Abstract
The aim of the study was to examine the ability of Göttingen minipigs to acquire an olfaction-based operant conditioning task and to determine the detection threshold for ethyl acetate and ethanol. We used an automated olfactometer developed for rodents to train and test 14 pigs. Odor sampling and reliable responding were obtained after three to fifteen 160-trial sessions. Successful transfer of the task from ethyl acetate to ethanol was achieved in 1–4 sessions. Detection threshold for ethyl acetate varied between 10−2% and 10−6% v/v and for ethanol between 0.1% and 5 × 10−6% v/v. The results provide evidence that minipigs can successfully acquire 2-odorant discrimination using a food-rewarded instrumental conditioning paradigm for testing olfactory function. This olfactory discrimination paradigm provides reliable measures of olfactory sensitivity and thereby enables detection of changes in olfaction in a porcine model of Alzheimer's disease currently being developed.
Keywords: odor sensitivity, olfaction, operant conditioning, pigs
Introduction
Impaired olfaction is an early symptom in Alzheimer's disease (AD) (Graves et al. 1999; Devanand et al. 2000; Wilson et al. 2009) and correlates with the presence of senile plaques and neurofibrillary tangles in the olfactory bulbs which are the neuropathological hallmarks of AD (Attems et al. 2005). Among transgenic murine models of AD, none present all characteristic neuropathological lesions and behavioral deviations of AD (Duyckaerts et al. 2008), and the phenotype is often unpredictable and diverges between different genetic lines (Gotz et al. 2004).
The pig (Sus scrofa domesticus) may provide a better animal model as it is genetically and physiologically more closely related to humans than rodents are. To this end, we demonstrated the use of a transgene insertion to induce expression of the AD-causing dominant mutation APPsw in the brain of cloned Göttingen minipigs (Kragh et al. 2009). To examine early phenotypic changes, we have initiated a research program to assess olfaction in the early stages of AD in Göttingen minipigs. Olfactory tasks may be particularly well suited to assess cognitive function (Slotnick 2001), and prior studies have demonstrated that large pig breeds are trainable using operant conditioning to discriminate odors (Meese et al. 1975; Dorries et al. 1995; Jones et al. 2001). However, there have been few behavioral studies using the Göttingen minipig, and its suitability for such conditioning studies is largely unknown. Thus, the aim of the present study was to examine the ability of Göttingen minipigs to acquire an olfactory discrimination task which can be used to assess olfactory learning and to obtain some baseline values for a future porcine model of AD.
Materials and methods
Animals, housing, and feeding
Fourteen Göttingen minipigs (Ellegaard Göttingen Minipigs A/S, Dalmose, Denmark), 8 females (ID F1–F8) and 6 males (ID M1–M6) (pairs of siblings from 7 litters), were used in the experiment. The pigs arrived at the research facility at 4–5 months of age. The introductory training (see below) was initiated at 8–9 months of age, when the pigs weighed 12–20 kg. Animals were housed in pairs in a temperature-controlled pig house in pens of 2.40 × 1.90 m and fed twice daily at 0700 and 1400 h with standard minipigs pellets (Special Diets Services) according to producer's recommendation. Water was supplied manually twice daily. The pig house was lit by electric lights from 0700 to 1900 h in addition to natural light. The animals were kept on wood shavings, and artificial environmental enrichment was provided (plastic balls, metal chains, wood bricks, chew rubber toys, etc.). The day before a training session pigs were fed 70% of their daily ration. This reduction is often used to enhance food as a reinforcer in pigs (Klopfer 1966; Kornum et al. 2007; Nielsen et al. 2009). Each pig was trained 1–2 times per week during 6 months. All animal experiments were performed in accordance with the European Communities Council Resolves of 24 November 1986 (86/609/ECC) and approved by the Danish Experimental Animal Inspectorate (journal number 2006/561−1156).
Apparatus
Animals were trained and tested using a liquid dilution olfactometer (Knosys Olfactometers Inc.) modified for minipigs. The 8-channel odor generator has been described in detail (Slotnick and Restrepo 2005). Briefly, each channel consisted of a 200 mL PVC bottle whose input and output C-flex lines were controlled by pinch valves. Odors were generated by passing 50 cc/min of air over the odorant material within the bottle and adding that output to a 1950 cc/min flow of clean air. The operant chamber (116 × 66 × 70 cm) had a hinged back wall for introducing the subject. The front wall contained a plexiglas odor sampling port, a response lever, and a food tray. The 8 × 8 cm odor sampling port was mounted on the outside of the chamber and an 8 cm diameter hole cut through the back wall and sampling port allowed access for the animal's snout. Snout insertions into the sampling port were monitored by a photocell. A lever could be operated by the pig by raising it with the snout. During the initial training, it was mounted 5 cm beneath the odor sampling port. During the rest of the testing, it was moved 22 cm to the left (see later). The reward for correct responses was a 0.9 g chocolate pellet delivered by a pellet dispenser (Med Associates Inc.). A light located near the sampling port was used to signal the end of the intertrial interval to the pig.
Stimuli
Ethyl acetate (Sigma-Aldrich Danmark A/S) and ethanol (VWR—Bie & Berntsen A/S) were used as odorants and were of the highest purity available. Both odorants have been used in prior animal olfactometric studies (e.g., Laska 1990; Laska and Seibt 2002a; Doty et al. 2003; Slotnick 2007), and ethyl acetate has been widely used as a training stimulus (e.g., Doty and Ferguson-Segall 1987; Bodyak and Slotnick 1999; Joly et al. 2004). Odorants were diluted v/v with purified and UV photooxidated water (Millipore A/S) to the desired concentration, and 10 mL solution was used as the odorant source in the odor saturation bottles. Bottles were changed daily and cleaned in ion-exchanged water and 70% ethanol. Odorant concentrations are presented as the liquid dilution of the odorant in the saturator tubes. The 50 cc/min odorant vapor from the saturator tube was manifolded with 1950 cc/min of clean air before being introduced to the sampling port and, therefore, the odor concentration delivered to the animal sampling port was approximately 2.5% of the concentration of the headspace above the liquid odorant. The odorant concentration of the headspace above the liquid solution is not known, but gas chromatographic analyses indicate that headspace concentrations of a wide variety of hydrocarbons from mineral oil dilutions are proportional to their liquid dilution (Cometto-Muniz et al. 2003).
Training and test procedures
Initial training
Subjects were transported individually from their pen to the operant chamber in a trolley. In initial sessions, food rewards (chocolate pellets) were delivered to the reinforcement tray every 20 s for 10 min on day one and for 20 min on day 2. Pellet delivery was signaled by the brief onset of a buzzer. Next, the minipig's behavior was shaped by reinforcing successive approximations to lever pressing with its snout. Training was continued until the pig responded by pressing the lever for two 60-trial sessions. Next, the minipigs were shaped to insert the snout into the odor sampling port and, in the last stage of this training, to insert its snout into the odor sampling port and then operate the lever for a reward (two 60-trial sessions). The first snout insertion at the end of a 5-s intertrial interval resulted in presentation of the positive (S+) odor stimulus (ethyl acetate 1% v/v) in the sampling port. To obtain a reward, the minipig was required to keep its snout in the odor sampling port for at least 1 s and then respond by raising the lever within 6 s. Initial training was terminated when the minipig responded reliably in 6 sessions of 100 of these S+-only trials.
Discrimination task
Procedures during discrimination training were identical except that both positive (ethyl acetate 1% v/v) and negative (water) trials were presented, and the use of a time-out punishment of 10 s for responding during an S− trial. S+ and S− trials were presented in a modified random order ensuring an equal number of each in each block of 20 trials.
The go/no-go discrimination method described by Slotnick and Restrepo (2005) was used. Making a criterion response (lever press) within 6 s after delivery of the S+ was rewarded by delivery of a chocolate pellet and was scored as a hit. Making a criterion response after delivery of the S− was not rewarded and was scored as a false alarm. Not responding to an S+ trial was scored as a miss, and not responding to an S− trial was scored as a correct rejection. The termination of each trial initiated the intertrial interval. Accuracy scores (mean percent correct responses) were computed for each block of 20 trials (((Hits + Correct rejections)/20) × 100). The session was terminated when the pig had completed 8 blocks (160 trials).
Transfer task
To investigate the ability of pigs to perform the task with a novel odorant, ethyl acetate was replaced by 95% ethanol (v/v).
Olfactory detection threshold
After completion of the initial training, pigs were tested for their ability to detect sequential dilutions of ethyl acetate (n = 7) or ethanol (n = 4). Thus, pigs were exposed to successively lower concentrations in each session. The session was terminated when the pig had reached 85% or more correct responses in 5 blocks or when the pig had completed 8 blocks; thus, the number of trials per session varied between 100 and 160 trials. If criterion performance was not achieved, training at that concentration was continued until criterion performance was obtained or for a maximum of 3 sessions. The concentrations of ethyl acetate used in these tests were log10 steps from 1 to 1 × 10−10 (percent v/v), whereas concentrations of ethanol used were approximately binary dilutions of the odorant (from 10% to 10−6%) because log10 steps showed to be too large intervals.
Data analysis
The criterion for olfactory discrimination was retrospectively set at a mean of 75% correct responses in a 160-trial session during initial training and discrimination task. This criterion is statistically highly significant according to the 2-tailed binomial probability test. Actually, 93 correct responses in 160 trials (corresponding to 58% correct responses) entail a probability of P < 0.05. However, because we were interested in a high degree of sensitivity in order to detect even subtle changes in olfactory functioning, we decided on a relatively strict criterion of success. In the olfactory detection tasks, the criterion for olfactory discrimination was set at a mean of 75% correct responses in sessions of 100–160 trials, as described previously. The percentage of correct responses was calculated for each individual. Results are expressed as mean percentage of correct responses per session.
Results
Discrimination task
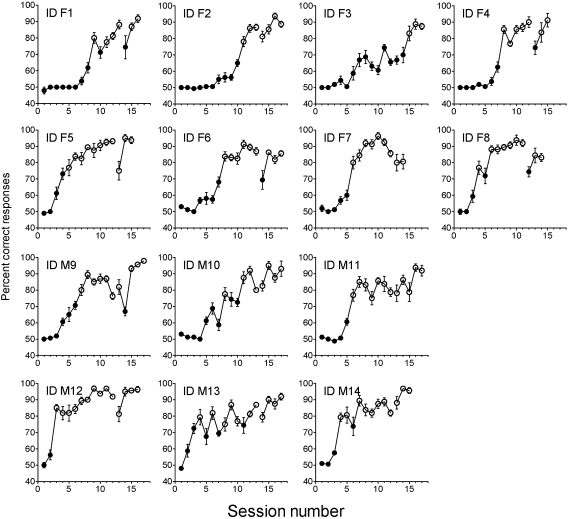
Acquisition functions for 14 minipigs trained to discriminate between 1% ethyl acetate and water are presented in Figure 1. The first session in this series followed initial training using only presentation of S+ and was the first session in which both S+ and S− were used (see Materials and methods section). As shown in Figure 1, minipigs required multiple sessions of training on the olfactory discrimination task before reaching criterion performance. On average, pigs made 633.3 errors (standard deviation, 204.5) in achieving criterion, almost all of which (98%) were false alarms. ID M12 had the most rapid learning and achieved criterion in the third session, making 325 errors. The slowest learner (ID F3) performed at chance (scores of 45–55% correct responses) on each block of trials in 4 training sessions before achieving block scores of 65% and 70%. However, this level of accuracy was not sustained and criterion was not achieved until session 15 (1023 errors). Two patterns of acquisition were observed: most pigs performed at or near chance on essentially all blocks of trials before a sudden increase in accuracy occurred within a session. This pattern is also reflected in the mean session scores shown in Figure 1 (e.g., ID F1, F2, F4, and M14). Only 4 pigs showed what might be described as a gradual acquisition of the task (e.g., ID F6, M9, and M13 in Figure 1).
Figure 1.
Learning curves regarding olfactory discrimination for each of 14 individual minipigs. Mean percent correct responding (±standard error of the mean) on each 160-trial session. S+ was a 1% aqueous solution of ethyl acetate, and S- was water solvent. Gaps in lines denote a 1-month pause in testing during which the apparatus was optimized by moderate modifications. To master the task, individuals must obtain a minimum 75% correct responses in one 160-trial session. Success in reaching criterion is denoted by a blank circle. Females (ID F1-F8), males (ID M9-M14).
Once criterion performance was achieved, 8 of the pigs continued to perform at high levels above criterion. However, performance accuracy of 6 pigs decreased to below criterion after criterion was reached for one session (ID F1 session 10; ID F8 session 5; ID M9 session 14; and ID M14 session 6), 2 sessions (ID M10 sessions 9 and 10), and 3 sessions (ID M13 sessions 5, 7, and 11) but then improved and maintained high accuracy scores.
Transfer task
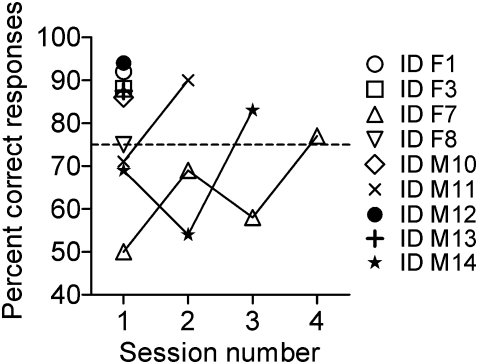
Following completion of training on 1% ethyl acetate, 9 pigs were trained using 95% ethanol as S+ (Figure 2). ID F1, F3, F8, M10, M12, and M13 mastered the task in the first session. One subject (ID M11) reached criterion of success in session 2, whereas one subject (ID M14) used 3 sessions to succeed and one subject (ID F7) achieved the criterion in session 4. The performance of one animal (ID F6) was unstable but almost reached success criterion after 6 sessions (data not shown).
Figure 2.
Learning curves regarding olfactory discrimination of a novel odorant for 9 minipigs. Mean percent correct responding on each 160-trial session. S+ was a 95% aqueous solution of ethanol, and S- was the water solvent. Horizontal reference line (y = 75) denotes criterion of success. To master the task, individuals should reach at least 75% correct responses in one 160-trial session.
Ethyl acetate detection threshold
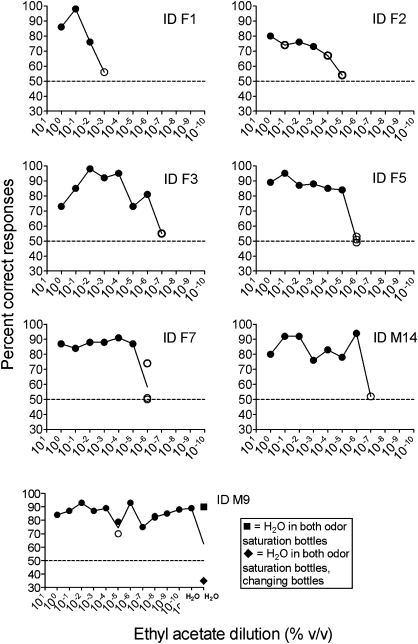
Results from 7 minipigs tested with continuously weaker concentrations of ethyl acetate are shown in Figure 3. The odor detection threshold for olfactory ethyl acetate detection was found to be 10−6% for 2 animals (ID F3 and M14), 10−5% for 2 animals (ID F5 and F7), 10−3% for one animal (ID F2), and 10−2% for one animal (ID F1). Regarding one animal (ID M9), it was not possible to establish the olfactory detection threshold within the concentration range (10−10%) used in the test.
Figure 3.
Performance of minipigs in detecting descending concentrations of ethyl acetate. Each data point represents mean percentage correct choices from 5 to 8 blocks of 20 trials comprising a total of 100-160 decisions. In case of more than one replicate at one concentration, a line is drawn at the mean value (ID F5, F7, and M9). Horizontal reference line (y = 50) denotes chance level. To master the task, individuals should reach a mean of minimum 75% correct responses in one 100- to 160-trial session. Failure in reaching success criterion is denoted by a blank circle. ID M9 showed a peculiar performance as it was not readily possible to determine the olfactory detection threshold for ethyl acetate. However, when exchanging S+ with water in odor saturator bottle number one (▪), the pig still performed perfectly, whereas the performance reached a below chance level (35% correct response) when odor saturator bottle number 1 and 2 both containing water were interchanged (♦).
Ethanol detection threshold
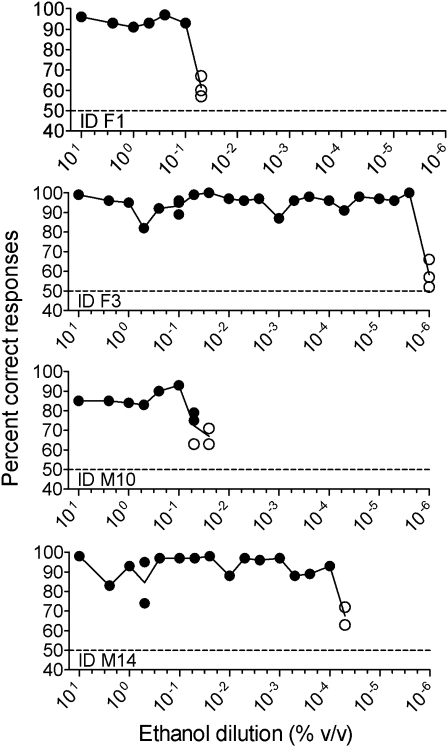
Of 4 animals tested with successively lower concentrations of ethanol, one (ID F1) reached olfactory detection threshold at 0.1% ethanol, one (ID M10) reached olfactory detection threshold at 0.025%, one (ID M14) reached threshold at 10−4%, and threshold for one (ID F3) was reached at 5 × 10−6% (Figure 4).
Figure 4.
Determination of ethanol detection threshold for 4 minipigs. Each data point represents mean percentage correct choices from 5 to 8 blocks of 20 trials comprising a total of 100-160 decisions. In case of more than one replicate at one concentration, a line is drawn at the mean value. Horizontal reference line (y = 50) denotes chance level. To master the task, individuals should reach a mean of minimum 75% correct responses in one 100- to 160-trial session. Failure in reaching success criterion is denoted by a blank circle.
Discussion
This is the first study demonstrating odor discrimination learning and detection in the Göttingen minipig, which strongly rely on their sense of smell in various behavioral contexts. The olfactometer designed for use with rodents was suitable for the minipig with only small modifications for accommodating a larger animal species. Thus, the olfactometer enabled investigation of olfactory abilities with a high degree of control with respect to stimulus presentation and operant behavior. Although intra- and interindividual variation was present, indications regarding the olfactory detection threshold values (ethyl acetate and ethanol) for minipigs were obtained, thus enabling estimations of a baseline for a future porcine model of AD.
Discrimination task
All 14 animals were able to acquire the odor discrimination task based on the principles of go/no-go, according to which the animal learns to press a lever when presented with an S+ (odor) and to abstain from pressing the lever in case of an S− (solvent). However, the learning speed varied considerably between animals, with some minipigs acquiring the task more rapidly (e.g., ID F5, F8, M12, M13, and M14; Figure 1) compared with others (e.g., ID F2 and F3). This variation was partly abated by separating the reinforcement delivery tray and the operant lever by a larger distance. Acquisition of the task depends on the ability to attend to the stimulus, associate the odor with the food reward, and abstain from responding to the S−. Various reasons may account for the individually unstable performance because several factors including learning ability, memory capabilities, sensory functioning, and stress level of an animal undoubtedly can influence the behavior in a given situation.
Comparing the minipigs' performance with other species trained on 2-odor discrimination tasks using food-rewarded operant conditioning procedures shows that the speed of acquiring the task (480–2340 stimulus contacts till criterion) varies more than with fur seals (480–880 stimulus contacts) (Laska et al. 2008), spider monkeys (660–720 stimulus contacts) (Laska et al. 2003), or pigtail macaques (960–1800 stimulus contacts) (Hubener and Laska 2001). In dogs (Lubow et al. 1973), rats (Slotnick et al. 1991), and mice (Bodyak and Slotnick 1999) the speed of acquiring the task is considerably higher compared with the findings of the present study. Noteworthy, the criterion used in the present study (75% correct responses in a 160-trial session) is rigid compared with other studies (e.g., Laska and Hudson 1993; Laska et al. 2003; Laska et al. 2008). Consequently, the session length may advantageously be reduced resulting in a P value closer to 0.05 in future studies with minipigs, especially because they demonstrated substantial difficulties inhibiting responses in case of S− presentation. In the present study, sessions typically lasted 30–40 min. In a study on olfactory function in the gray mouse lemur, motivation dropped after 20 min (Joly et al. 2004), which supports a reduction in number of trials per session in future studies with minipigs. The present learning speed is comparable with a study on acquisition of visually guided conditional associative tasks in Göttingen minipigs. Here, 11 of 14 minipigs reached the behavioral criterion (90% correct for each of 2 consecutive 100-trial sessions) on the conditional go/no-go task in 16 or less sessions (Moustgaard et al. 2005).
To avoid confounding effects of side preferences, the go/no-go paradigm was used. Yet, virtually all errors were false alarms, responding to S−, indicating that in this species, acquisition is almost completely a function of inhibiting responses to S− trials. When required not to act on a stimulus, some minipigs reacted with intense oral manipulation of the operant chamber inventory, which may be an indicator of frustration. It may be speculated that using a paradigm with separate response devises for S+ and S− requiring the animals to respond to both types of stimulus could eliminate the obstacles regarding inhibiting responses on S− trials.
Transfer task
Ethanol was used as a novel odor to investigate transfer of learning, that is, the influence of prior learning with ethyl acetate on performance in a new situation, where S+ was ethanol and S− was water solvent. Nine minipigs were able to make a transfer from one odorant to a novel S+ during one to four 160-trial sessions (Figure 2). Interestingly, individual minipigs may acquire the task relatively fast with one odor, although acquirement of the task when presented with another odor is more challenging. For instance, the ethyl acetate learning curve of ID F3 was relatively shallow (Figure 1) but acquired the task excellently when presented with ethanol (Figure 2). Reversely, ID F7 learned the ethyl acetate task comparatively fast (Figure 1), whereas acquisition of the ethanol task was slower (Figure 2).
Olfactory detection threshold
Our results concerning minipigs' ethyl acetate sensitivity indicate interindividual variability which is generally larger (10−2% to 10−6%) than the range of 1–2 orders of magnitude reported in studies on olfactory sensitivity in humans (Cometto-Muniz et al. 2008), and in short-tailed fruit bats in which interindividual variation was not observed to exceed one order of magnitude (Laska 1990) as well as in studies with squirrel monkeys and pigtail macaques (Salazar et al. 2003). Likewise, the minipigs' ethanol sensitivity varied more than 4 orders of magnitude between individuals, a variation which is large compared with, for instance, short-tailed fruit bats not exceeding one order of magnitude (Laska 1990) or squirrel monkeys and pigtail macaques (Laska and Seibt 2002a). The results are obtained with a small number of animals and are only an indication of the variability of the threshold levels for the 2 odorants within this species. Further studies are needed to establish a baseline for olfactory detection of minipigs of comparable age.
Despite marked variability between individuals in the Göttingen minipig, comparison with the olfactory detection threshold values found in other mammalian species using instrumental conditioning paradigms is interesting. Across-species comparisons call for caution due to the use of different methods. The lowest olfactory detection threshold values for ethyl acetate obtained with the minipigs (10−6%) is comparable with those of spider monkeys (Salazar et al. 2003), squirrel monkeys (Laska and Seibt 2002b), mice (Bodyak and Slotnick 1999), short-tailed fruit bats (Laska 1990), and vampire bats (Schmidt 1975). Pigtail macaques (Laska and Seibt 2002b) and humans (Cometto-Muniz and Cain 1991) have been found to be more sensitive to ethyl acetate compared with these minipig data, and the rat even more sensitive (Moulton 1960).
With ethanol, olfactory detection threshold data have been published in several species. The lower olfactory detection threshold values obtained with the Göttingen minipigs (5 × 10−6%) outperform species such as rats (Moulton and Eayrs 1960), humans (Cometto-Muniz and Cain 1991), squirrel monkeys, and pigtail macaques (Laska and Seibt 2002a). However, the sensitivity to ethanol of short-tailed fruit bats is higher (Laska 1990).
Olfactory detection threshold values have been obtained with large pig breeds for other odorants. Dorries et al. (1995) found that adult Large white × Landrace × Hampshire obtained an olfactory detection threshold for geraniol at 1.8 × 10−6 M. Jones et al. (2001) showed that juvenile Duroc × Landrace achieved an olfactory detection threshold for butanol at 2.09 parts per trillion.
To explain similarities or differences in olfactory performance among or within species, it is appropriate to consider whether given odorants or classes of odorant differ in their degree of behavioral relevance for a species, as discussed by for instance Laska and Seibt (2002a).
One individual minipig (ID M9) performed particularly strange in the ethyl acetate detection test. At one time, the performance approached chance level, but when retested at the same concentration (10−5%), performance exceeded criterion of success again. After passing 10−10%, we carried out a control procedure exchanging the odorant with water, thus presenting S− in both odor saturator bottles. Still, the subject performed above criterion of success. In the next session, we repeated the control procedure using S− in both bottles (still, odor saturation bottle number 1 was assigned as the S+ and odor saturation bottle number 2 was assigned as the S−). Again the minipig performed above criterion of success. After 80 trials, we interchanged the position of the 2 bottles in the olfactometer and following the subject's performance decreased to well below chance level (35% correct responses). It may be argued that the odor saturator bottle was contaminated. Alternatively, the animal seemed to have learned to use another sensory modality than olfaction to solve the task. It can be difficult to control an animal's attention or focus on a sensory stimulus. Animals may discover clues to the stimulus and, as discussed by Slotnick and Restrepo (2005), olfactory functioning is difficult to measure because of lack of control regarding stimulus presentation. In the present study, control procedures suggested that minipig ID M9 responded to the odor saturator bottle and not to the odorant or the valve. These findings point to the importance of being cautious because variations in response patterns should reflect changes in stimulus presentation and not be a consequence of an animal discovering other clues to the stimulus such as differences in the sounds of equipment, nonrandom stimulus presentation, and illumination differences (Arave 1996).
Conclusion
The results of the present study provide evidence that minipigs can successfully acquire 2-odorant discrimination using a food-rewarded instrumental conditioning paradigm for testing olfactory function. This olfactory discrimination paradigm allowed us to obtain reliable measures of olfactory sensitivity and discriminability and thereby potentially detect early behavioral changes in a future porcine model of AD.
Acknowledgments
This work was supported by the Danish Agency for Science Technology and Innovation [grant number 274-05-0197], and by the Institute of Clinical Medicine, Aarhus University.
References
- Arave CW. Assessing sensory capacity of animals using operant technology. J Anim Sci. 1996;74:1996–2009. doi: 10.2527/1996.7481996x. [DOI] [PubMed] [Google Scholar]
- Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer's disease: an autopsy study. J Alzheimers Dis. 2005;7:149–157. doi: 10.3233/jad-2005-7208. [DOI] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS. Nasal pungency, odor, and eye irritation thresholds for homologous acetates. Pharmacol Biochem Behav. 1991;39:983–989. doi: 10.1016/0091-3057(91)90063-8. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chem Senses. 2003;28:467–477. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS, Abraham MH, Gil-Lostes J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol Behav. 2008;95:658–667. doi: 10.1016/j.physbeh.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. Olfactory sensitivity to the pheromone, androstenone, is sexually dimorphic in the pig. Physiol Behav. 1995;57:255–259. doi: 10.1016/0031-9384(94)00225-t. [DOI] [PubMed] [Google Scholar]
- Doty RL, Bagla R, Misra R, Mueller E, Kerr KL. No influence of scopolamine hydrobromide on odor detection performance of rats. Chem Senses. 2003;28:761–765. doi: 10.1093/chemse/bjg067. [DOI] [PubMed] [Google Scholar]
- Doty RL, Ferguson-Segall M. Odor detection performance of rats following d-amphetamine treatment: a signal detection analysis. Psychopharmacology (Berl) 1987;93:87–93. doi: 10.1007/BF02439592. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Potier M-C, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinski P, Chen F. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Hubener F, Laska M. A two-choice discrimination method to assess olfactory performance in pigtailed macaques, Macaca nemestrina. Physiol Behav. 2001;72:511–519. doi: 10.1016/s0031-9384(00)00447-9. [DOI] [PubMed] [Google Scholar]
- Joly M, Michel B, Deputte B, Verdier JM. Odor discrimination assessment with an automated olfactometric method in a prosimian primate, Microcebus murinus. Physiol Behav. 2004;82:325–329. doi: 10.1016/j.physbeh.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Jones JB, Wathes CM, Persaud KC, White RP, Jones RB. Acute and chronic exposure to ammonia and olfactory acuity for n-butanol in the pig. Appl Anim Behav Sci. 2001;71:13–28. doi: 10.1016/s0168-1591(00)00168-4. [DOI] [PubMed] [Google Scholar]
- Klopfer FD. Visual learning in swine. In: Bustad LK, McClelland RO, editors. Swine in Biomedical Research. Washington (DC): Batelle Memorial Institute; 1966. [Google Scholar]
- Kornum BR, Thygesen KS, Nielsen TR, Knudsen GM, Lind NM. The effect of the inter-phase delay interval in the spontaneous object recognition test for pigs. Behav Brain Res. 2007;181:210–217. doi: 10.1016/j.bbr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bogh IB, Holm IE, Jakobsen JE, Johansen MG, et al. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer's disease-causing dominant mutation APPsw. Transgenic Res. 2009;18:545–558. doi: 10.1007/s11248-009-9245-4. [DOI] [PubMed] [Google Scholar]
- Laska M. Olfactory sensitivity to food odor components in the short-tailed fruit bat, Carollia perspicillata (Phyllostomatidae, Chiroptera) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1990;166:395–399. [Google Scholar]
- Laska M, Hudson R. Assessing olfactory performance in a New World primate, Saimiri sciureus. Physiol Behav. 1993;53:89–95. doi: 10.1016/0031-9384(93)90015-8. [DOI] [PubMed] [Google Scholar]
- Laska M, Salazar LT, Luna ER. Successful acquisition of an olfactory discrimination paradigm by spider monkeys, Ateles geoffroyi. Physiol Behav. 2003;78:321–329. doi: 10.1016/s0031-9384(02)00976-9. [DOI] [PubMed] [Google Scholar]
- Laska M, Seibt A. Olfactory sensitivity for aliphatic alcohols in squirrel monkeys and pigtail macaques. J Exp Biol. 2002a;205:1633–1643. doi: 10.1242/jeb.205.11.1633. [DOI] [PubMed] [Google Scholar]
- Laska M, Seibt A. Olfactory sensitivity for aliphatic esters in squirrel monkeys and pigtail macaques. Behav Brain Res. 2002b;134:165–174. doi: 10.1016/s0166-4328(01)00464-8. [DOI] [PubMed] [Google Scholar]
- Laska M, Svelander M, Amundin M. Successful acquisition of an olfactory discrimination paradigm by South African fur seals, Arctocephalus pusillus. Physiol Behav. 2008;93:1033–1038. doi: 10.1016/j.physbeh.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Kahn M, Frommer R. Information processing of olfactory stimuli by the dog: 1. The acquisition and retention of four odor-pair discriminations. Bull Psychon Soc. 1973;1:143–145. [Google Scholar]
- Meese GB, Conner DJ, Baldwin BA. Ability of the pig to distinguish between conspecific urine samples using olfaction. Physiol Behav. 1975;15:121–125. doi: 10.1016/0031-9384(75)90289-9. [DOI] [PubMed] [Google Scholar]
- Moulton DG. Studies in olfactory acuity: III. Relative detectability of n-aliphatic acetates by the rat. Quart J Exp Psychol. 1960;12:203–213. [Google Scholar]
- Moulton DG, Eayrs JT. Studies in olfactory acuity: II. Relative detectability of n-aliphatic alcohols by the rat. Quart J Exp Psychol. 1960;12:99–109. [Google Scholar]
- Moustgaard A, Arnfred SM, Lind NM, Hemmingsen R, Hansen AK. Acquisition of visually guided conditional associative tasks in Gottingen minipigs. Behav Processes. 2005;68:97–102. doi: 10.1016/j.beproc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Nielsen TR, Kornum BR, Moustgaard A, Gade A, Lind NM, Knudsen GM. A novel spatial Delayed Non-Match to Sample (DNMS) task in the Gottingen minipig. Behav Brain Res. 2009;196:93–98. doi: 10.1016/j.bbr.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Salazar LTH, Laska M, Luna ER. Olfactory sensitivity for aliphatic esters in spider monkeys (Ateles geoffroyi) Behav Neurosci. 2003;117:1142–1149. doi: 10.1037/0735-7044.117.6.1142. [DOI] [PubMed] [Google Scholar]
- Schmidt U. Vergleichende Riechschwellenbestimmungen bei neotropischen Chiropteren (Desmodus rotundus, Artibeus lituratus, Phyllostomus discolor) Z Säugetierkunde. 1975;40:269–298. [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends Cogn Sci. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Response accuracy and odor sampling time in mice trained to discriminate between enantiomers of carvone and those of terpinen-4-ol. Chem Senses. 2007;32:721–725. doi: 10.1093/chemse/bjm039. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Restrepo D. Olfactometry with mice. Current protocols in neuroscience, unit 8.20. 2005 doi: 10.1002/0471142301.ns0820s33. Hoboken (NJ): Wiley InterScience. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Kufera A, Silberberg AM. Olfactory learning and odor memory in the rat. Physiol Behav. 1991;50:555–561. doi: 10.1016/0031-9384(91)90545-y. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann NY Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]