Abstract
Neuroglobin has shown rich neuroprotective effects against cerebral hypoxia, and therefore has the potential to impact outcomes after traumatic brain injury (TBI). However, to date an association between genetic variation within the human neuroglobin (NGB) gene and recovery post-TBI has not been reported. The purpose of this study was to explore the relationship between NGB genotypes and outcomes (as assessed by the Glasgow Outcome Scale [GOS], the Disability Rating Scale [DRS], and the Neurobehavioral Rating Scale-Revised [NRS-R]) after severe TBI. Genotyping using TaqMan allele discrimination for two tagging single nucleotide polymorphisms (tSNPs) that represent the two haplotype blocks for NGB (rs3783988 and rs10133981) was completed on DNA obtained from 196 Caucasian patients recovering from severe TBI. Patients were dichotomized based on the presence of the variant allele for each tSNP. Chi-square and Fisher's exact tests were used to compare characteristics between groups. Multivariate linear regression was used to examine NGB tSNPs and recovery from severe TBI. Subjects with the TT genotype (wild-type) for rs3783988 were more likely to have better GOS and DRS scores at 3, 6, 12, and 24 months, while rs10133981 genotype was not significantly related to functional outcome. After controlling for age, gender, and Glasgow Coma Scale (GCS) score, those subjects with the rs3783988 TT genotype had more than a 2.65-times greater likelihood of better functional outcomes than individuals with genotypes harboring a variant allele. Data suggest that the haplotype block represented by rs3783988 in NGB appears to influence recovery after severe TBI. Represented within this haplotype block of NGB is the region that codes for the oxygen-binding portion of NGB.
Key words: functional outcomes, neuroglobin, single nucleotide polymorphisms, traumatic brain injury
Introduction
Morbidity and mortality following traumatic brain injury (TBI) result from both primary and secondary injuries that may cause permanent cognitive dysfunction. Secondary injury, which occurs in the hours to days after TBI, may cause decreased oxygen supply and inadequate cerebral blood flow, exacerbating cerebral hypoxia/ischemia and causing additional neurological disability (Camak, I. 2005; National Institute of Neurological Disorders and Stroke, 2009). Functional recovery after TBI is worsened with oxygen deprivation, and secondary injury results (Cullen et al., 2009). The Centers for Disease Control and Prevention estimate that at least 3.17 million Americans currently have a long-term or lifelong need for help in performing activities of daily living as a result of TBI (Thurman et al., 1999; Langlois et al., 2006). Therefore, finding a neuroprotective agent to treat both primary and secondary injury would greatly improve acute and chronic functional recovery in this TBI population.
Neuroglobin (Ngb) is a protein that may assist cerebral neurons in either combating or protecting against hypoxia and ischemia in the early acute phase after TBI. Ngb exists in neurons in both the central and peripheral nervous systems, as well as in the endocrine system and retina (Burmester et al., 2000; Moens and Dewilde, 2000). Research has linked Ngb with the following functions: (1) as a detoxifier/scavenger of reactive oxygen species, free radicals, and reactive nitrogen species (Couture et al., 2001; Dewilde et al., 2001; Van Doorslaer et al., 2003); (2) as a cytoplasmic enzyme with nicotinamide adenine dinucleotide hydrogenase (NADH) oxidases for glycolysis from adenosine triphosphate (Brunori et al., 2005; Dewilde et al., 2001; Hamdane et al., 2003a; Pesce et al., 2002; Van Doorslaer et al., 2003); (3) as an oxygen carrier or sensor that aids in the diffusion of oxygen to mitochondria under hypoxemic condition (Dewilde et al., 2001; Mammen et al., 2002; Moens and Dewilde, 2000; Gerry and Mammen, 2003); and (4) as a neuroprotective agent from ischemic insult and apoptosis within cellular death receptors or pathways (Fordel et al., 2004; Reuss et al., 2002; Sun et al., 2001; Van Doorslaer et al., 2003; Wakasugi et al., 2003). Ngb is sensitive to biochemical changes between the extracellular and the intracellular environment, and in the setting of hypoxic or ischemic conditions, is thought to transport oxygen across the blood–brain barrier (BBB), and/or increase the availability of oxygen to neuronal mitochondria (Burmester et al., 2000).
Given that both the duration and severity of neurological damage is a critical determinant of outcome after severe TBI, neuroglobin human (NGB) may therefore play an important role in impacting both TBI severity and recovery (Brown et al., 1998; Jiang et al., 2002; Narotam et al., 2009; van Santbrink et al., 1996). To date, a number of studies have described a relationship between Ngb and improvement of, or protection against, cerebral hypoxia/ischemia and neurological damage in animal models (Herold and Fago, 2005; Hundahl et al., 2006; Khan et al., 2007; Peroni et al., 2007; Wang et al., 2008). It is hypothesized that Ngb is not only involved in the cerebral hypoxia/ischemia pathway, but it also contributes to the neurological degeneration process. There is some evidence from the animal cerebral ischemia model that Ngb may be upregulated in response to cerebral hypoxia or ischemia, and that it assists with either oxygen transport/utilization, or the exchange with biotoxic agents (Duong et al., 2009; Smagghe et al., 2008). While the mechanism behind how Ngb provides neuroprotection in periods of poor oxygenation has not yet been identified, genetic variations within the human (NGB) gene may influence the efficacy of the neuroglobin protein product. Therefore, human NGB polymorphisms may be associated with variations in short- and long-term outcomes in individuals experiencing severe TBI.
Whether NGB is expressed or has a beneficial function in humans during brain ischemia has not yet been determined, nor has it been specifically studied in the TBI patient population. Because of the potentially neuroprotective role of the NGB protein, the NGB gene was chosen as a candidate for study. The purpose of this study was to determine if genetic variation in NGB contributes to functional outcomes following severe TBI.
Methods
Population and outcome measures
A total of 196 patients had both NGB genotype and outcome data available and met the following study entry criteria: (1) Glasgow Coma Scale (GCS) score ≤ 8 without paralytics or sedatives, (2) head CT scan positive for closed head injury, and (3) 16–75 years of age. The study excluded those with pre-existing neurologic deficits. The study was limited to Caucasians due to the significant allele frequency differences noted between Caucasians and non-Caucasians, and the insufficient sample size of non-Caucasians in the sample (n = 12). Care of all subjects was provided as a protocol based on the series of Guidelines for Care of the Traumatic Brain Injury Patient (Povlishock, 2000, 2004). The Institutional Review Board at the University of Pittsburgh approved this project, and informed consent was obtained prior to sample or data collection.
Demographic and clinical variables included age, gender, race, mechanism of injury, pathologic injury noted on radiographic examination, and study admission GCS. For ease of analysis, the GCS was dichotomized into two groups (GCS score 3–5 or 6–8). Data regarding mortality and the functional outcomes as measured by the Glasgow Outcome Score (GOS), Disability Rating Scale (DRS), and Neurobehavioral Rating Scale-Revised (NRS-R) at 3, 6, 12, and 24 months, were collected by experienced neuropsychologists through the Brain Trauma Research Center at the University of Pittsburgh Medical Center. The GOS, which assesses the patient's perception of their general function, has five categories, with the lowest score of one (deceased), and the highest score of five (good recovery; Wright, 2000). For the analyses, the GOS was also dichotomized into two groups (a GOS score of 1–3 was considered a poor outcome, and a score of 4–5 was considered a good outcome; inter-rater r = 0.95; kappa = 0.62–0.79; Jennett and Bond, 1975; Jennett et al., 1981). The DRS indicates the individual's perceived ability to carry out activities of daily living independently. The DRS has excellent reliability (r = 0.97–0.98) and validity (r = 0.35–0.78), compared to the GOS score (Rappaport et al., 1982) in individuals with moderate and severe TBI. The DRS maximum score is 29 (extreme vegetative state), while a score of 30 indicates death, and a score of 0 indicates no disability perceived by the patient (Rappaport et al., 1982). The NRS-R assesses the individual's perception of cognitive and behavioral parameters, using 27 items with each item scored on a four-point Likert scale (absent, mild, moderate, and severe; inter-rater r = 0.47–0.93; Vanier et al., 2000). The overall score of the NRS-R ranges from 108 (worst recovery possible) to 27 (good recovery; McCauley et al., 2001). Not all subjects completed every outcome measure at every time point. Table 1 shows the percentages of patients who completed each of the three functional outcome measures (GOS, DRS, and NRS-R), at 3, 6, 12, and 24 months. The NRS-R had the lowest completion rates at baseline and over time, possibility due to the longer time required to complete it (15–20 min), compared to the shorter GOS and DRS assessment times.
Table 1.
Numbers of Subjects with Outcome Evaluations at the Selected Time Points
| Functional outcomes | 3 months (n, %) | 6 months (n, %) | 12 months (n, %) | 24 months (n, %) |
|---|---|---|---|---|
| GOS | 158 (81%) | 152 (78%) | 133 (68%) | 100 (51%) |
| DRS | 155 (79%) | 151 (77%) | 131 (67%) | 101 (52%) |
| NRS-R | 59 (30%) | 74 (38%) | 70 (36%) | 38 (19%) |
GOS, Glasgow Outcome Scale; NRS, Neurobehavioral Rating Scale-Revised, DRS, Disability Rating Scale.
Polymorphism selection and genotyping
DNA was extracted from one of two sources for each subject: either whole blood or cerebrospinal fluid (CSF). Whole blood was collected in EDTA sample tubes, processed to retrieve the buffy coat, and the DNA was extracted using a simple salting-out procedure (Miller et al., 1988). CSF was collected by passive drainage according to the standard of care, and DNA was extracted using the QIAamp DNA extraction protocol for extraction from body fluids (Qiagen Corporation, 2007). Hardy-Weinberg Equilibrium testing and double blind genotype calling were utilized for quality control measures of our genotype data.
Tagging single nucleotide polymorphism (tSNPs) were selected for NGB using a minor allele frequency cutoff of ≥ 0.20, and an r2 cutoff of ≥ 0.80, in the Utah residents with northern and western European ancestry from the CEPH collection (CEU) population using HapMap Build 35, which covers the entire coding region of the gene, as well as 1 kb upstream and downstream of the gene. The rs3783988 and rs10133981 tSNPs were selected to represent the two major haplotype blocks representative of all variation within the human NGB in the gene. Genotype data were collected using 5′ exonuclease Assay-on-Demand TaqMan assays (Applied Biosystems, 2006). Amplification and genotype assignments were conducted using the ABI7000 and SDS 2.0 software (Applied Biosystems, 2006).
The sample was dichotomized based on the presence of the variant allele for each tSNP. rs3783988 has genotypes CC, TT, and CT. TT is the wild-type genotype (variant absent category), and CC and CT are genotypes harboring at least one copy of the variant allele (variant present category). rs10133981 has genotypes GG, TT, and GT. GG is the wild-type genotype (making up the variant absent category), while TT and GT are genotypes that have at least one copy of the variant allele (variant present category).
The sample size of 196 has 80% power to detect an effect size of 0.3 with a significance level (alpha) of 0.05. This corresponds to an odds ratio of 1.671, and with multiple regression obtained an r2 of 0.05, which showed the precision of the estimates of outcome statistics as 95% confidence limits based on the sample size of 196 in the two genotype groups with each tSNP. Independent Student's t-tests, Pearson's chi-square tests, Fisher's exact tests, and exact tests were used to analyze the between-group differences (variant present or variant absent) in continuous and categorical variables as appropriate. We also performed logistic and linear regression analyses to test the ability of the rs3783988 and rs10133981 for NGB to predict good outcome. All analyses were completed using SPSS version 15.0 (SPSS, 2008).
Results
tSNP frequency and clinical and demographic characteristics
tSNP rs10133981 was in Hardy-Weinberg equilibrium; however, tSNP rs3783988 was not (p = 0.01). This departure from equilibrium is due to a higher proportion of the variant allele in this TBI population than in the publically available databases (dbSNP and HapMap). This may be an indication that the TBI population studied in this project is not representative of the general population.
We then assessed the frequency of tSNP variant presence within the sample (Table 2). For rs3783988 (variant allele = C), almost two-thirds of the sample was characterized as TT (62%), followed in frequency by a little less than one-third being CT, and a very small minority being CC. These categories were then dichotomized into rs3783988 variant present genotype (36.2%; n = 71), and variant absent genotype (62.2%; n = 122). For rs10133981 (variant allele = T), the sample was overwhelmingly categorized as GG, with only a small percentage as GT, and only one subject as TT. These categories were then dichotomized into rs10133981 variant present genotype (6.6%; n = 13), and variant absent genotype (91.3%; n = 179).
Table 2.
Frequency and Percentage of Variants in rs3783988 and rs10133981 in 196 Caucasians
|
rs3783988 |
rs10133981 |
||||
|---|---|---|---|---|---|
| n | Percent (%) | n | Percent (%) | ||
| CC | 8 | 4.1 | GG | 179 | 91.3 |
| TT | 122 | 62.2 | TT | 1 | 0.5 |
| CT | 63 | 32.1 | GT | 12 | 6.1 |
| Present (CC + CT) | 71 | 36.2 | Present (TT + GT) | 13 | 6.6 |
| Absent (TT) | 122 | 62.2 | Absent (GG) | 179 | 91.3 |
| Missing | 3 | 1.5 | Missing | 4 | 2.0 |
The study evaluated potential differences in clinical and demographic characteristics based on the presence or absence of the variants on the two tSNPs (Table 3). These analyses revealed no significant difference in age, gender, admission GCS score, or mechanism of injury, based on genotype group for either tSNP. On initial CT scan, 57.7% (n = 113) of our sample had intracerebral hemorrhage, 45.9% (n = 90) had subarachnoid hemorrhage, 44.9% (n = 88) had subdural hematoma, 22.5% (n = 44) had intraventricular hemorrhage, and 11.2% (n = 22) had epidural hematoma. There were no significant differences in pathological injury noted on radiographic examination between groups based on genotype of either tSNP.
Table 3.
Demographics and Clinical Characteristics of 196 Caucasian Patients Based on the Presence or Absence of NGB Variants on rs3783988 and rs10133981
| |
|
rs3783988 |
rs10133981 |
||||
|---|---|---|---|---|---|---|---|
| Total n = 196 | Present (CC/CT) n = 71 | Absent (TT) n = 122 | p | Present (TT/GT) n = 13 | Absent (GG) n = 179 | p | |
| Age (mean + SD)T | 34 ± 14.6 | 34.4 ± 15.5 | 33.3 ± 14.0 | 0.36T | 32.2 ± 13.4 | 33.8 ± 14.5 | .70T |
| GenderC (n, %) | |||||||
| Male | 154 (78.6%) | 57 (80.3%) | 94 (78.3%) | 0.75C | 11 (84.6%) | 140 (79.15) | 1.00F |
| Female | 40 (20.4%) | 14 (19.7%) | 26 (21.7%) | 2 (15.4%) | 37 (19.5%) | ||
| Cause of injuryE (n, %) | 0.81E | 0.96E | |||||
| Motor vehicle crash | 89 (45.4%) | 31 (44.3%) | 57 (46.7%) | 7 (53.8%) | 82 (46.1%) | ||
| Motorcycle | 34 (17.3%) | 13 (18.6%) | 20 (16.4%) | 2 (15.4%) | 31 (17.4%) | ||
| Fall | 31 (15.8%) | 13 (18.6%) | 18 (14.8%) | 2 (15.4%) | 26 (14.6%) | ||
| All-terrain vehicle | 11 (5.6%) | 3 (4.3%) | 8 (6.6%) | 1 (7.7%) | 10 (5.2%) | ||
| Pedestrians | 10 (5.1%) | 2 (2.9%) | 8 (6.6%) | 1 (7.7%) | 9 (5.1%) | ||
| Assault | 4 (2.0%) | 1 (1.4%) | 3 (2.5%) | 0 (0.0%) | 4 (2.2%) | ||
| Other | 15 (7.7%) | 7 (10.0%) | 7 (5.7%) | 0 (0.0%) | 15 (8.4%) | ||
| Admission GCS scoreC (n, %) | 0.11C | 0.77C | |||||
| Poor (3–5) | 83 (42.3%) | 36 (50.7%) | 47 (38.8%) | 5 (38.5%) | 76 (42.7%) | ||
| Better (6–8) | 113 (57.7%) | 35 (49.3%) | 74 (61.2%) | 8 (61.5%) | 102 (57.3%) | ||
GCS, Glasgow Outcome Scale; C, chi-square test; E, exact test; F, Fisher's exact test; T, independent t-test; SD, standard deviation.
Functional outcomes
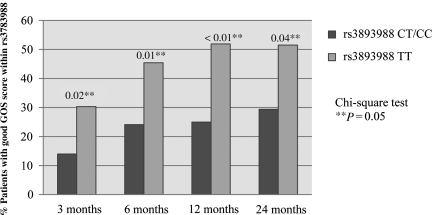
The next step in the analysis was to determine whether variation in clinical outcome following TBI was associated with NGB tSNPs. We first examined between-group differences in the GOS (Table 4). When GOS was examined as a dichotomous variable (good [score 4–5] versus poor [score 1–3]), persons with variant absent genotype (TT) at rs3783988 had significantly better outcomes at 3 (p = 0.02), 6 (p = 0.01), 12 (p < 0.01), and 24 (p = 0.04) months following injury (Fig. 1). GOS scores did not significantly differ by presence or absence of the variant allele for rs10133981. When examining between-group differences in DRS (Table 5), patients with the variant absent at rs3783988 also had significantly better outcomes at 3 (p < 0.01), 6 (p < 0.01), 12 (p = 0.01), and 24 (p = 0.02) months. DRS scores did not significantly differ at any time point by the presence or absence of the variant allele for rs10133981. Finally, no significant differences in outcomes on the NRS-R were found when evaluating either NGB tSNPs (Table 5), although the numbers of subjects who completed the NRS-R was small.
Table 4.
Functional Outcome of Glasgow Outcome Score from 3 to 24 Months Based on NGB Genotypes on rs3783988 and rs10133981
| |
rs3783988 |
rs10133981 |
||||
|---|---|---|---|---|---|---|
| Present (CC/CT) | Absent (TT) | p | Present (TT/GT) | Absent (GG) | p | |
| 3 Months | 0.02E | 0.16E | ||||
| 1 | 23 (40.4%) | 19 (19.2%) | 2 (20.0%) | 38 (26.4%) | ||
| 2 | 5 (8.8%) | 9 (9.1%) | 3 (30.0%) | 11 (7.6%) | ||
| 3 | 21 (36.8%) | 41 (41.4%) | 3 (30.0%) | 60 (41.7%) | ||
| 4 | 8 (14.0%) | 22 (22.2%) | 1 (10.0%) | 29 (20.1%) | ||
| 5 | 0 (0.0%) | 8 (8.1%) | 1 (10.0%) | 6 (4.2%) | ||
| Poor (1–3) | 49 (86.0%) | 69 (69.7%) | 0.02C | 8 (80.0%) | 109 (75.7%) | 1.00F |
| Good (4–5) | 8 (14.0%) | 30 (30.3%) | 2 (20.0%) | 35 (24.3%) | ||
| 6 Months | 0.11E | 0.42E | ||||
| 1 | 20 (37.0%) | 21 (21.6%) | 3 (25.0%) | 36 (26.5%) | ||
| 2 | 4 (7.4%) | 6 (6.2%) | 2 (16.7%) | 8 (5.9%) | ||
| 3 | 17 (31.5%) | 26 (26.8%) | 4 (33.3%) | 39 (28.7%) | ||
| 4 | 8 (14.8%) | 26 (26.8%) | 3 (25.0%) | 31 (22.8%) | ||
| 5 | 5 (9.3%) | 18 (18.6%) | 0 (0.0%) | 22 (16.2%) | ||
| Poor (1–3) | 41 (75.9%) | 53 (54.6%) | 0.01C | 9 (75.0%) | 83 (61.0%) | 0.54F |
| Good (4–5) | 13 (24.1%) | 44 (45.4%) | 3 (25.0%) | 53 (39.0%) | ||
| 12 Months | 0.02E | |||||
| 1 | 17 (32.7%) | 19 (23.5%) | 1 (10.0%) | 34 (28.1%) | 0.33E | |
| 2 | 4 (7.7%) | 5 (6.2%) | 1 (10.0%) | 8 (6.6%) | ||
| 3 | 18 (34.6%) | 15 (18.5%) | 5 (50.0%) | 28 (23.1%) | ||
| 4 | 9 (17.3%) | 19 (23.5%) | 2 (20.0%) | 25 (20.7%) | ||
| 5 | 4 (7.7%) | 23 (28.4%) | 1 (10.0%) | 26 (21.5%) | ||
| Poor (1–3) | 39 (75.0%) | 39 (48.1%) | <0.01C | 7 (70.0%) | 70 (57.9%) | 0.52F |
| Good (4–5) | 13 (25.0%) | 42 (51.9%) | 3 (30.0%) | 51 (42.1%) | ||
| 24 Months | 0.24E | 0.34E | ||||
| 1 | 16 (47.1%) | 18 (27.3%) | 1(14.3%) | 32 (35.2%) | ||
| 2 | 1 (2.9%) | 3 (4.5%) | 1 (14.3%) | 3 (3.3%) | ||
| 3 | 7 (20.6%) | 11 (16.7%) | 2 (28.6%) | 16 (17.6%) | ||
| 4 | 4 (11.8%) | 12 (18.2%) | 2 (28.6%) | 14 (15.4%) | ||
| 5 | 6 (17.6%) | 22 (33.3%) | 1 (14.3%) | 26 (28.6%) | ||
| Poor (1–3) | 24 (70.6%) | 32 (48.5%) | 0.04C | 4 (57.1%) | 51 (56.0%) | 1.00F |
| Good (4–5) | 10 (29.4%) | 34 (51.5%) | 3 (42.9%) | 40 (44.0%) | ||
F, Fisher's exact test; C, chi-square test; E, exact test.
FIG. 1.
Association between rs3783988 variants and good outcome by Glasgow Outcome Score (GOS) over time.
Table 5.
Functional Outcomes on the DRS and NRS-R Based on the Genotypes of NGB on SNPs
|
Disability Ranking Scale (DRS) | ||||||
|---|---|---|---|---|---|---|
| |
rs3783988 |
rs10133981 |
||||
| CC + CT (mean ± SD) | TT (mean ± SD) | p | TT + GT (mean ± SD) | GG (mean ± SD) | p | |
| 3 Months | 19.70 ± 10.88 | 12.82 ± 11.30 | (n = 153) < 0.01T* | 18.80 ± 10.27 | 14.96 ± 11.61 | (n = 151) 0.31T |
| 6 Months | 17.90 ± 11.87 | 11.44 ± 11.88 | (n = 150) < 0.01T* | 16.75 ± 11.52 | 13.04 ± 12.15 | (n = 147) 0.31T |
| 12 Months | 16.31 ± 11.95 | 10.81 ± 12.48 | (n = 131) 0.01T | 12.00 ± 11.27 | 13.00 ± 12.61 | (n = 129) 0.81T |
| 24 Months | 18.06 ± 13.23 | 11.47 ± 12.89 | (n = 101) 0.02T | 13.57 ± 12.33 | 13.74 ± 13.41 | (n = 99) 0.98T |
|
Neurobehavioral Rating Scale-Revised (NRS-R) | ||||||
|---|---|---|---|---|---|---|
| |
rs3783988 |
rs10133981 |
||||
| CC + CT (mean ± SD) | TT (mean ± SD) | p | TT + GT (mean ± SD) | GG (mean ± SD) | p | |
| 3 Months | 41.80 ± 11.12 | 39.58 ± 9.61 | (n = 58) 0.46T | 31.50 ± 0.71 | 40.55 ± 10.01 | (n = 58) 0.21T |
| 6 Months | 40.90 ± 8.58 | 41.00 ± 9.42 | (n = 74) 0.97T | 41.75 ± 13.35 | 41.03 ± 9.08 | (n = 72) 0.88T |
| 12 Months | 45.33 ± 15.01 | 40.02 ± 11.74 | (n = 70) 0.12T | 35.50 ± 6.46 | 42.15 ± 13.19 | (n = 69) 0.32T |
| 24 Months | 40.36 ± 9.11 | 41.59 ± 9.42 | (n = 38) 0.72T | 36.67 ± 3.22 | 41.94 ± 9.45 | (n = 37) 0.35T |
T, independent t-test; SD, standard deviation; SNP, single nucleotide polymorphism.
p < 0.05.
Regression analysis
Based on several significant associations between tSNPs and outcomes, linear regression analyses were performed to examine the impact of tSNPs on GOS and DRS, while controlling for age, gender, and admission GCS score. The logistics regression results for prediction of the GOS score, listed in Table 6, indicate that rs3783988 (TT) variant absent genotype significantly predicted a good GOS outcome at the 12-month time point only (OR = 2.65; 95% CI = 1.11, 6.30). Thus individuals with the rs3783988 TT genotype have more than a 2.65-times greater likelihood of better functional outcomes compared with individuals with genotypes harboring a variant allele. rs10133981 was not significantly associated with GOS at any time point (3 months p = 1.0, 6 months p = 0.54, 12 months p = 0.52, and 24 months p = 1.0). In the linear regression analysis, rs3783988 (TT) had a positive association with DRS at 3 and 6 months (p < 0.01). Again, rs10133981 was not significantly predictive of outcomes. There was no predictive association found between NRS-R and either of the two tSNPs.
Table 6.
Summary of Logistic Regression between Predictors (Age, Gender, GCS [Dichotomous], and rs3783988) with GOS for Months 3–24
| |
3 Months |
6 Months |
12 Months |
24 Months |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 0.93 | 0.89, 0.97 | 0.92** | 0.89, 0.96 | 0.94** | 0.92, 0.98 | 0.94* | 0.91, 0.97 |
| Gender | ||||||||
| Male | 1.00 | 0.32, 2.34 | 1.00 | 0.18, 1.29 | 1.00 | 0.30, 1.96 | 1.00 | 0.15, 1.31 |
| Female | 0.86 | 0.49 | 0.76 | 0.44 | ||||
| GCS | ||||||||
| Poor | 1.00 | 1.11, 6.76 | 1.00 | 2.71, 16.78 | 1.00 | 2.80, 17.91 | 1.00 | 2.09, 16.49 |
| Better | 2.74 | 6.74** | 7.08** | 5.87 | ||||
| SNP rs3783988 | ||||||||
| CC + CT | 1.00 | 0.79, 5.10 | 1.00 | 0.82, 4.61 | 1.00 | 1.11, 6.30 | 1.00 | 0.77, 5.94 |
| TT | 2.01 | 1.94 | 2.65* | 2.15 | ||||
OR, odds ratio; CI, confidence interval; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; SNP, single nucleotide polymorphism.
p < 0.05; **p < 0.001.
Discussion
This is the first study performed to date to examine the relationships between tSNP sequence genotypes for NGB and functional outcomes in persons with severe TBI. This study had two major findings: (1) wild-type tSNP rs3783988 genotype was positively associated with better outcomes as measured by the GOS and DRS, while rs10133981 was not related to outcomes; and (2) there were no significant differences in clinical and demographic characteristics by either tSNP genotype.
Our data suggest that rs3783988 genotype in NGB may play a role in functional recovery following TBI. We found positive associations between the presence of the rs3783988 wild-type allele and all outcome measures after severe TBI. The proposed biological role of NGB may be enhanced in individuals with this genotype. While little research has explored the isoform-specific variations in NGB function, the variant isoform may not function as efficiently as the wild-type thereby leading to variability in long-term outcomes. Our findings support the hypothesis that there may be genotypic variation in NGB function that impacts recovery after severe TBI and other pathologies. Lin and colleagues (2008) found that there were differences in the incidence of ischemic stroke based on NGB genotypes; however, they used different SNPs for their analysis. Their study sequenced the entire gene, identified two intronic SNPs, and compared the frequency of these SNPs in subjects with ischemic stroke. The SNPs identified as influencing the development of ischemic stroke are different from the tSNPs used in this study; however, the region represented in rs3783988 includes the SNP significantly associated with stroke. It is possible that the characteristics of the region tagged by rs3783988 within NGB between 76804 and 76805 bp (based on the International HapMap Project build 35; International HapMap Project, 2009) may impact the brain's response to injury. Hence it seems the region tagged by rs3783988 might have a potential function, particularly in the CD-D region, where a histidine at position E7 (sixth coordinated position) binds with exogenous ligands, such as oxygen (Hamdane et al., 2003b; Pesce, et al., 2003; Wakasugi et al., 2005; Wakasugi and Morishima, 2005). During cerebral ischemia, iron in Ngb changes from a ferric to a ferrous form, making Ngb better able to bind with oxygen at the proximal (His96-F8) and distal (His64-E7) sites. Then, as neurons start to die and toxic metabolites (such as free radicals) are released, Ngb releases the oxygen in the tissues to bind with the more toxic substances. Furthermore, unique imidazole rings, disulfide bonds (S—S), and cysteines (Hamdane et al., 2003a, 2004; Lardinois et al., 2008; Vinck et al., 2006; Walker, 2006; Xu et al., 2009; Hankeln et al., 2005), located at position E7 increase the rate of oxygen release to ischemic tissue. It will be interesting to explore whether the region tagged by rs3783988 may have a functional influence on recovery from TBI. In summary, our data suggest that the functional effect of the haplotype block tagged by rs3783988 in NGB (e.g., NGB quantity, protein composition, or characteristics) impacts recovery after TBI, although the mechanism behind this association is still not described and must be further explored.
The 3-month time point showed the most dramatic difference in terms of patient improvement between groups based on NGB genotype. Those patients who had the rs3783988 TT genotype seemed to have better responses to TBI. NGB may help neuronal survival in the early phase of recovery; however, there may be longer-term effects influencing long-term recovery. In the long-term outcomes of this study, rs3783988 genotype was associated with better GOS and DRS; however, more specific neuropsychological testing is necessary to evaluate the sensitivity of recovery of the TBI patient. Further, the dampening of the statistical association between genotype and long-term outcomes found when controlling for covariates may be indicative that NGB genotype is only neuroprotective for select individuals, specifically older, more severely-injured patients.
There are a few limitations to this study. A sample size of 196 may not be large enough to identify associations with a small effect size. The à priori power analysis suggests that this study was adequately powered to detect a moderate effect size, and significant findings should not be dismissed due to sample size. The sample size did, however, prevent a more detailed analysis of the role of potential confounding factors on the relationship between NGB genotype and outcome after TBI. The limited data related to pathology limit interpretation of these findings. Further research, including measures of cerebral ischemia and more extensive pathologies, could explain the mechanisms through which NGB exerts its effects. Finally, because the study sample was limited to Caucasians, the external validity for populations of other ethnicities is unknown.
This is the first study to demonstrate a relationship between genetic variants in NGB and the severity of injury and functional outcomes in humans. These data are valuable for providing the framework and justification for further research aimed at describing the potential role and pathological mechanisms that NGB plays in protecting individuals from secondary brain injury, and at developing pathways to utilize NGB treatment strategies.
Acknowledgments
We sincerely thank Dr. Mary E. Kerr, Deputy Director of the National Institute of Nursing Research, National Institutes of Health, for introducing us to this topic and for guiding us in our research. Support for the project was provided by grants from the National Institutes of Health (R01NR04801, R01NR008424, and P50NS30318).
Author Disclosure Statement
No competing financial interests exist.
References
- Applied Biosystems. Rev.C, May. Foster City, CA: 2006. TaqMan SNP Genotyping Assays. Protocol. Applied Biosystems. Part no. 4332856. [Google Scholar]
- Brown J.I. Moulton R.J. Konasiewixz S.J. Baker A.J. Cerebral oxidative metabolism and evoked potential deterioration after severe brain injury: new evidence of early posttraumatic ischemia. Neurosurgery. 1998;42:1057–1063. doi: 10.1097/00006123-199805000-00060. [DOI] [PubMed] [Google Scholar]
- Brunori M. Giuffre A. Nienhaus K. Nienhaus G.U. Scandurra F.M. Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester T. Hankeln T. Neuroglobin: a respiratory protein of the nervous system. News Physiol. Sci. 2004;19:110–113. doi: 10.1152/nips.01513.2003. [DOI] [PubMed] [Google Scholar]
- Burmester T. Weich B. Reinhardt S. Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture M. Burmester T. Hankeln T. Rousseau D.L. The heme environment of mouse neuroglobin. Evidence for the presence of two conformations of the heme pocket. J. Biol. Chem. 2001;276:36377–36382. doi: 10.1074/jbc.M103907200. [DOI] [PubMed] [Google Scholar]
- Cullen N.K. Crescini C. Bayley M.T. Rehabilitation outcomes after anoxic brain injury: a case-controlled comparison with traumatic brain injury. PM R. 2009;1:1069–1076. doi: 10.1016/j.pmrj.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Dewilde S. Kiger L. Burmester T. Hankeln T. Baudin-Creuza V. Aerts T. Marden M.C. Caubergs R. Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- Duong T.T. Witting P.K. Antao S.T. Parry S.N. Kennerson M. Lai B. Vogt S. Lay P.A. Harris H. H. Multiple protective activities of neuroglobin in cultured neuronal cells exposed to hypoxia re-oxygenation injury. J. Neurochem. 2009;108:1143–1154. doi: 10.1111/j.1471-4159.2008.05846.x. [DOI] [PubMed] [Google Scholar]
- Fordel E. Geuens E. Dewilde S. De Coen W. Moens L. Hypoxia/ischemia and the regulation of neuroglobin and cytoglobin expression. IUBMB Life. 2004;56:681–687. doi: 10.1080/15216540500037406. [DOI] [PubMed] [Google Scholar]
- Garry D.J. Mammen P.P. Neuroprotection and the role of neuroglobin. Lancet. 2003;362:342–343. doi: 10.1016/S0140-6736(03)14055-X. [DOI] [PubMed] [Google Scholar]
- Hamdane D. Kiger L. Dewilde S. Green B.N. Pesce A. Uzan J. Burmester T. Hankeln T. Bolognesi M. Moens L. Marden M.C. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 2003;278:51713–51721. doi: 10.1074/jbc.M309396200. [DOI] [PubMed] [Google Scholar]
- Hamdane D. Kiger L. Dewilde S. Green B.N. Pesce A. Uzan J. Burmeister T. Hankeln T. Bolognesi M. Moens L. Marden M.C. Coupling of the heme and an internal disulfide bond in human neuroglobin. Micron. 2004;35:59–62. doi: 10.1016/j.micron.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Hankeln T. Ebner B. Fuchs C. Gerlach F. Haberkamp M. Laufs T.L. Roesner A. Schmidt M. Weich B. Wystub S. Saaler-Reinhardt S. Reuss S. Bolognesi M. De Sanctis D. Marden M.C. Kiger L. Moens L. Dewilde S. Nevo E. Avivi A. Weber R.E. Fago A. Burmester T. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J. Inorg. Biochem. 2005;99:110–119. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Herold S. Fago A. Reactions of peroxynitrite with globin proteins and their possible physiological role. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:124–129. doi: 10.1016/j.cbpb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Hundahl C. Kelsen J. Kjaer K. Ronn L.C. Weber R.E. Geuens E. Hay-Schmidt A. Nyengaard J.R. Does neuroglobin protect neurons from ischemic insult? A quantitative investigation of neuroglobin expression following transient MCAo in spontaneously hypertensive rats. Brain Res. 2006;1085:19–27. doi: 10.1016/j.brainres.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Hundahl C. Kelsen J. Kjaer K. Ronn L.C. Weber R.E. Geuens E. Hay-Schmidt A. Nyengaard J.R. Does neuroglobin protect neurons from ischemic insult? A quantitative investigation of neuroglobin expression following transient MCAo in spontaneously hypertensive rats. Brain Res. 2006;1085:19–27. doi: 10.1016/j.brainres.2006.02.040. [DOI] [PubMed] [Google Scholar]
- International HapMap Project. From the HapMap Genome Browser (Phase 1, 2, and 3-merged genotypes and frequencies) Retrieved July, 20, 2009:2009. [Google Scholar]
- Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Jennett B. Snoek J. Bond M.R. Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.Y. Gao G.Y. Li. W.P. Yu. M.K. Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Khan A.A. Mao X.O. Banwait S. Jin K. Greenberg D.A. Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19114–19119. doi: 10.1073/pnas.0706167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. Centers for Disease Control and Prevention. National Center for Injury Prevention and Control; Atlanta, GA: 2006. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. [Google Scholar]
- Lardinois O.M. Tomer K. B. Mason R. P. Deterding L.J. Identification of protein radicals formed in the human neuroglobin-H2O2 reaction using immuno-spin trapping and mass spectrometry. Biochemistry. 2008;47:10440–10448. doi: 10.1021/bi800771k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Fang L. Xue X.H. Murong S.X. Wang N. Wu Z.Y. Association between Ngb polymorphisms and ischemic stroke in the Southern Chinese Han population. BMC Med. Genet. 2008;9:110. doi: 10.1186/1471-2350-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen P.P. Shelton J.M. Goetsch S.C. Williams S.C. Richardson J.A. Garry M.G. Garry D.J. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J. Histochem. Cytochem. 2002;50:1591–1598. doi: 10.1177/002215540205001203. [DOI] [PubMed] [Google Scholar]
- McCauley S. Levin H. Vanier M. Mazaux J. Boake C. Goldfader P. Rockers D. Butters M. Kareken D. Lambert J. Clifton G. The neurobehavioral rating scale-revised; sensitivity and validity in closed head injury assessment. J. Neurol. Neurosurg. Psychiatry. 2001;71:643–651. doi: 10.1136/jnnp.71.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.A. Dyskes D.D. Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res. 1998;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L. Dewilde S. Globins in the brain. Nature. 2000;407:461–462. doi: 10.1038/35035181. [DOI] [PubMed] [Google Scholar]
- Narotam P.K. Morrison J.F. Nathoon N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J. Neurosurg. 2009;111:672–682. doi: 10.3171/2009.4.JNS081150. [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke. Traumatic brain injury: hope through research. NINDS; Bethesda, MD: 2009. publication no.02-2478. [Google Scholar]
- Pesce A. Bolognesi M. Bocedi A. Ascenzi P. Dewilde S. Moens L. Hankeln T. Burmester T. Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin family. EMBO Rep. 2002a;3:1146–1151. doi: 10.1093/embo-reports/kvf248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce A. Dewilde S. Nardini M. Moens L. Ascenzi P. Hankeln T. Burmester T. Bolognesi M. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure. 2003;11:1087–1095. doi: 10.1016/s0969-2126(03)00166-7. [DOI] [PubMed] [Google Scholar]
- Pesce A. Nardini M. Dewilde S. Ascenzi P. Burmester T. Hankeln T. Moens L. Bolognesi M. Human neuroglobin: crystals and preliminary X-ray diffraction analysis. Acta Crystallogr. D Biol. Crystallogr. 2002b;58:1848–1850. doi: 10.1107/s090744490201260x. [DOI] [PubMed] [Google Scholar]
- Peroni D. Negro A. Bahr M. Dietz G.P. Intracellular delivery of neuroglobin using HIV-1 TAT protein transduction domain fails to protect against oxygen and glucose deprivation. Neurosci. Lett. 2007;421:110–114. doi: 10.1016/j.neulet.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Guidelines for the management of severe head injury. J. Neurotrauma. 2000;17:451–627. [Google Scholar]
- Povlishock J.T. Guidelines for the management of severe TBI [Special Issue] J. Neurotrauma. 2004;24:S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Qiagen Corporation. Sample 7 Assay Technologies. Qiagen Inc.; Valencia, CA: 2007. [Google Scholar]
- Rappaport M. Hall K.M. Hopkins K. Belleza T. Cope D.N. Disability rating scale for severe head trauma patients: coma to community. Arch. Phys. Med. Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- Reuss S. Saaler-Reinhardt S. Weich B. Wystub S. Reuss M.H. Burmester T. Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- Smagghe B.J. Trent J.T., 3rd Hargrove M.S. NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo. PLoS One. 2008;3:e2039. doi: 10.1371/journal.pone.0002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. SPSS Base 15.0 for Windows user's guide. SPSS Inc.; Chicago: 2008. [Google Scholar]
- Sun Y. Jin K. Mao X.O. Zhu Y. Greenberg D.A. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl. Acad. Sci. U.S.A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman D. Alverson C. Dunn K. Guerrero J. Sniezek J. Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Van Doorslaer S. Dewilde S. Kiger L. Nistor S.V. Goovaerts E. Marden M.C. Moens L. Nitric oxide binding properties of neuroglobin. A characterization by EPR and flash photolysis. J. Biol. Chem. 2003;278:4919–4925. doi: 10.1074/jbc.M210617200. [DOI] [PubMed] [Google Scholar]
- Vanier M. Mazaux J.-M. Lambert J. Dassa C. Levin H. Assessment of neuropsychologic impairments after head injury: interrater reliability and factorial and criterion validity of the neurobehavioral rating scale-revised. Arch. Phys. Med. Rehabil. 2000;81:796–806. doi: 10.1016/s0003-9993(00)90114-x. [DOI] [PubMed] [Google Scholar]
- van Santbrink H. Maas A.L. Avezaat C.J. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996;38:21–31. doi: 10.1097/00006123-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Vinck E. Van Doorslaer S. Dewilde S. Mitrikas G. Schweiger A. Moens L. Analyzing heme proteins using EPR techniques: the heme-pocket structure of ferric mouse neuroglobin. J. Biol. Inorg. Chem. 2006;11:467–475. doi: 10.1007/s00775-006-0100-2. [DOI] [PubMed] [Google Scholar]
- Wakasugi K. Morishima I. Identification of residues in human neuroglobin crucial for guanine nucleotide dissociation inhibitor activity. Biochemistry. 2005;44:2943–2948. doi: 10.1021/bi0477539. [DOI] [PubMed] [Google Scholar]
- Wakasugi K. Kitatsuji C. Morishima I. Possible neuroprotective mechanism of human neuroglobin. Ann. N.Y. Acad. Sci. 2005;1053:220–230. doi: 10.1196/annals.1344.020. [DOI] [PubMed] [Google Scholar]
- Wakasugi K. Nakano T. Morishima I. Oxidized human neuroglobin acts as a heterotrimeric G alpha protein guanine nucleotide dissociation inhibitor. J. Biol. Chem. 2003;278:36505–36512. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]
- Walker F.A. The heme environment of mouse neuroglobin: histidine imidazole plane orientations obtained from solution NMR and EPR spectroscopy as compared with x-ray crystallography. J. Biol. Inorg. Chem. 2006;11:391–397. doi: 10.1007/s00775-006-0095-8. [DOI] [PubMed] [Google Scholar]
- Wang X. Liu J. Zhu H. Tejima E. Tsuji K. Murata Y. Atochin D.N. Huang P.L. Zhang C. Lo E.H. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. The Glasgow Outcome Scale. The Center for Outcome Measurement in Brain Injury (COMBI) A project funded by the National Institute on Disability and Rehabilitation Research. 2000. Dec 30,
- Xu J. Li L. Yin G. Li H. Du W. Ligand orientation of human neuroglobin obtained from solution NMR and molecular dynamics simulation as compared with x-ray crystallography. J. Inorg. Biochem. 2009;103:1693–1701. doi: 10.1016/j.jinorgbio.2009.09.016. [DOI] [PubMed] [Google Scholar]