Abstract
Background
Receptor tyrosine kinases (RTK) act through dimerization. Previously it was thought that only bivalent ligands could be agonistic, whereas monovalent ligands should be antagonistic. This notion changed after the demonstration that monovalent ligands can be agonistic, including our report of a small molecule monovalent ligand “D3” that is a partial agonist of the NGF receptor TrkA. A bivalent “D3-linker-D3” was expected to increase agonism.
Methods
Dimeric analogs were synthesized and tested in binding, biochemical, and biological assays.
Results
One analog, 1-ss, binds TrkA with higher affinity than D3 and induces or stabilizes receptor dimers. However, 1-ss exhibited antagonistic activity, through two mechanisms. One mechanism is that 1-ss blocks NGF binding, unlike D3 which is non-competitive. Inhibition of NGF binding may be due to the linker of 1-ss filling the inter-receptor space that NGF traverses before docking. In a second mechanism, 1-ss acts as a pure antagonist, inhibiting NGF-independent TrkA activity in cells over-expressing receptors. Inhibition is likely due to 1-ss “freezing” the TrkA dimer in the inactive state.
Conclusions
Dimerization of an RTK can result in antagonism, through two independent mechanisms.
General Significance
we report a small molecule monovalent agonist being converted to a bivalent antagonist.
Keywords: neurotrophin, ligand, tyrosine kinase receptor, linker, agonist, antagonist
1. Introduction
The high affinity receptor for Nerve Growth Factor (NGF), TrkA, is a member of the receptor tyrosine kinase (RTK) family. TrkA, and RTKs in general, act through receptor dimers where each receptor trans-activates the juxtaposed intracellular tyrosine kinase domain of its neighbor [1]. Because NGF is a dimeric ligand, it is thought to induce ligand-dependent TrkA receptor dimerization or to stabilize preformed receptor dimers. This lead to the generally accepted notion that only dimeric ligands could be agonistic [2, 3]. Indeed modification of a ligand from a monovalent to a bivalent form could generate agonists: this was documented for TrkA [4, 5] and for other RTKs [6, 7].
However, bivalency is not absolutely required. Indeed, monovalent and monomeric agonists have been reported for several RTKs [8, 9], including TrkA [4, 10, 11]. Activation by monovalent ligands can take place through conformational changes in pre-formed receptor dimers [12]. However, to date structural analyses have not demonstrated conformational changes in TrkA even after the binding of protein ligands such as NGF [13]. Thus, the correlation between ligand-induced RTK dimerization, the activation state of a receptor, and agonistic activity is questionable for the natural protein ligands; and it is even less certain for receptor activation caused by small molecule ligands.
Here, we tested whether dimerization of an agonistic monovalent ligand could generate an antagonist. We generated homobivalent analogs of a previously reported NGF peptidomimetic D3 (hereafter termed “A”). Mimetic D3 is a monovalent and monomeric ligand of TrkA, and acts as an agonist to activate this receptor. D3 has intrinsic agonistic activity at TrkA, in the absence of NGF. Moreover, D3 binding to TrkA is non-competitive with respect to NGF. Thus, NGF and D3 can bind TrkA concomitantly, and in fact D3 potentiates NGF activity [4, 10].
The bivalent analogs generated are two D3 units (A-A) linked through a series of progressively longer and relatively rigid linkers, resulting in various lengths that approximate the geometry of the NGF dimer. Unlike the parental D3 molecule, the dimeric A-A antagonizes NGF binding and NGF-dependent function. Moreover, although dimeric A-A induces or stabilizes TrkA dimers, it actually antagonizes receptor function in TrkA over-expressing cells. Thus, non-covalent cross-linking of the receptor pair by a bivalent ligand could “freeze” the receptor dimer in an inactive state, resulting in an antagonist.
Switching the action of a small molecule ligand from an agonist to an antagonist can provide strategies to discover a new class of inhibitors or activators of NGF receptors, and the concept could be expanded to other members of the RTK family.
2. Methods
2.1 Preparation of the Bivalent Derivatives
The chemical synthesis and purification of non-tagged and the dye-tagged peptidomimetics is described in the Supplemental methods. A scheme of the reactions is given in Supplemental Figure 1. A scheme of the reactions for homodimerization is given in Supplemental Figure 2. All the final purified compounds were analyzed by analytical HPLC again and MALDI-MS as shown in Supplemental Table 1.
2.2 Cell Lines
NIH-TrkA cells are NIH3T3 fibroblasts transfected with human trkA cDNA. NIH-TrkC cells are NIH3T3 fibroblasts transfected with human trkC cDNA. NIH-IGF-1R cells are NIH3T3 fibroblasts transfected with human IGF-1R cDNA. All cells are stably transfected subclones that express high levels of their receptor. Cells are grown under drug selection (0.5 mg/ml G418) and are routinely screened for receptor expression by FACScan using monoclonal antibodies directed to the receptor extracellular domains. PC12 cells are a rat pheochromocytoma expressing low levels of endogenous TrkA (∼2,000 receptors/cell) and high levels of endogenous p75 (∼200,000 receptors/cell). 4-3.6 cells are stabily transfected rat neuroblastoma expressing endogenous p75 (∼40,000 receptors/cell) and human TrkA (∼30,000 receptors/cell) [4, 10].
2.3 Antibodies
mouse anti-TrkA mAb 5C3, mouse anti-TrkC mAb 2B7, mouse anti-p75 mAb MC912, and mouse anti-IGF-1R mAb alphaIR3 were purified using protein G-Sepharose (Pharmacia, Baie d'Urfe, Quebec, Canada). They have all been described [4, 14].
2.4 FACScan analysis
Cells (2 × 105) in 100 μl of FACScan binding buffer (BB: phosphate-buffered saline, 0.5% BSA, and 0.1% NaN3) were immunostained as described. Saturating mAbs, or control non-binding mouse IgGs were added to cells for 20 min at 4 °C, excess primary antibody was washed off, and cells were immunostained with fluoresceinated goat anti-mouse IgG (FITC-G-α-M) secondary antibody. As cellular controls NIH-3T3 cells not expressing NGF receptors were used (e.g. NIH-IGF-1R). Cells were acquired on a FACScan, and bell-shaped histograms were analyzed using LYSIS II and the CellQuest-pro program as described [4].
2.5 FACScan analysis of peptidomimetics
Peptidomimetics labeled with fluorescein were used in FACScan binding studies, as described in section 2.4. The assay was slightly modified to extend the incubation of the “primary” reagent to 40 minutes, followed by two washes to remove unbound material. There was no secondary reagent added, as the compounds are directly labeled.
2.6 FACScan binding competition assays
Blocking to the binding of FITC-labeled peptidomimetics were studied by pre-incubation of the cells with increasing concentrations of TrkA ligands anti-TrkA mAb 5C3, for 30 min at 4°C. Then, the FITC-labeled peptidomimetic was added, without previous washing, and the study progressed as described in section 2.5.
2.7 Ligand Binding
125I[NGF] (73.1 mCi/mg; NEN Life Science Products) binding assays were done on cells as described [15]. NIH-TrkA cells (1×106 per point) were added to serial dilutions of 125I[NGF] in BB at 4°C, that was prepared in the presence or absence of a molar excess of test peptidomimetic or control cold NGF. During the serial dilutions, the test peptidomimetic or control cold NGF remain at the indicated constant fold-molar excess over 125I[NGF]. NIH-3T3 wild type cells not expressing NGF receptors were used to assess nonspecific background (<15% of total binding). Scatchard plot analysis of the data was performed.
In related binding assays, increasing concentrations of the test peptidomimetic or control cold NGF were added to a constant dose of 125I[NGF].
2.8 Survival Assays
Cells (5,000–10,000 cells/well) were added to 96-well plates and cultured either in media containing 5% fetal bovine serum or in serum free media with 0.1% BSA (SFM). Ligands consisted of serial dilutions of neurotrophins or peptidomimetics were then added. FITC-tagged and TEG-tagged mimetics were tested, but only the data for TEG-tagged mimetics are shown. A suboptimal dose of neurotrophins (0.1 – 0.2 nM, affording ∼25% of survival) was used to test the effect of combination of NGF with peptidomimetics. The proliferative/survival profile of the cells was quantitated using the tetrazolium salt reagent (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma) and optical density (OD) readings as described. Assays were done 4-7 times, each assay n 4–8.
2.9 Receptor Dimerization Assays
Receptor cross-linking assays were carried out as reported elsewhere [10]. Here, NIH-TrkA cells (106 cells/ml each group) were exposed to the indicated ligands (untreated control, NGF 10 nM, 1-ss-fluorescein (20 μM), or 1-ss-TEG (20 μM)) for 30 min at 4°C. Following washing, cells were chemically cross-linked with 1 mM final disuccinimidyl suberate (DSS, Pierce) for 7 min. Un-reacted DSS was quenched with a 5-molar excess of ammonium acetate and the cells were washed two times with HBSS at 4°C. Then each cell pellet was detergent solubilized (1% NP40 containing protease inhibitors). Protein concentration for each of the cleared lysates were determined using a detergent-compatible BioRad kit. Equal protein (20 μg/lane) of each sample were resolved by SDS-PAGE, and after Western transfer the membranes were analyzed by western blotting with anti-TrkA mAbs. Equal loading was further verified by Coomassie blue staining of the gels.
2.10 Receptor Activation Assays
NIH-TrkA cells were cultured at 37°C in SFM for 12 hours to reduce their baseline tyrosine phosphorylation. Then the cells were exposed to the indicated ligand (NGF, 1-ss, untreated control) at 37°C for 20 minutes or for 2 hours. After washing in PBS, cells were detergent solubilized, and equal protein (20 μg/lane) of each of the clear lysates were resolved by SDS-PAGE, and after Western transfer the membranes were analyzed by western blotting with anti-pTyr mAb 4G10, or with anti-phospho-Akt as previously reported [16]. As controls, TrkA loading was verified using anti-TrkA mAb 5C3 and protein with anti-actin [17, 18].
3. Results
3.1 Synthesis of D3-homodimers
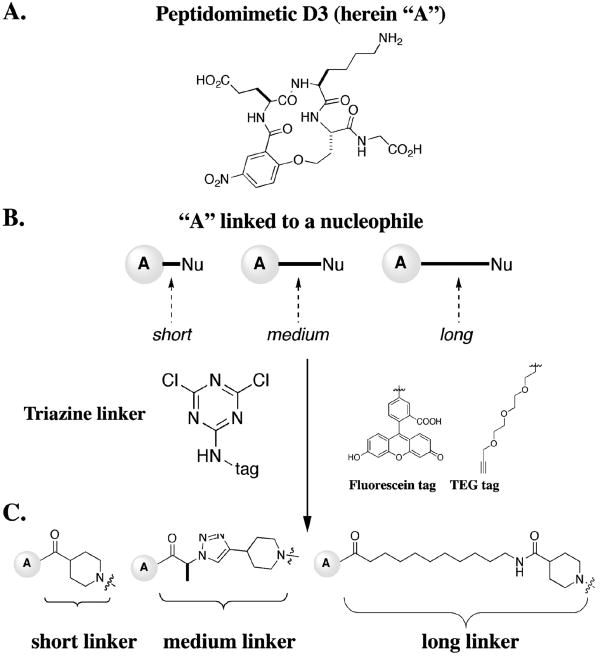
Previously we reported the design, synthesis, and activity of small molecule mimics of NGF [10, 19-22]. One of the early leads identified, originally reported as peptidomimetic D3 is herein termed compound “A” for simplicity. Biochemical and cellular assays showed that peptidomimetic “A” is a monovalent TrkA agonist binding at the D5 domain of TrkA [10], and in vivo is effective to activate TrkA and afford neuroproteciton [23-25].
The precursor to peptidomimetic “A” (Figure 1A) was assembled to make bivalent molecules through a modification of the literature procedure [26]. Assembly of the monovalent molecules into the bivalent ones was achieved using a triazine-based linker (Figure 1B) [27]. The monovalent building blocks “A” represent the pharmacophore and were purposely not modified, other than the nitro-aryl moiety which is not relevant for binding. However, the triazine and the linker parts were varied in two ways.
FIGURE 1. Synthetic scheme for a homobivalent library.
(A) Lead compound D3 compound (herein A for simplicity) was used to make a set of bivalent molecules using a combinatorial approach. (B) Mimetic A was attached to a short s, medium m, or long linker l bearing a nucleophile. (C) These were then paired on a triazine scaffold to which a tag (either a fluorescein (FITC) or a TEG) were incorporated.
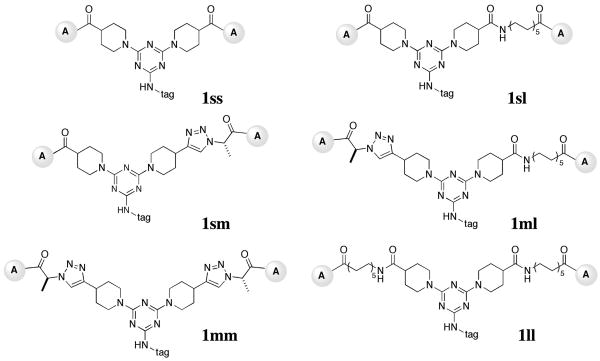
First, we used a combinatorial approach to attach mimetic A to a short (s), a medium (m), or a long linker (l) (Figure 1C). These were then paired on the triazine scaffold to yield a family of homobivalent compounds 1 representing combinations of linkers: 1-ss, 1-sm, 1-sl, 1-mm, 1-ml, and 1-ll (see structures in Figure 2). Second, the triazine had one of two different “tags”. Fluorescein isothiocyanate (FITC) was used to add a single fluorescein label to compounds to facilitate screening via fluorescence activated cell scanning (FACScan). A flexible triethylene glycol (TEG) tag was also added, and had no function except to substitute the fluorescein and to increase the water solubility of the mimics (Figure 1B). The purity and the composition of the bivalent molecules were confirmed by HPLC/MS (see supplemental Table 1).
FIGURE 2. Structure of the compounds.
1-ss, 1-sm, 1-sl, 1-mm, 1-ml, and 1-ll. The “ss” indicates a short-short linker length combination, “sm” a short-medium linker length combination, “sl” indicates a short-long linker length combination etc. Each of these structures can have a FITC tag or a TEG tag. Compounds were purified and their correct masses were verified.
3.2 Binding studies with labeled peptidomimetics
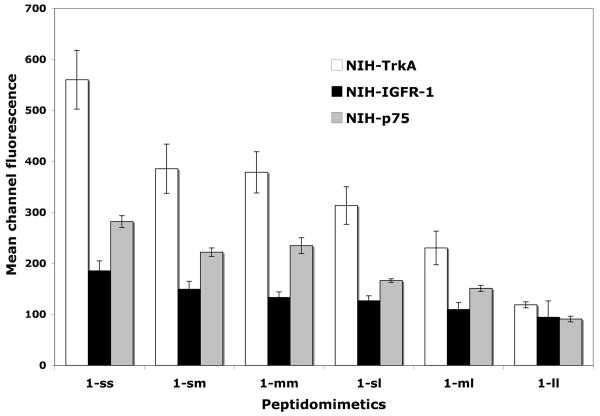
Direct FACScan-based binding assays were performed using fluorescein-labeled compounds, and NIH-3T3 cells stably transfected to express the neurotrophin receptor TrkA (NIH-TrkA), or controls expressing the p75 neurotrophin receptor (NIH-p75), or the IGF-1R receptor (NIH-IGF-1R) to test for selectivity (Figure 3), or the TrkC neurotrophin receptor (NIH-TrkC) (data not shown).
FIGURE 3. Direct binding FACScan assay with FITC-peptidomimetics.
Cells expressing the indicated receptor were bound with test ligand (20 μM) at 4°C. After washing, data was acquired and analyzed by FACScan/CellQuest with background subtracted. Mean channel fluorescence ± sem, n =3-6 independent experiments.
Peptidomimetic 1-ss bound TrkA selectively, and better than any other dimer. Using linkers based on the same triazine backbone but which are of longer length resulted in molecules with lower efficacy of TrkA binding. Progressively longer peptidomimetics 1-sm, 1-mm, 1-sl, 1-ml, and 1-ll exhibited significantly lower TrkA binding than 1-ss. These studies were replicated using at least two independent syntheses of the compounds, with each synthesis being tested independently at least three times.
3.3 1-ss inhibits the binding of NGF
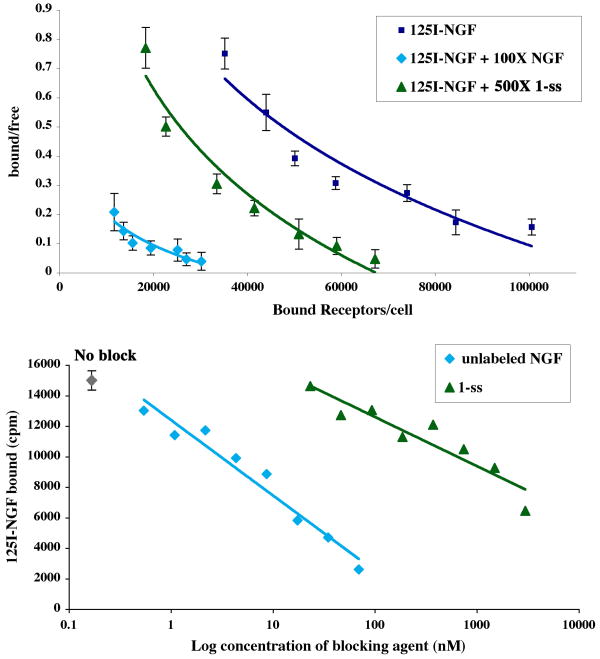
Binding experiments tested 1-ss effects on the binding of 125I[NGF] to TrkA-expressing NIH-3T3 cells (Figure 4). These cells do not express the p75 co-receptor, thus TrkA accounts for all the specific binding. Unlabeled NGF was used as control competitor. Scatchard plot analyses of 125I[NGF] binding data showed the expected ∼150,000 receptors/cell on the surface (Figure 4A). A constant 500-fold excess of peptidomimetic 1-ss reduced the binding of 125I[NGF] by ∼50%, without changing the affinity of the residual 125I[NGF] bound to TrkA. In a positive control, 100-fold excess unlabeled NGF as competitor blocked nearly all the binding of 125I[NGF]. The background cpms were determined by using the same concentration range of 125I[NGF] binding to NIH-wild type cells (not expressing TrkA). In all assays, background ranged from 5-15% of the total binding, and in each case these background cpms were subtracted.
FIGURE 4. Inhibition of NGF binding to TrkA.
NIH-TrkA cells were studied. (A) Scatchard plot analysis of high affinity 125I[NGF] binding data. Binding assays were carried out using a range of 125I[NGF] and a 100-fold excess of unlabeled NGF or a 500-fold excess of mimetic 1-ss as inhibitors. (B) Displacement of a constant concentration of 125I[NGF] (∼15,000 cpms) by increasing doses of unlabeled NGF or by mimetic 1-ss. In all assays NIH-3T3 wild type cells were used to assess nonspecific background (<15% of total binding).
Similar binding assays were carried out using a constant 125I[NGF] concentration (2 nM, resulting in maximal 16,500 cpms bound); and a dose-range of the inhibitors 1-ss or unlabeled NGF as control. The inhibitors reduced 125I[NGF] binding in a dose-dependent manner (Figure 4B). Averaged from three independent assays ± sem the inhibition of 125I[NGF] by cold NGF is IC50 9 ± 5 nM; and the 1-ss IC50 is 5,000 ± 420 nM (e.g. ∼550-fold higher than NGF competing itself). We estimate that 1-ss has a Kd ∼105 nM, which is improved compared to the affinity estimated for the monovalent parental compound A (Kd ∼ 10 μM). This ∼100-fold improved affinity was expected from 1-ss bivalency.
3.4 NGF does not block the binding of 1-ss
We attempted the converse experiment, to block the binding of 1-ss-fluorescein by pre-incubation of cells with NGF. In these FACScan-based binding assays NGF did not affect the binding of 1-ss-fluorescein (data not shown), even at 200 nM NGF concentrations, known to saturate TrkA. These data suggest that 1-ss binds to site of TrkA that is not overlapping with that of NGF. This is consistent with previous reports showing that parental compound A (from which 1-ss is made) binds TrkA in a way that does not compete with NGF binding [10]. It is therefore possible that NGF and 1-ss could bind a TrkA receptor at the same time.
3.5 1-ss Induces or Stabilizes Receptor Dimers
The increased affinity in 1-ss (compared to parental monovalent A) is likely due to its bivalent binding. Thus, we performed chemical cross-linking experiments to address the hypothesis that 1-ss binds to a TrkA-TrkA homodimer. NIH-TrkA cells were exposed to test ligands or controls, followed by chemical cross-linking to stabilize receptor dimers on the cell surface. Then cell lysates were resolved in denaturing SDS-PAGE and analyzed by western blotting with highly specific anti-TrkA antibodies (Figure 5).
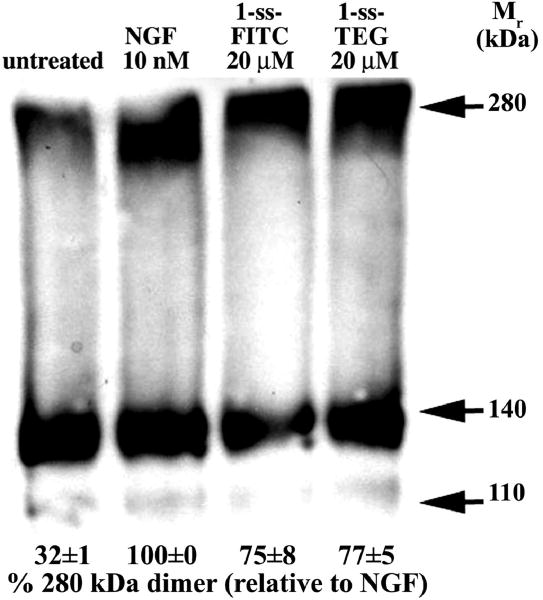
FIGURE 5. NGF and 1-ss induce/stabilize cross-linking of TrkA dimers.
NIH-TrkA cells were studied. After exposure to the indicated ligand (30 min at 4°C), or to no ligand, cells were chemically cross-linked with DSS. After washing, cells were detergent solubilized and samples (20 μg protein/lane) were studied by western blotting with a highly specific anti-TrkA mAb 5C3. The amount of putative TrkA dimer (280 kDa) relative to optimal NGF-induced dimer (100%) is indicated. Note that untreated NIH-TrkA cells have ∼32% of the level of cross-linked TrkA dimers fond in NGF-treated cells.
Lysaes from unliganded cross-linked NIH-TrkA cells show a band at p140 (cell surface TrkA monomer) and a less intense p110 band previously reported to be intracellular TrkA with a lower degree of glycosylation. A band at ∼280 kDa is also detected. This is consistent with ligand-independent pre-formed homodimers in receptor over-expressing cells [12]. Exposure of NIH-TrkA cells to ligands 1-ss or NGF significantly increased detection of the 280 kDa band using highly specific anti-TrkA mAbs. This would be consistent with a ligand-mediated increase in TrkA dimers. Standardization of the TrkA dimers detected after exposure of cells to 10 nM NGF as 100% show that 1-ss-fluorescein affords 75±8%, and 1-ss-TEG affords 77±5%. Previous cross-linking data reported for the parental monovalent A compound showed that it affords 21±4% of TrkA dimers relative to 10 nM NGF [10]. Controls performed without chemical cross-linking result is no detection of ∼280 kDa bands, because pre-formed homodimers are non-covalent and fall out in denaturing SDS-PAGE (data not shown).
Overall, the data indicate that NGF and 1-ss have the ability to induce or to stabilize TrkA dimers at the cell surface, and that 1-ss has improved ability to induce TrkA dimers compared to the parental monovalent agent.
3.6 1-ss Antagonizes NGF-promoted cell survival
The parental monovalent A compound is a partial TrkA agonist. Thus, the bioactivity of the bivalent peptidomimetics was tested in quantitative MTT assays testing receptor-mediated cell survival, using NIH-3T3 cells expressing TrkA, or as controls NIH-3T3 cells expressing TrkC, or IGF-1R.
When cells are cultured in serum-free media (SFM) they undergo apoptosis. In these conditions, cells can be protected by their appropriate growth factor (NIH-TrkA is protected by NGF, NIH-TrkC is protected by NT-3, NIH-IGF-1R is protected by IGF-1). Growth factor protection from apoptosis is dose-dependent, and suboptimal doses of growth factor can be used that result quantitatively and consistently in reduced survival (25-50% of maximal).
In initial assays the fluoresceinated peptidomimetic 1-ss was used. These assays proved that 1-ss antagonizes NGF (data not shown). However, using a fluorescein-labeled compound in bioassays is not desirable. Thus, the assays were repeated using analogs of 1-ss, 1-mm, and 1-ll that contain a TEG derivative to substitute the fluorescein moiety. This was done to verify that the fluorescein moiety was not relevant for antagonistic function.
Mimetic 1-ss-TEG was antagonistic of NGF bioactivity, measured as a reduction of NGF-mediated protection of NIH-TrkA cells undergoing apoptosis after serum deprivation (Figure 6A). Antagonism was selective because mimetic 1-ss-TEG did not antagonize the protective function of NT-3 upon NIH-TrkC cells (Figure 6B), nor the protective function of IGF-1 upon NIH-IGF-1R (data not shown). In control assays, mimetics 1-mm-TEG, and 1-ll-TEG had no significant effect on the survival-promoting effect of any growth factor, NGF (Figure 6A), NT3 (Figure 6B), or IGF-1 (data not shown). These inactive mimetics are highly related to mimetic 1-ss-TEG and are therefore crucial controls.
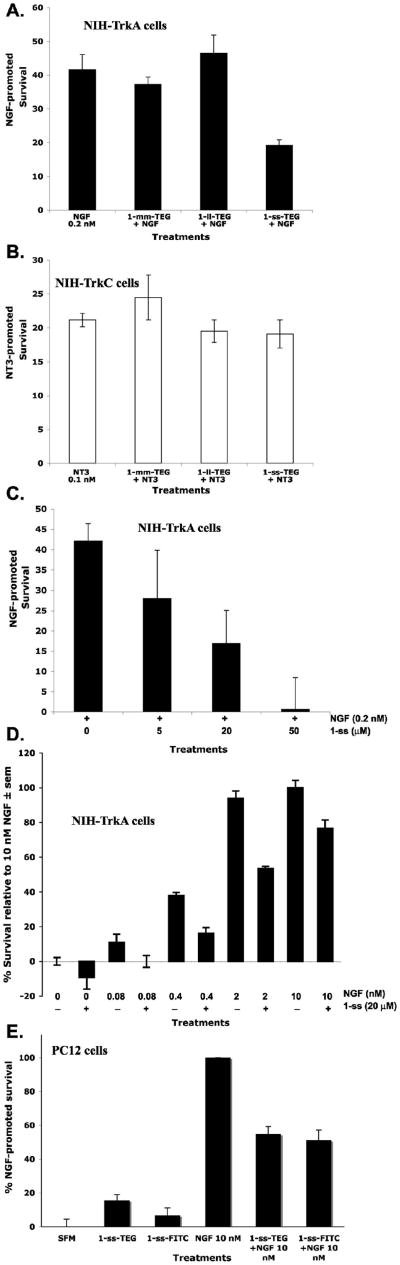
FIGURE 6. Mimetic 1-ss-TEG and 1-ss-fluorescein antagonize NGF and TrkA in cell survival assays.
NIH-3T3 cells expressing TrkA or TrkC, or PC12 cells were cultured in SFM alone or supplemented with growth factor (NGF for TrkA-expressing cells, NT-3 for TrkC-expressing cells). Survival was measured by MTT assays, and was calculated relative to optimal neurotrophin (100% protection). Suboptimal concentrations of growth factor (0.2 nM or 0.1 nM) were used to achieve limited survival. Results shown are average ± SEM, from at least three independent experiments (n = 4 per experiment). (A) Mimetic 1-ss-TEG (20 μM) antagonizes 0.2 nM NGF; but not (B) 0.1 nM NT-3. (C) Dose-dependent antagonism of TrkA-NGF by increasing doses of 1-ss-TEG in NIH-TkA TkA cells over-expressing TrkA. (D) Increasing doses of NGF oppose the antagonism of a constant dose of 1-ss-TEG (20 μM) in NIH-TkA cells over-expressing TrkA. (E) Antagonism of optimal (10 nM) NGF–induced survival in PC12 cells, expressing low levels of TrkA, using 20 μM 1-ss-TEG or 1-ss-fluorescein.
Antagonism of NGF action by 1-ss-TEG was dose-dependent. The survival promoted by 0.2 nM NGF (∼40% of maximal survival) was reduced by 1-ss-TEG. The mimetic at 5 μM, 20 μM, and 50 μM respectively reduced the survival promoted by NGF from ∼40% to ∼25% (non-significant), and to a statistically significant ∼15%, and ∼0% survival (Figure 6C).
In the converse experiment, increasing doses of NGF could partially overcome the antagonism of 20 μM 1-ss (Figure 6D). In the NIH-TrkA cells that over-express TrkA, 20 μM 1-ss inhibited NGF-mediated survival with efficacy ranging from ∼100% inhibition (0.08 nM NGF) to ∼70% inhibition (0.4 nM NGF) to ∼50% inhibition (2 nM NGF) to ∼25% inhibition (10 nM NGF).
Lastly, we tested 1-ss-fluorescein and 1-ss-TEG antagonism of NGF activity in PC12 cells because they express very low levels of TrkA. Mimetics 1-ss-fluorescein and 1-ss-TEG (20 μM) antagonized ∼50% of the survival promoted by 10 nM NGF in serum-free media (Figure 6E). Similar antagonism of NGF activity was obtained using the 4-3.6 cell line that expresses medium levels of TrkA (data not shown).
Together, these data demonstrate that 1-ss is an antagonist of NGF-dependent TrkA function, and that antagonism is dependent on the stoichiometry of 1-ss/NGF as well as on the relative levels of cell surface TrkA.
3.7 1-ss Antagonizes NGF-independent TrkA activity
Over-expressed TrkA in NIH-TrkA cells has spontaneous ligand-independent trophic functions. Indeed, high baseline ligand-independent activity has been reported in cells over-expressing TrkA as well as for many other RTKs, and this activity is oncogenic [2, 3]. Indeed, in the NIH-TrkA cells TrkA-dimers were observed in the absence of ligand (e.g. see Figure 5). For that reason, we hypothesized that mimetic 1-ss could accelerate the death of NIH-TrkA cells in SFM, in the absence of NGF. This would reflect antagonism of baseline receptor activity, which is ligand-independent.
Mimetic 1-ss-TEG significantly accelerated the death of NIH-TrkA cells in SFM, in the absence of NGF (Figure 7A). This effect was TrkA-selective, as it was not observed in control NIH-TrkC cells (Figure 7A) or NIH-IGF-1R cells (data not shown). Both of these control cell lines over-expressing RTKs also have high baseline receptor activity. In assays that control for compound, mimetics 1-mm-TEG, and 1-ll-TEG did not have a significant effect on ligand-independent activity of any of the three receptors (Figure 7A).
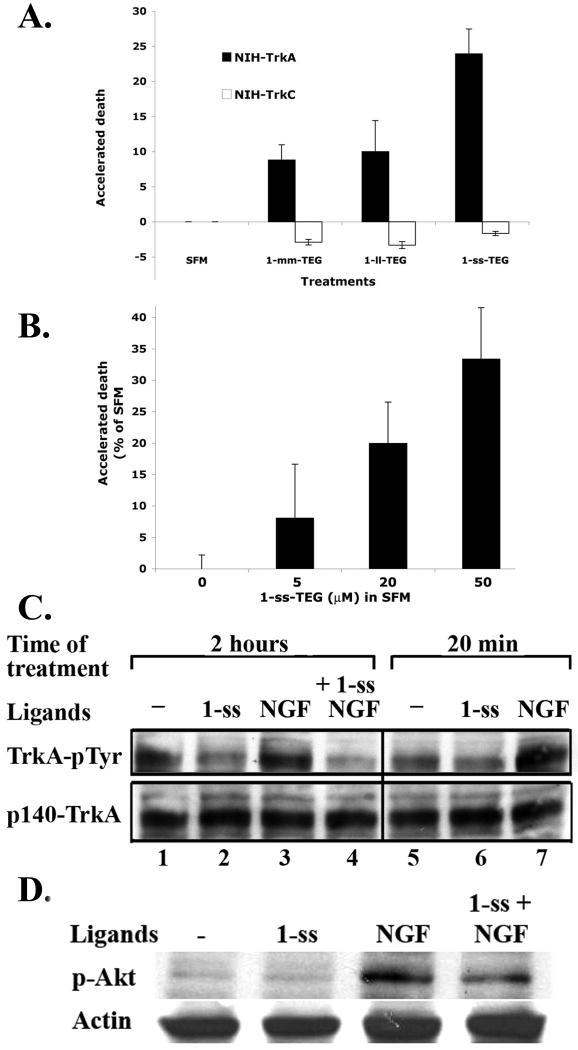
FIGURE 7. Mimetic 1-ss-TEG antagonizes baseline (ligand-independent) and ligand-dependent TrkA activity.
(A) selective inhibition of ligand-independent TrkA baseline activity, but not TrkC baseline activity, accelerates the death of NIH-TrkA in SFM, (B) dose-dependent inhibition of TrkA baseline activity. (C) NIH-TrkA cells were cultured in SFM ± the indicated ligands at 37°C in a cell incubator (NGF 2 nM; 1-ss 20 μM). After detergent solubilization the phosphotyrosine levels of the samples were studied by western blotting with anti-pTyr mAb 4G10. Total TrkA loading was verified on the same membranes with a highly specific anti-TrkA mAb 5C3. (D) Same as in (C) except that samples were studied by western blotting with anti-p-Akt mAb, standardized versus actin loading control, after treatment with the indicated ligands for 20 minutes.
Accelerated death of NIH-TrkA cells in SFM by 1-ss-TEG was dose dependent and significant at concentrations of 20 μM and 50 μM (Figure 7B). The actual OD490 readings from MTT are shown in Table 1.
TABLE 1.
Representative 3-day MTT assay of cell survival. The indicated cells were cultured in SFM in 96-well plates (n=6 wells/condition). The indicated wells were supplemented with serum (normal culture: growth + survival), or neurotrophin (NTF: NGF for TrkA-expressing cells, NT-3 for TrkC-expressing cells); with or without 1-ss-TEG (20 μM). The relative resistance to death in SFM is reduced by 1-ss-TEG in NIH-TrkA but not in NIH-TrkC. Ligand-independent survival is not seen in PC12 cells, as these cells do not over-express TrkA. Furthermore, the survival promoted by NGF is reduced by 1-ss-TEG in NIH-TrkA and PC12 cells, but the survival promoted by NT-3 is not reduced in NIH-TrkC.
| TREATMENT | OD490 (×1000) values | ||
|---|---|---|---|
| TrkA-NIH | TrkC-NIH | PC12 | |
| 5% serum | 609 ± 28 | 706 ± 61 | 403 ± 13 |
| 5% serum + 1-ss-TEG | 611 ± 22 | 700 ± 34 | 398 ± 10 |
| NTF optimal (10 nM) | 464 ± 12 | 535 ± 16 | 309 ± 8 |
| NTF suboptimal (0.2 or 0.1 nM) | 243 ± 17 | 269 ± 23 | 145 ± 25 |
| SMF | 127 ± 7 | 153 ± 4 | 29 ± 4 |
| 1-ss-TEG | 59 ± 5 | 156 ± 5 | 32 ± 5 |
| NTF suboptimal + 1-ss-TEG | 155 ± 11 | 274 ± 19 | 42 ± 6 |
These data show the relatively high resistance of NIH-TrkA and NIH-TrkC cells to death in SFM, because they over-express a tyrosine kinase receptor and have a degree of ligand-independent activity. This relatively high background survival is not seen in cells that express lower TrkA density (e.g. PC12 cells) (Table 1). It is noteworthy that there was no detectable toxicity in MTT assays for any cell type (over-expressing NIH transfectants, PC12, or 4-3.6 cells) when they were cultured in serum containing media + the mimetics (see Table 1).
In sum, cells over-expressing RTKs exhibit a relative resistance to death in SFM. In NIH-TrkA cells the resistance is reduced by 1-ss-TEG, resulting in the accelerated death of these cells. In contrast, the NIH-TrkC resistance is unaffected by 1-ss-TEG. In addition, as reported in Figure 6, the survival promoted by NGF in NIH-TrkA and PC12 cells is reduced by 1-ss-TEG. In contrast, the survival promoted by NT-3 in NIH-TrkC is unaffected by 1-ss-TEG.
3.8 Antagonism of biochemical signals by 1-ss
We further characterized ligand-dependent and ligand-independent antagonism. We carried out biochemical analyses of receptor-tyrosine phosphorylation (pTyr) in untreated or ligand treated cells by western blotting using anti-phosphotyrosine antibodies (Figure 7). To show equal receptor loading the membranes were stripped and re-blotted with anti-TrkA mAb 5C3.
Untreated NIH-TrkA cells have significant basal TrkA-pTyr (Figure 7C lane 1), consistent with the ligand-independent receptor dimers reported in Figure 5 and ligand-independent survival activity reported in Figure 7 and Table 1. Ligand-independent TrkA-pTyr decreases significantly after culture with 1-ss-TEG (20 μM) for 2 hours, and decreases even when treated for a relatively short 20 minutes (Figure 7C lane 1 versus 2; lane 5 versus 6).
Treatment with NGF (2 nM) results in significant and sustained increases in TrkA-pTyr lasting longer than 2 hours (Figure 7C lane 3, lane 7). In these conditions, 1-ss was able to reduce the level of TrkA-pTyr even in the continuous presence of NGF over a 2 hour period (Figure 7C, lane 4). Similar data were obtained when a downstream adaptor of activated TrkA, phospho-Akt (p-AKT), was studied (Figure 7D). NGF induces rapid p-Akt over baseline, which is inhibited by 1-ss.
The biochemical data are consistent with the biological data, and indicate that 1-ss has the ability to inhibit NGF-dependent, as well to inhibit the NGF-independent, activation of TrkA and signals downstream.
Conclusions
Here we address whether and how dimerization of an agonistic monovalent ligand could generate an antagonist. We rationalized that if an agonistic ligand must induce conformational changes in a receptor, a relatively rigid bivalent small molecule ligand could “freeze” the receptor dimer by non-covalently cross-linking a receptor pair, and in actuality prevent conformational changes that may be necessary for activation. This is important because conformational changes in TrkA take place upon the binding of its protein ligand NGF have not yet been demonstrated through structural analyses and this is the matter of current debate (e.g. see letters to editor in [13]).
Both the monovalent parental ligand D3 and the bivalent ligand 1-ss have high selectivity towards TrkA. While 1-ss has higher affinity and efficacy at inducing or stabilizing TrkA-homodimers, 1-ss also gained the ability to block NGF binding and to inhibit TrkA function. Inhibition of NGF binding is peculiar because the parental D3 molecule and NGF do not block each other, even though both bind to the D5 domain of TrkA [10]. In fact D3 potentiates NGF function [10]. For those reasons we expected that 1-ss and NGF should not block each other.
On the other hand, we found that while pre-bound 1-ss blocks NGF binding, pre-bound NGF does not block 1-ss binding. How does 1-ss block NGF binding to TrkA? Speculative in silico molecular docking (presented in the Supplemental Figure 3) suggest that the extended triazine linker bridge of 1-ss “fills” the inter-receptor space where NGF would access its docking sites on the TrkA dimers. This would conceivably result in NGF and 1-ss being able to bind simultaneously to TrkA, provided that NGF is bound first prior to 1-ss blocking its access to the receptor. The biological outcome of concomitant binding by NGF and 1-ss could result either in an overactive TrkA (i.e. NGF is “trapped”) or in an inactive TrkA (i.e. the dimer is “frozen” in the inactive state). Our biological and biochemical data supports the latter possibility, as 1-ss antagonizes ligand-dependent TrkA-pTyr, and prevents the survival signals arising from TrkA activation.
The simplest way to explain the differences in the intrinsic function of 1-ss (antagonist) compared to the parental D3 mimetic (agonist and potentiator of NGF) would be in their ability to affect the activation state of the receptor. For example, receptor topology and inter-domain interactions have been suggested to be critical for TrkA receptor activation [18, 28-30]. The specific receptor-receptor assembly required for activation may be affected by the linker of 1-ss, and this may be key for changing an agonist into an antagonist. This notion is further supported by the fact that 1-ss inhibits the ligand-independent (oncogenic) TrkA activation in receptor over-expressing cells, even though its binding results in increased detection of TrkA-TrkA dimers which are generally presumed to be the active form of receptor tyrosine kinases.
In sum, 1-ss docks onto TrkA and acts as an antagonist of NGF binding. Logically, blocking NGF binding results in antagonism of the trophic activity of NGF. However, it is important to emphasize that antagonism by 1-ss is not exclusively the result of blocking NGF binding, because functional antagonism can also take place in bioassays over long periods (48-72 hours) under conditions where the much higher affinity NGF ligand can bind to TrkA. The 1-ss mimetic also acts as a dimer-promoting receptor antagonist, reducing the baseline ligand-independent TrkA activity and tyrosine phosphorylation in TrkA over-expressing cells.
Ligand-independent trophic functions in RTK over-expressing cells is oncogenic [2, 3]. It would seem unusual that an antagonist would increase TrkA-TrkA dimers, because dimeric receptors are thought to be in the active state. There is a plausible albeit more complex explanation with precedents in the ErbB2 family of receptors. In that family, growth factor-induced dimerization causes receptor phosphorylation, then the phosphorylated receptors dissociate and each phosphorylated monomer can interact with a new (nonphosphorylated) receptor to form a secondary dimer that amplifies signals [31]. It is possible that 1-ss inhibits the monomerization of phosphorylated TrkA dimers, thereby inhibiting signal amplification. Also, because one ErbB2 monomer can interact with one of two possible complementary surfaces on a second ErbB2 monomer [32], differential juxtapositioning of the dimer can alter the signaling; and it is attractive to speculate that this may also apply to TrkA.
We report on an agonistic ligand being converted to an antagonist through dimerization. This model can provide strategies to discover a new class of inhibitors or activators of NGF receptors, and the concept could be expanded to other members of the receptor tyrosine kinase family.
Supplementary Material
Acknowledgments
Supported by the Canadian Institutes of Health Research (MOP 192060) and the Cancer Research Society (11138) to HUS, and the National Institute of Health (MH070040 and GM076261) to KB.
Non-standard abbreviations used
- FACScan
Fluoresence Activated Cell Scanner
- FITC
Fluorescein isothiocyanate
- TEG
Triethylene Glycol
- Trk
Tropomyosin receptor kinase family
- NGF
Nerve Growth Factor
- p75
Neurotrophin Receptor
- IGF-1
Insulin-like growth Factor-1
- IGF-1R
Insulin-like growth Factor-1 Receptor
- pTyr
Phosphotyrosine
- RTK
Receptor Tyrosine Kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fouad Brahimi, Lady Davis Institute-Jewish General Hospital, Pharmacology and Therapeutics, Oncology and the Cancer Center. McGill University.
Jing Liu, Department of Chemistry, Texas A&M University, Box 30012, College Station, TX 77841, USA.
Andrey Malakhov, Department of Chemistry, Texas A&M University, Box 30012, College Station, TX 77841, USA.
Shafinaz Chowdhury, Lady Davis Institute-Jewish General Hospital, Pharmacology and Therapeutics, Oncology and the Cancer Center. McGill University.
Enrico O. Purisima, Biotechnology Research Institute, National Research Council Canada, 6100 Royalmount Avenue, Montreal, Quebec H4P 2R2, Canada
Ljubica Ivanisevic, Lady Davis Institute-Jewish General Hospital, Pharmacology and Therapeutics, Oncology and the Cancer Center. McGill University.
Antoine Caron, Lady Davis Institute-Jewish General Hospital, Pharmacology and Therapeutics, Oncology and the Cancer Center. McGill University.
Kevin Burgess, Department of Chemistry, Texas A&M University, Box 30012, College Station, TX 77841, USA.
H. Uri Saragovi, Lady Davis Institute-Jewish General Hospital, Pharmacology and Therapeutics, Oncology and the Cancer Center. McGill University.
References
- 1.Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 2.Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 4.Maliartchouk S, Debeir T, Beglova N, Cuello AC, Gehring K, Saragovi HU. Genuine monovalent ligands of TrkA nerve growth factor receptors reveal a novel pharmacological mechanism of action. J Biol Chem. 2000;275:9946–9956. doi: 10.1074/jbc.275.14.9946. [DOI] [PubMed] [Google Scholar]
- 5.Maliartchouk S, Saragovi HU. Optimal nerve growth factor trophic signals mediated by synergy of TrkA and p75 receptor-specific ligands. J Neurosci. 1997;17:6031–6037. doi: 10.1523/JNEUROSCI.17-16-06031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Tom JYK, Oare D, Yen R, Fairbrother WJ, Wells JA, Cunningham BC. Minimization of a Polypeptide Hormone. Science. 1995;270:1657–1660. doi: 10.1126/science.270.5242.1657. [DOI] [PubMed] [Google Scholar]
- 7.Wells JA. Hormone Mimicry. Science. 1996;273:449–450. doi: 10.1126/science.273.5274.449. [DOI] [PubMed] [Google Scholar]
- 8.McInnes C, Sykes BD. Growth factor receptors: structure, mechanism, and drug discovery. Biopolymers. 1997;43:339–366. doi: 10.1002/(SICI)1097-0282(1997)43:5<339::AID-BIP2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Lin B, Li Z, Park K, Deng L, Pai A, Zhong L, Pirrung MC, Webster NJ. Identification of novel orally available small molecule insulin mimetics. J Pharmacol Exp Ther. 2007;323:579–585. doi: 10.1124/jpet.107.126102. [DOI] [PubMed] [Google Scholar]
- 10.Maliartchouk S, Feng Y, Ivanisevic L, Debeir T, Cuello AC, Burgess K, Saragovi HU. A designed peptidomimetic agonistic ligand of TrkA nerve growth factor receptors. Mol Pharmacol. 2000;57:385–391. [PubMed] [Google Scholar]
- 11.Jang SW, Okada M, Sayeed I, Xiao G, Stein D, Jin P, Ye K. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc Natl Acad Sci U S A. 2007;104:16329–16334. doi: 10.1073/pnas.0706662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mischel PS, Umbach JA, Eskandari S, Smith SG, Gundersen CB, Zampighi GA. Nerve growth factor signals via preexisting TrkA receptor oligomers. Biophys J. 2002;83:968–976. doi: 10.1016/S0006-3495(02)75222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Guillemard V, Ivanisevic L, Garcia AG, Scholten V, Lazo OM, Bronfman FC, Saragovi HU. An agonistic mAb directed to the TrkC receptor juxtamembrane region defines a trophic hot spot, and interactions with p75 co-receptors. Dev Neurobiol. 2009 doi: 10.1002/dneu.20776. in press. [DOI] [PubMed] [Google Scholar]
- 15.Saragovi HU, Zheng W, Maliartchouk S, DiGugliemo GM, Mawal YR, Kamen A, Woo SB, Cuello AC, Debeir T, Neet KE. A TrkA-selective, Fast Internalizing Nerve Growth Factor-Antibody Complex Induces Trophic but Not Neuritogenic Signals. J Biol Chem. 1998;273:34933–34940. doi: 10.1074/jbc.273.52.34933. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Brahimi F, Angell Y, Li YC, Moscowicz J, Saragovi HU, Burgess K. Bivalent peptidomimetic ligands of TrkC are biased agonists and selectively induce neuritogenesis or potentiate neurotrophin-3 trophic signals. ACS chemical biology. 2009;4:769–781. doi: 10.1021/cb9001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanisevic L, Zheng W, Woo SB, Neet KE, Saragovi HU. TrkA receptor “hot spots” for binding of NT-3 as a heterologous ligand. J Biol Chem. 2007;282:16754–16763. doi: 10.1074/jbc.M701996200. [DOI] [PubMed] [Google Scholar]
- 18.Zaccaro MC, Ivanisevic L, Perez P, Meakin SO, Saragovi HU. p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J Biol Chem. 2001;276:31023–31029. doi: 10.1074/jbc.M104630200. [DOI] [PubMed] [Google Scholar]
- 19.LeSauteur L, Wei L, Gibbs B, Saragovi HU. Small Peptide Mimics of Nerve Growth Factor Bind TrkA Receptors and Affect Biological Responses. J Biol Chem. 1995;270:6564–6569. doi: 10.1074/jbc.270.12.6564. [DOI] [PubMed] [Google Scholar]
- 20.Zaccaro MC, Lee HB, Pattarawarapan M, Xia Z, Caron A, L'Heureux PJ, Bengio Y, Burgess K, Saragovi HU. Selective small molecule peptidomimetic ligands of TrkC and TrkA receptors afford discrete or complete neurotrophic activities. Chem Biol. 2005;12:1015–1028. doi: 10.1016/j.chembiol.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Pattarawarapan M, Chen J, Steffensen M, Burgess K. A Rigid Linker-Scaffold for Solid Phase Synthesis of Dimeric Pharmacophores. J Comb Chem. 2001;3:102–116. doi: 10.1021/cc000081v. [DOI] [PubMed] [Google Scholar]
- 22.Pattarawarapan M, Zaccaro MC, Saragovi U, Burgess K. New Templates for Synthesis of Ring-fused C10 β-Turn Peptidomimetics Leading To The First Reported Small Molecule Mimic of Neurotrophin-3. J Med Chem. 2002;45:4387–4390. doi: 10.1021/jm0255421. [DOI] [PubMed] [Google Scholar]
- 23.Shi Z, Birman E, Saragovi HU. Neurotrophic rationale in glaucoma: a TrkA agonist, but not NGF or a p75 antagonist, protects retinal ganglion cells in vivo. Dev Neurobiol. 2007;67:884–894. doi: 10.1002/dneu.20360. [DOI] [PubMed] [Google Scholar]
- 24.Bruno MA, Clarke PB, Seltzer A, Quirion R, Burgess K, Cuello AC, Saragovi HU. Long-lasting rescue of age-associated deficits in cognition and the CNS cholinergic phenotype by a partial agonist peptidomimetic ligand of TrkA. J Neurosci. 2004;24:8009–8018. doi: 10.1523/JNEUROSCI.1508-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebrun-Julien F, Morquette B, Douillette A, Saragovi HU, Di Polo A. Inhibition of p75(NTR) in glia potentiates TrkA-mediated survival of injured retinal ganglion cells. Mol Cell Neurosci. 2009;40:410–420. doi: 10.1016/j.mcn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y, Burgess K. Solid Phase SNAr Macrocyclizations to Give Turn-extended-turn Peptidomimetics. Chemistry - A European J. 1999;5:3261–3272. [Google Scholar]
- 27.Angell Y, Chen D, Brahimi F, Saragovi HU, Burgess K. A combinatorial method for solution-phase synthesis of labeled bivalent beta-turn mimics. J Am Chem Soc. 2008;130:556–565. doi: 10.1021/ja074717z. [DOI] [PubMed] [Google Scholar]
- 28.Arevalo J, Conde B, Hempstead B, Chao M, Martin-Zanca D, Perez P. TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of the receptor. Mol Cell Biol. 2000;20:5908–5916. doi: 10.1128/mcb.20.16.5908-5916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arevalo J, Conde B, Hempstead B, Chao M, Martin-Zanca D, Perez P. A novel mutation within the extracellular domain of TrkA causes constitutive receptor activation. Oncogene. 2001;20:1229–1234. doi: 10.1038/sj.onc.1204215. [DOI] [PubMed] [Google Scholar]
- 30.Aller P, Garnier N, Genest M. Transmembrane helix packing of ErbB/Neu receptor in membrane environment: a molecular dynamics study. J Biomol Struct Dyn. 2006;24:209–228. doi: 10.1080/07391102.2006.10507114. [DOI] [PubMed] [Google Scholar]
- 31.Gamett DC, Pearson G, Cerione RA, Friedberg I. Secondary dimerization between members of the epidermal growth factor receptor family. J Biol Chem. 1997;272:12052–12056. doi: 10.1074/jbc.272.18.12052. [DOI] [PubMed] [Google Scholar]
- 32.Groenen LC, Walker F, Burgess AW, Treutlein HR. A model for the activation of the epidermal growth factor receptor kinase involvement of an asymmetric dimer? Biochemistry. 1997;36:3826–3836. doi: 10.1021/bi9614141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.