Abstract
In the last 15 years, our understanding of the cellular basis of gastrointestinal function has been altered irreversibly by the discovery that normal gastrointestinal motility requires interstitial cells of Cajal (ICC). Research in this relatively short time period has modified our original concept that the core unit that controls motility is made up of nerves and smooth muscle, to one that now includes ICC. This concept has now expanded to beyond the gastrointestinal tract, suggesting that it may be a fundamental property of the regulation of smooth muscle function that requires rhythmic contraction. ICC are distributed throughout the gastrointestinal tract, have important functions in the control of gastrointestinal motility and are often abnormal in diseased states. Recently, significant steps forward have been made in our understanding of the physiology of ICC as well as mechanisms of injury and recovery. These advances will be the focus of this review.
The physiology of ICC
Unique motor patterns are intrinsic to every organ of the gastrointestinal tract, which suit their functions related to mixing, absorption and anally directed movement. ICC are an integral part of the control of these motor activities. The distribution of ICC throughout the musculature is associated with nerve structures. ICC surround the Auerbach’s or myenteric plexus and are associated with nerve varicosities throughout the muscle layers, the so called intramuscular ICC (Figures 1,2). Other subpopulations of ICC are associated with non-ganglionated plexuses of nerve varicosities at the inner borders of the circular muscle layers in the intestine and colon (Figures 1,2). The best understood function is that of pacemaker activity in the stomach and small intestine where the ICC generate a periodic depolarization at a characteristic frequency in each of these organs that is called the slow wave or pacemaker activity. This involves rhythmic oscillations of intracellular calcium and activation of membrane ion channels that causes depolarization. Ion channels viewed as important in the generation of pacemaker activity include non-selective cat-ion channels 1 calcium-activated chloride channels 2–4 and sodium channels 5,
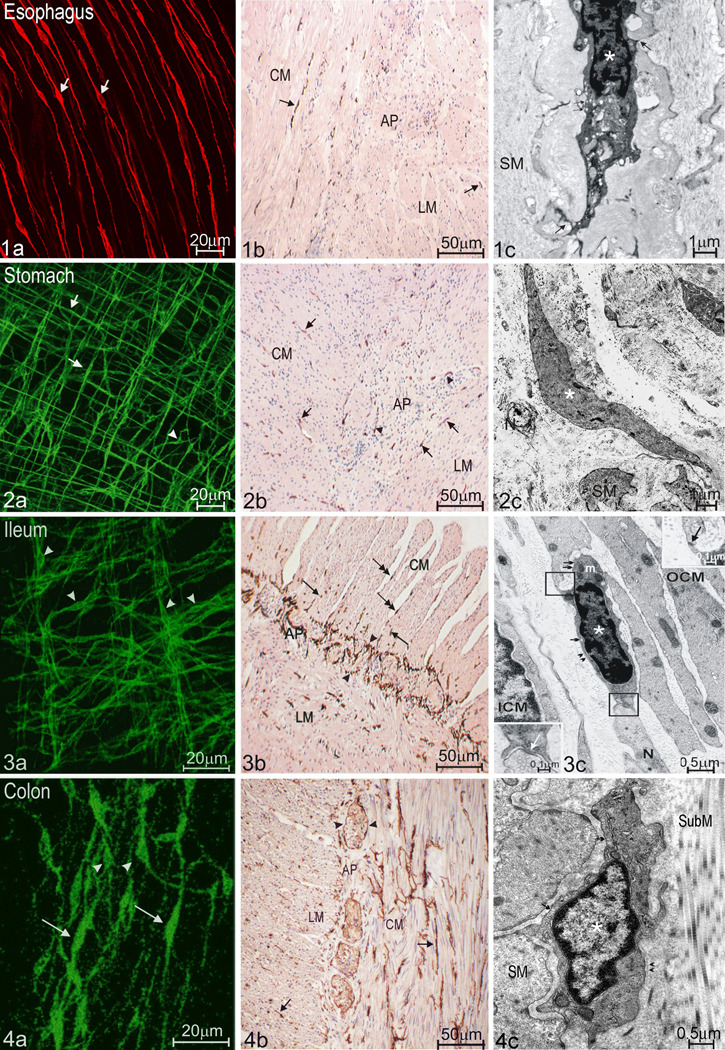
Figure 1. Subtypes and distribution of ICC along the GI tract as shown by various morphological techniques.
a: whole mount light microscopy
b: cross sections light microscopy
c: electron microscopy
1. The esophagus has only ICC-IM (1a from mouse LES, 1b&c from human esophagus). These ICC-IM (arrows in 1a&b) exhibit a spindle shaped body with processes emerging from both cellular extremes (1a). Ultrastructural characteristics of ICC (* in 1c) are: electron dense cytoplasm, oval nucleus with heterochromatin distributed in the periphery and abundant mitochondria. ICC in the esophagus can establish close connections (arrows in 1c) with nearby smooth muscle cells (SM). Small arrows: caveolae.
2. There are two main classes of ICC networks in the stomach: ICC-IM, including septal ICC in larger animals, and ICC associated with the myenteric (Auerbach’s) plexus (2a from mouse corpus; 2b&c from human stomach). 2a shows ICC-IM with typical spindle shaped bodies (arrows) and myenteric pacemaker ICC with characteristic stellar bodies (arrowheads) in the wholemount preparation. 2b demonstrates ICC (arrowheads) and ICC-IM (arrows) scattered among smooth muscle cells in both muscle layers. 2c shows a longitudinal section of a myenteric ICC (*). N: nerves; SM: smooth muscle cells.
3. There are three main ICC populations in the small bowel (3a–c, all from human): ICC-IM (arrows in 3b), myenteric pacemaker ICC (arrowheads in 3a and 3b) and ICC-DMP, which are ICC, associated with the deep muscular plexus located between the outer and inner circular muscle layer (OCM&ICM). ICC-IM are distributed both within the musculature (arrows) and in the septa (double arrows) in 3a. ICC-DMP show weak Kit staining compared to other ICC and can be better identified by electron microscopy (3c). An ICC-DMP (*) in 3c establishes simultaneous close contact with a nearby nerve terminal (N) and a neighboring outer circular smooth muscle cell (OCM). Upper right inset in 3c shows a contact with the smooth muscle cell and lower left inset shows a synapse like contact with the nerve terminal. ICM: inner circular muscle. Small arrows in 3c: caveolae; Arrowheads in 3c: basal lamina.
4. Three different subtypes of ICC are present in the colon (4a–c, all from human). ICC-IM (arrows in 4a&b), myenteric pacemaker ICC (arrowheads 4a&b) and ICC-SM (* in 4c), which are ICC, associated with the submuscular plexus. Arrows in 4c: caveolae along the membrane; Arrowheads in 4c: basal lamina.
CM: circular muscle cells; LM: longitudinal muscle cells; AP: Auerbach's plexus.
Acknowledgements: Figure 4c was obtained with permission from Shigeko Torihashi 64. Figure 2c was obtained with permission from Simonetta Faussone-Pellegrini 65.
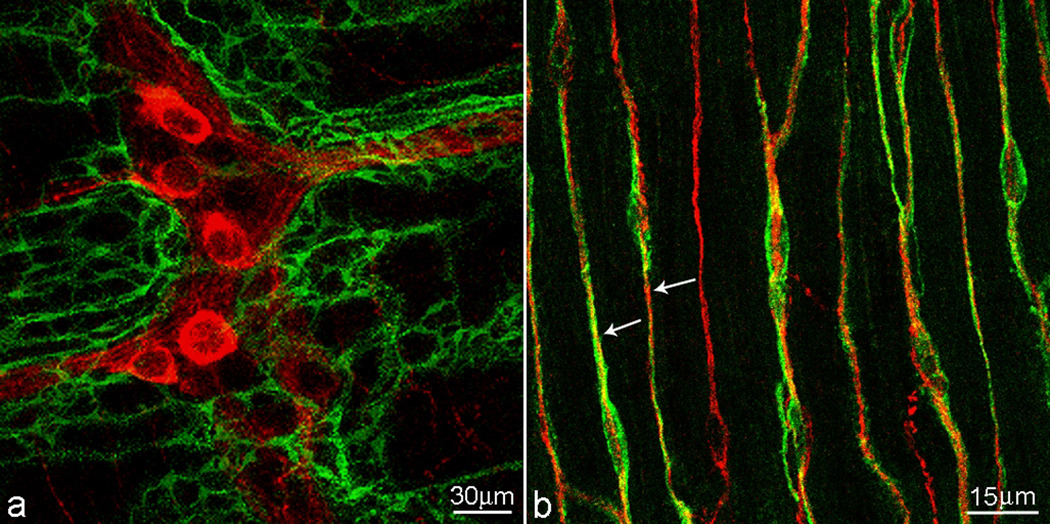
Figure 2. ICC are associated with the enteric nervous system.
a. A dense network of myenteric pacemaker ICC surrounds the myenteric plexus ganglia in the small intestine. b. ICC of the deep muscular plexus in the small intestine are bipolar and aligned with smooth muscle cells. Many ICC are fully aligned with enteric nerves (shown here are nitrergic nerves). The confocal scanning thickness was 4 µm.
C-kit staining of ICC (green), neural plexus is stained with nNOS antibody (red).
Interstitial cells of Cajal are not unique to the gut; they are present in other rhythmically active structures, such as the portal vein 6 and the bladder 7. This phenomenon, however, leads to a discussion on properties of cells that are essential to give them the identity of ICC 8. Genetic abnormalities to the Kit receptor that lead to loss of ICC in the gut, may not equally influence ICC in other organs 9.
The rhythmic depolarization generated by pacemaker ICC propagates into the circular and longitudinal muscle layers, resulting in periods of low and high excitability of the smooth muscle cells at the pacemaker frequency. Under unstimulated conditions, i.e. much of the nocturnal period, this generally does not result in muscle contraction. However, under stimulated conditions, such as a meal, distention and/or neural excitation, smooth muscle cells will generate action potentials during the depolarized portion of the slow wave. Thus, the resulting characteristic motor patterns will have the unique frequency of the ICC pacemaker system. The pacemaker frequency decreases aborally, slow waves occur simultaneously along the circumference and spread in aboral direction, hence, excitation results in a ring of circular muscle contraction that propagates anally 10. In the antrum this results in powerful peristalsis that also serves to mix and grind the stomach content. In the small intestine this results in peristaltic activity propagating over variable distances. Pacemaker activity also results in rhythmic pendular movements of the longitudinal muscle layer 11 that maximizes mixing of content, optimizing digestion and absorption.
Our current understanding of the role of ICC in peristalsis requires a distinction between peristalsis and the peristaltic reflex 10. The peristaltic reflex is a specific motor pattern that is evoked by a bolus and involves excitation oral to and inhibition anal to the bolus, programmed by the enteric nervous system 12, 13. There is a remarkable variety of motor activities, including peristalsis that are controlled to varying degrees by the central and the enteric nervous system, the ICC and hormonal and myogenic mechanisms. Expression of various motor patterns is generally not the consequence of independent actions of different control systems but rather, depending upon specific stimuli, varying domination of one or more of the control activities. This intermingling of control systems is probably the main reason why the exact role of ICC in gastrointestinal motor disorders has been controversial, perhaps unnecessarily so. It is the perspective of the authors that the question should not be whether a particular cell type is 100% responsible for a particular function; rather, it should be how different cell types integrate their functions to coordinate gastrointestinal function. Demonstration of the importance of the ICC pacemaker system can be found in the motor activity of the gastro-pyloro-duodenal junction where one finds a continuous musculature, but a discontinuity of the ICC pacemaker network 14. Consequently, the stomach and duodenum have their own independent peristaltic activities and the pylorus can act independently and be controlled by the enteric nervous system to perform sphincter function. In the stomach, strong evidence exists that the intramuscular ICC provide secondary pacemaker activity and Purkinje fiber-like conduction pathways 15. In the colon, ICC situated at the submucosal border of the circular muscle are carrying out pacemaker function as shown in the canine and human colon 16.
A dominant characteristic of ICC is their extensive innervation (Figure 2, table 1). While smooth muscle cells are innervated primarily through non-synaptic neurotransmission, ICC appear to have synapse-like contact with varicosities of the intrinsic nervous system. This is supported by the presence of proteins involved in neuro-vesicle docking to presynaptic membranes in nerve fibers in close apposition to ICC and the expression of postsynaptic density proteins by ICC 17. There is substantial evidence for the notion that enteric motor neurons innervate ICC and regulate the slow wave frequency 18 and ICC excitability 19, 20, and thus, indirectly affect smooth muscle function. In the esophagus and fundus it has been demonstrated conclusively that ICC are associated with vagal afferent nerve endings 21. The synaptic innervation of ICC brought forth the suggestion that ICC are a primary target of nerves and possibly a preferred pathway for inhibitory and excitatory neural innervation of smooth muscle cells 22, but this remains controversial. Purinergic and peptidergic innervations, appear to follow diffusion of neurotransmitters directly to receptors on smooth muscle cells. For nitrergic and cholinergic innervation however, evidence for an intermediary function of ICC comes from apparent lack of smooth muscle responses to enteric nerve stimulation in the fundus, lower esophageal and pyloric sphincters and colon of mice with hypomorphic mutations in Kit protein (W/Wv) or Kit ligand (Sl/Sld mice) where ICC networks are disrupted 23, 24 and from the small intestines of mice where ICC networks had been depleted by injections of neutralizing anti-Kit antibodies 25. However, lack of nitrergic innervation in the absence of intramuscular ICC has not been found consistently by other investigators in the fundus or lower esophageal, pyloric and internal anal sphincters or the whole stomach of W/Wv mice and in Ws/Ws fundus 26–29. Direct and indirect innervation of smooth muscle may exist side by side. In summary, smooth muscle activity is not the consequence of a single cascade of events but rather parallel streams of influences. The primary ones are the following: 1) Intrinsic activity of smooth muscle cells (secondary pacemaker activity, action potential generation, direct responses to depolarizing stimuli and distention); 2) Non-synaptic neurotransmission from excitatory and inhibitory motor neurons; and 3) Primary pacemaker activity from ICC that can be modified by synaptic innervation of the ICC.
Table 1. Animal models affecting interstitial cells of Cajal.
Pacemaker ICC refers to ICC located in the myenteric plexus. Only a limited number of references are included because of space limitations.
| Animal | Type | Name | Alteration | Status of ICC | Functional changes observed |
|---|---|---|---|---|---|
| Mouse | Models which primarily target the ICC population | W/Wv | Mutation of the proto-oncogene c-kit reduces tyrosine kinase activity | Loss of ICC-IM in stomach and sphincters24 ; loss of pacemaker ICC in the intestine78,79; reduced ICC in the colon | Increased gastric compliance24; altered 24 or no change 28 in neurotransmission; increased fundic muscle excitability ; abnormal intestinal motility due to loss of pacemaker activity |
| Sld /Sld | Mutation of the Steel locus produces an abnormal, ineffective membrane bound SCF, the ligand for Kit | Loss of ICC-IM in stomach and sphincters66 ; loss of pacemaker ICC in the intestine67 | Altered fundus neurotransmission66, abnormal intestinal motility due to loss of pacemaker activity | ||
| WZsGreen/+ | Insertion of green fluorescent protein sequence, ZsGreen, into the first exon of the c-kit gene | Expresses a fluorescent tag in KIT-expressing cells68 | Allows morphological identification of Kit-expressing cells | ||
| Pharmacological blockage of tyrosine kinase activity | In vivo blockage of Kit receptor by i.p injection of antibodies | Severe disruption of ICC populations53,69 | Intestinal ileus with loss of pacemaker activity and ineffective neurotransmission | ||
| Murine partial bowel obstruction | Mechanical obstruction achieved by placing a clip on the small intestine | Disruption of ICC networks proximal to the obstruction site; recovery after clip removal 35 | Loss of electrical slow waves and responses to enteric nerve stimulation proximal to obstruction site; recovery after clip removal | ||
| Diabetes models exhibiting ICC alterations | Db/db | Model of Type 2 diabetes | Reduced ICC population in antrum, small intestine and colon70 | Gastroparesis, intestinal dysmotility, decreased whole gut transit | |
| NOD/LtJ | Model of human Type 1 diabetes | Reduction of antral and body myenteric ICC and ICC-IM; loss of close connection with nerve terminals; reduced SCF production 71 | Gastroparesis with impairment in pacemaker activity and altered neurotransmission | ||
| Models of enteric agangliosis | Ls/ls | Homozygous for the lethal spotted (ls) allele display a loss of function mutation in the endothelin-3 gene. Model of short Hirschsprung’s disease | Aganglionosis in the terminal regions of the large intestine. Distribution and density of ICC populations seems unaltered72 | Loss of spontaneous electrical activity and postjunctional neuronal responses; unlikely to depend on changes in ICC | |
| GDNF−/− mice | Glial cell line-derived neurotrophic factor (GDNF) knockout mice. Model for long segment Hirschsprung’s disease | Aganglionosis along most of the GI tract. Distribution and density of ICC populations seems unaltered73 | Normal pacemaker activity | ||
| Rat | Model which primarily targets the ICC population | Ws/Ws | Deletion at the tyrosine kinase domain of Kit due to a mutation in the proto-oncogene c-kit | Loss of ICC-IM in the esophagus 27; reduction or loss of ICC populations in the stomach and colon74 ; loss of pacemaker ICC in the small intestine 75 | Neurotransmission relatively preserved, increased spontaneous contractile activity in esophagus27 and colon; altered colonic pacemaker activity in the colon 76 |
| Diabetes model exhibiting ICC alterations | STZ-DM | Streptozotocin-induced diabetes mellitus in Wistar rat. Model of human Type 1 diabetes | Reduced antral and colonic ICC-IM and ICC-SMP77 | Gastroparesis; increased amplitude of stretch induced colonic contractions | |
| Models of enteric agangliosis | ETB receptor null rat | Endothelin receptor null rat possess an autosomal recessive gene (sl) that leads to aganglionosis. Model for long segment Hirschsprung’s disease | Severe disruption of pacemaker ICC network41 | Irregular spontaneous phasic contractile activity. |
ICC are situated ideally to monitor the contractile state of the musculature and transmit this to the extrinsic and/or intrinsic nervous system, hence function as mechanoreceptors by virtue of their distribution throughout the musculature and their multiple processes that contact many smooth muscle cells. There is good evidence for interactions between vagal afferent nerves and ICC in the esophagus and fundus 21. Although the functional consequences are still to be elucidated, loss of ICC-IM is associated with loss of vagal afferent nerve endings, thereby suggesting a survival dependency 30. One of the more advanced hypotheses is that mechanical distortion of human ICC activates a sodium channel that depolarizes the ICC and increases pacemaker frequency 5. Mutations in this sodium channel macromolecular complex may lead to gastrointestinal symptoms 31 and may be associated with irritable bowel syndrome 32 and intestinal pseudoobstruction 33. A very interesting hypothesis that has not yet been adequately explored is that mechanical interaction between ICC and smooth muscle cells involves dynamic creation of peg and socket junctions 34.
ICC and the pathophysiology of gastrointestinal motility disorders
Acknowledgment of the importance of ICC for the integrity of the motor function of the gastrointestinal tract prompted interest in the fate of ICC in gut motor disorders. Damage to ICC and/or reduction of its population has been described in almost every gastrointestinal motility disorder from the esophagus to the rectum. There is already a significant body of evidence for the involvement of ICC in the pathophysiology of gastroparesis and constipation, but ICC abnormalities are also present in acquired conditions such as achalasia, Chagas disease, intestinal pseudo-obstruction, and the inflammatory bowel disorders as well as congenital diseases such as Hirschsprung’s and congenital hypertrophic pyloric stenosis (CHPS). All these conditions exhibit abnormalities of motor activity leading to impaired regional transit and symptoms. ICC loss or disruption is associated often with concomitant neuronal and smooth muscle changes suggesting a close interdependence between these cell types. It is still unclear for most of these motility disorders if the disruption in ICC networks is primary or secondary and it will be important to resolve this question.
Mechanisms underlying abnormalities in the populations of ICC are currently incompletely understood but various factors are likely to influence the fate of ICC: 1) a variable degree of regional obstruction and subsequent proximal dilation, 2) injury to the nervous system, 3) the immune system and 4) ICC plasticity.
Obstruction and plasticity
Studies in animal models show that ICC viability and function are compromised in dilated bowel segments proximal to an area of partial obstruction. The degree of disruption of the ICC network is a function of the distance from the obstruction and it is reversible after the obstruction is removed 35 (table 1), highlighting a remarkable degree of plasticity. This could explain the recovery of the pyloric ICC-IM population in patients with CHPS after pyloromyotomy, a procedure that resolves the mechanical and functional obstruction to gastric emptying associated with this condition. It could also explain the frequently observed lack of correlation between the degree of ICC loss and the duration of the disease. Timing and type of treatments varies between patients, influencing the degree of intraluminal distension. This intrinsic ICC plasticity is important as it opens a window for recovery of the ICC phenotype and function if the underlying insult is addressed, although it is still unclear if human ICC are as susceptible to distension as they are in smaller species.
Injury to the nervous system
ICC appear to develop independently from the enteric nervous system and an apparently normal ICC network was observed in a newborn without an enteric nervous system 36 as well as in mouse models. Nevertheless, one should not conclude that there is no interaction between nerves and ICC related to survival as demonstrated for intramuscular ICC in the stomach and afferent vagal nerves 30. It is currently unknown if ICC and nerves, in diseased states, simultaneously are injured by the same mechanism or if injury to ICC can be secondary to the loss of neural structures. Interestingly, NOS containing nerves contain membrane bound stem cell factor but it is unclear if ICC have access to neuronally produced stem cell factor 37. nNOS derived NO promotes ICC proliferation in vitro and nNOS knockout mice have altered ICC networks 38. Thus, ICC could be particularly susceptible to damage to NOS containing nerves, particularly relevant for achalasia, diabetic gastroparesis and CHPS.
Relationship with the immune system
Damage to neural structures in Crohn’s disease or ulcerative colitis is to a large part mediated by the inflammatory infiltrate, which also appears also to contribute to the development of achalasia and a subset of idiopathic intestinal pseudo-obstruction. The role of an inflammatory infiltrate in the fate of ICC in these conditions is currently unknown and the available information is limited to morphological descriptions on the spatial relationship between ICC and different inflammatory cells infiltrating the gut wall. Interestingly, susceptibility of the different ICC subpopulations appears to depend on upon their proximity to blood vessels that carry immune cells into the tissue, as deduced from animal models of gut inflammation. Membrane to membrane contacts have been observed between ICC and both macrophages and mast cells in Crohn’s disease 39; intimate contacts with the latter have also been described in achalasia 40. Secretory products from macrophages can have a deleterious influence on ICC. iNOS derived NO produced by resident macrophages has been proposed in the disruption of ICC networks in the endothelin-B receptor null rat 41, considered a model for long-segment Hirschsprung’s disease. By contrast, different types of macrophages appear to be cytoprotective to ICC, as in a mouse model of diabetic gastroparesis, likely due to upregulation of heme oxygenase 1 and subsequent reduction in oxidative stress 38.
Our knowledge about functional consequences of loss or injury of ICC is still rudimentary. This is in part due to the fact that ICC are rarely the only affected cell type in a motor disorder and furthermore, it is not known how much damage ICC networks can withstand before functional abnormalities develop. Studies are needed to assess the state of pacemaker activity, distension-induced peristalsis and neurotransmission in human diseases with abnormal ICC.
Although the assessment of ICC in gastrointestinal motility disorders has entered clinical practice, the published literature is conflicting. There are several reasons for this, including the inability to visualize all ICC from formalin fixed, paraffin embedded tissue despite optimal antigen retrieval 42, the need to correctly handle human tissue as ICC appear to be particularly susceptible to ischemia 43, the general lack of data that quantify the normal ICC population 44 and the reliance upon Kit antibodies as the sole method of identifying ICC. In this regard, antibodies to Ano-1 may be of use to determine or confirm abnormalities in ICC in human tissue, with the additional advantage that Ano-1, unlike Kit, is not expressed on mast cells 4. Establishment of normal values and standardization of tissue collection, fixation and ICC visualization are required in order for the field to progress.
ICC injury, death and recovery
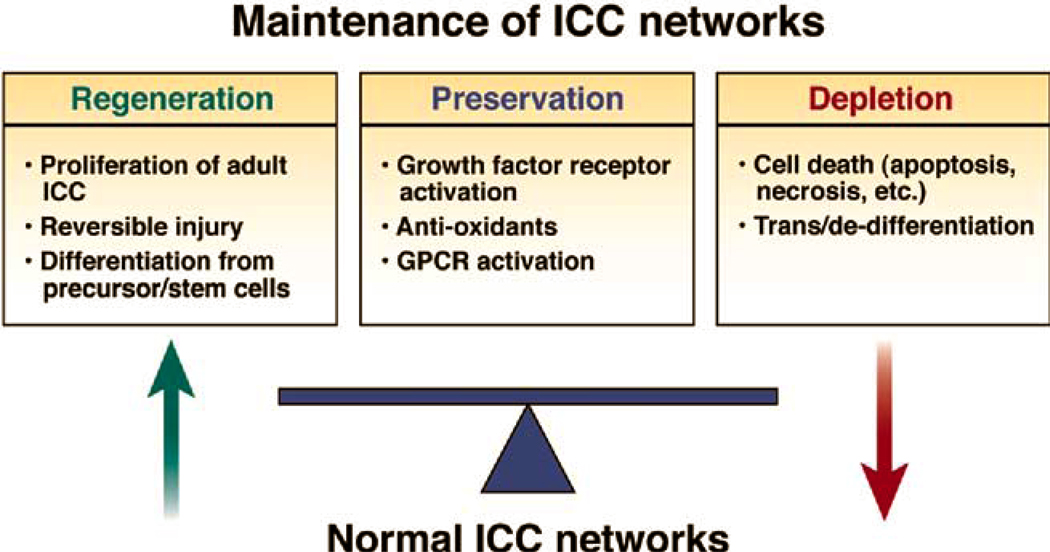
ICC are reduced or otherwise affected in several motility disorders as outlined above but resolution of these disorders in animal models (Table 1) has been demonstrated to result in repopulation of ICC networks underscoring their plasticity as described above. This occurrs in partial small bowel obstruction 35, inflammation 45 46, surgical transection and anastomosis 46, 47. In health, ICC numbers are dynamic 48, 49. Conservative estimates indicate human colonic ICC turn over completely in months and similar kinetics appear to be present in other regions of the gastrointestinal tract. Therefore, to maintain functional ICC networks, ICC turnover needs to be tightly controlled with processes that regulate both ICC loss and ICC replacement. Mechanisms shown to contribute to ICC loss include apoptosis 49 and trans/de-differentiation 50, 51, while repair from injury, proliferation from adult ICC 43, 48 and replenishment from ICC stem cell precursors 52 contribute to ICC replacement (Figure 3).
Figure 3. Proposed paradigm for the control of ICC networks.
Recent data suggest that adult ICC proliferate 43, 48 53. The Kit signaling pathway, activated by the Kit-ligand stem cell factor, was the first pathway associated with the control of ICC survival and proliferation. We now know that several other signaling pathways also contribute to ICC survival and proliferation, including neuronally derived nitric oxide 38, serotonin signaling through the 5-HT2B receptor 54, interleukin 9 55 insulin and IGF-1 signaling through stem cell factor 56 and heme oxygenase 1 57. 5-HT appears to play an unexpected role in the maintenance of ICC networks. ICC express several 5-HT receptors, including the 5-HT2B receptor. Activation of the 5-HT2B receptor causes an increase in ICC proliferation and activation of PKCγ. This pathway appears to be active in vivo as 5-HT2B knockout mice have markedly reduced ICC networks. ICC appear also to be particularly susceptible to oxidative stress. Data from diabetic non-obese diabetic (NOD) mice reveal that maintenance of ICC in diabetes is dependent upon diabetes-induced upregulation of heme oxygenase-1 (HO-1), an enzyme that generates the cytoprotective gaseous mediator carbon monoxide in tissue macrophages 57. Mice that had high levels of HO-1 had normal Kit expression while mice that could not sustain elevated levels of HO-1 had high levels of oxidative stress and lost Kit expression. These data suggest that HO-1 plays a central role in protecting ICC from oxidative damage. Future work needs to be directed towards determining if these diverse factors interact and whether there are common intracellular signaling pathways that may offer therapeutic targets.
ICC replenishment can also occur through local precursor cells. The discovery of a candidate ICC progenitor or stem cell 52 suggests the possibility that manipulation or transplantation of cells with stem-cell properties is a new therapeutic approach to diseases associated with ICC loss. These studies in postnatal murine gastric muscle have revealed rare cells that express up to 10 times less Kit on their surface than mature ICC. They also express CD44, CD34, Ano-1 and receptors for insulin and IGF-1 52, 58. Unlike adult ICC, which require membrane bound stem cell factor, their proliferation can be sustained with soluble stem cell factor and by the direct action of IGF-I. Interestingly, differentiation of these KitlowCD44+CD34+Insr+Igf1r+ cells gave rise to not only ICC but also smooth muscle and other cell types, which may have the additional advantage of providing the correct milieu for survival and maintenance of mature ICC networks.
While a decrease in ICC replenishment from either adult ICC or ICC precursors is associated with several gastrointestinal motility disorders, an uncontrolled increase may result in gastrointestinal stromal tumors (GISTs). Most GISTs arise from ICC lineage as a result of activating mutations in Kit 59, 60, and occasionally in PDGFR 61, 62. Treatment of advanced-stage GISTs with imitinab, a tyrosine kinase inhibitor, has increased survival but is rarely curative.
Different mechanisms have been proposed for ICC loss. Ultrastructural studies have suggested that in diseased states, ICC might dedifferentiate into an earlier developmental stage, more reminiscent of smooth muscle cells or fibroblasts from an ultrastructural viewpoint 50, 51, 63. Whether this changed phenotype is reversible into ICC or is destined to be lost is not yet known. As outlined above, ICC can be lost through apoptosis. Apoptotic ICC, as detected by activated caspase-3 or TUNEL, can be detected in all layers of the human colonic muscle 49.
ICC, together with the enteric nervous system, provide a critical control system for gastrointestinal motility. ICC are affected by inflammation, oxidative stress and obstruction and their function and survival markedly influenced by various growth factors. Development of an understanding of the factors that regulate maintenance of ICC networks is well underway although much more remains to be discovered. The new paradigm (Figure 3) of a tightly controlled ongoing balance between pro-survival and pro-loss factors is of significance because it indicates that strategies to stop ICC loss or reverse loss can be directed to both sides of the equation, decrease ICC loss or increase ICC proliferation by specific interventions on ICC signaling pathways, and/or restoration from precursors.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research (MOP 12874), the Canadian Association of Gastroenterology and the National Institutes of Health (DK 57061 and DK68055). Dr. Xuan-Yu Wang was instrumental in creating figures 1 and 2.
Footnotes
The authors disclose no conflicts.
REFERENCES
- 1.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513(Pt 1):203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 3.d'Antonio C, Wang B, McKay C, Huizinga JD. Substance P activates a non-selective cation channel in murine pacemaker ICC. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–G1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 6.Harhun MI, Gordienko DV, Povstyan OV, Moss RF, Bolton TB. Function of interstitial cells of Cajal in the rabbit portal vein. Circ Res. 2004;95:619–626. doi: 10.1161/01.RES.0000143014.04535.a3. [DOI] [PubMed] [Google Scholar]
- 7.Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WF, Wendt I, Parkington HC. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695–705. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huizinga JD, Faussone-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468–473. doi: 10.1111/j.1582-4934.2005.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCloskey KD, Anderson UA, Davidson RA, Bayguinov YR, Sanders KM, Ward SM. Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice. Br J Pharmacol. 2009;156:273–283. doi: 10.1111/j.1476-5381.2008.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–G8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 11.Lammers WJ. Spatial and temporal coupling between slow waves and pendular contractions. Am J Physiol Gastrointest Liver Physiol. 2005;289:G898–G903. doi: 10.1152/ajpgi.00070.2005. [DOI] [PubMed] [Google Scholar]
- 12.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa M, Brookes SH. Architecture of enteric neural circuits involved in intestinal motility. Eur Rev Med Pharmacol Sci. 2008;12 Suppl 1:3–19. [PubMed] [Google Scholar]
- 14.Wang XY, Lammers WJ, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G539–G549. doi: 10.1152/ajpgi.00046.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hirst GD, Garcia-Londono AP, Edwards FR. Propagation of slow waves in the guinea-pig gastric antrum. J Physiol. 2006;571:165–177. doi: 10.1113/jphysiol.2005.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rae MG, Fleming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol. 1998;510(Pt 1):309–320. doi: 10.1111/j.1469-7793.1998.309bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- 18.Kim TW, Koh SD, Ordog T, Ward SM, Sanders KM. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J Physiol. 2003;546:415–425. doi: 10.1113/jphysiol.2002.028977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huizinga JD, Golden CM, Zhu Y, White EJ. Ion channels in interstitial cells of Cajal as targets for neurotransmitter action. Neurogastroenterol Motil. 2004;16 Suppl 1:106–111. doi: 10.1111/j.1743-3150.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Huizinga JD. Nitric oxide decreases the excitability of interstitial cells of Cajal through activation of the BK channel. J Cell Mol Med. 2008;12:1718–1727. doi: 10.1111/j.1582-4934.2008.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 22.Ward SM. Interstitial cells of Cajal in enteric neurotransmission. Gut. 2000;47 Suppl 4:iv40–iv43. doi: 10.1136/gut.47.suppl_4.iv40. discussion iv52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckett EA, Ro S, Bayguinov Y, Sanders KM, Ward SM. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- 26.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 27.Farre R, Wang XY, Vidal E, Domenech A, Pumarola M, Clave P, Huizinga JD, Jimenez M. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil. 2007;19:484–496. doi: 10.1111/j.1365-2982.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 28.Huizinga JD, Liu LW, Fitzpatrick A, White E, Gill S, Wang XY, Zarate N, Krebs L, Choi C, Starret T, Dixit D, Ye J. Deficiency of intramuscular ICC increases fundic muscle excitability but does not impede nitrergic innervation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G589–G594. doi: 10.1152/ajpgi.00130.2007. [DOI] [PubMed] [Google Scholar]
- 29.De Lorijn F, De Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005;54:1107–1113. doi: 10.1136/gut.2004.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat Embryol (Berl) 2001;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- 31.Locke GR, 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol. 2006;101:1299–1304. doi: 10.1111/j.1572-0241.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 32.Saito YA, Strege PR, Tester DJ, Locke GR, 3rd, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ, Farrugia G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol. 2009;296:G211–G218. doi: 10.1152/ajpgi.90571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, Makielski JC, Stanghellini V, Gibbons SJ, Ackerman MJ, Farrugia G. A mutation in telethonin alters Nav1.5 function. J Biol Chem. 2008;283:16537–16544. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thuneberg L, Peters S. Toward a concept of stretch-coupling in smooth muscle. I. Anatomy of intestinal segmentation and sleeve contractions. Anat Rec. 2001;262:110–124. doi: 10.1002/1097-0185(20010101)262:1<110::AID-AR1016>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–568. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huizinga JD, Berezin I, Sircar K, Hewlett B, Donnelly G, Bercik P, Ross C, Algoufi T, Fitzgerald P, Der T, Riddell RH, Collins SM, Jacobson K. Development of interstitial cells of Cajal in a full-term infant without an enteric nervous system. Gastroenterology. 2001;120:561–567. doi: 10.1053/gast.2001.21200. [DOI] [PubMed] [Google Scholar]
- 37.Young HM, Torihashi S, Ciampoli D, Sanders KM. Identification of neurons that express stem cell factor in the mouse small intestine. Gastroenterology. 1998;115:898–908. doi: 10.1016/s0016-5085(98)70262-8. [DOI] [PubMed] [Google Scholar]
- 38.Choi KM, Gibbons SJ, Roeder JL, Lurken MS, Zhu J, Wouters MM, Miller SM, Szurszewski JH, Farrugia G. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–595. doi: 10.1111/j.1365-2982.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang XY, Zarate N, Soderholm JD, Bourgeois JM, Liu LW, Huizinga JD. Ultrastructural injury to interstitial cells of Cajal and communication with mast cells in Crohn's disease. Neurogastroenterol Motil. 2007;19:349–364. doi: 10.1111/j.1365-2982.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 40.Zarate N, Wang XY, Tougas G, Anvari M, Birch D, Mearin F, Malagelada JR, Huizinga JD. Intramuscular interstitial cells of Cajal associated with mast cells survive nitrergic nerves in achalasia. Neurogastroenterol Motil. 2006;18:556–568. doi: 10.1111/j.1365-2982.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Won KJ, Horiguchi K, Kinoshita K, Hori M, Torihashi S, Momotani E, Itoh K, Hirayama K, Ward SM, Sanders KM, Ozaki H. Muscularis inflammation and the loss of interstitial cells of Cajal in the endothelin ETB receptor null rat. Am J Physiol Gastrointest Liver Physiol. 2004;287:G638–G646. doi: 10.1152/ajpgi.00077.2004. [DOI] [PubMed] [Google Scholar]
- 42.Garrity MM, Gibbons SJ, Smyrk TC, Vanderwinden JM, Gomez-Pinilla PJ, Nehra A, Borg M, Farrugia G. Diagnostic challenges of motility disorders: optimal detection of CD117+ interstitial cells of Cajal. Histopathology. 2009;54:286–294. doi: 10.1111/j.1365-2559.2008.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20 Suppl 1:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 44.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Gershon MD, Hutson J, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 45.Wang XY, Vannucchi MG, Nieuwmeyer F, Ye J, Faussone-Pellegrini MS, Huizinga JD. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am J Pathol. 2005;167:437–453. doi: 10.1016/S0002-9440(10)62988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanagida H, Yanase H, Sanders KM, Ward SM. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004;127:1748–1759. doi: 10.1053/j.gastro.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 47.Mei F, Yu B, Ma H, Zhang HJ, Zhou DS. Interstitial cells of Cajal could regenerate and restore their normal distribution after disrupted by intestinal transection and anastomosis in the adult guinea pigs. Virchows Arch. 2006;449:348–357. doi: 10.1007/s00428-006-0258-6. [DOI] [PubMed] [Google Scholar]
- 48.Mei F, Zhu J, Guo S, Zhou DS, Han J, Yu B, Li SF, Jiang ZY, Xiong CJ. An age-dependent proliferation is involved in the postnatal development of interstitial cells of Cajal in the small intestine of mice. Histochem Cell Biol. 2009;131:43–53. doi: 10.1007/s00418-008-0515-7. [DOI] [PubMed] [Google Scholar]
- 49.Gibbons SJ, De Giorgio R, Pellegrini MS, Garrity-Park MM, Miller SM, Schmalz PF, Young-Fadok TM, Larson DW, Dozois EJ, Camilleri M, Stanghellini V, Szurszewski JH, Farrugia G. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol Motil. 2009;21:85–93. doi: 10.1111/j.1365-2982.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–148. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 51.Faussone-Pellegrini MS, Vannucchi MG, Ledder O, Huang TY, Hanani M. Plasticity of interstitial cells of Cajal: a study of mouse colon. Cell Tissue Res. 2006;325:211–217. doi: 10.1007/s00441-006-0174-8. [DOI] [PubMed] [Google Scholar]
- 52.Lorincz A, Redelman D, Horvath VJ, Bardsley MR, Chen H, Ordog T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 54.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Ye J, Zhu Y, Khan WI, Van Snick J, Huizinga JD. IL-9 enhances growth of ICC, maintains network structure and strengthens rhythmicity of contraction in culture. J Cell Mol Med. 2006;10:687–694. doi: 10.1111/j.1582-4934.2006.tb00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, Ordog T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 57.Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, Szurszewski JH, Farrugia G. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. 2064 e1–2064 e2. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Ordog T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492–509. doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- 59.Nakahara M, Isozaki K, Hirota S, Miyagawa J, Hase-Sawada N, Taniguchi M, Nishida T, Kanayama S, Kitamura Y, Shinomura Y, Matsuzawa Y. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology. 1998;115:1090–1095. doi: 10.1016/s0016-5085(98)70079-4. [DOI] [PubMed] [Google Scholar]
- 60.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 61.de Raedt T, Cools J, Debiec-Rychter M, Brems H, Mentens N, Sciot R, Himpens J, de Wever I, Schoffski P, Marynen P, Legius E. Intestinal neurofibromatosis is a subtype of familial GIST and results from a dominant activating mutation in PDGFRA. Gastroenterology. 2006;131:1907–1912. doi: 10.1053/j.gastro.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Weisberg E, Wright RD, Jiang J, Ray A, Moreno D, Manley PW, Fabbro D, Hall-Meyers E, Catley L, Podar K, Kung AL, Griffin JD. Effects of PKC412, nilotinib, and imatinib against GIST-associated PDGFRA mutants with differential imatinib sensitivity. Gastroenterology. 2006;131:1734–1742. doi: 10.1053/j.gastro.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang XY, Alberti E, White EJ, Mikkelsen HB, Larsen JO, Jimenez M, Huizinga JD. Igf1r+/CD34+ immature ICC are putative adult progenitor cells, identified ultrastructurally as fibroblast-like ICC in Ws/Ws rat colon. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 65.Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of Cajal of the human stomach. J Submicrosc Cytol Pathol. 1989;21:439–460. [PubMed] [Google Scholar]
- 66.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 68.Wouters M, Smans K, Vanderwinden JM. WZsGreen/+: a new green fluorescent protein knock-in mouse model for the study of KIT-expressing cells in gut and cerebellum. Physiol Genomics. 2005;22:412–421. doi: 10.1152/physiolgenomics.00105.2005. [DOI] [PubMed] [Google Scholar]
- 69.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660–667. doi: 10.1111/j.1440-1746.2008.05326.x. [DOI] [PubMed] [Google Scholar]
- 71.Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 72.Ward SM, Gershon MD, Keef K, Bayguinov YR, Nelson C, Sanders KM. Interstitial cells of Cajal and electrical activity in ganglionic and aganglionic colons of mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G445–G456. doi: 10.1152/ajpgi.00475.2001. [DOI] [PubMed] [Google Scholar]
- 73.Ward SM, Ordog T, Bayguinov JR, Horowitz B, Epperson A, Shen L, Westphal H, Sanders KM. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology. 1999;117:584–594. doi: 10.1016/s0016-5085(99)70451-8. [DOI] [PubMed] [Google Scholar]
- 74.Mitsui R, Komuro T. Distribution and ultrastructure of interstitial cells of Cajal in the gastric antrum of wild-type and Ws/Ws rats. Anat Embryol (Berl) 2003;206:453–460. doi: 10.1007/s00429-003-0323-8. [DOI] [PubMed] [Google Scholar]
- 75.Takeda M, Takayama I, Terada N, Baba T, Ward SM, Ohno S, Fujino MA. Immunoelectron-microscopic study of Kit-expressing cells in the jejunum of wildtype and Ws/Ws rats. Cell Tissue Res. 2001;304:21–30. doi: 10.1007/s004410000333. [DOI] [PubMed] [Google Scholar]
- 76.Alberti E, Mikkelsen HB, Wang XY, Diaz M, Larsen JO, Huizinga JD, Jimenez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–G1510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 77.Forrest A, Huizinga JD, Wang XY, Liu LW, Parsons M. Increase in stretch-induced rhythmic motor activity in the diabetic rat colon is associated with loss of ICC of the submuscular plexus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G315–G326. doi: 10.1152/ajpgi.00196.2007. [DOI] [PubMed] [Google Scholar]
- 78.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]