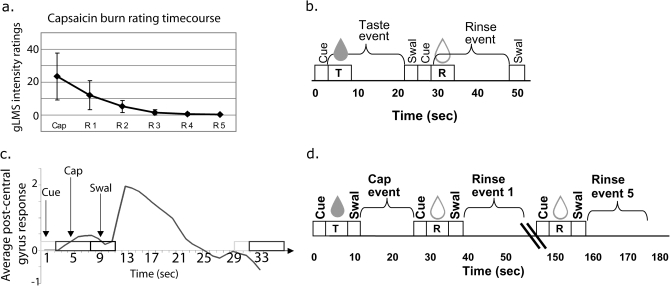
Figure 1.
(a) Burn intensity ratings: average perceived intensity of burning sensation (y axis) after delivery of capsaicin and tasteless rinses (x axis) in subjects included in fMRI analysis (n = 16). Burning sensation returns to zero by the fifth rinse for all subjects. Error bars represent 1 standard deviation. (b) Depiction of taste events: Cue = subject hears cue that liquid will be delivered. T = delivery of one of the 3 tastes over 3 s. Taste event = 18-s event of interest. Swal = time during which the tone plays signaling subject to swallow; R = delivery of the tasteless solution over 3 s. Rinse event = 18-s event modeled as tasteless baseline for fMRI analysis. (c) Signal change in response to capsaicin stimulation: Pilot data showing raw evoked response in percent signal change to a capsaicin event in the postcentral gyrus (y axis) in one subject plotted as a function of time (x axis, in seconds). Maximal hemodynamic response to capsaicin occurs 4 s after the swallow and resolves 13 s later. (d) Capsaicin event: Cap = capsaicin delivery over 3 s; For fMRI analysis, the capsaicin event is modeled as the event of interest, the tasteless5 as the baseline and the cue, swallow, and tasteless1–4 as events of no interest.
Stimuli
Stimuli were solutions of 1.0 M sucrose, 0.32 mM quinine, 0.56 M NaCl, and 44 μM capsaicin in distilled water. Rinses were dilutions of a 0.0125 M KCl and 0.00125 M NaHCO3 solution in distilled water, which mimics the ionic components of saliva (O'Doherty et al. 2001). Prior work in our laboratory (Small et al. 2003, 2008) and others (O'Doherty et al. 2001; de Araujo et al. 2003) has shown that the rinse solution, which is diluted to 3/4, 1/2, or 1/4 strength according to whether the subject perceived it as tasteless, is a good baseline stimulus to control for the somatosensory and motor effects of receiving and swallowing a liquid, without introducing the potentially confounding taste of water (de Araujo et al. 2003).
Stimulus delivery
Subjects received the solutions through an fMRI-compatible custom-designed gustometer (Veldhuizen et al. 2007). In brief, the gustometer consists of a series of programmable syringe pumps with 60-mL syringes and beverage tubing attached and leading to a mouthpiece (gustatory manifold) that is anchored to the fMRI head coil by a separate attachment piece (Veldhuizen et al. 2007). The same delivery system, which is controlled by Matlab, was used in a mock scanner during psychophysical testing prior to collection of fMRI data in the actual scanner. The mock scanner consists of a padded table on which the subject lays, a removable wooden replica of the fMRI head coil that is placed over the subject's head, and a plastic sheet that folds over the subject and simulates the bore of the scanner.
During each real and mock run, subjects experienced 10 stimulus events. Each taste event consisted of delivery of one of the 3 taste solutions as a 0.2 mL bolus over 3 s, followed by a rest period of 13–17 s (a jitter), a cue to swallow the solution, a 0.5 mL bolus rinse of tasteless solution (delivered over 3 s), and a second jitter and swallow cue (Figure 1b). Control events consisted of delivery of 0.2 mL of tasteless solution over 3 s, followed by a 13–17 s jitter and a swallow cue. For taste stimuli, perceptual responses rise to maximum intensity after delivery of the taste. Thus, in our standard taste trial, the subject receives the taste and holds it in the mouth until cued 15 s later to swallow. The swallow is delayed so that the blood oxygen level–dependent (BOLD) signal, which peaks 4–5 s after delivery, is not contaminated by movement artifacts related to swallowing.
Pilot testing
Because capsaicin's sensory effects can take several seconds to develop fully, pilot tests were conducted to determine its time course under the specific conditions of stimulation we created for use in the scanner. It was immediately clear that the burn from capsaicin was maximal after it had been swallowed. To formalize this impression, we collected intensity ratings for capsaicin on a single subject using our standard taste delivery procedure in the mock scanner (Veldhuizen et al. 2007; Small et al. 2008). Intensity ratings were collected using the general labeled magnitude scale (gLMS) (Green et al. 1996; Bartoshuk et al. 2002) 5 s after stimulus delivery, immediately after swallowing (after holding the capsaicin in the mouth for 15 s, according to our standard taste paradigm) and after swallowing a series of 3 tasteless rinses. Consistent with the investigators’ preliminary testing, intensity ratings collected for 16 events revealed that the maximum perception of capsaicin intensity occurred not following delivery (average intensity rating: 8.6 ± 8.7) but following the swallow (average intensity rating: 26.1 ± 5.0; P < 0.0001). To account for the different perceptual time course of capsaicin response compared with taste responses, the delivery paradigm for capsaicin was modified so that the swallow took place immediately after delivery and the 18-s period after the swallow was modeled as the event of interest (Figure 1d).
Further pilot testing was performed on a different subject to investigate the brain response to capsaicin under the modified capsaicin delivery paradigm so that the temporal course of the BOLD response could further inform paradigm design and data analysis. The psychophysical effect of delayed maximal intensity was echoed by the BOLD hemodynamic response function (HRF) of the pilot subject, which showed maximal response in the postcentral gyrus just after the swallow (Figure 1c). Although this may include capsaicin stimulation of the back of the throat as well as the tongue, we believed that capturing the moment of maximum perceived intensity was critical. Furthermore, because the BOLD signal rises after the swallow, this design allowed us to measure neural response to maximum perceived intensity without contamination by swallow-related movement.
The perceptual data collected from the first pilot subject also showed that the perceived burn from capsaicin persisted longer than the sensation from other tastes and that it was not removed by a standard tasteless rinse. Thus, in the imaging pilot test, we included 5 rinses following delivery of capsaicin rather than the usual 1 rinse (Figure 1d). We examined the unmodeled BOLD response to capsaicin in the pilot subject to determine whether the BOLD response in the insula and operculum were also sustained. This is an important issue because if the neural response were sustained (unlike gustatory responses), it would prevent us from using the predicted HRF employed by our neuroimaging software analysis package Statistical Parametric Mapping (SPM) (Welcome Department of Cognitive Neurology) to increase statistical sensitivity. The pilot neuroimaging data showed that the unmodeled BOLD response to capsaicin was not sustained. Rather, we found that the response conformed well to the canonical HRF with a 4–5 s lag and resolution within approximately 13 s. Although it is unclear why the neural response shows a different time course than the perceptual response and while we cannot rule out that other areas may show sustained responses to capsaicin, our primary areas of interest showed a canonical HRF, which gave us the confidence to use our standard design and analysis. Based upon these pilot data, we modeled both taste and capsaicin with the standard canonical HRF, as is standard procedure in SPM.
In order to ensure that our stimuli showed the expected differences in pleasantness but similar intensities, an additional group of 10 subjects were tested in the mock scanner. In this pilot testing, subjects underwent three 12-min runs modeled on the runs used during scanning (Figure 1). Each run included two 3-min capsaicin events (each defined as one capsaicin trial followed by 5 tasteless trials) and two 54-s events of each of the other tastes plus their respective tasteless baseline conditions. The order of events was randomized across subjects. After each stimulus delivery, subjects rated the pleasantness of the stimulus using the labeled hedonic scale (LHS) (Lim et al. 2009) and its intensity using the gLMS.
Intake and training session
Prior to scanning, all fMRI subjects were asked to attend an intake and training session. This session served to screen subjects, collect psychophysical data, familiarize subjects with the procedures and equipment used during the actual scan, and make sure that they were able to tolerate receiving the taste stimulus in a supine position. During this session, subjects rated the intensity of sweet, salty, sour, bitter, and burning sensations evoked by each stimulus. As expected (Green and Hayes 2003; Green and Schullery 2003), some subjects rated the capsaicin as bitter, and those who reported greater than “barely detectable” bitterness were excluded from the study. This was done to provide a clear test of the hypothesis that areas of the brain involved in perception of a burning sensation from capsaicin (rather than a bitter taste from capsaicin) overlap with areas of the brain involved in taste perception. Subjects then underwent 4 runs identical to those they would experience in the fMRI scanner.
Neuroimaging sessions
In order to obtain a sufficient number of trials for trial averaging, fMRI scanning took place on 2 separate days. Both days included 5 runs identical to those in the simulator session but without the psychophysical ratings. We elected not to collect ratings during scanning because data from our laboratory (Bender et al. 2009), as well as data from other laboratories show that performance of rating tasks alter gustatory responding (Grabenhorst et al. 2008). Each run included 2 trials for each stimulus, making a total of 20 events per stimulus per subject over the 2 scanning days.
We used a 3 T Trio scanner by Siemens to collect functional and anatomical images. Laterality for image processing was confirmed by taping a vitamin E capsule to the left temporal region in every subject. Echoplanar imaging was used to measure the BOLD signal as an indication of cerebral brain activation. A susceptibility-weighted single-shot echoplanar method was used to image the regional distribution of the BOLD signal with time repetition [TR] = 2000 ms, time echo [TE] = 20 ms, flip angle = 80°, matrix = 64 × 64, slice thickness = 3 mm, and acquisition of 40 contiguous slices. Slices were acquired in an interleaved mode to reduce the cross talk of the slice selection pulse. At the beginning of each functional run, the MR signal was allowed to equilibrate over 6 scans for a total of 12 s, which was excluded from analysis. The anatomical scan used a T1-weighted 3D FLASH sequence (TR/TE = 15/6 ms, flip angle 20°, matrix 256 with 160 one mm slices acquired in the same orientation as the functional data) with a saturation band placed over the neck to reduce distortion in the temporal lobes caused by blood flow.
Data analysis
The mean intensity rating for each stimulus within each subject was calculated. Because responses on the gLMS tend to be log-normally distributed across subjects (Green et al. 1996), the means were log-transformed prior to group statistical analysis. One-way repeated measures analysis of variance (ANOVA) was performed using SPSS to determine whether there were differences among perceived intensities of stimuli. Additionally, repeated measures ANOVA was performed to determine whether there were differences in LHS liking ratings or log-transformed gLMS intensity ratings between stimuli, using the mean ratings for each stimulus within each subject.
The neuroimaging data were pre- and postprocessed using SPM5 (Welcome Department of Cognitive Neurology) on Linux workstations running MATLAB (Mathworks, Inc.) using standard procedures (Friston et al. 1994; Worsley and Friston 1995; Veldhuizen et al. 2007). Functional images were time acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the scan immediately preceding the anatomical T1 image. When a subject's head movement was greater than 1 mm in any direction during a single run that run was excluded from analysis. If more than 4 runs needed to be discarded, the subject was excluded from data analysis. Images were normalized to the Montreal Neurological Institute template (MNI-305), smoothed with a 6-mm full-width at half-maximum isotropic Gaussian kernel, and highpass (128) filtered to remove noise. Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. Condition-specific effects at each voxel were estimated using the general linear model. The response to events was modeled by a canonical HRF, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 s and the subsequent undershoot (Friston et al. 1994). The temporal derivative of the hemodynamic function was also included as part of the basis set to account for small deviations in timing from the canonical HRF.
Analyses were based on random effects models in order to account for intersubject variability (Strange et al. 1999). Note that before we performed the planned contrast analyses (e.g., before we compared response with potentially nutritive vs. potentially harmful oral stimuli), the response to each stimulus was compared with a control tasteless solution. This was done to “subtract out” general effects of having a stimulus in the mouth. For each subject SPMs reflecting the smoothed response (parameter estimate) at each voxel across the whole volume were created for each stimulus minus its baseline. Individual SPMs were then entered into a multisubject repeated measures (flexible factorial) ANOVA with the factor “subject” (with one level for each subject) and “stimulus” (with one level each for each stimulus—capsaicin, sucrose, NaCl, and quinine) and contrasts were performed to test our specific predictions (e.g., nutritive vs. harmful). We also tested whether responses to the stimuli overlapped using conjunction analyses (Nichols et al. 2005). Conjunction analyses were based on the conjunction null hypothesis which stipulates that each component of the conjunction (e.g., responses to sucrose, NaCl, quinine, and capsaicin) must be individually significant (each stimulus must produce a significant response compared with its baseline) and not just jointly significant (Friston et al. 1999; Calvert 2001). As such, it is a conservative test. SPM maps were thresholded at P < 0.005 with a cluster criterion of 3 voxels.
Unpredicted peaks were considered significant at P < 0.05, false discovery rate (FDR)-corrected across the entire brain at the voxel level. For predicted peaks, we used a region-of-interest (ROI) approach in which we used WFU pickatlas (Maldjian et al. 2003, 2004) to create masks of predicted ROIs including the feeding- and taste-related areas: insula, orbitofrontal cortex, Rolandic operculum, frontal operculum, thalamus, and ventral striatum, hypothalamus, and ventral pallidum. Peaks within these masks were considered significant at P < 0.05, FDR-corrected across the voxels of the ROI.
In addition to conjunction analyses, we performed psychophysiological interactions (PPIs) (Gitelman et al. 2003). PPI analyses provide a way to study the interactions between the signal from a source or “seed” region and signals elsewhere in the brain as a function of sensory parameters such as taste quality (Friston et al. 1997; Friston 1998). To identify the exact locations from which to extract data from each subject's seed regions, we performed small volume searches centered on the insula peaks isolated in the group conjunction analysis (spheres with 15 mm diameter) in individual subject SPMs (thresholded at P = 0.01). We extracted the deconvolved time course of neural response from the insula seed region in each subject. We then calculated the product of this time-course data and the vector of the psychological variable of interest (e.g., potentially nutritive − potentially harmful) to create the PPI term. New SPMs were computed for each subject, including the interaction as a regressor. These maps thus display regions where neural response correlates with the interaction between activity in the seed region and the psychological variable. Individual PPI SPMs were then entered into a random-effects group analysis (thresholded at P < 0.005) with a cluster size of >3 voxels. We again used an ROI approach, adopting our previously created WFU pickatlas (Maldjian et al. 2003, 2004) masks of insula, orbitofrontal cortex, Rolandic operculum, frontal operculum, thalamus, and ventral striatum, hypothalamus, and ventral pallidum. Peaks within these masks were considered significant at P < 0.05, FDR-corrected across the voxels of the ROI. Thus, for example, a peak resulting from our PPI analysis represents an area where the time course of neural activity is more tightly correlated with the time course of insula (our seed region) activity during potentially nutritive versus potentially harmful stimulation.
Results
Psychophysical data
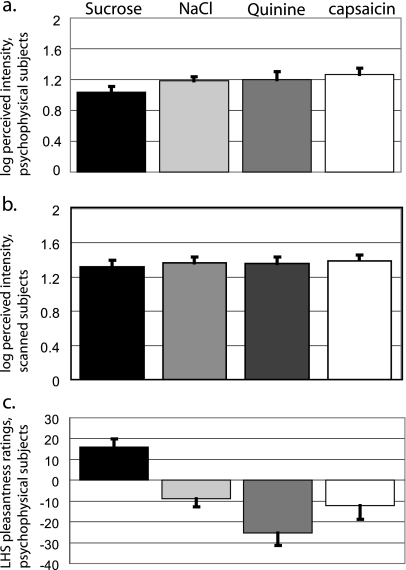
Among subjects in the psychophysical testing group, all stimuli were rated between “weak” and “strong” in intensity. A one-way repeated measures ANOVA showed an effect of stimulus (F3,27 = 3.084, P = 0.044) (Figure 2a), although pairwise comparisons revealed no significant differences among gLMS ratings of capsaicin (21.34 ± 3.65; log-transformed 1.265 ± 0.082; these and following values represent mean ± standard error of the mean), sucrose (12.62 ± 2.36; log-transformed 1.058 ± 0.08), NaCl (16.43 ± 1.93; log-transformed 1.187 ± 0.056), and quinine (19.73 ± 3.79; log-transformed 1.265 ± 0.082). With respect to hedonic ratings, all stimuli were rated between “liked moderately” and “disliked very much.” A repeated measures ANOVA showed a significant effect of stimulus on pleasantness ratings (F3,27 = 16.81, P = 0.0001) (Figure 2c). Pairwise comparisons revealed that liking ratings for sucrose (17.35 ± 3.7) were significantly higher than those for NaCl (−9.1 ± 6.1; P < 0.004), quinine (−26.9 ± 5.9; P < 0.0002), or capsaicin (−12.1 ± 6.8; P < 0.010) (Figure 2b). No other stimulus pairs were significantly different. No subject rated sucrose as unpleasant, and no subject rated quinine as pleasant.
Figure 2.
Stimulus ratings (a) Average intensity ratings of oral stimuli for psychophysical subjects. For a and b, the gLMS was used to collect ratings, and data were log-transformed before analysis. Error bars represent the standard error of the mean. (b) Average intensity ratings of oral stimuli for scanned subjects. (c) Average pleasantness ratings of oral stimuli for psychophysical subjects. The LHS was used to collect ratings. Error bars represent the standard error of the mean.
All subjects who were scanned reported the burn from capsaicin as returning to zero by the fifth rinse (Figure 1a). This was important to ensure that residual burn did not affect the perception of the following taste and to ensure that the tasteless rinse (i.e., the fifth rinse, which is used as the baseline condition for capsaicin) was devoid of burn. Among scanned subjects, all stimuli were rated between moderate and strong in intensity and a repeated measures ANOVA showed that the stimuli did not differ significantly in perceived intensity (F3,45 = 0.990, P = 0.406) (Figure 2b).
Neuroimaging data
Areas of overlapping representation
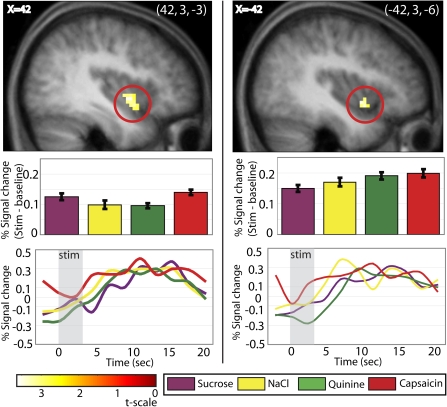
To determine what areas are commonly activated by all oral stimuli, we first performed a conjunction analysis between all the stimuli minus their control conditions (Figure 3, Table I). This isolated bilateral responses in the anterior ventral insula (−42, 3, −6; z = 3.33; P = 0.029; k = 12 and 42, 3, −3; z = 3.48; P = 0.029; k = 30).
Figure 3.
The insula responds to all stimuli. Response in the insula (sagittal sections at x = −42 on the left and x = 42 on the right) resulting from the conjunction analysis of [capsaicin + sweet + salty + bitter]. T-map is thresholded at P < 0.005 and k > 3 voxels. For this and successive pictures, bar graphs show activity in peak voxel within circled brain region in response to each stimulus (minus its baseline) in percent signal change, averaged over subjects. Error bars represent 2 standard error of the mean. Activations are significant at P < 0.05 FDR-corrected across regions of interest. The line graph displays the time course of the signal (stimulus minus baseline), extracted from the voxel responding maximally to each stimulus in this area (percent signal change, averaged over subjects). We note that the time-course data and bar graphs may not correspond exactly because the bar graphs reflect data fitted to the canonical HRF, whereas the time courses are extracted using a finite impulse response model in the RFXplots toolbox (Glascher 2009).
Table 1.
Results from fMRI analysis
| Brain region | x | y | z | z statistic | t-value | PFDR-corrected | k |
| (capsaicin + quinine + sucrose + NaCl) | |||||||
| Left insula | −42 | 3 | 6 | 3.33 | 3.44 | 0.029 | 12 |
| Right insula | 42 | 3 | −3 | 3.48 | 3.61 | 0.029 | 30 |
| (sucrose + NaCl) – (quinine + capsaicin) from left insula | |||||||
| Ventral striatum | −15 | 18 | −3 | 3.86 | 5.16 | 0.033 | 27 |
| Ventral pallidum | 18 | 0 | −6 | 3.46 | 4.37 | 0.027 | 11 |
| Hypothalamus | −3 | 0 | 0 | 3.99 | 5.45 | 0.005 | 18 |
| (sucrose + NaCl) – (quinine + capsaicin) from right insula | |||||||
| Hypothalamus | 0 | −6 | −9 | 3.64 | 4.72 | 0.019 | 27 |
| Right ventral pallidum | 18 | −3 | −6 | 3.98 | 5.74 | 0.007 | 17 |
| Left ventral pallidum | −21 | −3 | −6 | 3.50 | 4.64 | 0.015 | 8 |
| (quinine + capsaicin) − (sucrose + NaCl) from insula (−42, 0, 3) | |||||||
| No significant activations | |||||||
Physiological significance
To determine the influence of physiological significance, we contrasted response to potentially harmful versus potentially nutritive stimuli or [(capsaicin + quinine) – (sucrose + NaCl)] and [(sucrose + NaCl) – (capsaicin + quinine)]. These contrasts yielded no significant differential activation.
Influence of physiological significance on brain connectivity
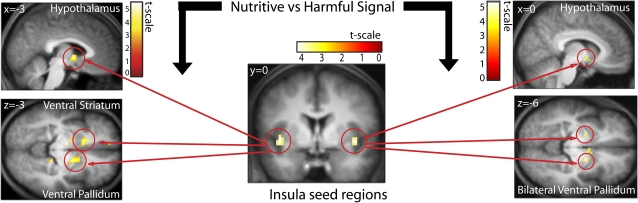
To determine whether physiological significance influenced connectivity between the commonly activated insular peak and other key flavor and feeding brain regions, we performed PPIs (Figure 4e, Table I). No areas showed greater connectivity with the insula under potentially harmful versus potentially nutritive. However, greater connectivity was found between the left insula and the hypothalamus (−3, 0, 0; z = 3.99; P = 0.005), ventral striatum (−15, 18, −3; z = 3.86; P = 0.033), and ventral pallidum (18, 0, −6; z = 3.46; P = 0.027) during sensation of the potentially nutritive compared with the potentially harmful stimuli. Greater connectivity was also found between the right insula and the hypothalamus (0, −6, −9; z = 3.64; P = 0.019) and bilateral ventral pallidum (18, −3, −6; z = 3.98, P = 0.007 and −21, −3, −6; z = 3.50; P = 0.015) during sensation of potentially nutritive compared with potentially harmful stimuli. In order to investigate whether this effect was influenced by the pleasantness of the taste, we performed a contrast of the connectivity strengths for sucrose versus NaCl, which differed significantly in rated pleasantness. We found no significant differences in connectivity as a function of taste. Moreover, enhanced connectivity between the insula and the hypothalamus, ventral pallidum, and ventral striatum were observed following independent PPIs for sucrose versus potentially harmful (0, 3, −6; z = 1.89, Punc = 0.029 and −15, 24, −3; z = 1.37; Punc = 0.086 and 18, −3, 3; z = 1.50, Punc = 0.067) and NaCl versus potentially harmful (3, −3, −3; z = 1.63, Punc = 0.052 and −6, 15, −3; z = 2.23; Punc = 0.013 and 27, 6, 3; z = 1.40, Punc = 0.081); although these effects did not survive correction for multiple comparisons. In sum, the connectivity analyses suggest greater connectivity between the insula and key feeding regions during perception of potentially nutritive tastes, irrespective of their perceived pleasantness.
Figure 4.
Effects of physiological significance. During sucrose and NaCl compared with during quinine and capsaicin, activity in the left insula (−42, 0, 3; shown is coronal section y = 0) is more correlated with the hypothalamus (shown is sagittal section x = −3), ventral striatum, and ventral pallidum (shown is axial section z = −3). Activity in the right insula (45, 3, −6) is more correlated with the hypothalamus (shown is sagittal section x = 6) and bilateral ventral striatum (shown is axial section z = −3). Based on contrast of [(sucrose + NaCl) – (quinine + capsaicin)].
Effect of modality
No effect of modality was observed. More specifically, contrasts of [capsaicin – (sucrose + NaCl + quinine)] and [(sucrose + NaCl + quinine) – capsaicin] yielded no significant differential activations. PPIs examining the influence of modality on connectivity of the insula also showed no significant effect of modality upon connectivity with other brain areas.
Unique activations
Additionally, contrasts of each individual stimulus minus all the others (e.g., [sucrose – (NaCl + quinine + capsaicin)] yielded no significant activations.
Discussion
The aims of this study were to determine the influence of modality and physiological significance upon brain representation of oral stimulation. Surprisingly, we found no evidence for an influence of modality. Rather, we identified a region of anterior ventral insula that showed significant responses to all the stimuli compared with their respective baseline conditions. In other words, we found that response in the anterior ventral insula occurred to all oral stimuli irrespective of their modality (chemesthetic or gustatory) or physiological significance (protentially nutritive or potentially harmful). However, physiological significance did influence the connectivity of the anterior ventral insula with key brain regions involved in feeding. More specifically, greater connectivity was observed between the insula and the feeding network when subjects sampled potentially nutritive tastes. Taken together, these results argue for a supramodal oral sensory system in the anterior ventral insula that preferentially interacts with areas orchestrating feeding behaviors and homeostasis when the oral stimulus is potentially nutritive. These results are discussed in detail below.
Supramodal responses in the anterior insula
Consistent with the existence of an integrated oral sensory system, we found overlapping responses in the anterior ventral insula to capsaicin versus a tasteless baseline condition and to the gustatory stimuli versus their respective tasteless baseline conditions. This overlap was identified using a very stringent statistical test that requires significant effects for each contrast entered into the analysis (Friston et al. 1999). Thus, it is the case that sucrose, quinine, NaCl, and capsaicin all resulted in significant responses compared with their baseline conditions in the same anterior ventral insular region. This finding agrees with work in monkeys showing that the anterior insular region contains both somatosensory-specific and taste-specific neurons, as well as bimodal neurons that respond to modalities (Plata-Salaman et al. 1996). This region also shows overlapping responses to taste and retronasal odors in humans (de Araujo et al. 2003; Small et al. 2004) and overlapping responses to sweet taste and fat (de Araujo and Rolls 2004). Moreover, supra-additive responses are observed to simultaneously presented tastes and odors compared with the sum of the response to their independent stimulation (Small et al. 2004). This suggests that not only is there overlapping representation of tastes and odors but also that these signals are being integrated. Previous neuroimaging results (Cerf-Ducastel et al. 2001) have also indicated that “somato-gustatory” stimuli (in this case, astringent aluminum potassium sulfate and pungent strong HCl) commonly activate the insula but show different patterns of activation in the Rolandic operculum compared with “pure” taste stimuli (NaCl, aspartame, quinine, and weak HCl). Our analyses did not reveal significant differences in brain response to chemesthetic versus taste stimuli in any region of the brain. The reason for the disagreement between studies is unclear. What is more important is that both studies found a relative paucity of modality-specific responses and strong multimodal responses in the insula (Cerf-Ducastel et al. 2001), which is consistent with the view that the gustatory and oral somatosensory systems are widely overlapping within the central nervous system (Simon et al. 2008) and with the possibility that the anterior ventral insula is an important site for the integration of these signals.
It is important to note, however, that the absence of identifiably different activations in the insula does not rule out the existence of separate circuits for taste and chemesthesis that were simply not discernable with the current protocol. It is possible, and even likely given work in animals (Katz et al. 2001; Roussin et al. 2008), that separate circuits function on finer grained spatial or temporal scales than were detectable with the resolution of the current study. Future work using techniques such as multivariate pattern analyses or fMRI adaptation, which allows for the disambiguation of spatially overlapping responses, are needed to resolve this issue.
Physiological significance
The second aim of our study was to determine if physiological significance influences brain response to oral stimuli. The results provided partial support for this hypothesis. Although we did not observe significantly different responses in the anterior ventral insula during the perception of potentially harmful versus nutritive oral stimuli (though subsignificant peaks were present), we were able to use the responses from this region to find evidence that physiological significance may modulate the influence of the anterior ventral insula on other areas of the brain that orchestrate feeding behavior. Employing the PPI analysis, we found that response in the anterior ventral insula better predicted response in the hypothalamus, ventral pallidum, and nucleus accumbens when subjects sampled potentially nutritive compared with potentially harmful oral stimuli. These areas each play critical roles in orchestrating feeding behavior by integrating sensory, affective, and homeostatic signals (Berridge 2007). Thus, even though physiological significance did not impact response “within” the anterior ventral insula, it appears that it modulates the “interaction” of this region with key regions of the feeding network. We suggest that this reflects the integration of sensory information about potentially nutritive flavors encoded in the insula with information about affective and homeostatic state, coded in the hypothalamus, pallidum, and ventral striatum so that adaptive feeding behaviors may be orchestrated.
We believe that the observed PPI cannot be accounted for by differences in the perceived pleasantness of the potentially nutritive and harmful stimuli. Capitalizing on the fact that in aqueous solution NaCl was experienced as unpleasant, whereas sucrose was experienced as pleasant, we tested the possibility that effective connectivity between the anterior insula and the feeding network would be greater during consumption of sucrose compared with NaCl. However, we found no significant PPI as a function of stimulus for the 2 potentially nutritive taste stimuli (NaCl vs. sucrose).
Additional considerations
An important feature of the current study is that we took steps to minimize the chance that confounding factors contributed to the chemesthetic response. First, to rule out the perception of suprathreshold bitterness as a component in the chemesthetic response, we excluded subjects for whom capsaicin evoked even a barely detectable taste. Even so, we cannot rule out the possibility that subthreshold bitter signals may have influenced the response to capsaicin. In theory, this possibility would be more convincingly addressed by measuring detection thresholds for capsaicin bitterness and ruling out subjects whose thresholds for bitterness were significantly below the concentration of the test concentration. But because capsaicin produces a readily detectable burning sensation, the threshold for perception of bitterness could not be measured using the most sensitive, criterion-free methods (e.g., 2-alternative forced-choice), which is based on the detection of any stimulus quality. A less-sensitive threshold method that would require subjects to specifically identify bitterness would not be demonstrably more sensitive than the method we used. However, given that the burning sensation from capsaicin was rated to be at least weak-to-moderate by all subjects, it is unlikely that imperceptible gustatory stimulation would significantly influence brain response to capsaicin.
Another consideration is the potential for painful sensations from capsaicin, as painful stimuli frequently activate the anterior insula (Treede et al. 2000). However, we are confident that the capsaicin stimulus was not perceived as painful as the low concentration that was chosen did not evoke pain during pilot testing, and similarly, intense stimulation has rarely been rated as painful in other psychophysical studies (Green and Hayes 2003, 2004; Green et al. 2005).
Summary
The present study produced 2 main findings. First, that there are overlapping responses in the anterior ventral insula to gustatory and to chemesthetic stimulation which are not dependent upon physiological significance or perceived pleasantness. Second, the BOLD response within the anterior ventral insula was associated with differences in connectivity between this region and other feeding-related regions. Specifically, when tasting a potentially nutritive substance, connectivity was greater than when tasting a potentially harmful substance. Taken together, these results support the existence of a supramodal oral sensory system that is sensitive to the physiological significance of oral stimuli.
Funding
This work was supported by grants from the National Institutes of Health; National Institute on Deafness and Communication Disorders [R01 DC005002] and [R01 DC006706].
Acknowledgments
Additional thanks to Jennifer Felsted for data collection and Dr Maria Veldhuizen for assistance in data processing.
References
- Bartoshuk L, Duffy V, Fast K, Green BG, Prutkin J, Snyder D. Labeled scales and invalid across-group comparisons. J Food Qual Prefer. 2002;14:125–138. [Google Scholar]
- Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- Bender G, Meltzer JA, Gitelman D, Small DM. Neural correlates of evaluative compared with passive tasting. Eur J Neurosci. 2009;30:327–328. doi: 10.1111/j.1460-9568.2009.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bradley RM, Smoke RH, Akin T, Najafi K. Functional regeneration of glossopharyngeal nerve through micromachined sieve electrode arrays. Brain Res. 1992;594:84–90. doi: 10.1016/0006-8993(92)91031-9. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Van de Moortele P-F, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses. 2001;26:371–383. doi: 10.1093/chemse/26.4.371. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr., Nicolelis MAL, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes. 2009;33:S23–S43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Imaging neuroscience: principles or maps? Proc Natl Acad Sci U S A. 1998;95:796–802. doi: 10.1073/pnas.95.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Glascher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- Green BG. Studying taste as a cutaneous sense. Food Qual Prefer. 2003;14:99–109. [Google Scholar]
- Green BG, Alvarez-Reeves M, George P, Akirav C. Chemesthesis and taste: evidence of independent processing of sensation intensity. Physiol Behav. 2005;86:526–637. doi: 10.1016/j.physbeh.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Green BG, Gelhard B. Salt as an oral irritant. Chem Senses. 1989;14:259–271. [Google Scholar]
- Green BG, Hayes JE. Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiol Behav. 2003;79:811–821. doi: 10.1016/s0031-9384(03)00213-0. [DOI] [PubMed] [Google Scholar]
- Green BG, Hayes JE. Individual differences in perception of bitterness from capsaicin, piperine and zingerone. Chem Senses. 2004;29:53–60. doi: 10.1093/chemse/bjh005. [DOI] [PubMed] [Google Scholar]
- Green BG, Lawless HT. The psychophysics of somatosensory chemoreception in the nose and mouth. In: Getchel TV, Doty RL, Bartoshuk L, Snow JB, editors. Smell and taste in health and disease. New York: Raven Press; 1991. pp. 235–253. [Google Scholar]
- Green BG, Mason JR, Kare MR. Chemical senses: irritation. New York: Marcel-Dekker, Inc; 1990. [Google Scholar]
- Green BG, Schullery MT. Stimulation of bitterness by capsaicin and menthol: differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem Senses. 2003;28:45–55. doi: 10.1093/chemse/28.1.45. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Rolls ET, Verhagen JV. Orbitofrontal cortex: neuronal representation of oral temperature and capsaicin in addition to taste and texture. Neuroscience. 2004;127:207–221. doi: 10.1016/j.neuroscience.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MAL. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M. The role of TRP channels in sensory neurons. Novartis Found Symp. 2004;260:206–213. [PubMed] [Google Scholar]
- Lim J, Green BG. The psychophysical relationship between bitter taste and burning sensation: evidence of qualitative similarity. Chem Senses. 2007;32:31–39. doi: 10.1093/chemse/bjl033. [DOI] [PubMed] [Google Scholar]
- Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34:739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, Kraft R. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;2003:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Roufogalis B. ThermoTRP channels in nociceptors: taking a lead from capsaicin receptor TRPV1. Curr Neuropharmacol. 2008;6:21–38. doi: 10.2174/157015908783769680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Anderson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ito S, Nomura T. Two distinct projection areas from tongue nerves in the frontal operculum of macaque monkeys as revealed with evoked potential mapping. Neurosci Res. 1985;2:447–459. doi: 10.1016/0168-0102(85)90017-3. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Scott TR, Smith-Swintosky VL. Gustatory neural coding in the monkey cortex: l-amino acids. J Neurophysiol. 1992;67:1552–1561. doi: 10.1152/jn.1992.67.6.1552. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Scott TR, Smith-Swintosky VL. Gustatory neural coding in the monkey cortex: mixtures. J Neurophysiol. 1996;75:2369–2379. doi: 10.1152/jn.1996.75.6.2369. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Norgren R. Neural coding of gustatory information in the thalamus of Macaca mulatta. J Neurophysiol. 1989;61:1–14. doi: 10.1152/jn.1989.61.1.1. [DOI] [PubMed] [Google Scholar]
- Roussin AT, Victor JD, Chen J-Y, Di Lorenzo PM. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. J Neurophysiol. 2008;99:644–655. doi: 10.1152/jn.00920.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Stapleton JR, Nicolelis MAL. Multisensory processing of gustatory stimuli. Chem Percept. 2008;1:95–102. doi: 10.1007/s12078-008-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;13:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92:1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Plata-Salaman CR, Scott TR. Gustatory neural coding in the monkey cortex: stimulus quality. J Neurophysiol. 1991;66:1156–1165. doi: 10.1152/jn.1991.66.4.1156. [DOI] [PubMed] [Google Scholar]
- Strange BA, Portas C, Dolan R, Holmes AP, Friston KJ. Random effects analysis for event-related fMRI. Neuroimage. 1999;9:1053–1089. [Google Scholar]
- Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res. 1988;457:1–11. doi: 10.1016/0006-8993(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Treede R-D, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87:113–119. doi: 10.1016/S0304-3959(00)00350-X. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Subdivisions and neuron types of the nucleus of the solitary tract that project to the parabrachial nucleus in the hamster. J Comp Neurol. 1990;301:554–574. doi: 10.1002/cne.903010406. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol. 1983;220:378–395. doi: 10.1002/cne.902200403. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yamomoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats: information processing of taste quality. J Neurophysiol. 1985;53:1370–1386. doi: 10.1152/jn.1985.53.6.1356. [DOI] [PubMed] [Google Scholar]
- Zanotto K, Merrill A, Carstens M, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. Neurophysiology. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]