Abstract
Resveratrol (3,5,4′-trihydroxystilbene) is a plant-derived small molecule that is protective against multiple neurological and systemic insults. To date, no studies have explored the potential for resveratrol to provide behavioral protection in adult animals in the setting of traumatic brain injury (TBI). Using 50 male Sprague-Dawley rats, we employed the controlled cortical impact (CCI) model to ascertain whether post-injury administration of resveratrol would reduce the severity of the well-described cognitive and motor deficits associated with the model. Contusion volumes and hippocampal neuronal numbers were also measured to characterize the tissue and neuronal-sparing properties, respectively, of resveratrol. We found that 100 mg/kg, but not 10 mg/kg, of intraperitoneal resveratrol administered after injury provides significant behavioral protection in rats sustaining CCI. Specifically, rodents treated with 100 mg/kg of resveratrol showed improvements in motor performance (beam balance and beam walking) and testing of visuospatial memory (Morris water maze). Behavioral protection was correlated with significantly reduced contusion volumes, preservation of CA1 and CA3 hippocampal neurons, and protection from overt hippocampal loss as a result of incorporation into the overlying cortical contusion in resveratrol-treated animals. Although the mechanisms by which resveratrol mediates its neuroprotection is unclear, the current study adds to the growing literature identifying resveratrol as a potential therapy for human brain injury.
Key words: controlled cortical impact; contusion; neuroprotection; resveratrol (3,5,4′-trihydroxystilbene); traumatic brain injury
Introduction
Traumatic brain injury (TBI) is an important clinical problem that causes significant mortality and a broad spectrum of morbidity. To date, the reported efficacy of numerous preclinical experimental therapies has not been replicated in human clinical trials (Saatman et al., 2008). It is therefore important to continue characterizing therapeutics that may prove helpful in attenuating the deleterious consequences of this condition.
Resveratrol is one such substance that has shown promise in treating a broad range of pathologies, including those of the central nervous system (Baur and Sinclair, 2006). This compound, also known as 3,5,4′-trihydroxystilbene, is a naturally-occurring phytoalexin that is found in multiple plant sources (Baur and Sinclair, 2006). In multiple models of neurological injury including stroke (Wang et al., 2002; West et al., 2007), spinal cord injury (Ates et al., 2006; Kaplan et al., 2005; Kiziltepe et al., 2004; Yang and Piao, 2003), epilepsy (Gupta et al., 2002a; 2001; 2002b; Wu et al., 2009), and Huntington's disease (Parker et al., 2005), resveratrol has demonstrated efficacy in reducing neuropathological and behavioral sequelae. Some studies have also found that traumatic brain injury (TBI) in both adult (Ates et al., 2007) and immature (Sonmez et al., 2007) animals is amenable to treatment with resveratrol, although behavioral protection has not yet been demonstrated in adult models of TBI. In addition, evidence correlating histopathological protection with improved behavioral outcomes is lacking.
Using the well characterized controlled cortical impact (CCI) model of TBI (Dixon et al., 1991), we confirm and extend these previous findings and demonstrate for the first time that post-injury administration of resveratrol attenuates both motor and cognitive deficits in adult TBI. The behavioral protection afforded by resveratrol occurred in the setting of reduced contusion volumes, preservation of neurons in both the CA1 and CA3 regions of the ipsilateral hippocampus, and protection of hippocampal morphology underlying the cortical contusion site.
Methods
Animals
In this study we used 50 adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 300–400 g, that were housed in steel wire mesh cages in a facility with a 12-h light-dark cycle. The rats had access to chow and water ad libitum. The experimental animal protocol was approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and conformed to the guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996).
Traumatic brain injury
The controlled cortical impact (CCI) model used in this study has been previously described (Dixon et al., 1991; Kline and Dixon, 2001). Briefly, the rats were anesthetized in 5% isoflurane (IsoFlo®; Abbott Laboratories, North Chicago, IL) carried in a 2:1 ratio of nitrous oxide:oxygen, endotracheally intubated, and maintained on a small-animal ventilator (Harvard Rodent Ventilator, Model 683; Harvard Apparatus, Holliston, MA) with 1–2% isoflurane in the same carrier gas mixture. Anesthesia was then maintained for the duration of the procedure. Body temperature was maintained via the use of a warming pad. After placement in a stereotactic frame (David Kopf Instruments, Tujunga, CA), the head was shaved and then prepped with povidone-iodine. A midline incision was made to expose from bregma to lambda, and a self-retaining retractor was placed. A right parietal craniectomy was performed using a dental drill. The size of the craniectomy was large enough to allow the 6-mm impactor tip to strike the brain without touching the surrounding bone. The impactor tip was leveled with the surface of the brain, and the injury was administered (impact speed: 4 m/sec, injury depth: 2.6 mm, dwell time: 50 msec). The injury settings were consistent with previous studies and designed to generate a brain injury with reproducible motor and cognitive deficits, as well as underlying cortical and hippocampal tissue loss (Dixon et al., 1991, 2003). Following injury the anesthesia was stopped, the incision was closed with interrupted silk sutures, and the animals were ventilated on 100% oxygen until spontaneous return of respiration, at which point the endotracheal tube was removed. Sham injury utilized the same procedure with the exception that the button to trigger activation of the injury device was not depressed. Animal recovery was monitored by recording the times until the return of the following reflexes: corneal, pinna, tail pinch, toe pinch, and righting. All injured animals had significantly delayed righting times compared to sham injury (data not shown). Following this, the rats were monitored until fully recovered from anesthesia, and they were then returned to the housing facility.
Drug preparation and administration
Resveratrol was prepared in a vehicle solution which was generated by dissolving Solutol HS15 (BASF, Ludwigshafen, Germany) in preservative-free 0.9% saline in a 30% weight:volume ratio. This solution was sterilized via autoclave, and then resveratrol (product #R5010; Sigma-Aldrich, St. Louis, MO) was added to a final concentration of 5 mg/cc or 50 mg/cc. The final vehicle and resveratrol solutions were then transferred to sterile vials, which were then stoppered and capped. The resveratrol was protected from light as much as possible, as it converts bioactive trans-resveratrol to the inactive cis-resveratrol isomer. Separate vials of vehicle solution (30% Solutol HS15 in 0.9% saline) were also prepared. The vials were wrapped in aluminum foil and stored in the dark at 4°C until use. Prior to use the solutions were warmed for 10 min in a 37°C water bath, and vigorously vortexed for 1 min to ensure uniform suspension.
The drugs were first administered via intraperitoneal (IP) injection 5 min after injury or sham injury. The animals received two more doses on post-injury days 1 and 2 after the motor testing was completed. All experimental groups (n = 10 per group) received 2 cc/kg of the respective drug or vehicle solution with each injection. Sham-injured animals received vehicle, while injury groups were injected with either vehicle or resveratrol to a final dose of either 10 mg/kg (using the 5-mg/cc stock), or 100 mg/kg (using the 50-mg/cc stock) per injection. Two different doses of resveratrol were utilized to increase the chances of identifying a significant behavioral effect. The 100-mg/kg dose was based on previous work showing the efficacy of resveratrol in reducing the histopathological consequences of TBI, as well as markers of lipid peroxidation and nitric oxide (Ates et al., 2007). The lower dose was utilized due to other studies demonstrating the potential for reduced efficacy of resveratrol at higher doses (Raval et al., 2006). Three daily doses of the drug were given for several reasons. First, the toxicity of resveratrol is very low and no adverse events have been reported for doses lower than 300 mg/kg/d (Crowell et al., 2004). Next, other studies of neural injury have reported positive results with both pre- and post-insult resveratrol administered as multiple doses (Dong et al., 2008; Li et al., 2010; Sinha et al., 2002; Wang et al., 2002). Last, extended post-injury administration of the medication is potentially more applicable to a clinical setting.
Randomization
The experiment was performed in a randomized, vehicle-controlled fashion. All animals were assigned to one of the four treatment groups (sham injury + vehicle, CCI + vehicle, CCI + 10 mg/kg resveratrol, and CCI + 100 mg/kg resveratrol), using a four-block randomization table. Surgery/injury, behavioral testing, and tissue analyses were all performed by different individuals. Behavioral testing and tissue analyses were performed by individuals blinded to treatment group.
Motor testing (post-injury days 1–5)
Gross vestibulomotor function was assessed on a beam-balance task in which the time the animal remained on an elevated, 1.5-cm-wide wooden beam was recorded (up to a maximum of 60 sec). Three trials were performed per animal per day. Training/pre-assessment was completed on the day prior to surgery/injury. Spinning on the beam was counted as a fall. The animals fell onto a cushioned pad to prevent injury.
Finer components of vestibulomotor function and coordination were assessed using a modified beam-walking task (Feeney et al., 1982). On the day prior to injury the rats were trained to escape a bright light and loud white noise (model #15800C; Lafayette Instruments, Inc., Lafayette, IN), by traversing a narrow wooden beam (2.5 × 100 cm), and entering a darkened goal box at the opposite end. The noise and light were terminated when the rat entered the goal box. Four pegs (3 mm diameter and 4 cm high) were equally spaced along the center of the beam to increase the difficulty of the task. Performance was assessed by measuring the latency to traverse the beam. The rats remained in the goal box for 30 sec between trials. If the rats did not cross the beam in 60 sec or fell off, the light and noise were stopped and the rat was placed in the goal box. Data for each session consisted of the mean of three trials.
The rats were also given a beam-walking score to denote progression along the beam during the beam walk test. Animals successfully reaching the goal box were assigned 5 points. Rats not reaching the goal box were assigned lesser scores, depending on either their final spot on the beam when 60 sec had elapsed, or the spot at which they fell off. Reaching between the fourth peg and goal box = 4 points, between the third and fourth peg = 3 points, between the second and third peg = 2 points, between the first and second peg = 1 point, and not passing the first peg = 0 points. The final daily beam-walking score was the mean score of the three beam-walking trials.
Cognitive testing (post-injury days 14–20)
We employed a variant of the Morris water maze (MWM; Morris, 1984), that has been shown to be sensitive to cognitive function/dysfunction following TBI (Dixon et al., 1994, 1999a; Hamm et al., 1992; Scheff et al., 1997). This water maze task was used to compare rates of acquisition between injured and sham groups. The Morris water maze consisted of a dark blue plastic pool 180 cm in diameter and 60 cm in depth. The pool was filled with water to a depth of 28 cm and a clear acrylic glass platform 10 cm in diameter and 26 cm high (i.e., 2 cm below the surface) was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each trial. Testing for spatial learning began on postoperative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for 5 consecutive days (days 14–18) to locate the platform when it was submerged 2 cm below the water's surface (i.e., invisible to the rat). Two additional days (days 19–20) were provided to locate the platform when it was raised 2 cm above the water's surface (i.e., visible to the rat). The visible platform task was used as a control procedure to determine the contributions of non-spatial factors (e.g., sensorimotor performance, motivation, and visual acuity) on MWM performance. For each daily block of trials, the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The mean latency scores of the four trials for each rat were used in the statistical analyses. Swim speed and distance was recorded with a video analysis system (Chromatrack; San Diego Instruments, San Diego, CA). The pool was located in a 2.5 × 2.5-meter room with numerous extra-maze visual cues that remained constant throughout the experiment.
Animal sacrifice and tissue processing
After completion of behavioral testing (post-injury day 21), the animals were terminally anesthetized with an IP injection of sodium pentobarbital (100 mg/kg) and intracardially perfused with 0.9% saline followed by 10% phosphate-buffered formalin. After fixation, the brains were removed and then post-fixed in 10% phosphate-buffered formalin for 5 days. The brains were then blocked to remove the hindbrain, and then the forebrain was paraffin-embedded and sectioned on a microtome (Leitz 1512; Leica Microsystems, Wetzlar, Germany). Sections 7 μm thick were cut every 500 μm and deparaffinized, serially hydrated, stained with cresyl violet, and then cover-slipped for tissue analysis.
Tissue analyses
Using a macro objective (Nikon 1X; Nikon, Tokyo, Japan), a photomicrograph (Nikon Eclipse E600) was obtained of each coronal section taken at 500-μm increments throughout the forebrain of each animal. Image analysis software (ImageJ; National Institutes of Health) was used to outline the contusion, if present, and to calculate the area for each section. Using Cavalieri's principle (Mouton, 2002) an estimate of total contusion volume per animal was calculated in cubic millimeters.
Neuronal counting in the selectively vulnerable CA1 and CA3 regions of the dorsal hippocampus underlying the area of the contusion was also performed using coronal sections 3.5 mm posterior to the bregma (Dixon et al., 1999b, 2003). The pyramidal cell layer from CA1 to the dentate gyrus of each hippocampus was sequentially imaged using a 10 × objective (Nikon), digitally reconstructed into a montage (ImageJ), and the CA1 and CA3 subfields were identified. CA1 was identified as the layer consisting of small pyramidal neurons in the dorsal hippocampus; CA3 was identified as the layer of large pyramidal neurons in the ventral hippocampus extending from the dentate gyrus to the transition zone adjacent to CA1. Manual counting of the neurons in each subfield was then performed using ImageJ software. Only histologically normal-appearing neurons with a clearly defined cell body and nucleus were counted. Thus neurons only partially seen due to the level of sectioning were not included. Two adjacent sections per animal were counted and the results were averaged.
Statistical analysis
MWM, beam-balance, beam-walking, and beam-walk score data were analyzed using separate repeated-measures analysis of variance (ANOVA) tests (group × days). If a significant effect was found, group comparisons were performed using a one-tailed Dunnett's post-hoc test comparing each group with the CCI + vehicle group. Swim speed, contusion volume, and hippocampal CA1/CA3 neuronal counts were assessed using a one-way ANOVA. If a significant effect was found by the ANOVA, group comparison was performed using a one-tailed Dunnett's post-hoc test to compare each group with the CCI + vehicle group. Analyses were performed using PASW Statistics 18 (SPSS). Data are expressed as the group mean ± standard error (SE) of the mean. A significance level of p < 0.05 was used for all tests.
Results
Motor performance
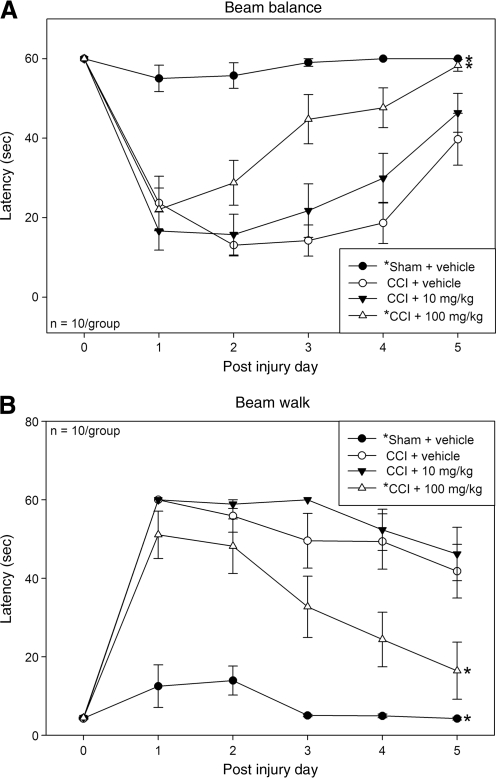
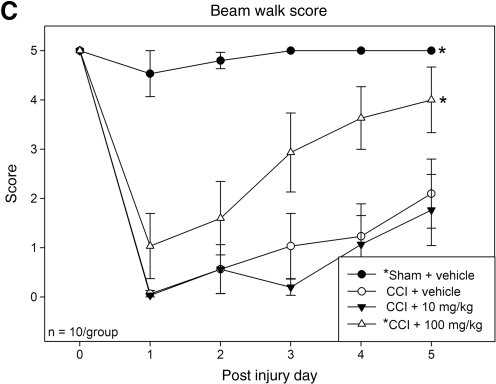
Statistically significant between-group effects were noted in the beam balance (F3,36 = 22.44; p < 0.001), beam walk (F3,36 = 33.09; p < 0.001), and beam walk score (F3,36 = 26.16; p < 0.001) tests. Among all groups both the sham + vehicle and CCI + 100 mg/kg resveratrol groups showed statistically higher beam-balance latencies (Fig. 1A), lower beam-walk latencies (Fig. 1B), and higher beam-walk scores (Fig. 1C), compared to the CCI + vehicle group (p < 0.01 for all motor tests). The CCI + 10 mg/kg group did not statistically differ from the CCI + vehicle group in any test.
FIG. 1.
Motor testing. In the beam-balance test (A), beam-walk test (B), and beam-walk score (C), rodents in the sham injury + vehicle (solid circles), and CCI + 100 mg/kg resveratrol (open triangles) groups showed significantly improved performance compared to the CCI + vehicle group (open circles). Animals in the CCI + 10 mg/kg resveratrol group (solid triangles) did not show significant improvement compared to the CCI + vehicle group (*p < 0.01 compared to the CCI + vehicle group; CCI, controlled cortical impact).
Cognitive performance
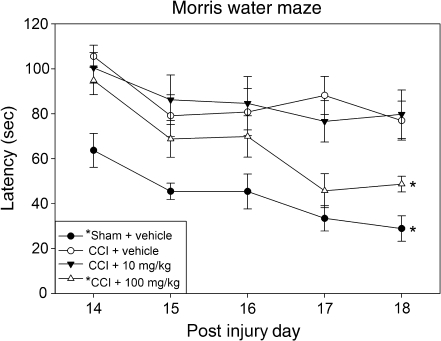
In testing of spatial memory, a statistically significant between-group effect on the latency to reach the submerged platform was noted in the MWM test (F3,36 = 11.70; p < 0.001). Post-hoc testing demonstrated significantly reduced latencies to find the submerged platform in the sham + vehicle (p < 0.001) and CCI + 100 mg/kg resveratrol (p < 0.05) groups, relative to the CCI + vehicle group (Fig. 2). The CCI + 10 mg/kg resveratrol group did not significantly differ from CCI + vehicle. Swim speed did not significantly differ between the sham and injury groups (F3,36 = 1.16; p = 0.336; data not shown). When comparing latency to reach the platform on the last day of the hidden platform trials (post-injury day 18) versus the last day of the visible platform trials (post-injury day 20), all groups demonstrated significant improvement when the platform was visible (p < 0.01). Between-group comparisons revealed greater, but not significant, improvement in the CCI + 10 mg/kg resveratrol and CCI + vehicle groups compared to the CCI + 100 mg/kg resveratrol and sham + vehicle groups; no significant difference existed between the groups on the last day of visible platform testing (data not shown). The visible platform task helps to control for many neuroanatomical and cognitive aspects of the MWM that are not related to spatial memory. As such, increased improvement in the visible platform portion of the MWM relative to performance in the hidden platform trials is an indirect indicator of spatial memory deficits (Vorhees and Williams, 2006).
FIG. 2.
Morris water maze (MWM) testing. Rodents in the sham injury + vehicle (solid circles) and CCI + 100 mg/kg resveratrol (open triangles) groups showed significantly improved performance on the MWM task compared to the CCI + vehicle group (open circles). Animals in the CCI + 10 mg/kg resveratrol group (solid triangles) did not show significant improvement compared to CCI + vehicle group (*p < 0.05 compared to the CCI + vehicle group; CCI, controlled cortical impact).
Tissue analyses
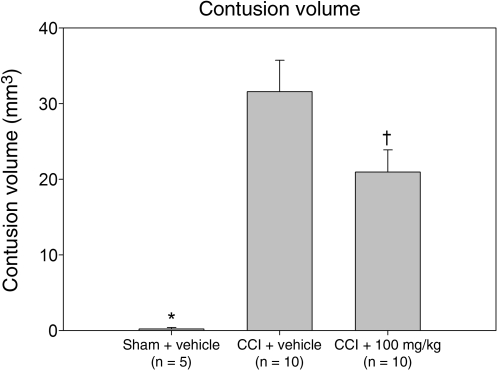
Given the demonstrated lack of efficacy of the 10-mg/kg resveratrol dose in providing behavioral neuroprotection, brain sections from these animals were excluded from the tissue analyses. In the evaluation of contusion volume (Fig. 3), a significant between-group difference was found (F2,22 = 15.712; p < 0.001). Compared to vehicle-treated injured animals, animals receiving 100 mg/kg resveratrol after injury demonstrated significantly reduced mean contusion volume (31.6 ± 4.2 versus 21.0 ± 2.9 mm3, respectively; p = 0.028). As expected, sham-injured animals did not develop contusions and were significantly different from both the CCI + 100 mg/kg resveratrol and CCI + vehicle groups (p < 0.001).
FIG. 3.
Contusion volume. In comparison to the CCI + vehicle group, injured animals receiving 100 mg/kg resveratrol showed significantly decreased mean contusion volume. Sham-injured animals did not develop brain contusions (†p < 0.05 compared to the CCI + vehicle group; *p < 0.001 compared to the CCI + vehicle group and the CCI + 100 mg/kg resveratrol group; CCI, controlled cortical impact).
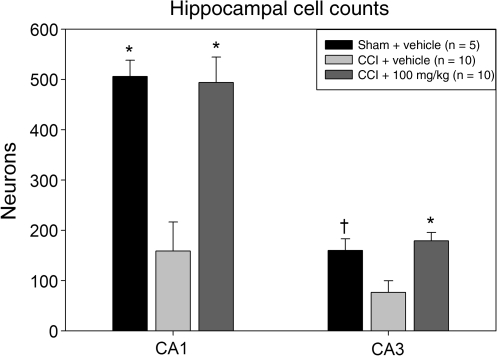
The analysis of selectively vulnerable hippocampal regions adjacent to the contusion (Fig. 4) revealed significant between-group effects in both the CA1 (F2,22 = 13.720; p < 0.001) and CA3 (F2,22 = 7.519; p = 0.003) regions. In the CA1 sector, compared to the number of neurons in the CCI + vehicle group (158.9 ± 57.7), the sham-injured (505.8 ± 32.4) and CCI + 100 mg/kg resveratrol groups (493.8 ± 50.8) demonstrated significantly more cells (p = 0.001 and p < 0.001, respectively). Similar findings were noted in the CA3 region, where in comparison to the CCI + vehicle group (76.5 ± 23.0), the sham-injured (160.0 ± 23.2) and CCI + 100 mg/kg resveratrol groups (179.0 ± 16.5) showed significantly more hippocampal neurons (p = 0.02 and p = 0.001, respectively). The CA1 and CA3 regions in the contralateral hippocampus were also evaluated and no significant differences were noted between the groups (data not shown).
FIG. 4.
Hippocampal neuronal counts. Compared to the CCI + vehicle group, animals treated with 100 mg/kg resveratrol after CCI showed preserved neuronal numbers in both the CA1 and CA3 hippocampal subfields (†p < 0.05 and *p < 0.001 compared to the CCI + vehicle group; CCI, controlled cortical impact).
It should be noted that in the CCI + vehicle group, multiple animals showed complete or near-complete absence of both the CA1 and CA3 regions due to overt loss of the majority of the dorsal hippocampus (Table 1). In these cases, the overlying cortical contusion extended more deeply into the subcortical regions, incorporating a large portion or all of the hippocampus (Fig. 5). Given that the CA1 and CA3 loss in these animals likely occurred as a result of overt tissue disruption and infarction rather than loss of selectively vulnerable neurons, these data were re-analyzed and the results from these five animals were excluded. The number of neurons in the CCI + vehicle animals increased as a result in both the CA1 (287.8 ± 76.1) and CA3 (135.8 ± 18.1) regions. In CA1, the difference between the number of neurons in the sham + vehicle and CCI + 100 mg/kg resveratrol groups compared to the CCI + vehicle group remained significant (p = 0.028 and p = 0.018, respectively), whereas in CA3, the difference was no longer significant (p = 0.336 and p = 0.108, respectively).
Table 1.
Status of the Ipsilateral Hippocampus 21 Days after Controlled Cortical Impact Injury
| Group | Hippocampus intact | <50% Hippocampal loss | >50% Hippocampal loss |
|---|---|---|---|
| Sham + vehicle | 5 | 0 | 0 |
| TBI + 100 mg/kg resveratrol | 9 | 1 | 0 |
| TBI + vehicle | 2 | 3 | 5 |
TBI, traumatic brain injury.
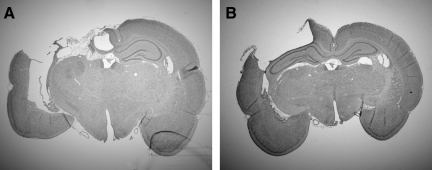
FIG. 5.
Resveratrol treatment reduces contusion volume and protects hippocampal integrity. Coronal brain sections at the level of brain impact demonstrate a large contusion with overt loss of the majority of the dorsal hippocampus in a representative vehicle-treated animal (A), and reduced contusion volume with hippocampal preservation in an animal treated with 100 mg/kg resveratrol (B).
The loss of > 50% of the hippocampal structure seen in some of the CCI + vehicle animals was not present in any animals in the CCI + 100 mg/kg resveratrol group (Table 1). To explore the possibility that treatment with resveratrol was associated with protection from marked hippocampal degeneration and incorporation into the overlying contusion, animals from each group were categorized as demonstrating loss of greater than or less than 50% of the dorsal hippocampus. These data were analyzed using Fisher's exact test, which revealed that resveratrol treatment was significantly associated with hippocampal preservation (p = 0.033). When these groups were re-categorized into having an intact hippocampus versus any loss and re-analyzed, the association of resveratrol treatment with hippocampal preservation remained significant (p = 0.005).
Discussion
Resveratrol has been previously shown to afford protection against a variety of pathological conditions, including brain injury (Ates et al., 2007; Sonmez et al., 2007). In the current study, we extend these previous findings to show that 100 mg/kg, but not 10 mg/kg, of IP resveratrol delivered as three post-injury doses provides robust behavioral protection from the deleterious effects of TBI. The improvement in motor and cognitive end-points correlates with significant cortical and hippocampal tissue preservation. Specifically, resveratrol treatment attenuates contusion volume and protects the underlying hippocampus from incorporation into the overlying contusion, while also reducing secondary hippocampal neuronal degeneration. When resveratrol administration begins shortly after TBI, the onset of behavioral neuroprotection becomes apparent over the first 3 days post-injury. During this time, which occurs within the motor testing period, performance by rodents treated with 100 mg/kg resveratrol showed progressively more improvement than the CCI + 10 mg/kg resveratrol and CCI + vehicle groups. Although specific intra-day analyses of group differences were not performed as a part of this study, the CCI + 100 mg/kg resveratrol group appears to become distinct from the other two TBI groups by day 2 post-injury in the beam-balance test, and day 3 post-injury in the beam-walk and beam-walk score. Given that this is the first study to evaluate the efficacy of multiple doses of post-injury resveratrol in TBI, it is not known if the time course of its neuroprotective effects would be modified by a different dosing regimen. Similarly, further study will be necessary to determine if extending the duration of resveratrol treatment results in additional improvement in the Morris water maze results, which was conducted 2 weeks after injury.
The specific mechanisms by which resveratrol exerts its neuroprotective activity remain unclear. Previous work in rodents has shown that the drug is able to cross the blood–brain barrier (Wang et al., 2002). Resveratrol has been shown have antioxidant and free-radical scavenging properties. This activity occurs directly, as a result of the polyphenolic nature of the compound (Frankel et al., 1993; Karlsson et al., 2000), as well as indirectly via inhibition of cyclooxygenase 1 and 2 (Jang et al., 1997), quinine reductase 2 (Buryanovskyy et al., 2004), and cytochrome P450 (Chun et al., 1999; Ciolino et al., 1998; Piver et al., 2001; Yu et al., 2003), and activation of catalase (Floreani et al., 2003), heme oxygenase (Kaga et al., 2005), and phase 2 enzymes in the drug metabolism pathway (Jang et al., 1997). The overall effect of these enzymatic changes is to increase cellular antioxidative and free-radical scavenging capacity. Resveratrol is also a phytoestrogen that has been shown to bind with low affinity to the estrogen receptor (Freyberger and Schmuck, 2005; Gehm et al., 1997). Despite this, resveratrol has been shown to activate the estrogen receptor significantly more than estrogen in transactivation assays (Freyberger and Schmuck, 2005). Given that estrogen has well-documented neuroprotective effects (Bramlett and Dietrich, 2001; Dubal et al., 2001; Emerson et al., 1993; Roof and Hall, 2000; Wagner et al., 2004), this activity may also mediate some of resveratrol's overall protection. Neuroprotection may also arise from SIRT1 activation, which induces pro-survival cellular changes during times of stress and/or starvation (Baur and Sinclair, 2006; Howitz et al., 2003; Raval et al., 2006). Some of these changes that may prove helpful in modulating the effects of TBI include increasing mitochondrial number (Baur et al., 2006) and function (Lagouge et al., 2006), while also improving insulin sensitivity and lowering fasting blood glucose levels (Baur et al., 2006). Numerous other described activities of resveratrol, including downregulation of p53 (Howitz et al., 2003), induction of the unfolded protein response (Liu et al., 2009; Viswanathan et al., 2005), prevention of mitochondrial uncoupling (Della-Morte et al., 2009), and blockade of inflammation (Manna et al., 2000; Tsai et al., 1999) may also contribute to its neuroprotective activity.
It should be noted that this study was not designed a priori as a dose-response experiment. We cannot directly state that the 10 mg/kg dose is less efficacious than 100 mg/kg. The post-hoc analyses, however, reveal that the 10 mg/kg dose provided no improvement in behavior when compared to CCI + vehicle-treated animals, whereas the 100 mg/kg dose did. The reason for this difference is unclear. There is a discrepancy in the literature regarding effective dosing of resveratrol to obtain protective activity. Some studies have found increasing doses of the drug to be less effective (Howitz et al., 2003; Raval et al., 2006), which would suggest the presence of lower-affinity binding sites that either result in downregulation of protective activities, or induction of other systems that have deleterious consequences. In the current study, however, the lack of effect of the lower dose suggests that activities mediated by high-affinity resveratrol binding partners are less important in mediating its neuroprotective activities than lower-affinity targets. Binding characteristics are not known for many of the targets of resveratrol; however, it is known that the highest-affinity binding partner is QR2, which is thought to mediate some of the indirect antioxidant/free-radical scavenging activities of the drug (Buryanovskyy et al., 2004). Binding to SIRT1 has been found to occur at higher drug concentrations (Kaeberlein et al., 2005), suggesting that downstream effects of SIRT1 may be more important in resveratrol-mediated neuroprotection than antioxidative effects. To date, measurements of SIRT1 activity in the setting of experimental TBI, and the extent to which that activity is modified by resveratrol treatment, have not been performed and will form the basis for future investigations in our laboratory.
After data analysis, it became evident that contusion-related loss of CA1/CA3 hippocampal neurons occurred for one of two possible reasons: either loss of selectively vulnerable neurons in the setting of preserved hippocampal structure, or overt loss of the dorsal hippocampus due to incorporation into the overlying contusion. The loss of significant portions of the hippocampus in some rodents in the vehicle-treated group did impact the counts of CA1 and CA3 neurons. Due to the a priori experimental design, however, direct contusion-related loss of either hippocampal field was counted as zero neurons in that area, resulting in the finding of significant neuroprotection of resveratrol in both regions. To attempt a more accurate accounting of the effect of resveratrol treatment on survival of CA1 and CA3 neurons due to the secondary pathological cascades set into motion as a result of the injury and not as a consequence of overwhelming direct contusion-related hippocampal infarction and loss, we retrospectively reanalyzed the data, excluding those animals showing significant loss of hippocampal structure. The effect of resveratrol was as a result diminished, yet still significant in the CA1 region, but not CA3. Similarly, the finding of preservation of gross hippocampal structure in resveratrol-treated animals compared to the CCI + vehicle group was also based on unblinded data analysis. The results from both of these retrospective examinations of the data carry with them the caveats and biases of post-hoc data analysis.
In sum, for the first time we have been able to demonstrate behavioral neuroprotection afforded by post-injury resveratrol treatment in the setting of experimental adult TBI. In addition to its beneficial motor and cognitive effects, resveratrol also significantly reduces contusion volume and preserves hippocampal integrity. At the cellular level, treatment with the drug also protects selectively vulnerable neurons in the CA1 and CA3 regions, although preservation of overall hippocampal structure may account for the protection afforded to the CA3 region.
Acknowledgments
The authors would like to thank Xiecheng Ma for her help with animal preparation and injury, Sherman Culver and Kristin Macfarlane for their help with animal behavioral testing, and Yue-Fang Chang, Ph.D., for her assistance with statistical analyses. This work was supported by National Institutes of Health-National Institute of Neurological Disorders and Stroke grants NS030318 and NS060672.
Author Disclosure Statement
No competing financial interests exist.
References
- Ates O. Cayli S. Altinoz E. Gurses I. Yucel N. Kocak A. Yologlu S. Turkoz Y. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol. Sin. 2006;27:1317–1325. doi: 10.1111/j.1745-7254.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Ates O. Cayli S. Altinoz E. Gurses I. Yucel N. Sener M. Kocak A. Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- Baur J.A. Pearson K.J. Price N.L. Jamieson H.A. Lerin C. Kalra A. Prabhu V.V. Allard J.S. Lopez-Lluch G. Lewis K. Pistell P.J. Poosala S. Becker K.G. Boss O. Gwinn D. Wang M. Ramaswamy S. Fishbein K.W. Spencer R.G. Lakatta E.G. Le Couteur D. Shaw R.J. Navas P. Puigserver P. Ingram D.K. de Cabo R. Sinclair D.A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J.A. Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Buryanovskyy L. Fu Y. Boyd M. Ma Y. Hsieh T.C. Wu J.M. Zhang Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Y.J. Kim M.Y. Guengerich F.P. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem. Biophys. Res. Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- Ciolino H.P. Daschner P.J. Yeh G.C. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- Crowell J.A. Korytko P.J. Morrissey R.L. Booth T.D. Levine B.S. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- Della-Morte D. Dave K.R. DeFazio R.A. Bao Y.C. Raval A.P. Perez-Pinzon M.A. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Clifton G.L. Lighthall J.W. Yaghmai A.A. Hayes R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Hamm R.J. Taft W.C. Hayes R.L. Increased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the rat. J. Neurotrauma. 1994;11:275–287. doi: 10.1089/neu.1994.11.275. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Kochanek P.M. Yan H.Q. Schiding J.K. Griffith R.G. Baum E. Marion D.W. DeKosky S.T. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma. 1999a;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Kraus M.F. Kline A.E. Ma X. Yan H.Q. Griffith R.G. Wolfson B.M. Marion D.W. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 1999b;14:285–294. [PubMed] [Google Scholar]
- Dixon C.E. Ma X. Kline A.E. Yan H.Q. Ferimer H. Kochanek P.M. Wisniewski S.R. Jenkins L.W. Marion D.W. Acute etomidate treatment reduces cognitive deficits and histopathology in rats with traumatic brain injury. Crit. Care Med. 2003;31:2222–2227. doi: 10.1097/01.CCM.0000080493.04978.73. [DOI] [PubMed] [Google Scholar]
- Dong W. Li N. Gao D. Zhen H. Zhang X. Li F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J. Vasc. Surg. 2008;48:709–714. doi: 10.1016/j.jvs.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Dubal D.B. Zhu H. Yu J. Rau S.W. Shughrue P.J. Merchenthaler I. Kindy M.S. Wise P.M. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson C.S. Headrick J.P. Vink R. Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res. 1993;608:95–100. doi: 10.1016/0006-8993(93)90778-l. [DOI] [PubMed] [Google Scholar]
- Feeney D.M. Gonzalez A. Law W.A. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Floreani M. Napoli E. Quintieri L. Palatini P. Oral administration of trans-resveratrol to guinea pigs increases cardiac DT-diaphorase and catalase activities, and protects isolated atria from menadione toxicity. Life Sci. 2003;72:2741–2750. doi: 10.1016/s0024-3205(03)00179-6. [DOI] [PubMed] [Google Scholar]
- Frankel E.N. Waterhouse A.L. Kinsella J.E. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- Freyberger A. Schmuck G. Screening for estrogenicity and anti-estrogenicity: a critical evaluation of an MVLN cell-based transactivation assay. Toxicol. Lett. 2005;155:1–13. doi: 10.1016/j.toxlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Gehm B.D. McAndrews J.M. Chien P.Y. Jameson J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y.K. Briyal S. Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol. Biochem. Behav. 2002a;71:245–249. doi: 10.1016/s0091-3057(01)00663-3. [DOI] [PubMed] [Google Scholar]
- Gupta Y.K. Chaudhary G. Sinha K. Srivastava A.K. Protective effect of resveratrol against intracortical FeCl3-induced model of posttraumatic seizures in rats. Methods Find. Exp. Clin. Pharmacol. 2001;23:241–244. doi: 10.1358/mf.2001.23.5.662120. [DOI] [PubMed] [Google Scholar]
- Gupta Y.K. Chaudhary G. Srivastava A.K. Protective effect of resveratrol against pentylenetetrazole-induced seizures and its modulation by an adenosinergic system. Pharmacology. 2002b;65:170–174. doi: 10.1159/000058044. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Howitz K.T. Bitterman K.J. Cohen H.Y. Lamming D.W. Lavu S. Wood J.G. Zipkin R.E. Chung P. Kisielewski A. Zhang L.L. Scherer B. Sinclair D.A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Jang M. Cai L. Udeani G.O. Slowing K.V. Thomas C.F. Beecher C.W. Fong H.H. Farnsworth N.R. Kinghorn A.D. Mehta R.G. Moon R.C. Pezzuto J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M. McDonagh T. Heltweg B. Hixon J. Westman E.A. Caldwell S.D. Napper A. Curtis R. DiStefano P.S. Fields S. Bedalov A. Kennedy B.K. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kaga S. Zhan L. Matsumoto M. Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kaplan S. Bisleri G. Morgan J.A. Cheema F.H. Oz M.C. Resveratrol, a natural red wine polyphenol, reduces ischemia-reperfusion-induced spinal cord injury. Ann. Thorac. Surg. 2005;80:2242–2249. doi: 10.1016/j.athoracsur.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Karlsson J. Emgard M. Brundin P. Burkitt M.J. Trans-resveratrol protects embryonic mesencephalic cells from tert-butyl hydroperoxide: electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. J. Neurochem. 2000;75:141–150. doi: 10.1046/j.1471-4159.2000.0750141.x. [DOI] [PubMed] [Google Scholar]
- Kiziltepe U. Turan N.N. Han U. Ulus A.T. Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia-reperfusion injury. J. Vasc. Surg. 2004;40:138–145. doi: 10.1016/j.jvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Kline A.E. Dixon C.E. Contemporery in vivo models of brain trauma and a comparison of injury responses. In: Miller L.P., editor; Hayes R.L., editor. Head trauma: Basic, preclinical, and Clinical Directions. John Wiley and Sons; New York: 2001. pp. 65–84. [Google Scholar]
- Lagouge M. Argmann C. Gerhart-Hines Z. Meziane H. Lerin C. Daussin F. Messadeq N. Milne J. Lambert P. Elliott P. Geny B. Laakso M. Puigserver P. Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Li C. Yan Z. Yang J. Chen H. Li H. Jiang Y. Zhang Z. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochem. Int. 2010;56:495–500. doi: 10.1016/j.neuint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Liu B.Q. Gao Y.Y. Niu X.F. Xie J.S. Meng X. Guan Y. Wang H.Q. Implication of unfolded protein response in resveratrol-induced inhibition of K562 cell proliferation. Biochem. Biophys. Res. Commun. 2009;391:778–782. doi: 10.1016/j.bbrc.2009.11.137. [DOI] [PubMed] [Google Scholar]
- Manna S.K. Mukhopadhyay A. Aggarwal B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mouton P.R. Principles and Practices of Unbiased Stereology. Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- National Academy Press. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Parker J.A. Arango M. Abderrahmane S. Lambert E. Tourette C. Catoire H. Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Piver B. Berthou F. Dreano Y. Lucas D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol. Lett. 2001;125:83–91. doi: 10.1016/s0378-4274(01)00418-0. [DOI] [PubMed] [Google Scholar]
- Raval A.P. Dave K.R. Perez-Pinzon M.A. Resveratrol mimics ischemic preconditioning in the brain. J. Cereb. Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Hall E.D. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Saatman K.E. Duhaime A.C. Bullock R. Maas A.I. Valadka A. Manley G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Sinha K. Chaudhary G. Gupta Y.K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- Sonmez U. Sonmez A. Erbil G. Tekmen I. Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci. Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Tsai S.H. Lin-Shiau S.Y. Lin J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br. J. Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M. Kim S.K. Berdichevsky A. Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V. Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.K. Willard L.A. Kline A.E. Wenger M.K. Bolinger B.D. Ren D. Zafonte R.D. Dixon C.E. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Wang Q. Xu J. Rottinghaus G.E. Simonyi A. Lubahn D. Sun G.Y. Sun A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- West T. Atzeva M. Holtzman D.M. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev. Neurosci. 2007;29:363–372. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. Xu Q. Zhang L. Kong D. Ma R. Wang L. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem. Res. 2009;34:1393–1400. doi: 10.1007/s11064-009-9920-0. [DOI] [PubMed] [Google Scholar]
- Yang Y.B. Piao Y.J. Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol. Sin. 2003;24:703–710. [PubMed] [Google Scholar]
- Yu C. Shin Y.G. Kosmeder J.W. Pezzuto J.M. van Breemen R.B. Liquid chromatography/tandem mass spectrometric determination of inhibition of human cytochrome P450 isozymes by resveratrol and resveratrol-3-sulfate. Rapid Commun. Mass Spectrom. 2003;17:307–313. doi: 10.1002/rcm.918. [DOI] [PubMed] [Google Scholar]