Abstract
Objective
The extent to which the apolipoprotein E (APOE) ε4 allele is a susceptibility gene for late-onset Alzheimer's disease (AD) in Latino individuals continues to be clarified. In this study, fluorodeoxyglucose positron emission tomography (PET) was used to investigate whether regional reductions in the cerebral metabolic rate for glucose (CMRgl) previously found in cognitively normal late-middle-aged APOE ε4 carriers extends to members of the Latino Mexican-American community.
Methods
A brain mapping algorithm (SPM5) was used to compare cross-sectional regional CMRgl in Latino APOE ε4 carriers versus noncarriers.
Subjects
11 APOE ε4 carriers and 16 noncarriers from Arizona's Latino community (mean age 54.6±6.4 years) matched for sex, mean age and educational level, and who were predominantly of self-designated Mexican origin.
Results
Participant groups had similar distributions for age, gender, education, family history of dementia, clinical ratings and neuropsychological test scores. Latino APOE ε4 carriers had lower CMRgl than the noncarriers in posterior cingulate, precuneus and parietal regions previously found to be preferentially affected in AD patients and cognitively normal non-Latino APOE ε4 carriers. Additionally, the Latino APOE ε4 carriers had lower CMRgl in middle and anterior cingulate cortex, hippocampus and thalamus.
Conclusions
This study provides support for the relationship between APOE ε4 and risk of AD in Latinos. It illustrates the role of PET as a presymptomatic endophenotype for the assessment of AD risk factors, and supports the inclusion of Latino APOE ε4 carriers in proof-of-concept studies using FDG PET to evaluate promising presymptomatic treatments in cognitively normal carriers of this common AD susceptibility gene.
Introduction
Alzheimer's disease (AD) is the most common form of dementia in the elderly.1 Over the next few decades, it is projected that there will be dramatic increases in the number of elderly individuals, their racial and ethnic diversity2 and the number of patients afflicted by AD.3 Latino individuals appear to have a higher incidence of AD,4 which may be partly attributable to suggested AD risk factors including diabetes, obesity, cardiovascular disease, and hypertension, coupled with an earlier mean age of onset,5 and a longer post-diagnosis survival time.6
Next to age, the apolipoprotein E (APOE) ε4 allele is the best established risk factor for late-onset AD.7 While this association has been confirmed in numerous case-control studies in Europe and the Americas, the association in different Latino groups continues to be clarified.8-12 For instance, the association between AD and APOE ε4 is inconsistent for individuals from the Caribbean, perhaps due to differing levels of African admixture among these populations.10;12-16 Moreover, some studies suggest that the strength of the association in Latinos is weaker compared to that in non-Latino Caucasians.8;17 The differences could be due to a multitude of factors, including whether there are differences in the frequency of APOE alleles by ethnic group,10 whether only APOE ε4 homozygosity increases the risk for AD in certain ethnicities or races,13 or whether ε4 is only associated with late-onset familial AD rather than sporadic AD in certain populations.11 Additionally, the accuracy of the self-designated origin, racial or ethnic classifications and the homogeneity of the Latino groupings may be factors, as some Latinos are of African origin and the association between APOE ε4 and AD is inconsistent in Nigerians and African Americans.10;18;19 Lastly, other genetic variants, including variants in SORL1, may be associated with AD in certain Latino populations as well as non-Latino Caucasians.20;21
Fluorodeoxyglucose positron emission tomography (PET) studies find that AD patients have preferential and progressive reductions in) in the cerebral metabolic rate for glucose (CMRgl) in posterior cingulate, precuneus, parietal, temporal, and prefrontal brain regions.22-29 We and others have found that cognitively normal APOE ε4 carriers exhibit CMRgl reductions in these AD-affected regions,30-34 leading us to propose that FDG PET could be used as a promising biomarker (but not yet validated surrogate endpoint) for the evaluation of promising presymptomatic AD treatments in cognitively normal APOE ε4 carriers.33;35 Based on the finding that the CMRgl reductions in AD-affected regions are associated with the number of ε4 alleles in a person's APOE genotype (i.e., three levels of genetic risk for AD),36 we proposed using FDG PET to provide a quantitative presymptomatic endophenotype – a measurable feature that is more closely related to disease susceptibility than the clinical syndrome itself – to help assess the individual and aggregate effects of putative modifiers of AD risk.33
This study compared FDG PET measurements of regional CMRgl in cognitively normal late-middle-aged APOE ε4 carriers and noncarriers from the United States of America's rapidly growing Latino community.2 In particular, it sought to determine whether cognitively normal Latino APOE ε4 carriers have lower CMRgl than noncarriers in AD-affected regions, to provide presymptomatic endophenotypic evidence for the role of the APOE ε4 allele as a late-onset AD susceptibility gene in this population, and to support the inclusion of Latino ε4 carriers in our proposed proof-of-concept AD prevention trials. Moreover, in order to address the potential heterogeneity within the Latino cohort, the cohort was tested for stratification between APOE ε4 carriers and non-carriers.
Methods
Participants
To identify Latino APOE ε4 carriers, a newspaper article about AD-research and accompanying advertisement, as well as other outreach activities permitted us to recruit 81 (68 women, and 13 men) cognitively normal volunteers from Arizona's English-speaking Latino community, 47 to 68 years of age, irrespective of their reported family history of AD. Nearly all respondents were the result of the newspaper article and accompanying advertisement. Respondents understood that they would not receive any information about their APOE genotype, provided their informed consent, and were studied under guidelines approved by the human subjects committees at Banner Good Samaritan Medical Center and the Mayo Clinic. Venous blood samples were drawn and APOE genotypes characterized with analysis involving restriction fragment length polymorphisms.37
The distribution of APOE genotypes in the Latino respondents is noted in Table 1. While 22 ε4 carriers were identified, eight declined to participate in the imaging studies and three did not meet our selection criteria for imaging study enrollment due to co-morbid medical conditions (diabetes, stroke, cancer). Of the 59 noncarriers, 14 declined to participate in the imaging studies and 11 did not meet our selection criteria due to diabetes or stroke. The 11 ε4 carriers who agreed to participate in the imaging studies (1 with the ε4/ε4 allele, 1 with the ε2/ε4 allele, and 9 with the ε3/ε4 allele) were matched to 16 ε4 noncarriers (1 with the ε2/ε3 allele and 15 with the ε3/ε3 allele) for sex, mean age and educational level. Investigators who were unaware of the participants' APOE genotypes obtained data from medical and family histories as previously described,31 including a neurological examination, a structured psychiatric interview, the Mini-Mental State Examination (MMSE),38 the Hamilton Depression Rating Scale (HAM-D),39 a battery of neuropsychological tests, and brain imaging studies.
Table 1.
Distribution of Apolipoprotein E Genotypes in 81 Latino Respondents, 47-68 years of age
| Genotype | No. of Respondents (%) |
|---|---|
| ε2/ε2 | 0 (0) |
| ε2/ε3 | 3 (3.7) |
| ε2/ε4 | 2 (2.5) |
| ε3/ε3 | 56 (69.1) |
| ε3/ε4 | 18 (22.2) |
| ε4/ε4 | 2 (2.5) |
The 27 participants in the imaging portion of the study were predominantly self-designated Mexican-Americans (1 participant self-designated being of South American origin [Peru] and 2 self-designated being of Puerto Rican origin) and 10 reported a first degree family history of dementia. At the time of their initial visit, all participants denied having impairment in memory or other cognitive skills, did not satisfy criteria for a current psychiatric disorder other than depression or anxiety, had no known cardiovascular or cerebrovascular disease, had scores of at least 28 on the MMSE, had a normal neurological exam, and all identified English as their primary language. In order to allow for inclusion of this underrepresented cohort into a study of this nature, we broadened our usual criteria to allow for self-reported depression or anxiety and pharmaceutical treatments of these disorders and did not require a first-degree family history of AD. Five participants reported using medication to treat depression or anxiety; however, all HAM-D scores were within normal limits. In addition, 6 participants reported a history of hypertension or hypercholesterolemia.
Brain Imaging
Volumetric T1-weighted MRI, performed to rule out structural lesions, and fluorodeoxyglucose PET were performed as previously described.31;36 For six participants (2 ε4 carriers and 4 noncarriers), PET was performed with an older 951/31 scanner (Siemens, Knoxville, TN). This scanner records in 2D mode with intravenous injection of about 10 mCi of FDG. The remaining 21 participants (9 ε4 carriers and 12 noncarriers) were studies with an HR+ scanner (Siemens, Knoxville, TN). The HR+ scanner simultaneously records data in a 3D mode with the intravenous injection of 5-8 mCi of FDG, and permits the reconstruction of images consisting of 63 horizontal slices with a center-to-center slice separation of 2.46 mm, an axial field of view of 15.5 cm, an in-plane resolution of 4.2-5.1 mm full width at half-maximum (FWHM), and an axial resolution of 4.6-6.0 mm FWHM. Regardless of scanner type, a 60-minute dynamic sequence of emission scans was acquired from each participant, who had fasted for at least 4 hours, and was instructed to lay quietly with eyes closed in a darkened room. The emission image was reconstructed with measured attenuation correction and a 0.40-cycle per pixel Hanning filter, resulting in a final in-plane resolution of 10.5mm full width half maximum. Regional analyses were performed using the PET images (counts relative to the whole brain uptake) acquired during the last 30 minutes.
PET data analysis
An automated algorithm (SPM5, Wellcome Department of Cognitive Neurology, London, U.K.) was used to linearly and non-linearly deform each person's PET image into the coordinates of a standard brain atlas. Images were further smoothed using a three-dimensional Gaussian filter to a spatial resolution of 12 mm full-width-at-half-maximum. The images were normalized for the variation in whole brain measurements by using proportionate scaling. Two-sample t-tests were used to examine the differences in CMRgl between the Latino ε4 carriers and noncarriers (p < 0.005, uncorrected for multiple comparisons) on a voxel-by-voxel basis. The statistical map was superimposed onto a map of CMRgl reductions in previously studied patients with AD 22 and a spatially standardized, volume-rendered MRI. Significance levels were then adjusted for the number of resolution elements in the AD-affected posterior cingulate, precuneus, parietotemporal, and frontal brain regions using the small-volume correction (SVC) procedure in SPM (p<0.05, corrected for multiple comparisons). Findings in other brain regions were not corrected for multiple comparisons and are considered exploratory. The statistical analysis was first performed using all data, covarying for the coding of the two scanners. A post-hoc analysis was conducted using only the data (n = 21) from the HR+ scanner to confirm findings independent of any potential confounds associated with the use of the two different scanners. Post-hoc voxel based analysis was conducted using data from a predominately non-Latino Caucasian cohort 40 (11 ε4 heterozygotes and 22 noncarriers) to confirm findings independent of an interaction between APOE ε4 carrier/noncarrier status and Latino/non-Latino status. Additional post-hoc anlayses were conducted. First, a voxel-wise post-hoc analyses to determine the effect of positive first-degree family history of dementia on CMRgl reductions, given that this may impart additional risk above APOE.41-43 Second, an analysis to examine whether the observed CMRgl reductions were associated with performance on the Rey Auditory Verbal Learning Test – Long Term Memory 44 (AVLT-LTM), a measure we have shown to be the most sensitive to age-related memory decline in APOE ε4 carriers.45 For the later, data from the voxel with the most significant reduction of CMRgl (one AD-predicted region and one additional region) was extracted from each subject and used to calculate Pearson correlation coefficients.
Population structure analysis
25 of the 27 Latino samples were successfully genotyped with the Affymetrix 6.0 array using standard methods and Birdsuite46 was used to call SNP genotypes from CEL files. The Latino cohort was tested for stratification between APOE ε4 carriers and non-carriers in PLINK v1.0647 using a clustering approach utilizing pairwise identity-by-state (IBS) distance measures. For this analysis we removed SNPs with Hardy-Weinberg Equilibrium p <= 0.001, missing genotyped > 10%, and minor allele frequencies < 1% for a total of 764,108 SNPs used for stratification analysis. 10,000 label-swapping permutations were performed between APOE ε4 carriers/non-carriers and similarity p-values were calculated.
Results
The distribution of apolipoprotein E genotypes in the 81 subjects is shown in Table 1. The APOE genotype frequencies are similar to those found in other samples of Mexican-Americans,48;49 and the percentage of ε4 homozygotes is similar to that found in the general population.50 The characteristics of the ε4 carriers and noncarriers enrolled in the imaging study are shown in Table 2. The ε4 carriers and noncarrier groups did not differ significantly in their sex, reported family history of dementia, mean age, educational level, MMSE score, other clinical ratings and neuropsychological test scores, or in the scanners used to acquire their FDG PET data.
Table 2.
Characteristics, clinical ratings and neuropsychological scores of the subjects studied with PET a
| noncarriers (n=16) | ε4 carriers (n=11) | P value b | |
|---|---|---|---|
| Age (years) | 53.9±4.7 | 55.5±8.1 | 0.53 |
| Sex (F/M) | 12/4 | 10/1 | 0.25 |
| Education (years) | 14.9±1.6 | 15.8±2.9 | 0.29 |
| First Degree Family History of AD | 44% | 27% | 0.22 |
| MMSE | 29.4±0.5 | 29.4±0.7 | 0.94 |
| HAM-D | 2.8±3.6 | 1.3±1.6 | 0.21 |
| AVLT | |||
| Total Learning | 47.7±11.6 | 51.8±10.3 | 0.35 |
| Short Term Memory | 9.8±2.2 | 10.2±2.9 | 0.66 |
| Long Term Memory | 9.9±2.9 | 9.0±3.6 | 0.48 |
| Complex Figure Test | |||
| Copy | 34.3±2.1 | 32.4±4.3 | 0.12 |
| Recall | 14.7±5.5 | 15.9±7.3 | 0.63 |
| Boston Naming Test | 53.0±5.3 | 54.4±4.3 | 0.48 |
| WAIS-R | |||
| Information | 10.6±1.6 | 10.5±2.1 | 0.92 |
| Digit Span | 9.6±2.5 | 10.8±2.7 | 0.22 |
| Block Design | 10.6±1.8 | 10.8±2.6 | 0.76 |
| Arithmetic | 9.5±1.6 | 9.6±2.2 | 0.86 |
| Similarities | 10.8±1.7 | 11.8±1.8 | 0.12 |
| COWAT | 45.1±9.7 | 48.5±9.8 | 0.38 |
| WMS-R Orientation | 13.8±0.4 | 13.9±0.3 | 0.31 |
Abbreviations: MMSE, Mini-Mental State Exam; HAM-D, Hamilton Depression Rating Scale; AVLT, Auditory Verbal Learning Test; WAIS-R, Wechsler Adult Intelligence Scale-Revised; COWAT, Controlled Oral Word Association Test; WMS-R, Wechsler Memory Scale-Revised.
Data are given as mean ± standard deviation unless otherwise indicated
P values for continuous variables are from t tests. P values for categorical data are from Fisher's exact tests.
As predicted, Latino ε4 carriers had significantly lower CMRgl relative to the noncarriers bilaterally in brain regions previously found to be preferentially affected by AD, including the posterior cingulate (p<0.05, corrected for multiple comparisons using small volume correction), precuneus, and parietal cortex (p<0.005, uncorrected for multiple comparisons) (Table 3, Figure 1). In addition, compared to noncarriers, the ε4 carriers had CMRgl reductions in the middle and anterior cingulate, hippocampus, and thalamus (p<0.005, uncorrected for multiple comparisons) (Table 3, Figure 1). The latter findings should be considered exploratory since the findings were not predicted and were not subject to SVC. For each of the locations specified in Table 3, the mean CMRgl was 6.9 to 16.0% lower in the ε4 carriers than in the noncarriers.
Table 3.
Location and magnitude of most significant CMRgl reductions among Latino APOE ε4 carriers compared to noncarriers
| Brain region | Atlas Coordinates (mm) a | Percent Reduction | T-value | P-value b | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Predicted Regions | |||||||
| Posterior Cingulate | Left | -4 | -30 | 31 | 13.3 | 3.2 | 2.0 × 10-3 c |
| Right | 6 | -33 | 29 | 12.9 | 2.8 | 5.0 × 10-3 c | |
| Precuneus | Left | -16 | -41 | 46 | 9.3 | 4.9 | 2.9 × 10-5 |
| Right | 16 | -43 | 43 | 10.8 | 3.9 | 3.8 × 10-4 | |
| Parietal | Left | -65 | -37 | 39 | 6.9 | 3.2 | 1.8 × 10-3 |
| Right | 69 | -25 | 42 | 7.7 | 3.8 | 4.8 × 10-4 | |
| Additional Regions | |||||||
| Mid Cingulate | Left | -8 | 8 | 33 | 9.3 | 3.6 | 6.7 × 10-4 |
| Right | 12 | 13 | 25 | 14.1 | 6.4 | 6.7 × 10 -7 | |
| Anterior cingulate | Left | -10 | 31 | 2 | 7.1 | 3.9 | 3.1 × 10-3 |
| Right | 10 | 13 | 21 | 16.4 | 6.1 | 1.3 × 10-6 | |
| Hippocampus | Left | -26 | -33 | 9 | 10.8 | 3.1 | 2.5 × 10-3 |
| Thalamus | Right | 14 | -25 | 12 | 16.0 | 3.6 | 8.0 × 10-4 |
For AD-related predicted search regions, the data were extracted from voxels associated with the most significant correlations in each of the brain regions previously found to be associated with abnormally low CMRgl in patients with AD.22
The coordinates were obtained from Talairach and Tournoux.54 X is the distance to the right (+) or left (-) of the midline, Y is the distance anterior (+) or posterior (-) to the anterior commissure, and Z is the distance superior (+) or inferior (-) to a horizontal plane through the anterior and posterior commissures.
The reported significance levels are uncorrected for multiple comparisons.
Remained significant (p<0.05) after correcting for multiple comparisons in the relevant AD-related predicted search regions.
Figure 1.
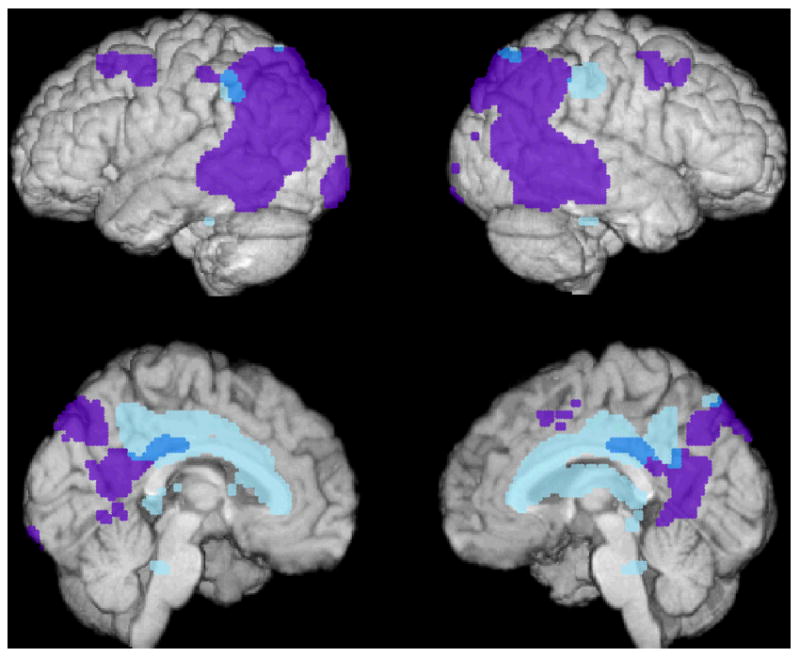
Significantly lower CMRgl in cognitively normal Latino APOE ε4 carriers than noncarriers (shown in blue, p<0.005, uncorrected). Reductions are shown in relationship to brain regions preferentially affected in an earlier PET study of patients with AD22 (shown in purple).
A post-hoc voxel based analysis failed to identify an interaction between APOE ε4 carrier/noncarrier status and Latino/non-Latino status, as the CMRgl reductions observed in the Latino APOE ε4 carriers compared to noncarriers were not significantly different from CMRgl reductions in non-Latino Caucasian APOE ε4 heterozygotes compared to noncarriers. Findings from the post-hoc analysis of the data from the 21 participants scanned with the HR+ scanner was nearly identical to the results obtained from all 27 participants who were scanned on either the HR+ scanner or 951/31 scanner, indicating that the findings are not attributable to any confounds associated with the use of two scanners. Similarly, the post-hoc voxel based analyses which controlled for first-degree family history of dementia were nearly identical to those shown in Table 3 and Figure 1, indicating that the findings are not solely attributable to this confound (data not shown). Lastly, reduction in CMRgl was not correlated with AVLT-LTM, nor was there a significant interaction with APOE ε4 carrier status.
Pairwise IBS clustering across 764,108 SNPs showed no significant similarly or difference between APOE ε4 carriers or non-carriers (p = 0.169 less similar and p = 0.831 more similar). Additionally using the IBS approach we confirmed the 25 genotyped Latinos clustered into a single cluster with no significant outliers based on nearest neighbor calculations.
Discussion
In our previous FDG PET studies, we found that cognitively normal late-middle APOE ε4 carriers have significantly lower CMRgl than noncarriers in brain regions preferentially affected by AD.31;33;36 The present study extends our findings to a cohort of Latino Mexican-Americans, and supports the relationship between the APOE ε4 allele and the risk of AD in this rapidly growing North American community. Furthermore, the genetic findings confirmed the relative homogeneity of our Latino cohort, with no stratification between the APOE ε4 carriers and non-carriers.
Among certain Latino groups, the association between the ε4 allele and AD continues to be clarified, particularly for those of Caribbean origin.10;12-14 Some studies suggest that the strength of the association in Latinos is weaker compared to that in non-Latino Caucasians,8;17 or that the degree of association among Latinos may be intermediate between that in African Americans and that in non-Latino Caucasians,12;51 consistent with the African admixture in Latinos of Caribbean origin.52 If inconsistent findings persist, they may be attributable to AD-related environmental and genetic differences (e.g., dietary differences or African versus European origin) within the heterogeneous characterization of “Latino”, perhaps warranting the use of genome-wide genetic analyses in future studies, with the caveat that there may be cultural sensitivities to the issue specific to different ethnic groups.
In addition to the predicted CMRgl reductions in AD-related brain regions, the Latino ε4 carriers exhibited hypometabolism in the middle and anterior cingulate cortex, hippocampus and thalamus. This pattern of hypometabolism is somewhat different then what we previously observed in a predominately non-Latino cohort of ε4 carriers.31;40 However, in a post-hoc comparison of the interaction between Latino/non-Latino status and ε4 carrier/noncarrier status, these CMRgl reductions in the Latinos were not significantly greater than those in the predominately non-Latino cohort, and so are of uncertain significance. Accordingly, there is a need for additional studies with larger a Latino sample size to confirm that the more extensive regional findings remain significant. If they do, there are other differences besides Latino status that may account for the findings. For instance, there is no requirement for family history of AD in our Latino cohort as there is in our predominately non-Latino cohort, thereby potentially causing us to slightly underestimate changes related to AD risk in the non-Latino cohort.
This study has some limitations. First, since our findings are restricted to participants who identified English as their primary language, were predominately self-designated Mexican-American, were recruited using targeted outreach efforts, had relatively high levels of education, included only 11 APOE ε4 carriers, and included more females than males, additional population-based studies are needed to determine the extent to which they are generalizable to other Latino subjects and communities. Still, our findings are likely to be relevant to those Mexican-American individuals who express interest in proof-of-concept pre-symptomatic treatment studies, discussed below. Second, we have not yet sought to determine the extent to which our findings are solely attributable to the combined effects of atrophy and partial-volume-averaging, MRI white-matter intensities, or vascular risk factors. Still, we previously demonstrated that APOE ε4-related CMRgl reductions are not solely attributable to brain atrophy, subjects in our imaging studies do not have clinically significant MRI abnormalities, and the carriers and noncarriers did not differ significantly in their reported vascular risk factors.
We have proposed how FDG PET and other brain imaging measurements could be used in cognitively normal APOE ε4 carriers as quantitative presymptomatic endophenotypes – measurable features that are more closely related to disease-susceptibility than the clinical syndrome itself – to help evaluate the individual and aggregate effects of putative genetic and non-genetic modifiers of AD risk.36 We are currently investigating the possibility of utilizing multivariate statistical methods, including partial least squares and our recently reported multimodal partial least squares method53 to characterize the patterns of FDG PET and other imaging changes associated with APOE ε4 gene dose, characterize the pre-symptomatic brain-imaging phenotype in a single subject score that reflects this pattern, and use it to evaluate suggested genetic and non-genetic modifiers of AD risk using our pre-symptomatic brain imaging phenotype with superior statistical power and freedom from the Type I error associated with multiple comparisons.
We have also proposed how FDG PET and other brain imaging measurements could be used to evaluate the effectiveness of putative primary prevention therapies to slow down the progressive regional CMRgl declines in proof-of-concept studies without having to study thousands of healthy late-middle-aged persons or wait many years to determine whether or when persons in the clinical trial develop symptoms.35 As a complement to observational studies of older AD patients and controls, our proposed endophenotype could provide prospective evaluation of putative risk modifiers, help address the potentially confounding effects of differential survival related to the risk modifiers, provide information about the individual or aggregate effects of risk factors, and could permit the accurate measurement and real-time evaluation of a putative risk factor years before the onset of symptoms. As a complement to prospective cohort studies, this imaging endophenotype could potentially be utilized as a surrogate marker for preventative treatment development, decreasing the number of healthy participants needed or length of treatment needed to observe drug effects.
The present study illustrates the use of this endophenotype by showing an AD-related pattern of hypometabolism in APOE ε4 carriers from Arizona's Latino community. Consistent with previous findings in non-Latino Caucasians, the predominately Mexican-American APOE ε4 carriers have reduced glucose metabolism in AD-related brain regions which, coupled with the projected rapid increase in the racial and ethnic diversity of older adults,2 supports the inclusion of Latino ε4 carriers in proof-of-concept studies using FDG PET to evaluate promising presymptomatic treatments in cognitively normal people at increased risk for AD.
Acknowledgments
Portions of this study were presented in July 2008 at the 11th International Conference on Alzheimer's Disease (ICAD) in Chicago, IL. It was supported by the National Institute of Mental Health (R01MH57899 to EMR), the National Institute on Aging (R01AG031581 and P30AG19610 to EMR), the Evelyn G. McKnight Brain Institute (GEA), the state of Arizona (EMR, RJC, GEA, KC), and contributions from the Banner Alzheimer's Foundation and Mayo Clinic Foundation. We thank Dr. Richard Gerkin, Dr. Napatkamon Ayutyanont, Patti Aguilar, David Branch, Sandra Goodwin, Bruce Henslin, Debbie Intorcia, Jennifer Keppler, Xiaofen Liu, Anita Prouty, Oded Smilovici, Desiree Van Egmond, Justin Venditti, and Sandra Yee-Benedetto for their assistance.
Reference List
- 1.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 2.Day JC. Population projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. In: U.S.Bureau of the Census, editor. Current Population Reports. Washington,DC: U.S. Government Printing Office; 1996. pp. 25–1130. [Google Scholar]
- 3.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 4.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Clark CM, DeCarli C, Mungas D, et al. Earlier Onset of Alzheimer Disease Symptoms in Latino Individuals Compared With Anglo Individuals. Arch Neurol. 2005;62:774–778. doi: 10.1001/archneur.62.5.774. [DOI] [PubMed] [Google Scholar]
- 6.Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: A multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 8.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 9.Harwood DG, Barker WW, Ownby RL, Mullan M, Duara R. Apolipoprotein E polymorphism and cognitive impairment in a bi-ethnic community-dwelling elderly sample. Alzheimer Dis Assoc Disord. 2002;16:8–14. doi: 10.1097/00002093-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 11.Romas SN, Santana V, Williamson J, et al. Familial Alzheimer disease among Caribbean Hispanics: a reexamination of its association with APOE. Arch Neurol. 2002;59:87–91. doi: 10.1001/archneur.59.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Maestre G, Ottman R, Stern Y, et al. Apolipoprotein E and Alzheimer's disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37:254–259. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- 13.Tang MX, Maestre G, Tsai WY, et al. Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58:574–584. [PMC free article] [PubMed] [Google Scholar]
- 14.Olarte L, Schupf N, Lee JH, et al. Apolipoprotein E epsilon4 and age at onset of sporadic and familial Alzheimer disease in Caribbean Hispanics. Arch Neurol. 2006;63:1586–1590. doi: 10.1001/archneur.63.11.1586. [DOI] [PubMed] [Google Scholar]
- 15.Arboleda GH, Yunis JJ, Pardo R, et al. Apolipoprotein E genotyping in a sample of Colombian patients with Alzheimer's disease. Neurosci Lett. 2001;305:135–138. doi: 10.1016/s0304-3940(01)01829-8. [DOI] [PubMed] [Google Scholar]
- 16.Sevush S, Peruyera G, Crawford F, Mullan M. Apolipoprotein-E epsilon 4 allele frequency and conferred risk for Cuban Americans with Alzheimer's disease. Am J Geriatr Psychiatry. 2000;8:254–256. [PubMed] [Google Scholar]
- 17.Harwood DG, Barker WW, Ownby RL, St George-Hyslop P, Mullan M, Duara R. Apolipoprotein E polymorphism and age of onset for Alzheimer's disease in a bi-ethnic sample. Int Psychogeriatr. 2004;16:317–326. doi: 10.1017/s104161020400033x. [DOI] [PubMed] [Google Scholar]
- 18.Hendrie HC, Hall KS, Hui S, et al. Apolipoprotein E genotypes and Alzheimer's disease in a community study of elderly African Americans. Ann Neurol. 1995;37:118–120. doi: 10.1002/ana.410370123. [DOI] [PubMed] [Google Scholar]
- 19.Osuntokun BO, Sahota A, Ogunniyi AO, et al. Lack of an association between apolipoprotein E epsilon 4 and Alzheimer's disease in elderly Nigerians. Ann Neurol. 1995;38:463–465. doi: 10.1002/ana.410380319. [DOI] [PubMed] [Google Scholar]
- 20.Tycko B, Lee JH, Ciappa A, et al. APOE and APOC1 promoter polymorphisms and the risk of Alzheimer disease in African American and Caribbean Hispanic individuals. Arch Neurol. 2004;61:1434–1439. doi: 10.1001/archneur.61.9.1434. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Cheng R, Schupf N, et al. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, community-based cohort. Arch Neurol. 2007;64:501–506. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: A potential outcome measure in Alzheimer's disease treatment studies. Am J Psychiatry. 2002;159:738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 23.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 24.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 25.Chase TN, Foster NL, Fedio P, Brooks R, Mansi L, Di Chiro G. Regional cortical dysfunction in Alzheimer's disease as determined by positron emission tomography. Ann Neurol. 1984;15 Suppl:S170–S174. doi: 10.1002/ana.410150732. [DOI] [PubMed] [Google Scholar]
- 26.Foster NL, Chase TN, Mansi L, et al. Cortical abnormalities in Alzheimer's disease. Ann Neurol. 1984;16:649–654. doi: 10.1002/ana.410160605. [DOI] [PubMed] [Google Scholar]
- 27.Jagust WJ, Friedland RP, Budinger TF, Koss E, Ober B. Longitudinal studies of regional cerebral metabolism in Alzheimer's disease. Neurology. 1988;38:909–912. doi: 10.1212/wnl.38.6.909. [DOI] [PubMed] [Google Scholar]
- 28.Mosconi L, Tsui WH, Herholz K, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nucl Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langbaum JBS, Chen K, Lee W, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45:1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 31.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 32.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E ε4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimajova M, Lenzo NP, Wu JS, et al. Fluoro-2-deoxy-D-glucose (FDG)-PET in APOE epsilon4 carriers in the Australian population. J Alzheimers Dis. 2008;13:137–146. doi: 10.3233/jad-2008-13203. [DOI] [PubMed] [Google Scholar]
- 35.Reiman EM, Langbaum JBS. Brain imaging in the evaluation of putative Alzheimer's disease slowing, risk-reducing and prevention therapies. In: Jagust WJ, D'Esposito M, editors. Imaging the Aging Brain. New York: Oxford University Press; 2009. pp. 319–350. [Google Scholar]
- 36.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods. 1994;53:125–127. doi: 10.1016/0165-0270(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton M. A rating scale for depression. J neurol neurosurg psychiatry. 1960:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiman EM, Caselli RJ, Alexander GE, Chen K. Tracking the decline in cerebral glucose metablolism in persons and laboratory animals at genetic risk for Alzheimer's disease. Clinical Neuroscience Research. 2001;1:194–206. [Google Scholar]
- 41.Mosconi L, Mistur R, Switalski R, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson SC, Schmitz TW, Trivedi MA, et al. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu G, McLaren DG, Ries ML, et al. The influence of parental history of Alzheimer's disease and apolipoprotein E epsilon4 on the BOLD signal during recognition memory. Brain. 2009;132:383–391. doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rey A. L'examen psychologique dans les cas d'encephalopathie tramatique. Archives de Psychologie. 1941;28:215–285. [Google Scholar]
- 45.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE ε4 effect. N Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mccarroll S, Kuruvilla F, Korn J, et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40 doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 47.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haffner SM, Stern MP, Miettinen H, Robbins D, Howard BV. Apolipoprotein E polymorphism and LDL size in a biethnic population. Arterioscler Thromb Vasc Biol. 1996;16:1184–1188. doi: 10.1161/01.atv.16.9.1184. [DOI] [PubMed] [Google Scholar]
- 49.Shriver MD, Boerwinkle E, Hewett-Emmett D, Hanis CL. Frequency and effects of apolipoprotein E polymorphism in Mexican-American NIDDM subjects. Diabetes. 1991;40:334–337. doi: 10.2337/diab.40.3.334. [DOI] [PubMed] [Google Scholar]
- 50.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 51.Mayeux R, Stern Y, Ottman R, et al. The apolipoprotein epsilon 4 allele in patients with Alzheimer's disease. Ann Neurol. 1993;34:752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- 52.Osborne LC, Mason JM. HLA-A/B haplotye frequencies among U.S. Hispanic and African-American populations. Hum Genet. 1993;91:326–332. doi: 10.1007/BF00217351. [DOI] [PubMed] [Google Scholar]
- 53.Chen K, Reiman EM, Huan Z, et al. Linking functional and structural brain images with multivariate network analyses: A novel application of the partial least square method. Neuroimage. 2009;47:602–610. doi: 10.1016/j.neuroimage.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]


