In the last 10 years, the assessment and treatment of pain have become a priority for health care organizations, especially after the introduction of regulatory standards and patient satisfaction surveys directly correlating pain control with a favorable satisfaction score. While much of the focus is on increasing the number of patients receiving adequate pain relief, there has been a subsequent increase in overaggressive attempts to ensure comfort (1).
Severe acute pain is best treated with intermittent, intravenous doses of opioids, which allow rapid titration of effect. Appropriately and accurately prescribed patient-controlled analgesia (PCA) is an effective and efficient method of controlling severe acute pain; the risk of oversedation is significantly reduced, and there is considerable potential to improve pain management for patients (2, 3). PCA allows patients to self-administer more frequent but smaller doses of analgesia than the traditional nurse-administered larger and less frequent bolus doses, thus making PCA a favorable choice to comply with standards and patients' goals for comfort. PCA offers advantages especially when protocols are in place to assess the level of pain and sedation; assess the rate, depth, and quality of respirations; perform a preprocedure cognitive assessment; and note whether the patient is opioid tolerant or naive.
However, serious unintended consequences such as oversedation, respiratory depression, and undertreated pain may occur from the use of PCA. Contributing factors that lead to these events include improper patient selection, inadequate patient monitoring, pump programming errors, PCA by proxy, patients' self-administration of home analgesics while receiving PCA, imprudent polypharmacy, and insufficient health care team member training or education about medications administered via PCA and their dosing and lockout periods. Adverse events due to one of these many contributing factors are preventable and can be significantly reduced with guidance for treatment team staff, patients, and family members.
Numerous adverse event reports totaling in the thousands and a few resulting in patient harm or fatality have occurred since the introduction of PCA (4). In response, the Institute for Safe Medication Practices (ISMP) and the Joint Commission have issued a number of PCA-related safety alerts to health care organizations (2, 3, 5–8). Based on these warnings, many organizations have formed focused, team-based approaches to review PCA-related events and design measures to address the contributing factors leading to these events. Much of the focus is on five areas: patient selection, prescribing errors, staff training, monitoring, and patient education. Developing criteria for selecting appropriate patients to receive PCA is one of the most overlooked but preventable mechanisms to significantly impact events. Well-documented risk factors for oversedation and respiratory depression include opioid-naive status, obesity, age, mental status, and multiple comorbid conditions such as intrinsic lung disease, obstructive sleep apnea (OSA), and renal and hepatic impairment.
OPIOID STATUS
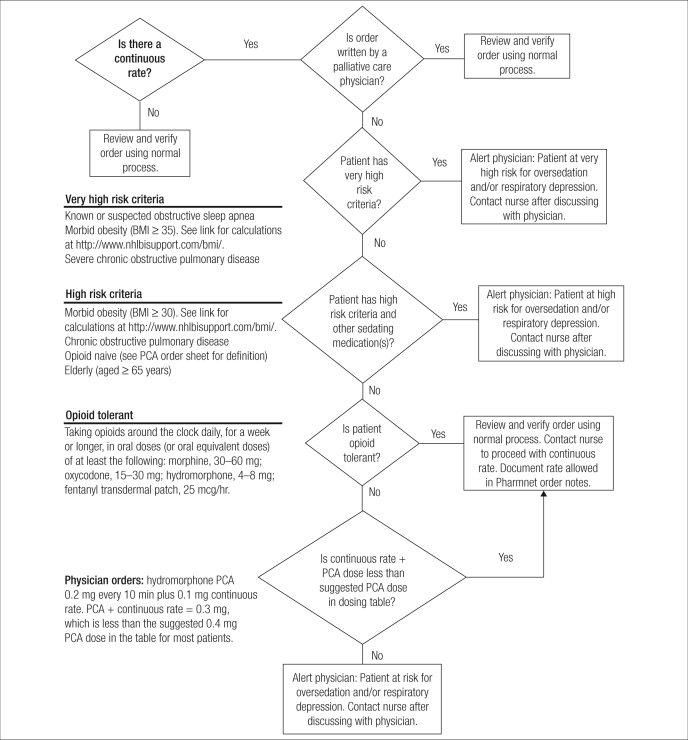
Patients are considered physiologically opioid tolerant if they receive at least 60 mg daily of oral morphine, at least 25 mcg/hr of transdermal fentanyl, at least 30 mg daily of oral oxycodone, at least 8 mg daily of oral hydromorphone, or an equianalgesic daily dose of another opioid for at least a week (9). Patients not meeting this definition would be considered opioid naive. Assessing a patient's opioid status may facilitate ordering the most appropriate initial dose. Most patients fall into the opioid-naive status and thus receive “typical” doses; tolerant patients will need higher supplemental and patient-controlled doses, with consideration given to a continuous infusion (Table 1). The use of a continuous infusion rate for opioid-naive patients has been eliminated at many facilities, aside for the rare exception for which additional safety steps are required before and during the infusion, such as a more detailed pharmacy risk review (Figure).
Table 1.
Recommended opioid doses∗
| Drug | Most patients | Patients over 64 yrs or with sleep apnea | Opioid-tolerantpatients |
| HYDROmorphone | |||
| PCA dose | 0.3 mg | 0.2 mg | 0.4 mg |
| Lockout interval | 10 min | 10 min | 10 min |
| Continuous dose | None | None | 0.3 mg/hr‡ |
| Maximum limit in 4 hrs | 4 mg | 3 mg | 6 mg |
| Loading dose† | 0.6 mg | 0.4 mg | 1 mg |
| Morphine | |||
| PCA dose | 1 mg | 0.7 mg | 1.2 mg |
| Lockout interval | 10 min | 10 min | 10 min |
| Continuous dose | None | None | 2 mg/hr‡ |
| Maximum limit in 4 hrs | 20 mg | 15 mg | 30 mg |
| Loading dose† | 3 mg | 2 mg | 4 mg |
∗Reprinted with permission from Institute for Safe Medication Practices, 2009 (8).
†Repeat loading dose intravenously every 4 hours if needed for breakthrough pain.
‡With pulse oximetry.
Figure.
Pharmacy risk review for patient-controlled analgesia (PCA) used at Baylor University Medical Center at Dallas. BMI indicates body mass index.
COMORBIDITIES
Several patient conditions predispose patients to unwanted effects from opioids. Known, untreated, or unknown OSA poses a significant risk for respiratory depression. OSA screening tools such as Chung et al's STOP-BANG questionnaire (Table 2) (10), used as part of the admission history process, aid the identification process so respiratory therapists, pulmonologists, and treatment teams take appropriate precautions such as making continuous positive airway pressure or bilevel positive airway pressure ventilation devices available. Patients may be trained in the use of these devices to prevent opioid-induced sleep apnea, especially during the use of PCA.
Table 2.
STOP-BANG Scoring Model∗
| Factor | Description | Yes | No |
| 1. Snoring | Do you snore loudly (louder than talking or loud enough to be heard through closed doors)? | ||
| 2. Tired | Do you often feel tired, fatigued, or sleepy during daytime? | ||
| 3. Observed | Has anyone observed you stop breathing during your sleep? | ||
| 4. Blood pressure | Do you have or are you being treated for high blood pressure? | ||
| 5. Body mass index | BMI more than 35 kg/m2? | ||
| 6. Age | Age over 50 yr old? | ||
| 7. Neck circumference | Neck circumference greater than 40 cm? | ||
| 8. Gender | Gender male? |
∗Reprinted with permission from Chung F et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108(5):812–821. Answering yes to three or more items indicates a high risk of obstructive sleep apnea.
Respiratory disease, especially chronic obstructive pulmonary disease (COPD), also poses a significant opioid-related respiratory depression risk. Hypoxemia is a significant risk for both OSA and COPD patients during PCA therapy.
Since opioids are renally eliminated and metabolized by the liver, underlying renal and/or hepatic impairment may lead to opioid accumulation; thus, more pronounced respiratory depressant effects are likely.
OBESITY
The National Heart, Lung, and Blood Institute has defined body mass index categories (Table 3) (11). Obesity places patients at risk for sleep apnea and hypoxemia during PCA therapy.
Table 3.
Classification of overweight and obesity by body mass index (BMI)
| Classification | Class | BMI (kg/m2) |
| Underweight | <18.5 | |
| Normal | 18.5–24.9 | |
| Overweight | 25.0–29.9 | |
| Obesity | I | 30.0–34.9 |
| II | 35.0–39.9 | |
| Extreme obesity | III | ≥40 |
AGE
Metabolic pathways, renal elimination, decreased muscle mass, drug-drug interactions with analgesics, and chronic medications predispose the aging generation to greater opioid analgesic effects, especially oversedation and mental status changes. General principles when approaching the use of PCA in this population are starting low and going slow, plus assessing a patient's mental state and level of consciousness; patients with confusion or dementia cannot safely use PCA. Despite these issues, PCA use should not be withheld based on age alone.
SEX-BASED DIFFERENCES
With more and more knowledge gained from the study of sex-based disparities, we have learned about potential pharmacotherapy differences. To name a few differences, women possess less metabolic function, less muscle mass, less lung capacity, and higher opioid receptor density, which translates into differing reactions to many medications prescribed at typical doses (12). Most clinical trials enroll male subjects or do not differentiate men from women in data analysis, which leads to bias when translating the impact of a medication's known therapeutic and adverse effect profile to women. A clear relationship has been shown between adverse effects and the female gender. Research suggests that sex-based dosing of analgesia may be part of daily practice in the future (12).
PRESCRIBING
Prescribing initial doses or dose adjustments that are too high for opioid-naive patients or for patients with OSA, intrinsic lung disease, renal and/or hepatic impairment, obesity, age ≥ 60 years, and even female gender may result in an increased risk for respiratory depression. Understanding the risks associated with these groups is crucial when prescribing PCA. Facilities should support the use of screening methods to guide prescribers to assess a patient's risk and prescribe doses based on the known risk. Incorporating dosing guidelines along with risk categories as part of the PCA ordering process as seen in Table 1 promotes appropriate dose selection. The choice of opioid is equally important. Hydromorphone has at least five times more analgesic potency than morphine. Initial doses should be significantly lower for hydromorphone on a milligram basis (Table 4).
Table 4.
Opioid equianalgesic doses
| Medication | Dose |
| Morphine | 10 mg |
| Hydromorphone (with repeated intravenous dosing) | 2 mg |
| Fentanyl | 100 mcg |
| Meperidine | 75 mg |
For several reasons, prescribers should avoid utilizing meperidine for pain management in general but more importantly as a PCA option. Meperidine is less effective than most opioids; also, it is metabolized to an active form that tends to accumulate and thus may lead to confusion, central nervous system excitement, and seizures. Many facilities limit meperidine use to the short-term treatment of shivering and rigors.
PUMP PROGRAMMING
Use of capital letters (HYDROmorphone) when prescribing also decreases look-alike, sound-alike confusion during order verification and pump programming. Limiting or standardizing drug concentrations enhances patient safety by preventing the misfilling of a higher concentration when a lower concentration is ordered (2). Smart pumps with barcode scanning are instrumental in preventing these errors.
In addition, doses should always be ordered in milligrams for morphine and hydromorphone or micrograms for fentanyl, or safety is sacrificed. Calculation errors occur when ordering PCA doses in milliliters, as pumps are programmed in milligrams or micrograms; another serious error related to the wrong unit of measure, milliliters, occurs when concentrations do not match between physician orders, nurse programming, and pharmacy stock. For example, if a physician orders morphine 1 mg/mL PCA dose with 1 mL every 5 minutes and the pharmacy stocks only morphine 5 mg/mL, the patient may receive five times more medication than ordered. For these reasons, double checks are put in place to ensure proper pump programming.
The order in which PCA settings are written can also impact safety. PCA orders should be written knowing the order for programming the pump settings. If the pump requests the loading dose settings first, the written order should start with the loading dose.
DOSE AND LOCKOUT INTERVALS
Having too frequent dosing intervals leads to stacking of opioid effects. Appropriate dose intervals should take into account the medication's onset and peak times. In general, the onset of action for most intravenous opioids is 5 minutes, with peak effects in 7 to 15 minutes. (Fentanyl has a slightly quicker onset of action and peak time due to greater lipophilic properties.) Six minutes is the typical dose interval used in practice; however, this interval allows a patient to redose before the peak effect has been reached. The measure of safety is reduced as a patient continues pressing the button to achieve analgesia while the accumulation of effect leads to oversedation. With appropriate dose intervals, usually an appreciable analgesic effect and/or mild sedation is achieved before the patient can assess the next dose, and thus there is a lower chance for oversedation and respiratory depression. A sedated patient will not push the PCA button to give additional doses. Employing a 10-minute dose interval simplifies the ordering process.
Implementing 4-hour lockout intervals to improve safety has led to disappointing pain control; thus, many organizations no longer support a 4-hour lockout. Patients may exhaust all the allotted milligrams early and then have to wait a long time before they receive medication. A 1-hour lockout interval allows for timely monitoring and frequent dose adjustments if needed.
ISMP safe practice recommendations include the development of standardized, preprinted PCA order sets with dosing guidelines for most patients, high-risk patients, and opioid-tolerant patients; such order sets have had a substantial impact on improved analgesia plus the added benefit of reducing adverse events (5). Use of conservative initial doses and subsequent opioid dose adjustments is advisable for all high-risk populations.
POLYPHARMACY
Polypharmacy is the use of multiple drugs or routes to treat one or a limited number of conditions, where the combination poses a significant safety risk, such as increased sedation or respiratory depression. The practice of multimodal pain management involves the addition of multiple medications with differing mechanisms of action and routes in a sensible manner. Yet, in many situations involving PCA, the additive sedating effects from a plethora of irrationally prescribed opioid analgesics and sedating adjuvants (promethazine, diphenhydramine, muscle relaxants, anxiolytics, and sedatives) lead to oversedation. Concomitant use of oral, transdermal, and rectal routes along with PCA may be appropriate in rare circumstances such as during rotation to a nonintravenous pain management strategy or during acute pain episodes in patients with a history of chronic pain, but most scenarios do not support this technique.
PAIN SCALE–BASED RANGE ORDERS
An equally serious problem is linking a patient's self-reported pain score to treatment protocols. PCA pain regimens may include statements such as “adjust patient bolus dose by 25% for pain score 5 to 7” or “adjust patient bolus dose by 50% for pain score 8 to 10.” Patients with a high tolerance for pain may underreport their pain score and thus be undertreated, while patients with a low tolerance for pain may receive too much medication (7). Clinical assessment and evaluation of a patient's overall response to analgesia and reported pain level together produce a safer environment. Modifying PCA settings should not be limited to a reported pain score alone.
POSTOPERATIVE CONCERNS
The cumulative effects of opioids given in the intraoperative and postoperative period and/or in another unit must be ascertained to avoid overdosing with the PCA (7). Delaying the initiation of PCA for high-risk postoperative patients experiencing prolonged oversedation or respiratory issues in the postoperative care unit led to a reduction in events at one facility.
MONITORING
Finally, careful monitoring is a valuable tool to facilitate the safer use of PCA. The limitations of the pain score as described above have led to the development of analgesia-minded monitoring techniques, where respiratory rates are assessed along with respiratory depth and quality plus levels of sedation. Assessment of sedation level is a more reliable way of detecting early opioid-induced respiratory depression than a decreased respiratory rate since hypoxemic episodes often occur in the absence of a low respiratory rate (5). Oversedated patients may respond to aggressive stimulation, which increases respiratory rate and level of consciousness momentarily, so health care providers should not assume the situation is all clear (6). Minimal spoken and tactile stimulation would be preferred during assessment (Table 5) (13).
Table 5.
Pasero Opioid Sedation Scale (POSS)∗
| Value | Action |
| S = Sleep, easy to arouse | Acceptable; no action necessary; may administer opioid if needed. |
| 1 = Awake and alert | Acceptable; no action necessary; may administer opioid dose if needed. |
| 2 = Slightly drowsy, easily aroused | Acceptable; no action necessary; may administer opioid dose if needed. |
| 3 = Frequently drowsy, arousable, drifts off during conversation | Unacceptable; do not administer opioid dose; notify prescriber for orders; suggest administration of a nonsedating, opioid-sparing nonopioid, such as acetaminophen or a nonsteroidal antiinflammatory drug; monitor respiratory status and sedation level closely until sedation level is <3 and respiratory status is satisfactory. When opioid administration is resumed, decrease the initial dose by 50%. |
| 4 = Somnolent, minimal or no response to physical stimulation | Unacceptable; stop opioid administration; consider administering naloxone (Narcan); notify prescriber and house officer or first-response team for orders; monitor respiratory status and sedation level closely until sedation level is <3 and respiratory status is satisfactory. When opioid is resumed, decrease the initial dose by 50%. |
∗Reprinted with permission from Pasero C, Manworren RC, McCaffery M. PAIN control: IV opioid range orders for acute pain management. Am J Nurs 2007;107(2):52–59.
The first 24 hours after surgery represent a high-risk period for a respiratory event, and sedation is highest within the first 12 hours postoperatively, not to mention concerns for nocturnal hypoxemia (6). After identifying “at-risk” groups, ISMP suggests increased monitoring, such as pulse oximetry and/or capnography, with continued assessment of vital signs and alertness (5). As with all treatment, the health care team should regularly reassess the appropriateness of therapy.
PATIENT EDUCATION
Patients and family members should receive both written and verbal instruction about the proper use of PCA during the preoperative period when patients are more alert; repeat teaching is also advisable postoperatively, when patients are not under the influence of intraoperative and postoperative medications. PCA by proxy is a serious danger for the patient. Only the patient should press the PCA button. Place a PCA warning sign at the head of the bed or attach one to the pump.
CONCLUSION
While prescribing PCA is complex and can seem like a burden, with consideration of all patient factors, appropriate prescribing, and effective monitoring, PCA provides timely, safe, and useful analgesia.
References
- 1.Vila H, Jr, Smith RA, Augustyniak MJ, Nagi PA, Soto RG, Ross TW, Cantor AB, Strickland JM, Miguel RV. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101(2):474–480. doi: 10.1213/01.ANE.0000155970.45321.A8. [DOI] [PubMed] [Google Scholar]
- 2.The Joint Commission Focus on five. Preventing patient-controlled analgesia overdose: let eligible patients keep control. Joint Commission Perspectives on Patient Safety. 2005;5(10):11. [Google Scholar]
- 3.The Joint Commission. Patient controlled analgesia by proxy. Sentinel event alert, December 20, 2004. Available at http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_33.htm; accessed July 24, 2010. [PubMed]
- 4.Taylor S, Voytovich AE, Kozol RA. Has the pendulum swung too far in postoperative pain control? Am J Surg. 2003;186(5):472–475. doi: 10.1016/j.amjsurg.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Institute for Safe Medication Practices. More on avoiding opiate toxicity with PCA by proxy. ISMP Medication Safety Alert! May 29, 2002. Available at http://www.ismp.org/newsletters/acutecare/articles/20020529.asp; accessed July 24, 2010.
- 6.Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia. Part I—how errors occur. ISMP Medication Safety Alert! July 10, 2003. Available at http://www.ismp.org/newsletters/acutecare/articles/20030710.asp; accessed July 24, 2010.
- 7.Institute for Safe Medication Practices. Pain scales don't weigh every risk. ISMP Medication Safety Alert! July 24, 2002. Available at http://www.ismp.org/newsletters/acutecare/articles/20020724.asp; accessed July 23, 2010.
- 8.Institute for Safe Medication Practices. Beware of basal opioid infusions with PCA therapy. ISMP Medication Safety Alert! March 12, 2009. Available at http://www.ismp.org/Newsletters/acutecare/articles/20090312.asp; accessed July 23, 2010.
- 9.Ortho-McNeil-Janssen Pharmaceuticals, Inc. Duragesic¯ (Fentanyl Transdermal System): Full US Prescribing Information, revised July 2009. Available at http://www.duragesic.com/sites/default/files/pdf/duragesic_0.pdf; accessed July 24, 2010.
- 10.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 11.National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults [NIH Publication No. 98-4083]. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health. Available at http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf; accessed July 23, 2010.
- 12.Overholser BR. Sex-related differences in disease and pharmacotherapy. In: Richardson MM, Chant C, Cheng JWM, et al., editors. Women's and Men's Health. Pharmacotherapy Self-Assessment Program. 6th ed. Kansas City, MO: American College of Clinical Pharmacy; 2008. pp. 1–18. [Google Scholar]
- 13.Pasero C, Manworren RC, McCaffery M. PAIN control: IV opioid range orders for acute pain management. Am J Nurs. 2007;107(2):52–59. doi: 10.1097/00000446-200702000-00023. [DOI] [PubMed] [Google Scholar]