Abstract
Sinus bradycardia can be defined as a sinus rhythm with a resting heart rate of 60 beats per minute or less. While it is assumed that increased autonomic parasympathetic activity is associated with sinus bradycardia, such an association has yet to be proven. The aims of this study were to compute a number of heart rate variability (HRV) parameters in healthy individuals with sinus bradycardia and determine whether there was a significant vagal component to sinus bradycardia. Forty-three healthy adults with normal sinus rhythm and 25 healthy adults with sinus bradycardia had an electrocardiogram recorded for 20 minutes, from which HRV indices were calculated. Results showed significant increases in SDNN (standard deviation of NN intervals) (P < 0.05), RMSDD (square root of the mean squared differences of successive NN intervals) (P < 0.05), and DFA32 (detrended fluctuation analysis) (P < 0.05) in bradycardic subjects compared with subjects with normal sinus rhythm. There were no significant differences in sympathetic frequency domain indices between the two groups. In conclusion, there were significant increases in total heart variability and increased parasympathetic drive in subjects with bradycardia. Clinically, bradycardia is likely to be cardioprotective in aging populations based upon these HRV findings.
Sinus bradycardia can be defined as a sinus rhythm with a resting heart rate (HR) of 60 beats per minute (bpm) or less. However, few patients actually become symptomatic until their heart rate drops to <50 bpm (1). That is, even while resting HR tends to diminish with age (2), physiological regulatory mechanisms maintain normal cardiac output by increasing stroke output both at rest and during exercise (2).
Sinus bradycardia is not commonly included among relevant biomarkers precipitating overt cardiovascular disease (1); a low resting sinus rate even appears to be a protecting factor against heart failure, but a high sinus rate emerged as an independent predictor of mortality in prospective studies carried out in the general population (3) and in selected groups of patients with hypertension (4) or myocardial infarction (5).
Commonly, sinus bradycardia is an incidental finding in otherwise healthy individuals, particularly in adults or sleeping patients. However, it remains unknown whether the autonomic nervous system has a significant influence on the maintenance of sinus bradycardia. Although it is assumed that the vagal system is contributory toward sinus bradycardia (2), actual data relating to cardiac autonomic indices of asymptomatic patients with resting heart rates of <60 bpm are lacking. However, considerable effort has gone into describing resting HR being modulated by a balance between sympathetic and parasympathetic tone with a predominance of the latter (6, 7). On this basis, some reports state that an increased vagal tonus is the main mechanism for the bradycardia induced by aerobic and/or endurance physical training (8). However, several other studies have failed to demonstrate differences in vagal tone between trained and untrained subjects (9, 10). Thus, our aims were to study healthy asymptomatic subjects with sinus bradycardia, calculate their cardiac heart rate variability (HRV) indices, and contrast these results with those of a group of subjects with normal sinus HR.
METHODS
To be included in this study, human subjects had to be at least 45 years old with no known diabetes or other cardiovascular health problems. Participants were excluded from the analysis if any cardiovascular anomaly was reported or identified on the electrocardiogram (ECG) trace (apart from sinus bradycardia) as well as if the ECG trace contained excessive noise. A standard three-lead ECG was obtained from 43 subjects (15 men and 28 women; mean age 54 ± 11 years SD) who had normal sinus rhythm and from 25 subjects (8 men and 17 women; mean age 57 ± 11 years SD) with sinus bradycardia. ECG data were acquired using a Maclab system (AD Instruments) and Chart (version 5). The ECG sampling rate was 400 samples per second with filters set to recommended levels to minimize baseline noise. A 20-minute ECG was recorded while the subject was in a resting supine position.
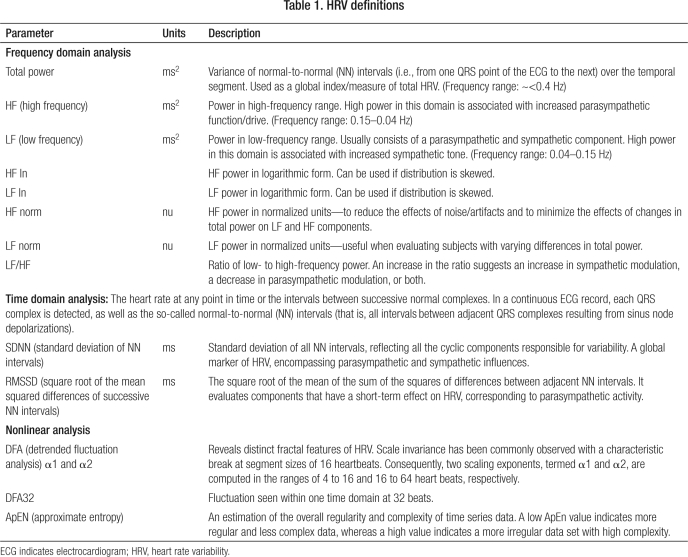
HRV was analyzed using the program Soft-ECG (copyright Herbert Jelinek). Before the Macintosh ECG recording was converted into Soft-ECG format, the digital ECG trace was manually edited to remove any movement artifacts and ectopic beats (11). Soft-ECG then converts the raw ECG trace into an R-R interval graph for analysis. R-R intervals are determined by using the criteria for detecting the fiducial point of the QRS wave (12). Once converted into an R-R graph, further intervals greater and smaller than 200 ms from the mean interval length were removed, as these were deemed to reflect ectopic beats or noise. The HRV parameters calculated include frequency domain analysis, time domain analysis, and nonlinear analysis measures (Table 1). The results were statistically analyzed using a t test or a Kruskal-Wallis test if the HRV data between sample comparisons were nonparametric. Results were considered significant if the P value was <0.05.
Table 1.
HRV definitions
RESULTS
Statistically significant increases in time domain analysis HRV parameters (SDNN, RMSDD) were found in the sinus bradycardia group when compared with normal sinus rate subjects (P < 0.05) (Table 2). SDNN reflects both sympathetic and parasympathetic activity and therefore provides an index of total HRV (6). RMSSD estimates the short-term components of HRV and provides an estimate of vagal nerve activity (6). Thus, in sinus bradycardia, total HRV is increased, and there is likely an increase in vagal activity that is also likely responsible for slowing the HR. It is difficult to interpret if sympathetic drive is changed using time domain analysis, although nonlinear analysis measures of HRV revealed no significant sympathetic differences between subjects with sinus bradycardia and normal sinus rate. Additionally, the nonlinear measure of HRV DFA32 was significantly increased in bradycardia subjects compared with normal sinus subjects, reflecting the increased complexity of the ECG and hence increased total HRV in bradycardia.
Table 2.
Summary statistics for frequency domain, time domain, and nonlinear heart rate variability across the two heart rate groups
| Mean value ± standard deviation |
||||
| Parameter | Normal HR (n = 43) | Bradycardia HR (n = 25) | P valueKruskal-Wallis test | P valuet test |
| Frequency domain analysis | ||||
| Total power | 1817 ± 1460 | 2411 ± 1724 | 0.113 | 0.155 |
| HF | 367 ± 530 | 401 ± 290 | 0.038∗ | 0.34 |
| LF | 448 ± 404 | 621 ± 572 | 0.135 | 0.19 |
| HF ln | 5.27 ± 1.09 | 5.720 ± 0.827 | 0.038∗ | 0.06 |
| LF ln | 5.695 ± 0.960 | 6.086 ± 0.831 | 0.135 | 0.082 |
| HF norm | 47.7 ± 65.0 | 40.7 ± 12.6 | 0.488 | 0.498 |
| LF norm | 56.3 ± 19.0 | 56.8 ± 12.8 | 0.975 | 0.898 |
| LF/HF | 2.24 ± 2.10 | 1.75 ± 1.36 | 0.661 | 0.249 |
| Time domain analysis | ||||
| SDNN | 40.5 ± 15.4 | 48.7 ± 15.1 | 0.038∗ | 0.038∗ |
| RMSSD | 26.2 ± 14.5 | 33.6 ± 13.0 | 0.007∗ | 0.034∗ |
| Nonlinear analysis | ||||
| DFA α1 | 1.013 ± 0.296 | 1.057 ± 0.247 | 0.537 | 0.513 |
| DFA α2 | 0.938 ± 0.178 | 0.923 ± 0.132 | 0.642 | 0.688 |
| DFA32 | 91.4 ± 42.2 | 129.0 ± 72.1 | 0.01∗ | 0.023∗ |
| ApEN | 1.112 ± 0.236 | 1.181 ± 0.199 | 0.242 | 0.203 |
∗Significant at P < 0.05.
HR indicates heart rate; for other abbreviations, see Table 1.
DISCUSSION
This study reflects two important findings. First, bradycardia is associated with increased total power, as measured in the frequency domain. Thus, a reduced nonpathological HR is likely to confer cardiac protection. Indeed, it has been shown that decreased HRV is associated with advancing age, which carries an increased risk of cardiac-related events in clinically disease-free patients (13); however, this is not likely to be the case in aging populations with reduced HR. Also, studies have demonstrated that a decreased HRV provides a poor prognosis for postinfarction patients and heart failure patients (14).
Second, we can confirm that “normal” sinus bradycardia in our study population is due to an increase in vagal drive, as revealed by time domain measures of HRV, i.e., an increase in RMSDD. It is incorrect to assume that increased vagal drive must be responsible for all cases of sinus bradycardia. For example, enhanced cardiac vagal efferent activity does not explain sports endurance training–induced bradycardia compared with age-matched controls. Finally, it has been shown that there is a decline in total HRV with aging mainly, but not exclusively, due to a decline in parasympathetic tonus (15). Thus, in an aging population, a slow heart rate is likely to confer cardioprotective benefits due to an associated increase in parasympathetic autonomic tone and also an associated increase in total HRV.
References
- 1.Alboni P, Brignole M, Menozzi C, Scarfò S. Is sinus bradycardia a factor facilitating overt heart failure? Eur Heart J. 1999;20(4):252–255. doi: 10.1053/euhj.1998.1347. [DOI] [PubMed] [Google Scholar]
- 2.Agruss NS, Rosin EY, Adolph RJ, Fowler NO. Significance of chronic sinus bradycardia in elderly people. Circulation. 1972;46(5):924–930. doi: 10.1161/01.cir.46.5.924. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 4.Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125(4):1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 5.Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112(6):736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 6.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 7.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 8.Catai AM, Chacon-Mikahil MP, Martinelli FS, Forti VA, Silva E, Golfetti R, Martins LE, Szrajer JS, Wanderley JS, Lima-Filho EC, Milan LA, Marin-Neto JA, Maciel BC, Gallo-Junior L. Effects of aerobic exercise training on heart rate variability during wakefulness and sleep and cardiorespiratory responses of young and middle-aged healthy men. Braz J Med Biol Res. 2002;35(6):741–752. doi: 10.1590/s0100-879x2002000600016. [DOI] [PubMed] [Google Scholar]
- 9.Perrault H, Gagnon MC, Johnson D, Mokrane A, Nadeau RA. An enhanced vagal influence does not explain training-induced bradycardia. Physiologist. 1996;39:A20. [Google Scholar]
- 10.Scott AS, Eberhard A, Ofir D, Benchetrit G, Dinh TP, Calabrese P, Lesiuk V, Perrault H. Enhanced cardiac vagal efferent activity does not explain training-induced bradycardia. Auton Neurosci. 2004;112(1–2):60–68. doi: 10.1016/j.autneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Touma F, Chew VS, Chua WC, Jelinek H, Wong PT, Spence I, McLachlan CS. Chronic high dose captopril decreases total heart rate variability and increases heart rate in C57BL/6J mice. Int J Cardiol. 2009;136(2):211–213. doi: 10.1016/j.ijcard.2008.03.082. [DOI] [PubMed] [Google Scholar]
- 12.Tompkins WJ. Biomedical Digital Signal Processing: C-Language Examples and Laboratory Experiments for the IBM PC. Englewood Cliffs, NJ: Prentice Hall; 1993. [Google Scholar]
- 13.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28(6):1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- 14.de Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, van Bemmel JH, Grobbee DE. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol. 1999;150(12):1282–1288. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- 15.Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. Am J Physiol. 1987;253(4 Pt 2):H874–H877. doi: 10.1152/ajpheart.1987.253.4.H874. [DOI] [PubMed] [Google Scholar]