Abstract
Matrix metalloproteinase-9 (MMP-9) and MMP-2 are important for recovery following direct traumatic injury within the central nervous system (CNS). However, most CNS injury models include both direct trauma and neuronal deafferentation. This limits the ability to determine if these MMPs are important to one or both components of injury. To establish if MMPs play a role in the deafferentation processes, we investigated MMP-9 and MMP-2 in the olfactory bulb following methyl bromide gas exposure. This injury model lesions neurons within the olfactory epithelium and thereby leads to deafferentation of the bulb without damaging it directly. We measured the response of MMP-9 and MMP-2 in the olfactory bulb from 1 to 60 days during neuronal deafferentation and recovery. MMP-9 increased rapidly on day 5 and remained elevated for 10 days. MMP-2 expression levels were low compared with MMP-9. Immunohistological staining performed on days 1, 5, and 10 revealed MMP-9 was localized to inflammatory cells within the olfactory nerve and glomerular layers. Our results demonstrate MMP-9 is present in inflammatory cells during deafferentation processes in the olfactory bulb. Although MMP-9 is elevated in other CNS injury models, this is the first report to demonstrate an increase in MMP-9 associated with neuronal deafferentation in the absence of direct trauma.
Keywords: deafferentation; inflammation, matrix metalloproteinase; methyl bromide gas; olfactory bulb; olfactory epithelium
Introduction
Matrix metalloproteinases (MMPs) are a family of over 20 structurally related enzymes comprised of a propeptide and a Zn2+-binding catalytic domain. These enzymes are implicated in the degradation of extracellular matrix (ECM) components including laminin, collagen IV, and elastin (Yong et al. 2001). In normal physiological processes, such as angiogenesis, wound healing, learning and memory, MMPs serve an important role in the remodeling of the ECM (Nagase and Woessner 1999). Though their enzymatic action is necessary for tissue restructuring and repair, the MMPs are highly regulated due to their potential destructive capability toward the ECM. Most MMPs are secreted as zymogens and require cleavage of the prodomain and association of a Zn2+ in the catalytic domain to become fully activated. Studies have shown that uncontrolled activation of these enzymes can result in certain pathologies including arthritis, multiple sclerosis, and Alzheimer’s disease (Yong et al. 1998; Yoshihara et al. 2000).
In the central nervous system (CNS), several MMPs are modulated after neuronal injury. Two specific MMPs, MMP-9 and MMP-2, have defined temporal expression patterns following different models of neuronal injuries (Rosenberg et al. 1996; Romanic et al. 1998; Wang et al. 2000, 2002). For example, after spinal cord injury, MMP-9 expression increased within hours, whereas MMP-2 expression was delayed for up to a week (de et al. 2000; Goussev et al. 2003). The early presence of MMP-9 correlates with inflammation, degradation of blood vessel walls, edema, and neuronal death. The delay in MMP-2 expression is important for remodeling the ECM and breakdown of scar, ensuring the proper conditions necessary for neuronal recovery (Zuo et al. 1998; Hsu et al. 2006).
We have previously reported the expression of MMP-9 and MMP-2 following direct trauma to olfactory neurons using a nerve transection (NTx) injury model. In this model, the axons are lesioned as they penetrate through the cribriform plate before entering the olfactory bulb. The NTx injury is not limited to the axons alone but also injures other CNS structures including the anterior ventral portion of the bulb and blood vessels. Following NTx, there is a temporal expression of these two specific MMPs, similar to other CNS neuronal injuries. MMP-9 is elevated within hours following NTx, whereas MMP-2 levels did not increase until a week after injury (Costanzo et al. 2006; Costanzo and Perrino 2008). The temporal expression of these two enzymes provides insight into the molecular processes associated with olfactory degeneration and regeneration. Though the expression of MMP-9 and MMP-2 in CNS injury has been established, these models involve some component of trauma. It is unknown if MMP-9 and MMP-2 are upregulated in the absence of direct trauma or toxic injury, that is, by deafferentation alone.
Unlike most models of CNS injury, the olfactory system is unique in that the cell bodies of sensory neurons are located outside the CNS, in the peripheral olfactory epithelium and project their axons to the olfactory bulb, a CNS structure. This organization allows for the study of direct (NTx) injury to the CNS versus isolated deafferentation via the destruction of the neuronal population. The latter is achieved through methyl bromide (MeBr) gas exposure. MeBr is passively inhaled, filling the nasal cavity and leading to destruction of over 90% of cells in the olfactory epithelial, including olfactory neurons (Schwob et al. 1995). This leads to deafferentation of the olfactory bulb without direct trauma.
In this study, we examined the expression levels of MMP-9 and MMP-2 in the olfactory bulb following MeBr exposure to determine if these MMPs are associated with neuronal deafferentation in the absence of direct CNS trauma. A comparison of findings from both these indirect (MeBr) and direct (NTx) CNS injury models provides a new approach to investigating the role of MMPs in neuronal injury and recovery processes.
Materials and methods
MeBr gas exposure
Adult C57/BL6 mice were exposed to MeBr gas as previously described (Schwob et al. 1995; Chen et al. 2004). Mice were placed in a wire enclosure measuring 15 × 15 × 15 cm centered within a plexiglass box measuring 30 × 30 × 30 cm and exposed to 180 ppm MeBr gas in purified air at a flow rate of 10 L/h for 6 h. Mice exposed to room air served as controls. All procedures were approved by the Institutional Animal Care and Use Committees of Virginia Commonwealth University and Tufts University School of Medicine.
Tissue sampling and preparation
At 1, 3, 5, 7, 10, 15, 40, and 60 days after MeBr gas exposure, mice were anesthetized with sodium pentobarbital and sacrificed by rapid decapitation. The olfactory bulbs were removed from the skull, flash frozen in liquid nitrogen and processed as previously described (Costanzo and Perrino 2008). The anterior ventral portion of the bulbs and samples from frontal cortex were removed for protein measurements. All tissue samples were placed in protein extraction buffer (50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 1% sodium dodecylsulfate, 1% deoxycholic acid) and homogenized with a motor-driven plastic homogenizer. The tissue was incubated on a rotating platform at 4°C for 30 min, rehomogenized and centrifuged at 4°C for 30 min at 16 000 revolutions per minute. Samples were then placed in a minus 80°C freezer for storage. Solubilized proteins in the supernatant were quantified using DC protein assay reagent kit (Bio-Rad Laboratories) using bovine serum albumin as a standard. Protein measurements were made at 750 nm on a micro-Quant plate reader (BioTek Instruments Inc.). Equal amounts of protein (20 μg for MMP-9 and 40 μg for MMP-2) from the tissue extracts and purified murine MMP-9 and MMP-2 (R and D Systems) were loaded onto 4–12% Bis–Tris density gradient gels and separated using NuPAGE MES [2-(N-morpholino) ethane sulfonic acid] reducing buffer system (Invitrogen) for 1 h at 200 V and 4°C. Protein was transferred to nitrocellulose membrane for 2 h at 25 V and 4°C; 5% bovine, nonfat dry milk in Tris-buffered saline, and 0.05% Tween-20 was used to block nonspecific binding for 1 h. Primary antibodies against MMP-9, MMP-2 (1:200; R and D Systems), olfactory marker protein (1:20 000; Wako Chemicals), and glial fibrillary acidic protein (1:50 000; DakoCytomation) were used. Cyclophilin A (1:7000; Upstate) antibodies (CPA) were obtained to control for protein loading. Nitrocellulose membranes were incubated in the primary antibody overnight at 4°C. Membranes were then exposed to the appropriate species peroxidase-conjugated immunoglobulin G secondary antibody (Rockland) for 1 h. The membranes were incubated for 1 min in Western Lightning Plus reagent (Perkin Elmer) and exposed to Blue Sensitive Autoradiography film (ThermoScientific).
Protein measurements
Quantification of protein expression on the nitrocellulose membrane was performed using Quantity One Analysis software (Bio-Rad Laboratories). The protein bands were measured both by band density and band area. The density–area measurements for MMP-2, MMP-9, olfactory marker protein (OMP) and glial fibrillary acidic protein (GFAP) at each recovery time point were then divided by the corresponding density–area measurement for CPA in the same gel to adjust for differences in protein loading. These protein ratios were then divided by control samples to obtain normalized expression levels.
Immunohistochemistry
Immunohistochemical staining for MMP-9 and MMP-2 was performed on horizontal sections at three different time points after MeBr exposure: days 1, 5, and 10. After washing with Invitrogen phosphate-buffered saline (PBS) for 10 min, sections were placed in 0.01 M citric acid with steam for 10 min, and washed in PBS for 10 min. Sections were then immersed for 1-min intervals in a series of alcohol solutions (70%, 95%, 100%, 95%, and 70%) and placed in PBS wash for 5 min. This was followed by incubation with 10% normal rabbit serum, 4% bovine serum albumin, 5% nonfat dry milk, and 0.5% Triton X-100 in PBS for 1 h and placed with MMP-9 or MMP-2 primary antibody (1:10) overnight. Sections were then incubated in peroxidase-conjugated rabbit antigoat immunoglobulin (1:50) and exposed to 3,3′-diaminobenzidine (DAB; Vector Laboratories, Inc.). Sections were counterstained with Harris-modified hematoxylin (Fisher Scientific) and visualized on a Eclipse E600 microscope (Nikon Inc.).
MMP-9-labeled cell counts
Digital images of immunostained histological sections were used to obtain cell counts of MMP-9-positive cells located within different layers of the olfactory bulb. For each olfactory bulb section, the total number of MMP-9-positive cells was counted within each of four defined regions: the combined nerve and glomerular layer, the external plexiform layer, the combined mitral and internal plexiform layer, and the granular cell layer. The criteria used to define a MMP-9-labeled cell was positive DAB staining and visualization of the cell nucleus. Three separate bulb sections were used to obtain the mean number of cells for each region. We also measured the average area (mm2) for each bulb region using ImageJ analysis software (National Institutes of Health).
Statistical analysis
A comparison of protein levels at each of the different recovery time points relative to controls was performed using one-way analyses of variance and a Dunnett two-sided post hoc test. A probability of less than 0.05 was considered statistically significant. All statistical analysis was performed using SPSS software (IBM).
Results
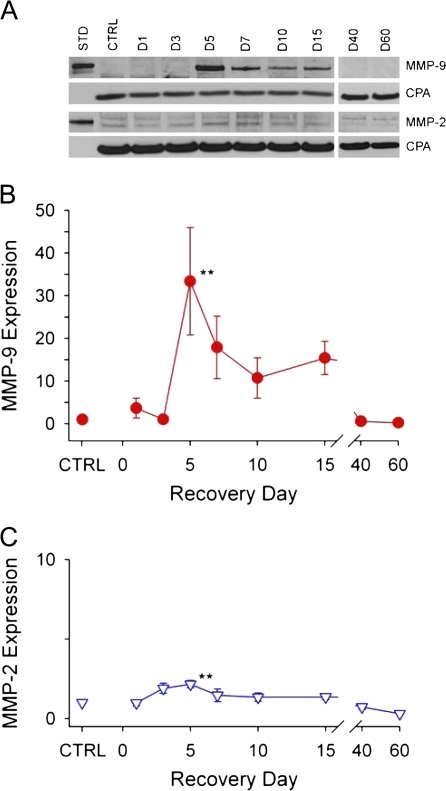
MMP-9 and MMP-2 protein expression was measured in the olfactory bulb shortly after MeBr gas exposure (day 1) and during neuronal degeneration (days 1–15) and regeneration (days 15–60) time periods. Western blot analysis of MMP-9 and MMP-2 expression following MeBr is shown in Figure 1. Density–area measurements of MMP protein bands were compared with that of CPA (Figure 1A) to determine the relative amounts of MMP-9 and MMP-2 in the bulb at each time point. The mean values normalized to control (CTRL) levels from four separate experiments are plotted in Figure 1B,C. In CTRL samples, MMP-9 expression was absent and MMP-2 levels were barely detectable. MMP-9 remained at CTRL levels during the first 3 days, increased rapidly between days 3 and 5, and reached maximum expression, 33 times higher than control values, at day 5. MMP-9 remained elevated for 2 weeks after MeBr and then returned to control levels by day 40.
Figure 1.
Changes in MMP-9 and MMP-2 protein expression in the olfactory bulb following MeBr gas injury. (A) Representative Western blots illustrating changes in MMP-9 and MMP-2 expression at different time points following injury. Lane 1 shows purified murine MMP-9 and MMP-2 standards (STD). Lane 2 shows an absence of MMP-9 and minimal detection of MMP-2 in control (CTRL) tissue. CPA bands for each lane were used as controls for protein loading. (B) and (C) Plots of the relative amounts of MMP-9 and MMP-2 expressed as a percentage of CPA and normalized to CTRL levels. MMP-9 increased rapidly on day 5, reaching 33 times the expression levels in control samples. MMP-9 remained elevated for 10 days and then returned to control levels by day 40. MMP-2 expression was detectable but at low levels following injury. At day 5, MMP-2 expression was increased 2-fold above background control levels. The doublet bands in the MMP-2 Western blot represent the pro and active forms of MMP-2. Data points represent the mean normalized to CTRL ± SEM (n = 4 for each time point; *P < 0.05; **P < 0.01). This figure appears in color in the online version of Chemical Senses.
MMP-2 expression was low in control animals and remained near control values following MeBr injury, though an increase in expression (2.1 times higher than control) was detected on day 5.
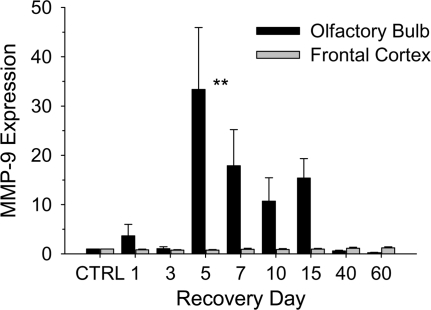
To determine if the MeBr injury leads to a widespread activation of MMP-9 in the CNS, we monitored MMP-9 expression in the frontal cortex (Figure 2). At each recovery time point, MMP-9 expression in frontal cortex remained at control levels, suggesting that the MeBr injury response was limited to the olfactory system in contrast with other systemically administered toxins (Colín-Barenque et al. 2008). This finding is consistent with other studies of MeBr injury (Schwob et al. 1995).
Figure 2.
Comparison of MMP-9 expression in the olfactory bulb and frontal cortex following MeBr gas (MeBr) exposure. Bar graphs represent the relative amount of MMP-9 expressed as a ratio of CPA normalized to control (CTRL) levels. The expression of MMP-9 in the olfactory bulb (black) remains at control levels through day 3 following MeBr. Between days 3 and 5, MMP-9 levels increase rapidly and reach maximum expression. MMP-9 levels begin to decrease by day 7 and return to CTRL levels by day 40. In the frontal cortex (gray), MMP-9 expression did not increase above CTRL levels. Data points represent the mean normalized to CTRL ± SEM (n = 4 for each time point; *P < 0.05;**P < 0.01).
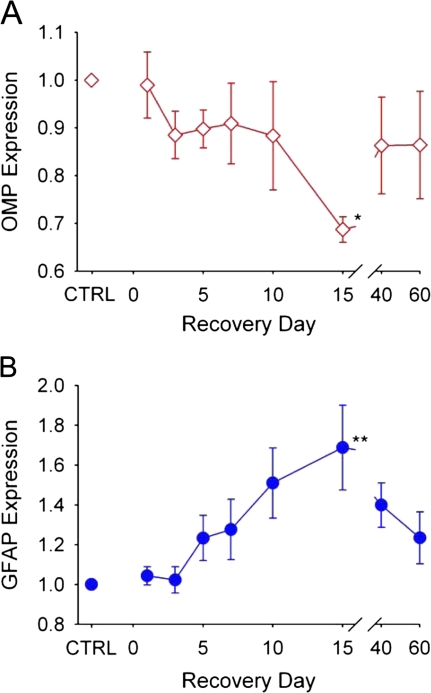
In addition to MMP expression, we monitored changes in OMP and GFAP within the olfactory bulb (Figure 3). OMP, a protein expressed in mature olfactory neurons, was used to monitor deafferentation and subsequent reinnervation of the bulb. The mean OMP values from four separate experiments normalized to CTRL levels are plotted in Figure 3A. OMP reached its lowest levels by day 15, corresponding to deafferentation of the bulb. By day 40, OMP levels had increased approaching CTRL levels, indicating reinnervation of the bulb by regenerated olfactory neurons.
Figure 3.
Changes in OMP and GFAP in the olfactory bulb following MeBr gas exposure. Plots represent relative amount of protein for OMP and GFAP expressed as a ratio of CPA normalized to control (CTRL) levels in four separate experiments. (A) OMP is a marker for degeneration and regeneration of olfactory neurons. OMP levels declined slowly following MeBr injury, reaching a minimum at day 15, indicating deafferentation of the bulb. By day 40, OMP begins to increase toward control levels, corresponding to reinnervation of the bulb. (B) GFAP, representing astrocytic activation and gliosis, remained low until day 5, slowly increased and reached a maximum level at day 15. Data points represent the mean normalized to CTRL ± SEM (n = 4 for each time point). This figure appears in color in the online version of Chemical Senses.
Monitoring GFAP levels allowed for the assessment of reactive gliosis following injury. GFAP is an intermediate filament protein present within astrocytes that increases in response to CNS injury (Silver and Miller 2004). Mean values of GFAP normalized to CTRL levels from four separate experiments are plotted in Figure 3B. After MeBr gas exposure, GFAP expression remained near control levels through day 3. GFAP levels increased by day 10 and achieved maximal expression at day 15. By day 40, GFAP expression started to decrease, although at day 60 it was still slightly elevated.
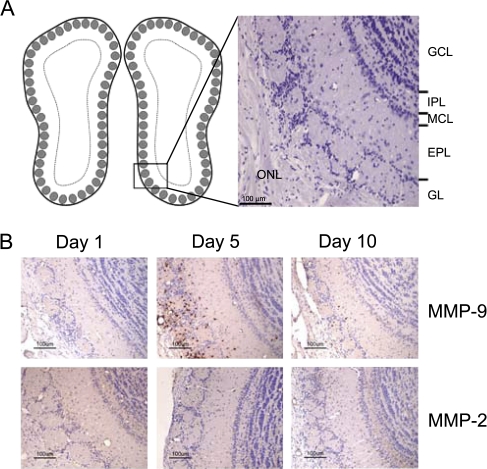
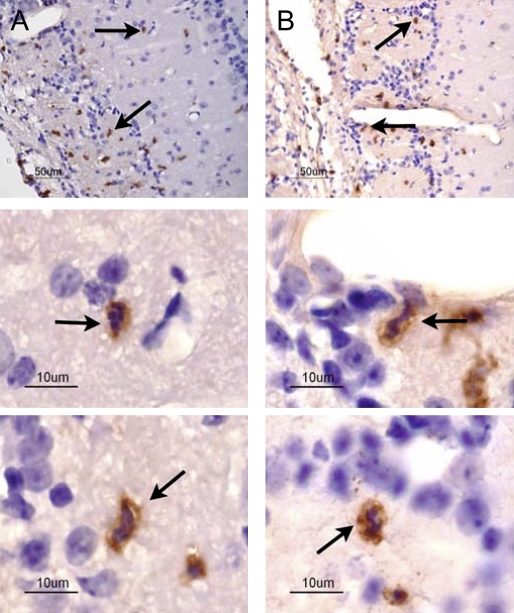
Histological sections of the olfactory bulb following MeBr injury are shown in Figure 4. The low-power image (Figure 4A) provides orientation to the distinct morphological layers of the bulb. Figure 4B shows immunohistochemical staining for MMP-9 and MMP-2 during the degeneration of pre-existing olfactory axons (days 1, 5, and 10). No MMP-9 signal was detected on day 1, confirming the Western blot analysis. At day 5, MMP-9 immunoreactivity was observed in cells in all regions of the bulb, though the cells labeled with MMP-9 were highly concentrated in the olfactory nerve and glomerular layers where the olfactory axons traverse and terminate, respectively (Figure 4B and Table 1). By day 10, MMP-9 immunoreactivity decreased although still detectable within the bulb. MMP-2-labeled cells were not observed at any of the three time points. The combination of DAB and hematoxylin staining (Figure 5 A,B) was used to demonstrate that MMP-9 was localized to neutrophils, which were identifiable on the basis of their lobulated nuclei.
Figure 4.
Coronal sections of the mouse olfactory bulb following MeBr gas exposure. (A) Diagram (left) illustrates approximate location of histological images. Image (right) shows the different anatomical layers of the olfactory bulb. (B) Images stained for MMP-9 and MMP-2 at recovery days 1, 5, and 10. MMP-9 expression was not observed at day 1. MMP-9-labeled cells were detected in all layers of the olfactory bulb, concentrated within the olfactory nerve and glomerular layers at day 5. Fewer MMP-9-labeled cells were observed at day 10. MMP-2 labeled cells were not observed at day 1, 5, or 10. GCL, granular cell layer; IPL, internal plexiform layer; MCL, mitral cell layer; EPL, external plexiform layer; GL, glomerular layer; ONL, olfactory nerve layer. This figure appears in color in the online version of Chemical Senses.
Table 1.
Average number of MMP-9-positive cells and area measurements for olfactory bulb layers
| Bulb layer(s) | Mean ± SD |
|||
| Day 1 | Day 5 | Day 10 | Area (mm2) | |
| ONL and GL | 15.0 ± 3.0 | 236.3 ± 29.8 | 39.3 ± 11.8 | 0.171 ± 0.29 |
| EPL | 5.0 ± 1.7 | 72.0 ± 3.6 | 17.3 ± 10.5 | 0.163 ± 0.050 |
| ML and IPL | 1.7 ± 0.5 | 10.3 ± 2.1 | 3.3 ± 2.5 | 0.057 ± 0.025 |
| GCL | 2.7 ± 2.1 | 49.0 ± 8.9 | 9.7 ± 6.5 | 0.187 ± 0.096 |
ONL, olfactory nerve layer; GL, glomerular layer; EPL, external plexiform layer; ML, mitral layer; IPL, internal plexiform layer; GCL, granular cell layer.
Figure 5.
Localization of MMP-9 to inflammatory cells in the olfactory bulb at day 5. (A) and (B) At low magnification (40×), MMP-9-labeled cells are observed in the olfactory nerve and glomerular layers of the olfactory bulb. Lower panels: At high magnification (60×), MMP-9 labeling was observed in neutrophils, having the characteristic polymorphic nuclei. Arrows in (A) and (B) (low magnification) identify cells shown below at high magnification.
Discussion
Comparison of the two injury models
Following trauma, the CNS uses different mechanisms to prevent further injury and to initiate a recovery process. Most CNS injury models include both deafferentation and direct trauma and therefore are unable to distinguish between the two. This limits our understanding of how the CNS responds to and resolves deafferentation versus direct trauma. The olfactory system offers a unique opportunity to assess mechanisms following both deafferentation (MeBr) and direct traumatic injury (NTx) in the CNS. These two olfactory injury models result in deafferentation followed by a reinnervation of the olfactory bulb. MeBr is a peripheral deafferentation injury for which there is no direct lesion to the CNS since the olfactory epithelium is separated from the bulb by the bony cribriform plate. In contrast, NTx injury includes direct trauma to CNS tissue including the olfactory bulb and nerve layer in addition to bulb deafferentation. Comparing these two injury models allows for the identification of processes associated with deafferentation and/or trauma. Expression of MMPs in both injury models suggests they play a common role in deafferentation of the olfactory bulb.
MMPs in CNS injury
Recovery from CNS injury requires the remodeling of the ECM and breakdown of scar tissue for successful recovery. MMPs have become the focus of recent studies of neuronal injury due to their ability to degrade many prominent components of the ECM and scar. Previous reports demonstrated that MMP-9 and MMP-2 have temporal expression patterns following different CNS trauma models including olfactory NTx, spinal cord injury, and stroke suggesting an important role in neuronal injury and recovery (Romanic et al. 1998; de et al. 2000; Costanzo et al. 2006; Costanzo and Perrino 2008). MMP-9 expression increases early in response to these CNS injuries, corresponding to inflammation, vascular breakdown, edema, and neuronal death. The inflammatory leukocytes are colocalized with MMP-9. These cells may use MMP-9 to penetrate the blood vessel walls and migrate through the ECM to reach the site of injury (Fleming et al. 2006; Rosell et al. 2008; Busch et al. 2009). MMP-9 has also been shown to disrupt the ECM and neuronal connections, leading to neuronal degeneration and death (Siebert et al. 2001; Gu et al. 2002). MMP-2 expression increases within a week following many CNS injuries. This corresponds to the reparative phases of neuronal recovery when MMP-2 participates in the remodeling of the ECM, digests scar components, and participate in angiogenesis (Zuo et al. 1998; Montaner et al. 2001; Hsu et al. 2006).
The inhibition of these enzymes has demonstrated the importance of MMPs in the injury and recovery process. In the absence of MMP-9, there is improved recovery in stroke injury models, whereas the inhibition of MMP-2 is detrimental to neuronal recovery (Asahi et al. 2001; Noble et al. 2002; Lee et al. 2004; Hsu et al. 2006). The modulation and temporal expression of MMP-9 and MMP-2 during olfactory injury and recovery provides potential targets for therapeutic intervention and improved outcome.
MMPs in olfactory injury and recovery
In this report, we demonstrate increased MMP-9 and MMP-2 expression in the olfactory bulb after MeBr exposure. Between days 3 and 5, MMP-9 rose rapidly and reached expression levels 33 times higher than in control samples at day 5. This increase following MeBr is isolated to the olfactory system, as demonstrated by the absence of MMP-9 expression in the frontal cortex following MeBr exposure (Figure 2), confirming that MeBr injury does not have a diffuse MMP response in the CNS. The increase in MMP-9 expression in MeBr injury is delayed when compared with the 5-h increase with direct CNS injury such as olfactory NTx. This delay in MMP-9 expression may reflect the time necessary for the degeneration process to reach the axon terminals within the bulb. In contrast, the rapid response of MMP-9 following NTx could be due to components of direct injury, specifically vascular disruption and trauma to CNS structures such as the anterior ventral surface of the bulb. With vascular injury, components of clotting cascade are increased, including tissue plasminogen activator and urokinase plasminogen activator, both known regulators of MMP-9 expression (Menshikov et al. 2002; Wang et al. 2003). Since MeBr injury occurs within the olfactory epithelium, a site distant from the olfactory bulb, bleeding is unlikely to occur within the CNS and therefore vascular-induced MMP-9 expression is not expected.
The constellation of findings in the olfactory bulb after MeBr exposure are similar in many respects to direct CNS injury (i.e., NTx), and therefore, MMP-9 may be playing a common role. During the initial recovery period following neuronal injury, two processes that rely on MMP-9, glial scar formation and leukocyte infiltration, are increased at the site of injury (Hsu et al. 2008). We demonstrated that MMP-9 is localized to neutrophils concentrated in the region of the injured nerve axonal projections following MeBr (Figure 4 and 5 and Table 1). This suggests that the signal for MMP-9 expression is the deafferentation injury. MMP-9 may allow these inflammatory leukocytes to penetrate through the vascular wall and ECM to reach and degrade the injured axons. Likewise, following MeBr injury, astrocytic activation is observed within the bulb as demonstrated by high levels of GFAP at day 10 and in previous immunohistochemical analysis (Schwob et al. 1999). The increased expression of GFAP paralleled the elevated levels of MMP-9. This association is also demonstrated following olfactory NTx, where GFAP increased 3 days following injury during elevated MMP-9 expression. This suggests similar processes are involved in both MeBr and NTx, leading to increases in MMP-9 expression.
MMP-2 expression was detectable but low in both control and MeBr injury samples, though a 2-fold increase was observed on day 5. Low levels of MMP-2 detection may reflect its constitutive expression in the CNS (Rosenberg 2002). The response of MMP-2 following MeBr is minimal compared with NTx, where up to a 20-fold increase in expression has been reported (Costanzo et al. 2008). This dramatic increase in MMP-2 following NTx occurs 7 days after injury, corresponding to the transition between deafferentation and reinnervation of the bulb. In MeBr injury, this transformation was observed between days 15 and 40, as demonstrated by changes in OMP (Figure 3A). The transition between deafferentation and reinnervation of the bulb following MeBr is delayed compared with NTx and may explain the relatively low MMP-2 expression in our MeBr injury model. The small increase in MMP-2 at day 5 may represent changes in the olfactory bulb with second-order neurons, specifically the mitral and tuft cells. As mitral and tuft cells synaptic connections are lost in the glomerular layer, these neurons may secrete MMP-2 in order to modify the ECM in an attempt to create new synapses. Further work will be necessary to determine if MMP-2 is being expressed by the regenerated first-order neurons, or the second-order neurons, in an attempt to create new synaptic connections.
Conclusion
A comparison of MeBr and NTx injury models has proved useful in uncovering important information underlying neuronal injury and recovery in the olfactory system. In both injury models, we demonstrated an early expression of MMP-9 corresponding to inflammatory processes. This suggests that, regardless of the mechanism of injury, inflammation is a key component of neuronal injury and recovery. Though the importance of MMP-2 is still unclear, we did observe differences in the timing of MMP-2 expression between the two injury models. This is the first report demonstrating that MMP-9 expression is associated with deafferentation in the absence of direct trauma. Further studies are needed to determine the underlying mechanisms and contributions of MMP-9 and MMP-2 to neuronal injury and recovery processes.
Funding
This work was supported by National Institutes of Health [R01 DC00165 to R.M.C. and R01 DC002167 to J.E.S.].
References
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci. 2009;29:9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Colín-Barenque L, Martínez-Hernández MG, Baiza-Gutman LA, Avila-Costa MR, Ordóñez-Librado JL, Bizarro-Nevares P, Rodriguez-Lara V, Piñón-Zarate G, Rojas-Lemus M, Mussali-Galante P, et al. Matrix metalloproteinases 2 and 9 in central nervous system and their modification after vanadium inhalation. J Appl Toxicol. 2008;28:718–723. doi: 10.1002/jat.1326. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Perrino LA. Peak in matrix metaloproteinases-2 levels observed during recovery from olfactory nerve injury. Neuroreport. 2008;19:327–331. doi: 10.1097/WNR.0b013e3282f50c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo RM, Perrino LA, Kobayashi M. Response of matrix metalloproteinase-9 to olfactory nerve injury. Neuroreport. 2006;17:1787–1791. doi: 10.1097/WNR.0b013e32800fef87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de CR, Jr., Burns CL, McAdoo DJ, Romanic AM. Metalloproteinase increases in the injured rat spinal cord. Neuroreport. 2000;11:3551–3554. doi: 10.1097/00001756-200011090-00029. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Goussev S, Hsu JY, Lin Y, Tjoa T, Maida N, Werb Z, Noble-Haeusslein LJ. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J Neurosurg. 2003;99:188–197. doi: 10.3171/spi.2003.99.2.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, Cun CL, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28:13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Tsuji K, Lee SR, Lo EH. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci. 2004;24:671–678. doi: 10.1523/JNEUROSCI.4243-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshikov M, Elizarova E, Plakida K, Timofeeva A, Khaspekov G, Beabealashvilli R, Bobik A, Tkachuk V. Urokinase upregulates matrix metalloproteinase-9 expression in THP-1 monocytes via gene transcription and protein synthesis. Biochem J. 2002;367:833–839. doi: 10.1042/BJ20020663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J, varez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez MA, Monasterio J. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32:1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza R. The reinnervation of the rat olfactory bulb after methyl bromide-induced lesion of the olfactory epithelium. J Comp Neurol. 1999;412:439–457. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Siebert H, Dippel N, Mader M, Weber F, Bruck W. Matrix metalloproteinase expression and inhibition after sciatic nerve axotomy. J Neuropathol Exp Neurol. 2001;60:85–93. doi: 10.1093/jnen/60.1.85. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori T, Jung JC, Fini ME, Lo EH. Secretion of matrix metalloproteinase-2 and -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma. 2002;19:615–625. doi: 10.1089/089771502753754082. [DOI] [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]