Abstract
Purpose
Perinatally HIV-infected children, who are increasingly aging into adolescence and early adulthood, have significant rates of psychiatric co-morbidities, some of which are treated with second-generation antipsychotics (SGAs). SGAs have been associated with elevated total cholesterol (TC) in youth, but no studies have examined this association in perinatally HIV-infected youth. This study examined changes in TC levels of youth with perinatally acquired HIV infection and co-morbid psychiatric conditions treated with SGAs.
Patients and methods
Long-term changes in TC levels were examined using data from the US multisite prospective Pediatric AIDS Clinical Trials Group 219C cohort study. The change in TC levels from baseline to 12 months after initiating SGA use was compared between 52 SGA-exposed and 148 matched SGA-unexposed perinatally HIV-infected youth, using generalized estimating equation models adjusting for other covariates. The prevalence and time to incident hypercholesterolemia were also compared between these 2 groups.
Results
After adjustment for confounders, 52 youth with prescriptions for SGAs had a larger increase in TC levels than 148 matched youth without antipsychotic prescriptions (mean difference = 9 mg/dL, z = 1.96, df = 1, P = 0.0496). Among youth with TC below 220 mg/dL at baseline, 27% of SGA-exposed youth developed hypercholesterolemia (defined as two consecutive TC measurements ≥220 mg/dL), compared with 13% of SGA-unexposed patients (Fisher's exact test, P = 0.046).
Conclusions
Caution should be used in prescribing SGAs to perinatally HIV-infected youth with psychiatric co-morbidities due to increased risk of hypercholesterolemia. Patients should be monitored, and alternative evidence-based treatments considered when available.
Keywords: HIV-infected youth with psychiatric co-morbidities, second-generation antipsychotics in youth, cholesterol in youth
Introduction
As the cohort of perinatally HIV-infected youth in the United States is aging into adolescence and early adulthood, the need for long-term management of highly prevalent co-morbid psychiatric symptoms in this unique population is growing.1–5 As a result, physicians caring for these youth increasingly need to manage complex medication regimens that include not only highly active antiretroviral therapy (HAART), but also psychotropic medications, with limited safety data to use as guidance.
Second-generation antipsychotics (SGAs) are prescribed to treat a variety of psychiatric disorders in youth,6–8 and most of them are approved by the US Food and Drug Administration for use in youth 13 years and older.9–11 However, evidence suggests that the use of SGAs might be associated with increased total cholesterol (TC) levels in youth.12–14 Perinatally HIV-infected youth with psychiatric co-morbidities might be particularly vulnerable to this treatment complication and related health risks because: (1) they are more likely to receive psychotropic medications than their HIV-uninfected, sociodemographically matched peers;15 (2) the prevalence of metabolic syndrome among HIV-infected individuals is growing;16 and (3) treatment with protease inhibitors (PIs), a key component of most HAART regimens, has been associated with increased TC levels in perinatally HIV-infected youth.17 On the other hand, evidence also suggests that psychopharmacologic safety data from the general population are not necessarily applicable to HIV-infected individuals; for example, prescribed stimulant medications did not show expected adverse impact on physical growth in a US cohort of perinatally HIV-infected youth,18 and plasma concentrations of selective serotonin reuptake inhibitors (SSRIs) paroxetine and sertraline were found to be much lower in HIV-infected adults than in the general population, suggesting that HIV infection itself may alter pharmacokinetic profiles of these agents.19 Thus, safety data specific for the distinct population of perinatally HIV-infected youth are needed to help prescribing clinicians optimize psychopharmacologic regimens and set parameters for monitoring treatment-emergent complications.
This study was designed to investigate the relationship between prescribed SGAs and changes in TC levels in a US cohort of perinatally HIV-infected youth. The hypothesis was that SGA treatment would be associated with increased TC levels.
Methods
Study design
This study evaluated data from the Pediatric AIDS Clinical Trials Group (PACTG), Protocol 219C (P219C), a multicenter, longitudinal observational cohort study of youth with HIV infection, acquired either perinatally or behaviorally, conducted from September 2000 until May 2007. P219C was a revision of PACTG Protocol 219, initiated in 1993 to study long-term effects of in-utero exposure to antiretroviral therapy (ART) and complications of HIV infection. Local institutional review boards, at over 80 participating sites in the US and Puerto Rico, approved P219C. Informed consent and assent were obtained per local institutional guidelines. Upon enrollment, study nurses abstracted participants' medical records to obtain medical and treatment histories, including diagnoses, ART, and concomitant medications. Follow-up visits included physical examinations, laboratory studies, and self-reports from children and primary caregivers (PCGs) to update demographic information, medical and treatment history, and quality-of-life information.
Participants
SGA exposure was def ined as having a history of prescription for aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, or ziprazidone. The inclusion criteria for SGA -exposed youth from the P219C cohort were: perinatally HIV-infected, 3–22 years old at the time of SGA treatment, prescribed first SGA for at least 6 months, and had available baseline visit within 6 months prior to SGA initiation. Participants who were prescribed a conventional antipsychotic prior to SGA initiation were excluded. Each SGA-exposed participant was matched with up to 3 perinatally infected participants without SGA-exposure history. The matching was based on sex, birth date within 1 year, baseline Tanner stage category (Tanner 1–2, 3–4, or 5), and baseline TC within 15 mg/dL (roughly half of the standard deviation (SD) of TC measurements). The baseline visits for the SGA-exposed and matching unexposed participants were required to be within 3 months of each other.
Total cholesterol measurements
The TC measurements were scheduled to be conducted along with other routine laboratory studies at the time of each 3-month follow-up visit. Fasting was not required prior to these laboratory measurements. For SGA-exposed participants, the baseline TC measurement was the latest measurement prior to SGA initiation but no more than 6 months prior to SGA initiation. The follow-up TC measurement used for primary analysis was the measurement taken closest to 12 months after the baseline visit. This measurement was required to be obtained no less than 6 months after SGA initiation and within 18 months after the baseline visit, and to occur while the participant was still taking the first SGA. For the SGA-unexposed controls, the baseline measurement was the measurement taken closest to the baseline visit for the matching exposed participant. The follow-up measurement was the measurement taken closest to 12 months after the baseline visit and was between 6 and 18 months after the baseline visit.
Statistical methods
Primary analysis
The primary outcome was change in TC levels, as measured from a baseline visit to a follow-up visit (as described above). The primary analysis focused on comparison of change in TC levels for perinatally HIV-infected youth with SGA exposure to those without antipsychotic exposure. A subgroup analysis compared participants with risperidone exposure with those without antipsychotic exposure.
Either Fisher's exact test or Pearson's chi-square test was used to compare patient characteristics between the SGA-exposed versus the unexposed group. A 2-sample t-test was used to compare length of follow-up time between the 2 groups. A 1-sample t-test was used to determine whether there was a significant average change in TC from baseline to follow-up in each group.
Univariate and multivariate generalized estimating equation models were used to evaluate the effect of SGA exposure and other variables on change in TC. Participant characteristics shown in Table 1 were considered as potential confounders in the model selection process. For the purpose of modeling, baseline values of the following variables were dichotomized: CD4 percent (>25% versus ≤25%), HIV RNA (>400 versus ≤400 copies/mL), CDC class (CDC class C versus other), PCG (biological parent versus other), and education level of PCG (high school graduate versus other). Race/ethnicity was collapsed into 3 categories (black non-Hispanic, Hispanic regardless of race, or white/other). ART use at baseline was considered as a categorical variable (HAART with PI, HAART without PI, ART without HAART, and no ART). HAART was defined as at least 3 drugs from at least 2 drug classes.
Table 1.
Participant characteristics
| Characteristic | SGA exposure status |
||||
|---|---|---|---|---|---|
| Not exposed | Exposed | Total | P-value | ||
| Age at baseline visit | 3–6 years | 8 (5%) | 1 (2%) | 9 (5%) | 0.58a |
| >6–9 years | 31 (21%) | 10 (19%) | 41 (21%) | ||
| >9–12years | 41 (28%) | 16 (31%) | 57 (29%) | ||
| >12–15 years | 51 (34%) | 19 (37%) | 70 (35%) | ||
| >15–18 years | 8 (5%) | 3 (6%) | 11 (6%) | ||
| >18–22 years | 9 (6%) | 3 (6%) | 12 (6%) | ||
| gender | Male | 104 (70%) | 37 (71%) | 141 (71%) | 1.00b |
| Female | 44 (30%) | 15 (29%) | 59 (30%) | ||
| Tanner stage at baseline visit | 1 | 82 (55%) | 28 (54%) | 110 (55%) | 0.84a |
| 2 | 17 (11%) | 8 (15%) | 25 (13%) | ||
| 3 | 14 (9%) | 4 (8%) | 18 (9%) | ||
| 4 | 16 (11%) | 2 (4%) | 18 (9%) | ||
| 5 | 19 (13%) | 10 (19%) | 29 (15%) | ||
| Total cholesterol at baseline visit | 170 or less | 55 (37%) | 19 (37%) | 74 (37%) | 0.76a |
| >170–220 | 79 (53%) | 30 (58%) | 109 (55%) | ||
| >220 | 14(9%) | 3 (6%) | 17 (9%) | ||
| BMI at baseline visit | ≤16 | 26 (18%) | 9 (18%) | 35 (18%) | 0.99a |
| <16–19 | 56 (39%) | 19 (37%) | 75 (38%) | ||
| 19–22 | 32 (22%) | 13 (25%) | 45 (23%) | ||
| >22 | 31 (21%) | 10 (20%) | 41 (21%) | ||
| race/ethnicity | White non-Hispanic | 19 (13%) | 9 (17%) | 28 (14%) | 0.73c |
| Black non-Hispanic | 79 (53%) | 29 (56%) | 108 (54%) | ||
| Hispanic (regardless of race) | 44 (30%) | 14 (27%) | 58 (29%) | ||
| Asian, Pacific Islander | 2 (1%) | 0 (0%) | 2 (1%) | ||
| American Indian, Alaskan Native | 2 (1%) | 0 (0%) | 2 (1%) | ||
| subject does not know or other/unknown | 2 (1%) | 0 (0%) | 2 (1%) | ||
| CD4 percent | 0–14 | 8 (6%) | 3 (6%) | 11 (6%) | 0.87a |
| 15–25 | 31 (22%) | 10 (20%) | 41 (21%) | ||
| >25 | 104 (73%) | 38 (75%) | 142 (73%) | ||
| HIV RNA (cp/mL) | 0–400 | 75 (52%) | 30 (58%) | 105 (54%) | 0.65a |
| >400–10000 | 44 (31%) | 13 (25%) | 57 (29%) | ||
| >10000 | 25 (17%) | 9 (17%) | 34 (17%) | ||
| CDC class | A | 45 (30%) | 19 (37%) | 64 (32%) | 0.49a |
| B | 58 (39%) | 12 (23%) | 70 (35%) | ||
| C | 35 (24%) | 14 (27%) | 49 (25%) | ||
| N | 10 (7%) | 7 (13%) | 17 (9%) | ||
| ARV use at baseline | HAART with PI | 104 (70%) | 38 (73%) | 142 (71%) | 0.93c |
| HAART without PI | 16 (11%) | 4 (8%) | 20 (10%) | ||
| ART without HAART | 19 (13%) | 7 (13%) | 26 (13%) | ||
| No ART | 9 (6%) | 3 (6%) | 12 (6%) | ||
| ARV regimen contains at least 1 PI at baseline | No | 41 (28%) | 13 (25%) | 54 (27%) | 0.86b |
| Yes | 107 (72%) | 39 (75%) | 146 (73%) | ||
| PCG | self (subject) | 3 (2%) | 2 (4%) | 5 (3%) | 0.03c |
| Biological parent(s) | 62 (42%) | 17 (33%) | 79 (40%) | ||
| Other relative | 40 (27%) | 7 (13%) | 47 (24%) | ||
| Other adult | 41 (28%) | 23 (44%) | 64 (32%) | ||
| shelter/home | 2 (1%) | 3 (6%) | 5 (3%) | ||
| education level, PCG | Other/unknown | 14 (9%) | 7 (13%) | 21 (11%) | 0.87c |
| grade 1–5/illiterate/none | 35 (24%) | 13 (25%) | 48 (24%) | ||
| grade 6–8 | 37 (25%) | 14 (27%) | 51 (26%) | ||
| grade 9–11 | 40 (27%) | II (21%) | 51 (26%) | ||
| HS graduate | 22 (15%) | 7 (13%) | 29 (15%) | ||
| Had a recent stressful life event | 62 (44%) | 23 (45%) | 85 (44%) | 0.87b | |
| Activities limited by health | 8 (6%) | 9 (19%) | 17 (9%) | 0.02b | |
| Participated in school sports | 96 (70%) | 31 (63%) | 127(68%) | 0.38b | |
| At least one neurological diagnosis | 47 (32%) | 33 (63%) | 80 (40%) | <0.001b | |
Notes:
Mantel-Haenszel chi-square
Fisher's exact test
chi-square test.
Abbreviations: SGA, second-generation antipsychotic; BMI, body mass index; PCG, primary caregiver; HAART, highly active antiretroviral therapy; PI, protease inhibitors; ArT, antiretroviral therapy.
In selecting a multivariate model, all covariates with P-values <0.25 in either the univariate model or the full model, which contained all possible covariates, were considered to be candidates for inclusion. Covariates were removed using backward selection with a significance level of 0.10. ART use at baseline was included in the multivariate model regardless of significance because it has been identified as an important confounder in a similar study17 and its effect was of particular interest. Duration of follow-up was also included in the final multivariate model regardless of significance.
Hypercholesterolemia analyses
To evaluate clinical relevance of the findings from the primary analysis, analyses of prevalence and time to incident hypercholesterolemia by SGA exposure were conducted. Prevalence was assessed at baseline and 6, 12, 18, 24, and 30 months from baseline. Incidence was assessed from baseline until the date the participant was last seen in the study. Because fasting was not required, the definition of incident hypercholesterolemia was modified to decrease false positives17 as follows: the threshold TC value was raised from the usual 200 mg/dL to 220 mg/dL, and an incident case of hypercholesterolemia was defined as 2 consecutive values of 220 mg/dL or higher in a participant whose TC value was below 220 mg/dL at baseline. Incident hypercholesterolemia was assumed to occur at the time of the second TC measurement of 220 mg/dL and above. Fisher's exact test was used to compare incidence of hypercholesterolemia between the SGA-exposed and the unexposed group. Kaplan–Meier plots were constructed to estimate probabilities of hypercholesterolemia in each group.
Results
Of the 2589 perinatally HIV-infected youth in the P219C cohort, 119 (5%) had a history of receiving SGA prescriptions. Of these, 67 were excluded for the following reasons: incomplete drug history (n = 5), started SGA prior to study enrollment (n = 34), used first SGA for less than 6 months (n = 18), prescribed a conventional antipsychotic prior to or concurrently with an SGA (n = 1), discontinued the first SGA before a follow-up measurement was taken (n = 2), did not have either a baseline or a follow-up measurement within the specified time windows (n = 6), or did not have any matching controls available (n = 1). The remaining 52 SGA-exposed participants were included in the analysis. Matching up to 3 SGA-unexposed participants with each SGA-exposed participant resulted in 148 matched unexposed participants for a total sample size of 200. The risperidone subgroup analysis included 31 youth with risperidone prescriptions and 89 matched SGA-unexposed youth for a total sample size of 120.
Participant characteristics
Table 1 shows selected participant characteristics by SGA exposure, including demographic, disease, medication, and quality-of-life characteristics. Overall, the majority of participants were male (71%) and black non-Hispanic (54%). Most participants were in the 12–15 years age category (35%), followed by 9–12 (29%) and 6–9 years (21%). Compared with SGA non-exposed participants, the SGA-exposed cohort had a higher proportion of youth whose PCG was not their biological parent or relative (χ2 = 10.6, df = 4, P = 0.03), whose activities were limited by health (Fisher's exact test, P = 0.02), and who had at least one recorded neurologic or psychiatric diagnosis (documented as: “encephalopathy/cerebral palsy,” “hypotonia,” “microcephaly/failure to thrive,” “epilepsy/seizure/infantile spasm,” “ADHD/behavior disorder,” “depression/anxiety/bipolar disorder,” or “intellectual disabilities/developmental disorder”) (Fisher's exact test, P <0.001). There were no significant differences between the 2 cohorts with respect to race/ethnicity, CD4 percent, HIV RNA (cp/mL), CDC disease severity class, ART use at baseline, recent stressful life events, participation in school sports, and education level of the PCG.
The most common first-prescribed SGA was risperidone, which was prescribed to 31 (60%) participants, followed by aripiprazole (n = 8; 15%), olanzapine (n = 7; 13%), quetiapine (n = 5; 10%) and ziprazidone (n = 1; 2%).
Table 2 shows the distribution of psychiatric and neurologic diagnoses among the participants included in the analysis. The most common documented diagnostic category overall was “ADHD/behavioral disorder” (n = 42; 21%), followed by “intellectual disabilities/developmental disorder” (n = 22; 11%) and “depression/anxiety/bipolar disorder” (n = 19; 10%). Among 52 SGA-exposed participants, 33 (63%) had at least 1 documented neurologic or psychiatric diagnosis. The SGA-exposed cohort had a significantly higher proportion of participants with a diagnosis of “ADHD/behavioral disorder” (Fisher's exact test, P <0.001), “depression/anxiety/bipolar disorder” (Fisher's exact test, P <0.001), and at least 1 documented neurologic or psychiatric diagnosis (Fisher's exact test, P <0.001), as compared with the SGA-unexposed cohort.
Table 2.
Frequencies of neurological and psychiatric diagnoses by SGA-exposure status
| Unexposed (N= 148) | Exposed (N = 52) | Total (N = 200) | |
|---|---|---|---|
| Encephalopathy/cerebral palsy | 12 (8%) | 6 (12%) | 18 (9%) |
| Hypotonia | 15 (10%) | 3 (6%) | 18 (9%) |
| Microcephaly/failure to thrive | 8 (5%) | 1 (2%) | 9 (5%) |
| Epilepsy/seizure/infantile spasm | 5 (3%) | 4 (8%) | 9 (5%) |
| ADHD/behavior disorder | 17 (11%) | 25 (48%) | 42 (21%) |
| Depression/anxiety/bipolar disorder | 6 (4%) | 13 (25%) | 19 (10%) |
| Intellectual disabilities/developmental disorder | 16 (11%) | 6 (12%) | 22 (11%) |
| At least one of the above | 47 (32%) | 33 (63%) | 80 (40%) |
| None of the above | 101 (68%) | 19 (37%) | 120 (60%) |
Abbreviations: SGA, second-generation antipsychotic; ADHD, attention deficit/hyperactivity disorder.
Distributions of the baseline characteristics as well as psychiatric and neurologic diagnoses were similar among the risperidone-exposed participants (data available upon request).
Changes in total cholesterol (TC) levels
Mean and median TC increased in the SGA-exposed group, but decreased slightly in the unexposed group, resulting in a greater increase in TC in the SGA-exposed group (mean change = 10.4 mg/dL) than in the unexposed group (mean change = −0.8 mg/dL). 1-sample t-tests showed that the mean change in TC levels was significantly different from 0 in the SGA-exposed group (t = 2.32, df = 51, P = 0.02), but not in the SGA-unexposed group (t = −0.90, df = 147, P = 0.37). Variation in change in TC was higher in the exposed group (SD = 32.3) than in the unexposed group (SD = 10.4). This was due to some very large changes in TC values (outliers) observed in the exposed group. The distribution of follow-up times was comparable for the SGA-exposed and unexposed groups, with a mean length of follow-up of 11.9 months in the exposed group and 12.0 months in the unexposed group.
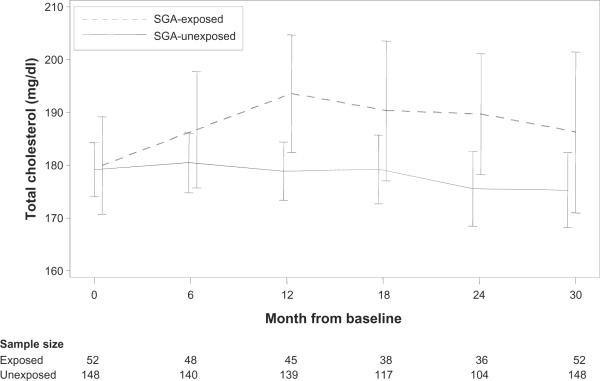
At baseline, mean TC and percentage of participants with elevated TC were comparable in the SGA-exposed and unexposed groups. During follow-up, mean TC and percentage with elevated TC were consistently higher in the SGA-exposed group (Figure 1).
Figure 1.
Mean total cholesterol over time, by SGA exposure, with 95% confidence intervals. The mean TC at a given time point included measurements that occurred within 3 months of that time point. If a participant had more than 1 measurement in this window, then the measurement closest to the time point was used.
Abbreviations: SGA, second-generation antipsychotic; TC, total cholesterol.
Relationships between SGA exposure and TC changes
Table 3 summarizes the results of the univariate and multivariate models for TC on SGA exposure. Unadjusted for potential confounders, SGA-exposed participants experienced an average increase in TC during follow-up that was approximately 11 mg/dL higher than those in the unexposed group (z = 2.45, df = 1, P = 0.01). ART use at baseline was predictive of change in TC levels (χ2 = 8.56, df = 3, P = 0.04). Both HAART without PI (z = −1.89, df = 1, P = 0.06) and no ART (z = −2.55, df = 1, P = 0.01) were associated with smaller increases in TC than HAART with PI. There was no significant difference in change in TC between the ART without HAART group and the HAART with PI group.
Table 3.
summary of estimated increase in total cholesterol over approximately 12 months for SGA (or risperidone)-exposed versus SGA (or risperidone)-unexposed youth
| Model | SGA exposure |
Risperidone exposure |
||||
|---|---|---|---|---|---|---|
| Estimate (mg/dL) | 95% confidence interval | P-value | Estimate (mg/dL) | 95% confidence interval | P-value | |
| Unadjusted | 11.12 | (2.21, 20.02) | 0.01 | 13.56 | (0.77, 26.35) | 0.04 |
| Multivariate modela | 8.68 | (0.01, 17.34) | 0.0496 | 13.02 | (−0.25, 26.30) | 0.055 |
Notes:
Adjusted for statistically significant covariates (race/ethnicity, primary caregiver, education level of the primary caregiver, limited activity due to health) and covariates chosen a priori as important confounders (4-category ArV use and length of follow-up).
Abbreviation: SGA, second-generation antipsychotic.
The effect of SGA exposure remained significant in the multivariate model. After adjustment for confounders, SGA-exposed participants had an average increase in TC during follow-up that was approximately 9 mg/dL higher than in the SGA-unexposed participants (z = 1.96, df = 1, P = 0.0496). ART use was not significant in the multivariate model.
Unadjusted for potential confounders, risperidone-exposed participants experienced an average change in TC during follow-up that was approximately 14 mg/dL higher than in the unexposed participants (z = 2.08, df = 1, P = 0.04). ART use at baseline was not significantly associated with TC levels. The effect of risperidone exposure was marginally significant in the multivariate model. After adjustment for confounders, patients exposed to risperidone had an average increase in TC during follow-up that was approximately 13 mg/dL higher than in the unexposed patients (z = 1.92, df = 1, P = 0.055).
Hypercholesterolemia analyses
Prevalence of hypercholesterolemia
The percentage of participants with elevated TC at baseline (defined as 220 mg/dL or higher) was comparable in the SGA-exposed and unexposed groups (6% versus 9%, Fisher's exact test, P = 0.57) (Figure 1).
Incidence of hypercholesterolemia
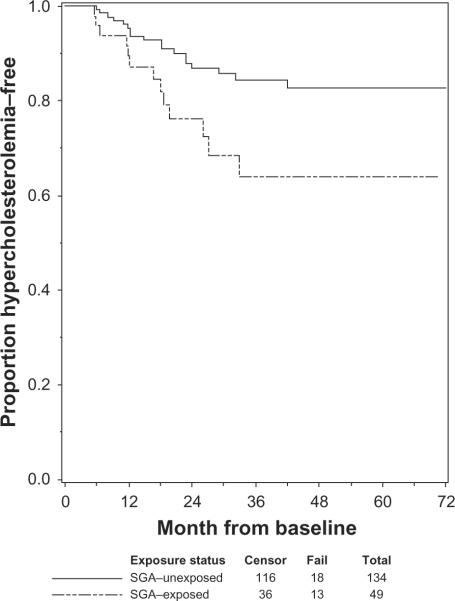
Of the 183 participants with TC below 220 mg/dL at baseline, 31 (17%) developed hypercholesterolemia during a median follow-up time of 28.9 months. Of the 134 unexposed participants with TC below 220 mg/dL at baseline, 18 (13%) developed hypercholesterolemia, compared with the 49 SGA-exposed patients with TC below 220 mg/dL at baseline, of whom 13 (27%) developed hypercholesterolemia (Fisher's exact test, P = 0.046). Figure 2 shows the Kaplan–Meier survival curves for the probability of staying hypercholesterolemia-free by exposure status; SGA-exposed participants had a lower probability of remaining hypercholesterolemia-free compared with unexposed participants. Among SGA-exposed participants who developed hypercholesterolemia, the median time to hypercholesterolemia was 16.8 months.
Figure 2.
Estimated probability of remaining free of hypercholesterolemia, by SGA-exposure.
Abbreviation: SGA, second-generation antipsychotic.
Discussion
This study examined relationships between prescribed SGAs and changes in TC levels in a cohort of perinatally HIV-infected youth. Exposure to SGAs as a class, as well as to risperidone alone, was associated with significantly increased TC levels over an average period of approximately 12 months. While the absolute values of the observed increases may not seem particularly high (9 mg/dL for SGA class and 13 mg/dL for risperidone alone), the statistical significance of these results, combined with matching of participants' sex, age, baseline Tanner stage category, and baseline TC, suggests that the increases are attributable to the SGA exposure alone. Furthermore, the incident analyses showed that SGA exposure was predictive of incident hypercholesterolemia, supporting the clinical significance of the TC increase.
Our data do not provide sufficient information to speculate about possible mechanisms of the observed associations. Findings from in vitro studies20 and model analyses21 suggest that SGA's affinity for histamine H1- and serotonin 5-HT2C receptors might affect hypothalamic control of appetite regulation and energy expenditure.20–22 Alternatively, in vitro data suggest that certain SGAs might induce stimulation of cellular lipogenesis, thus raising the risk of dyslipidemia.23 Similar mechanisms might be involved in SGA-associated increases in cholesterol in perinatally HIV-infected youth with psychiatric co-morbidities. Yet, in the latter group, these mechanisms might be further aggravated by the mechanisms of HAART-associated hypercholesterolemia that has been demonstrated in pediatric observational studies.17,24
Clinically, these findings should be viewed in the context of the already complex medical status of perinatally HIV-infected youth with psychiatric co-morbidities, including the growing rates of metabolic syndrome among HIV-infected adults.16 Cholesterol levels should be routinely monitored when SGAs are being prescribed to youth with perinatally acquired HIV infection. Close communication should be maintained between the consulting psychiatrists, pediatricians and/or infectious disease specialists involved in the child's care and nutritional counseling provided. The youth and their caregivers should be educated about the potential for SGA-related TC increase, as well as other complications previously reported in perinatally HIV-infected youth treated with SGAs, such as increased body mass index z-scores.25 Careful risk-benefit analysis should always be included in this process, given the known benefits of SGAs in treatment of certain psychiatric disorders in youth,8 and potential impact of untreated psychotic symptoms on their medical care, safety, and overall quality of life.
This study has several limitations. First, SGA treatment was not randomly assigned. Some participant characteristics that were in existence prior to SGA initiation, and were not included in the matching criteria, could have influenced the cholesterol outcomes. Randomization would have minimized such potential confounding effects. Next, because P219C did not include complete lipid profiles, the study outcome was reduced to TC alone. Not requiring fasting prior to TC measurement was another limitation, which we attempted to minimize by raising the threshold TC value well above 200 mg/dL and requiring this higher threshold to have been met at 2 consecutive study visits for defining hypercholesterolemia. However, as discussed at length in a related publication,7 elevated TC alone has been independently associated with a risk for early atherosclerotic disease,26 and non-fasting TC is considered a useful screening tool for pediatric dyslipidemia.27
In conclusion, the present study demonstrated a significant potential of SGAs to contribute to increased TC levels in perinatally HIV-infected youth with psychiatric co-morbidities. The issue has high clinical relevance against the backdrop of dramatically increased lifespan and high rates of psychiatric co-morbidities in this population, as well as growing trends of prescribing SGAs to youth with various psychiatric conditions. When considering prescribing an SGA, clinicians should exercise caution and consider other evidence-based treatments where appropriate.
Acknowledgments
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
We thank the children and families for their participation in PACTG 219C, and the individuals and institutions involved in the conduct of 219C as well as the leadership and participants of the P219/219C protocol team. We are grateful for the contributions of Joyce Kraimer, Barbara Heckman, Shirley Traite, and Nathan Tryon. The following institutions and clinical site investigators participated in PACTG 219/219C:
University of New Jersey Medical and Dental School – Department of Pediatrics, Division of Allergy, Immunology and Infectious Diseases: Dr James Oleske, Dr Arlene Bardeguez, Dr Arry Dieudonne, Linda Bettica, Juliette Johnson. Boston Medical Center, Division of Pediatric Infectious Diseases: Dr Stephen I Pelton, Dr Ellen R Cooper, Lauren Kay, Ann Marie Regan. Med, Children's Hospital LA – Department of Pediatrics, Division of Clinical Immunology and Allergy: Dr Joseph A Church, Theresa Dunaway. Long Beach Memorial Medical Center, Miller Children's Hospital: Dr Audra Deveikis, Dr Jagmohan Batra, Susan Marks, Ilaisanee Fineanganofo. Harbor – UCLA Medical Center – Department of Pediatrics, Division of Infectious Diseases: Dr Margaret A Keller, Dr Nasser Redjal, Spring Wettgen, Sheryl Sullivan. Johns Hopkins Hospital and Health System – Department of Pediatrics, Division of Infectious Diseases: Dr Nancy Hutton, Beth Griffith, Mary Joyner, Carolyn Keifer. University of Maryland Medical Center, Division of Pediatric Immunology and Rheumatology: Dr Douglas Watson, Dr John Farley. Texas Children's Hospital, Allergy and Immunology Clinic: Dr Mary E Paul, Chivon D Jackson, Faith Minglana, Dr Heidi Schwarzwald. Cook County Hospital: Dr Kenneth M Boyer, Dr Jamie Martinez, Dr James B McAuley, Maureen Haak. Children's Hospital of Columbus, Ohio: Dr Michael Brady, Dr Katalin Koranyi, Jane Hunkler, Charon Callaway. University of Miami Miller School of Medicine, Division of Pediatric Immunology and Infectious Disease: Dr Gwendolyn B Scott, Dr Charles D Mitchell, Dr Claudia Florez, Joan Gamber. University of California San Francisco School of Medicine, Department of Pediatrics: Dr Diane W Wara, Dr Ann Petru, Nicole Tilton, Mica Muscat. Children's Hospital and Research Center Oakland, Pediatric Clinical Research Center and Research Lab: Dr Ann Petru, Teresa Courville, Karen Gold, Katherine Eng. University of California San Diego Mother, Child and Adolescent HIV Program: Dr Stephen A Spector, Dr Rolando M Viani, Mary Caffery, Kimberly Norris. Duke University School of Medicine – Department of Pediatrics, Children's Health Center: Margaret Donnelly, Dr Kathleen McGann, Carole Mathison, John Swetnam. University of North Carolina at Chapel Hill School of Medicine – Department of Pediatrics, Division of Immunology and Infectious Diseases: Dr Tom Belhorn, Jean Eddleman, Betsy Pitkin. Schneider Children's Hospital: Dr Vincent R Bonagura, Dr Susan Schuval, Dr Blanka Kaplan, Dr Constance Colter. Harlem Hospital Center: Dr Elaine J Abrams, Maxine Frere, Delia Calo. New York University School of Medicine, Division of Pediatric Infectious Diseases: Dr William Borkowsky, Nagamah Deygoo, Maryam Minter, Seham Akleh. Children's National Medical Center, ACT: Diana Dobbins, Deidre Wimbley, Dr Lawrence D'Angelo, Hans Spiegel. University of Washington School of Medicine – Children's Hospital and Regional Medical Center: Dr Ann J Melvin, Kathleen M Mohan, Michele Acker, Suzanne Phelps. University of Illinois College of Medicine at Chicago, Department of Pediatrics: Dr Kenneth C Rich, Dr Karen Hayani, Julia Camacho. Yale University School of Medicine – Department of Pediatrics, Division of Infectious Disease: Dr Warren A Andiman, Leslie Hurst, Dr Janette de Jesus, Donna Schroeder. SUNY at Stony Brook School of Medicine, Division of Pediatric Infectious Diseases: Denise Ferraro, Jane Perillo, Michele Kelly. Howard University Hospital, Department of Pediatrics and Child Health: Dr Sohail Rana, Dr Helga Finke, Patricia Yu, Dr Jhoanna Roa. LA County/University of Southern California Medical Center: Dr Andrea Kovacs, Dr James Homans, Dr Michael Neely, Dr LaShonda Spencer. University of Florida Health Science Center Jacksonville, Division of Pediatric Infectious Disease and Immunology: Dr Mobeen H Rathore, Dr Ayesha Mirza, Kathy Thoma, Almer Mendoza. North Broward Hospital District, Children's Diagnostic and Treatment Center: Dr Ana M Puga, Dr Guillermo Talero, James Blood, Stefanie Juliano. University of Rochester Medical Center, Golisano Children's Hospital: Dr Geoffrey A Weinberg, Barbra Murante, Susan Laverty, Dr Francis Gigliotti. Medical College of Virginia: Dr Suzanne R Lavoie, Tima Y Smith. St Jude Children's Research Hospital, Department of Infectious Diseases: Dr Aditya Gaur, Dr Katherine Knapp, Dr Nehali Patel. Marion Donohoe, University of Puerto Rico, U Children's Hospital AIDS: Dr Irma L Febo, Dr Licette Lugo, Ruth Santos, Ibet Heyer. Children's Hospital of Philadelphia, Center for Pediatric and Adolescent AIDS: Dr Steven D Douglas, Dr Richard M Rutstein, Carol A Vincent, Patricia C Coburn. St Christopher's Hospital for Children/Drexel University College of Medicine: Dr Jill Foster, Dr Janet Chen, Dr Daniel Conway, Dr Roberta Laguerre. Bronx-Lebanon Hospital Center, Infectious Diseases: Dr Emma Stuard, Caroline Nubel, Dr Stefan Hagmann, Dr Murli Purswani. New York Medical College/Metropolitan Hospital Center: Dr Mahrukh Bamji, Dr Indu Pathak, Dr Savita Manwani, Dr Ekta Patel. University of Massachusetts Memorial Children's Medical School, Department of Pediatrics: Dr Katherine Luzuriaga, Dr Richard Moriarty. Baystate Health, Baystate Medical Center: Dr Barbara W Stechenberg, Dr Donna J Fisher, Dr Alicia M Johnston, Maripat Toye. Connecticut Children's Medical Center: Dr Juan C Salazar, Kirsten Fullerton, Gail Karas. Medical College of Georgia School of Medicine, Department of Pediatrics, Division of Infectious Disease: Dr Stuart Foshee, Dr Chitra S Mani, Dr Deniis L Murray, Dr Christopher White. University of South Alabama College of Medicine, Southeast Pediatric ACTU: Dr Mary Y Mancao, Dr Benjamin Estrada. LSU Health Sciences Center: Dr Ronald D Wilcox. Tulane University Health Sciences Center: Dr Margarita Silio, Dr Thomas Alchediak, Cheryl Borne, Shelia Bradford. St Josephs Hospital and Medical Center, Cooper University Hospital – Children's Hospital Boston, Division of Infectious Diseases, David Geffen School of Medicine at UCLA – Department of Pediatrics, Division of Infectious Diseases, Children's Hospital of Orange County, Children's Memorial Hospital – Department of Pediatrics, Division of Infectious Disease, University of Chicago – Department of Pediatrics, Division of Infectious Disease, Mt Sinai Hospital Medical Center – Chicago, Women's and Children's HIV Program, Columbia University Medical Center, Pediatric ACTU, Incarnation Children's Center, Cornell University, Division of Pediatric Infectious Diseases and Immunology, University of Miami Miller School of Medicine – Jackson Memorial Hospital, Bellevue Hospital (Pediatric), San Francisco General (Pediatric), Phoenix Children's Hospital, Metropolitan Hospital Center (NY), University of Cincinnati, SUNY Downstate Medical Center, Children's Hospital at Downstate, North Shore University Hospital, Jacobi Medical Center, University of South Florida – Department of Pediatrics, Division of Infectious Diseases, Cornell University, Oregon Health and Science University – Department of Pediatrics, Division of Infectious Diseases, Children's Hospital of the King's Daughters, Infectious Disease, Lincoln Medical and Mental Health Center, Mt Sinai School of Medicine, Division of Pediatric Infectious Diseases, Emory University Hospital, San Juan City Hospital, UMDNJ – Robert Wood Johnson, Ramon Ruiz Arnau University Hospital, Medical University of South Carolina, SUNY Upstate Medical University, Department of Pediatrics, Wayne State University School of Medicine, Children's Hospital of Michigan, Children's Hospital at Albany Medical Center, Children's Medical Center of Dallas, Children's Hospital – University of Colorado at Denver and Health Sciences, Center, Pediatric Infectious Diseases, Columbus Children's Hospital, University of Florida College of Medicine – Department of Pediatrics, Division of Immunology, Infectious Diseases and Allergy, University of Mississippi Medical Center, Palm Beach County Health Department, Children's Hospital LA – Department of Pediatrics, Division of Adolescent Medicine, Vanderbilt University Medical Center, Division of Pediatric Infectious Diseases, Washington University School of Medicine at St Louis, St Louis Children's Hospital, Children's Hospital and Medical Center, Seattle ACTU, Oregon Health Sciences University, St Luke's-Roosevelt Hospital Center, Montefiore Medical Center – Albert Einstein College of Medicine, Children's Hospital, Washington, DC, Children's Hospital of the King's Daughters, University of Alabama at Birmingham, Department of Pediatrics, Division of Infectious Diseases, Columbus Regional HealthCare System, The Medical Center, Sacred Heart Children's Hospital/CMS of Florida, Bronx Municipal Hospital Center/Jacobi Medical Center.
Footnotes
Disclosure None of the authors has any conflict of interest regarding this manuscript.
References
- 1.Lee GM, Gortmaker SL, McIntosh K, Hughes MD, Oleske JM. Quality of life for children and adolescents: Impact of HIV infection and anti-retroviral treatment. Pediatrics. 2006;117(2):273–283. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 2.Storm DS, Boland MG, Gortmaker SL, et al. Protease inhibitor combination therapy, severity of illness, and quality of life among children with perinatally acquired HIV-1 infection. Pediatrics. 2005;115(2):e173–e182. doi: 10.1542/peds.2004-1693. [DOI] [PubMed] [Google Scholar]
- 3.Butler A, Williams P, Howland L, Hutton A, Storm D, Seage GR., III Disclosure of HIV infection status and health-related quality of life in children with HIV infection. Pediatrics. 2009;123(3):935–943. doi: 10.1542/peds.2008-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children [Accessed May 1, 2010];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2008 July 29;:1–134. Available from: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 5.Mellins CA, Brackis-Cott E, Leu CS, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry. 2009;50(9):1131–1138. doi: 10.1111/j.1469-7610.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel NC, Crismon ML, Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44(6):548–556. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- 7.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63(6):679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 8.Kapetanovic S, Simpson GM. Review of antipsychotics in children and adolescents. Expert Opin Pharmacother. 2006;7(14):1871–1885. doi: 10.1517/14656566.7.14.1871. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration [Accessed May 1, 2010];FDA Approves the First Drug to Treat Irritability Associated with Autism, Risperdal. FDA News. 2006 Oct 6; Available from: http://www.fda.gov/bbs/topics/news/2006/new01485.html.
- 10.US Food and Drug Administration [Accessed May 1, 2010];FDA Approves Risperdal for Two Psychiatric Conditions in Children and Adolescents. FDA News. 2007 Aug 22; Available from: http://www.fda.gov/bbs/topics/news/2007/NEW01686.html.
- 11.Kuehn BM. FDA panel OKs 3 antipsychotic drugs for pediatric use, cautions against overuse. JAMA. 2009;302(8):833–834. doi: 10.1001/jama.2009.1152. [DOI] [PubMed] [Google Scholar]
- 12.Fraguas D, Merchán-Naranjo J, Laita P, et al. Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. J Clin Psychiatry. 2008;69(7):1166–1175. doi: 10.4088/jcp.v69n0717. [DOI] [PubMed] [Google Scholar]
- 13.Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165(11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PubMed] [Google Scholar]
- 14.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chernoff M, Nachman S, Williams P, et al. Mental health treatment patterns in perinatally HIV-infected youth and controls. Pediatrics. 2009;124(2):627–636. doi: 10.1542/peds.2008-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worm SW, Friis-Møller N, Bruyand M, et al. High prevalence of the metabolic syndrome in HIV-infected patients: impact of different definitions of the metabolic syndrome. AIDS. 2010;24(3):427–435. doi: 10.1097/QAD.0b013e328334344e. [DOI] [PubMed] [Google Scholar]
- 17.Tassiopoulos K, Williams PL, Seage GR, 3rd, Crain M, Oleske J, Farley J, for the International Maternal Pediatric Adolescent AIDS Clinical Trials 219C Team Association of hypercholesterolemia incidence with antiretroviral treatment, including protease inhibitors, among perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2008;47(5):607–614. doi: 10.1097/QAI.0b013e3181648e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirois PA, Montepiedra G, Kapetanovic S, et al. Impact of medications prescribed for treatment of attention-deficit hyperactivity disorder on physical growth in children and adolescents with HIV. J Dev Behav Pediatr. 2009;30(5):403–412. doi: 10.1097/dbp.0b013e3181ba0cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best BM, Letendre SL, Rossi SS, et al. Low exposure to paroxetine and sertraline, but not to citalopram, escitalopram or fluoxetine in HIV-infected patients. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 20.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 21.Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20:368–378. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- 22.Le Hellard S, Theisen FM, Haberhausen M, et al. Association between the insulin-induced gene 2 (INSIG2) and weight gain in a German sample of antipsychotic-treated schizophrenic patients: perturbation of SREBP-controlled lipogenesis in drug-related metabolic adverse effects? Mol Psychiatry. 2009;14(3):308–317. doi: 10.1038/sj.mp.4002133. [DOI] [PubMed] [Google Scholar]
- 23.Ferno J, Raeder MB, Vik-Mo AO, et al. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics. 2005;5:298–304. doi: 10.1038/sj.tpj.6500323. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Zaoutis T, Chu J, Zhao H, Rutstein R. Effects of highly active antiretroviral therapy (HAART) on cholesterol in HIV-1 infected children: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2009 Jul;18(7):589–594. doi: 10.1002/pds.1755. [DOI] [PubMed] [Google Scholar]
- 25.Kapetanovic S, Aaron L, Montepiedra G, et al. The use of second-generation antipsychotics and the changes in physical growth in children and adolescents with perinatally acquired HIV. AIDS Patient Care STDS. 2009;23(11):939–947. doi: 10.1089/apc.2009.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman W, Freedman D, Voors A, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis: the Bogalusa Heart Study. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 27.US Preventive Services Task Force Screening for lipid disorders in children: US Preventive Services Task Force recommendations statement. Pediatrics. 2007;120:e215–e219. doi: 10.1542/peds.2006-1812. [DOI] [PubMed] [Google Scholar]