Abstract
Hormonal dysfunction is a known consequence of moderate and severe traumatic brain injury (TBI). In this study we determined the incidence, time course, and clinical correlates of acute post-TBI gonadotroph and somatotroph dysfunction. Patients had daily measurement of serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, estradiol, growth hormone, and insulin-like growth factor-1 (IGF-1) for up to 10 days post-injury. Values below the fifth percentile of a healthy cohort were considered abnormal, as were non-measurable growth hormone (GH) values. Outcome measures were frequency and time course of hormonal suppression, injury characteristics, and Glasgow Outcome Scale (GOS) score. The cohort consisted of 101 patients (82% males; mean age 35 years; Glasgow Coma Scale [GCS] score ≤8 in 87%). In men, 100% had at least one low testosterone value, and 93% of all values were low; in premenopausal women, 43% had at least one low estradiol value, and 39% of all values were low. Non-measurable GH levels occurred in 38% of patients, while low IGF-1 levels were observed in 77% of patients, but tended to normalize within 10 days. Multivariate analysis revealed associations of younger age with low FSH and low IGF-1, acute anemia with low IGF-1, and older age and higher body mass index (BMI) with low GH. Hormonal suppression was not predictive of GOS score. These results indicate that within 10 days of complicated mild, moderate, and severe TBI, testosterone suppression occurs in all men and estrogen suppression occurs in over 40% of women. Transient somatotroph suppression occurs in over 75% of patients. Although this acute neuroendocrine dysfunction may not be TBI-specific, low gonadal steroids, IGF-1, and GH may be important given their putative neuroprotective functions.
Key words: estrogen, growth hormone, hypopituitarism, insulin-like growth factor-1, testosterone, traumatic brain injury
Introduction
Although traumatic brain injury (TBI) remains a leading cause of disability and death around the world (Consensus Conference, 1999; Adekoya et al., 2002; Ragnarsson, 2002), multiple clinical trials conducted over the last two decades have yielded no effective pharmacological therapies for brain injury (Consensus Conference, 1999; Maas et al., 2004; Narayan et al., 2002). One particular aspect of post-TBI pathophysiology that remains understudied but may be a potential target of hormonal or pharmacological intervention is acute neuroendocrine dysfunction. Our group recently showed that acute adrenal insufficiency occurs in 50% of moderate and severe TBI victims, and is associated with lower blood pressure and higher vasopressor requirements (Cohan et al., 2005). Acute thyroid dysfunction has also been demonstrated in TBI victims and in other critically ill patients in intensive care units (ICUs; Agha et al., 2004; Chiolero et al., 1988a, 1988b; Della Corte et al., 1998; Dimopoulou et al., 2004; Fleischer et al., 1978; Hackl et al., 1991; Heinen et al., 1981; Leon-Sanz et al., 1997; Slag et al., 1981; Van den Berghe et al., 2001; Woolf et al., 1988). Less is known about the acute functionality of the somatotroph and gonadotroph axes. Earlier studies have addressed this issue specifically in TBI patients, indicating that levels of growth hormone (GH), insulin-like growth factor-1 (IGF-1), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone in men and estradiol in women all decrease transiently during the acute post-injury period (Agha et al., 2004; Cernak et al., 1997; Chiolero et al., 1988a, 1988b; Della Corte et al., 1998; Hackl et al., 1991; Woolf et al., 1985, 1986). Although not considered critical for survival in times of acute stress such as multiple trauma or isolated head injury, the known roles of GH, IGF-1, estrogen, and testosterone in the healthy brain suggests their suppression acutely after TBI may have both acute and long-term consequences. There are abundant GH and IGF-1 receptors in the brain, with GH involved in vascular reactivity, vascular tone, and CNS repair processes, while IGF-1 appears to be important in re-myelination and prevention of demyelination (D'Ercole et al., 1996; Hana et al., 2002; Napoli et al., 2003; Scheepens et al., 2001, 2000; Silha et al., 2005; Ye and D'Ercole, 1999; Ye et al., 2002; Zhong et al., 2005). Similarly, there is evidence that estrogen in women and testosterone in men may be neuroprotective, and conversely, their absence or suppression in the acute post-injury period may be deleterious (Chisu et al., 2006; Garcia-Estrada et al., 1993; Hammond et al., 2001; Huppenbauer et al., 2005; Nguyen et al., 2005; Ramsden et al., 2003; Roof and Hall, 2000; Sawada et al., 1998; Sierra et al., 2003; Soustiel et al., 2005l; Stein, 2001; Stein and Hoffman, 2003; Toung et al., 1998; Zhang et al., 2004).
With these concepts in mind, as part of a prospective study of acute and chronic hypopituitarism after TBI, we sought to define the incidence and predictors of acute somatotroph and gonadotroph suppression in TBI patients, as well as in a cohort of non-head injured trauma patients. Relative to prior reports of acute post-TBI gonadotroph and somatotroph function, this study has the largest number of patients and samples analyzed to date, and is unique in using a multivariate analysis of predictors of hormone levels.
Methods
The institutional review boards of each participating center approved this research study. Informed written consent was provided by legal proxy within 48 h of admission at the Harbor-UCLA and UCLA Medical Centers, and within 24 h of injury at UC Davis Medical Center.
TBI cohort
Enrollment criteria for this prospective study included: (1) patients age 14–80 years admitted to the ICU of one of three Level 1 trauma centers (Harbor-UCLA, UCLA, and UC Davis Medical Centers) within 24 h of injury, (2) an initial head CT scan showing acute intracranial hemorrhage, (3) a post-resuscitation Glasgow Coma Scale (GCS) score of 3–14 or a deterioration to a GCS ≤ 14 within 24 h of admission, and (4) at least three consecutive days of hormonal data. Patients were further categorized as sustaining a complicated mild TBI (GCS score 13–14 with intracranial hemorrhage on acute CT; n = 5), moderate TBI (GCS score 9–12; n = 13), or severe TBI (GCS score 3–8; n = 88; Borgaro et al., 2003; Teasdale and Jennett, 1974).
Extracranial trauma cohort
Between June 2002 and February 2004, patients sustaining extracranial trauma (ECT) were prospectively enrolled in the study. Enrollment criteria included: (1) patients age 14–80 years and admitted to the ICU within 24 h of injury, (2) an Injury Severity Score (ISS) of ≥ 15, (3) a post-resuscitation GCS score of 14–15 without a discernible head injury, including no loss of consciousness by history and no intracranial injury or skull fracture on CT scan, and (4) at least two consecutive days of hormonal data. Injury types included chest, abdominal/pelvic, extremity, and neck. Of this original cohort of 45 ECT patients described in a previous article on adrenal insufficiency, 32 ECT subjects were enrolled at Harbor-UCLA Medical Center and had somatotroph and gonadotroph hormone assays performed (Cohan et al., 2005). Of these 32, four were excluded because their ISS was below 15 (n = 3), or they had less than 2 days of hormonal data (n = 1), leaving 28 patients for analysis. Since there were only 4 women among these 28 patients and two were post-menopausal, women were included only in the IGF-1 analysis and no data on female LH, FSH, or estradiol are presented.
Exclusion criteria for both the TBI and ECT patients included pregnancy, or diagnoses of cancer, AIDS, severe neurological or psychiatric illness, spinal cord injury, pre-existing adrenal or pituitary insufficiency, or having received glucocorticoids within 3 months of injury.
TBI patient management
All patients were admitted to the ICU after initial stabilization or after craniotomy for evacuation of an intracranial hematoma. Patient management was in accordance with the “Guidelines for the Management of Severe Head Injury,” and included an algorithm for maintaining intracranial pressure (ICP) less than 20 mm Hg, and cerebral perfusion pressure (CPP) above 60–70 mm Hg (Bullock et al., 1996).
Serial hormonal blood draws
During the acute post-injury period, TBI and ECT subjects had daily morning measurements of serum GH, IGF-1, LH, FSH, and estradiol (in women) and total testosterone (in men). The first hormone levels were drawn within 24 h of injury, with subsequent draws occurring at 6–7 am up to post-injury day 9, as long as the subjects remained in the ICU. As previously described, serum adrenocorticotropic hormone (ACTH) and cortisol levels were also drawn within 24 h of injury, with subsequent twice-daily draws occurring at 6 am and 4 pm (Cohan et al., 2005).
Normal healthy cohort
Healthy subjects were recruited by advertisements posted at Harbor-UCLA and UCLA Medical Centers. Those with previous diagnoses of endocrine diseases, currently on hormone replacement therapy, and pregnant females or those on birth control pills in the past 3 months were excluded. The subjects were studied as part of dynamic anterior pituitary function testing performed in the general clinical research center of their respective hospitals starting at 8 am after an overnight fast. Although not studied at the same phase of the menstrual cycle, all women reported a history of regular menses. In total, 101 healthy volunteer subjects were studied to define the normal ranges for basal morning LH, FSH, and testosterone (males), or estradiol (females), and for GH. Normal values for gonadotropins and sex steroids were divided to reflect gender disparities in hormone production. Normal ranges for IGF-1 were stratified by age range based on normative data from Quest Diagnostics (Lyndhurst, NJ), as it has been shown that IGF-1 secretion decreases with age. The IGF-1 age stratification used was: 16–24 years, 25–39 years, 40–54 years, and > 55 years.
Normal hormonal quantitative ranges were established, with the fifth percentile value of these healthy subjects representing the lower limit of normal for each hormonal axis, as described below. See Table 1 for normative values. Both TBI and ECT patient hormone levels were assessed according to these values, and any values below the fifth percentile cut-off were considered abnormally low.
Table 1.
Reference Range for Hormones from Healthy Men and Women
| Hormonal axis | Fifth percentile | 95th Percentile |
|---|---|---|
| Luteinizing hormone | ||
| Males | 1.4 IU/L | 6.9 IU/L |
| Females | 1.1 IU/L | 15.3 IU/L |
| Follicle-stimulating hormone | ||
| Males | 1.1 IU/L | 9.98 IU/L |
| Females | 1.3 IU/L | 11.1 IU/L |
| Testosterone (males) | 10.3 nmol/L | 28.1 nmol/L |
| Estradiol (females) | 101.2 pmol/L | 584.6 pmol/L |
| Growth hormone | 0.003 μg/La | 4.1 μg/La |
| Insulin-like growth factor-1 | ||
| Ages 16–24 | 26.8 nmol/L | 90.9 nmol/L |
| Ages 25–39 | 16.8 nmol/L | 57.3 nmol/L |
| Ages 40–54 | 13.2 nmol/L | 42.2 nmol/L |
| Age 55+ | 10.4 nmol/L | 33.9 nmol/L |
Lower limit of detection for growth hormone was ≤0.2 μg/L.
Serum samples for all hormones were stored at −20°C before assay by the GCRC Core Laboratory of the Harbor-UCLA Medical Center. All samples from the same patient were assayed in the same run whenever possible to reduce between-run variations. The hormones measured and assays used are described below.
Serum GH was measured by an enzymatically amplified two-step sandwich-type immunoassay with reagents obtained from Diagnostic Systems Laboratories (DSL-10-1900 hGH ELISA; Webster, TX), and validated at the Core Laboratory of the Harbor-UCLA Medical Center GCRC. The lower limit of quantization was 0.2 μg/L, and this was used to delineate the lower limit of normal in the TBI and ECT cohorts. The intra-assay and inter-assay coefficients of variation were less than 8% and 14%, respectively. The recovery of GH from serum spiked with 0.1–15 μg/L of GH was between 92% and 112% (Qu et al., 2005).
Serum IGF-1 was measured by RIA kits from Quest Diagnostics, after acid/ethanol extraction of serum. The normal adult ranges are presented in Table 1.
Serum FSH and LH were measured by fluoroimmunometric assays with reagents provided by Delfia (Wallac, Gaithersburg, MD). The adult normal male ranges are LH 1.0–8.1 IU/L, and FSH 1.0–6.9 IU/L.
Serum total testosterone was measured by RIA using reagents from Diagnostic Products Corporation (Torrance, CA) after extraction; the normal adult male range is 10.3–36.2 nmol/L (Qoubaitary et al., 2006).
Serum estradiol (E2) was measured by direct assay without extraction with reagents from ICN Pharmaceuticals (Costa Mesa, CA); the normal adult female range in the early follicular phase is 73.4–550.7 pmol/L.
The following clinical factors were assessed as being potentially related to acute hormonal suppression:
Injury factors. Age, post-resuscitation GCS score, post-resuscitation pupillary status (both normal, one abnormal, or both abnormal), ISS, BMI, length of ICU stay, 6-month Glasgow Outcome Scale (GOS) score, and Extended GOS (GOS-E) score were recorded for each subject (Jennett and Bond, 1975; Teasdale et al., 1998).
Ischemia factors. As previously described (Cohan et al., 2005), factors associated with a possible ischemic insult to the hypothalamic-pituitary axis included hypotension (systolic blood pressure <90 mm Hg) or severe anemia (hematocrit <25%) within 72 h of injury, hypoxia (Pao2 < 60 mm Hg or Sao2 < 90%) within 24 h of injury, or agonal respirations or apnea in the field (Chesnut et al., 1993). An ischemia score ranging from 0–3 was also calculated for each subject, with 1 point each for hypotension, hypoxia, or severe anemia (Cohan et al., 2005). While moderate anemia (hematocrit 25–35%) was also taken into account for the purpose of multivariate analysis, it was not considered a possible ischemic insult to the pituitary, and thus was not included in the ischemia score.
ICP and CPP. For patients in whom an ICP monitor was placed, mean ICP and CPP, total hours ICP was >20 mm Hg, and total hours CPP was <60 mm Hg were recorded (Jiang et al., 2002; Marmarou et al., 1991; Marshall et al., 1991a, 1991b; Sarrafzadeh et al., 2001).
Computed tomography findings. As previously described (Kelly et al., 2006), the following 10 findings of intracranial injury, all of which have been associated with worse long-term outcome after TBI, were recorded from patient first and second CT scans obtained within 24 h of injury: basilar cistern compression, diffuse brain swelling, midline shift >4 mm, evacuated acute subdural hematoma, evacuated intracerebral hematoma, multiple contusions, subarachnoid hemorrhage, hypothalamic hemorrhagic or swelling, diffuse punctuate (subcortical) hemorrhage (consistent with shearing injuries), and skull/facial fractures (calvarial, skull base, sphenoid, or facial; Eisenberg et al., 1990; Glenn et al., 2003; Kraus et al., 2003; Tomei et al., 1991). An aggregate CT score from 0–10 was calculated for each patient, with 1 point added for each of the above CT findings (Kelly et al., 2006).
Medication effects. Patients treated with etomidate and/or metabolic suppressive agents (e.g., pentobarbital and propofol) were identified. Etomidate, when given, was administered as a single dose immediately prior to intubation, and only the patients' first blood draw within 24 h of injury was considered to be potentially impacted by etomidate. Given that pentobarbital has a half-life of 15–48 h, any hormonal blood draws within 48 h of stopping the pentobarbital infusion were considered potentially influenced by this drug (Cormio et al., 1999; Gilman et al., 1980). Because propofol has a half-life of only 24–64 min, a hormonal blood draw was considered influenced by this drug only if the patient was receiving an infusion of at least 100 μg/kg/min at the time of the blood draw, which is generally considered the threshold rate to achieve EEG burst suppression (Albanese et al., 1990; Bailie et al., 1992; Illievich et al., 1993; Kelly et al., 1999; Vandesteene et al., 1988). Since dopamine is known to be associated with suppression of GH and IGF-1 levels, as well as LH and testosterone levels in critically-ill ICU patients, dopamine use was also assessed as a potential risk factor for suppression of these hormones (Van den Berghe and De Zegher, 1996).
Global outcome measurement. Neurological outcome was assessed at 6 months or more post-injury by staff blinded to hormonal status and other clinical data, using the Glasgow Outcome Scale (GOS), with favorable outcome defined as GOS score 5 or higher, versus GOS score 4 or lower (Choi et al., 1998).
Statistical analysis
Comparisons of the patient-level measures in the trauma and ECT cohorts were performed using exact Wilcoxon rank-sum tests (continuous measures), and Fisher's exact test (categorical measures).
Trends over time in hormones (FSH, LH, testosterone [men], estradiol [women], GH, and IGF-1) from morning blood draws for up to 10 days post-injury were assessed with random coefficient natural cubic spline regression models (Harrell et al., 1988; Oertel et al., 2005; Rice and Wu, 2001), with knots placed at the internal quintiles of the time axis. Each patient was categorized as acutely low for a hormone if any measurement was below the fifth percentile for that hormone based on the normal healthy cohort described above, except for GH, for which only non-measurable levels (≤0.2 μg/L) were characterized as low. The percentage of acutely low values and the percentage of patients with at least one acutely low value were calculated for each hormonal axis.
The association of multiple factors with the binary indicators of acutely low studies for each hormone was examined using the generalized estimating equation (GEE) method, with an exchangeable working correlation structure (Liang and Zeger, 1986). The GEE method is one of several possible approaches to accommodating within-subject correlation for longitudinal binary outcomes data; other possibilities include the mixed-effects logistic regression and Markov transition models (Neuhaus, 1992). Explanatory variables included gender, hypotension and hypoxia, CT score, hydrocortisone treatment or randomization, pupillary abnormalities, and anemia, as well as ischemia score, age, GCS, ISS, CT score, ICP (maximum, hours >20 mm Hg, and mean), and CPP (minimum, hours <50 mm Hg, and mean). Missing data for these predictor variables was handled using standard multiple imputation techniques (Schafer, 1999).
To examine the relationship of hormonal deficits to long-term outcome, logistic regression analysis was used with dichotomous GOS-E score (4 or less versus 5 or higher) as the outcome variable. Predictors included the severity (number) of acute hormonal deficits from 0–5: low gonadotroph hormones (lower than the reference range for testosterone or estradiol), low somatotrophs (lower than the reference range for age-adjusted IGF-1, and/or non-measurable GH), and acute adrenal insufficiency (moderate or severe). These measures were compared with known predictors of outcome, including age, GCS score, pupillary status, early hypotension or hypoxia, CT findings, and ICP and CPP time course (Chesnut et al., 1993; Jiang et al., 2002; Marmarou et al., 1991; Marshall et al., 1991a, 1991b; Sarrafzadeh et al., 2001; Ariza et al., 2004; Miller et al., 1978; Servadei et al., 2000; Vollmer et al., 1991).
All statistical analyses were conducted using SAS version 9.1, except for the random coefficient spline regressions, which were performed using the R language, version 2.5.1. No formal correction was made for multiple statistical testing, and significance was defined as p < 0.05.
Results
Between June 2002 and November 15, 2005, of 183 TBI patients enrolled in the study, 101 TBI patients are included in this analysis (82 male and 19 female patients; mean age 35 ± 17 years; median post-resuscitation GCS score 7, 87% with post-resuscitation or deterioration to GCS scores ≤ 8, 13% with GCS scores 9–12, and 5% with GCS scores 13–14). Mechanisms of patient injuries included 37 motor vehicle accidents, 20 falls, 14 pedestrians struck by automobiles, 11 blunt traumas, 10 motorcycle accidents, 8 gunshot wounds, and 1 other penetrating injury. The reason for excluding 82 patients from the original 183 patients were: prior medical conditions (4 subjects), glucocorticoid use (4 subjects), less than three consecutive days of hormonal blood data or insufficient serum available (21 subjects), and no hormonal data available (53 subjects), given that the blood collection protocol for this part of the study was started after the initiation of the overall study. As seen in Table 2, the 101 TBI patients and 28 ECT patients were similar in terms of age (35 ± 17 versus 35 ± 16 years), gender (82% men versus 86% men), and mean ISS (28 ± 10 versus 24 ± 9.5; p = 0.11).
Table 2.
Extracranial Trauma (ECT) and Traumatic Brain Injury (TBI) Subject Characteristics
| ECT | TBI | p Value | |
|---|---|---|---|
| Number of subjects | 28 | 101 | |
| Age (years) | 35 (18–62) | 35 (14–78) | 0.7 |
| Male | 24 (86%) | 82 (81%) | 0.6 |
| Injury Severity Score | 24 (16–25) | 28 (9–66) | 0.02 |
| Glasgow Coma Scale score | |||
| 14–15 | 15 (54%) | 0 (0%) | 0.0001 |
| 9–13 | 9 (32%) | 9 (11%) | |
| 3–8 | 4 (14%) | 75 (89%) | |
| Days in the intensive care unit | 5.3 (2–9) | 7.1 (2–10) | 0.001 |
Incidence and time course of somatotroph and gonadotroph suppression
Gonadotroph axis
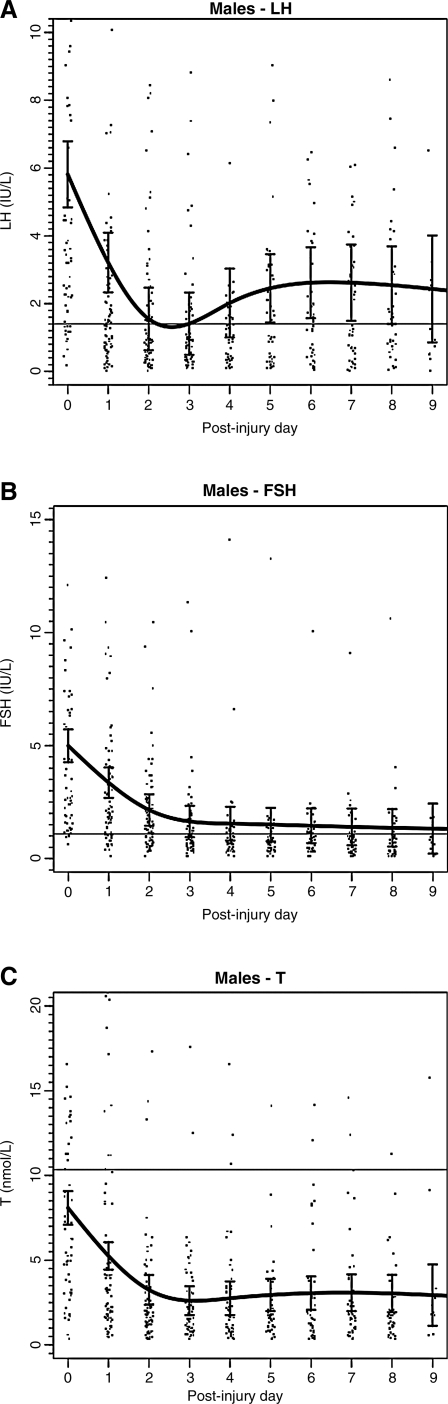
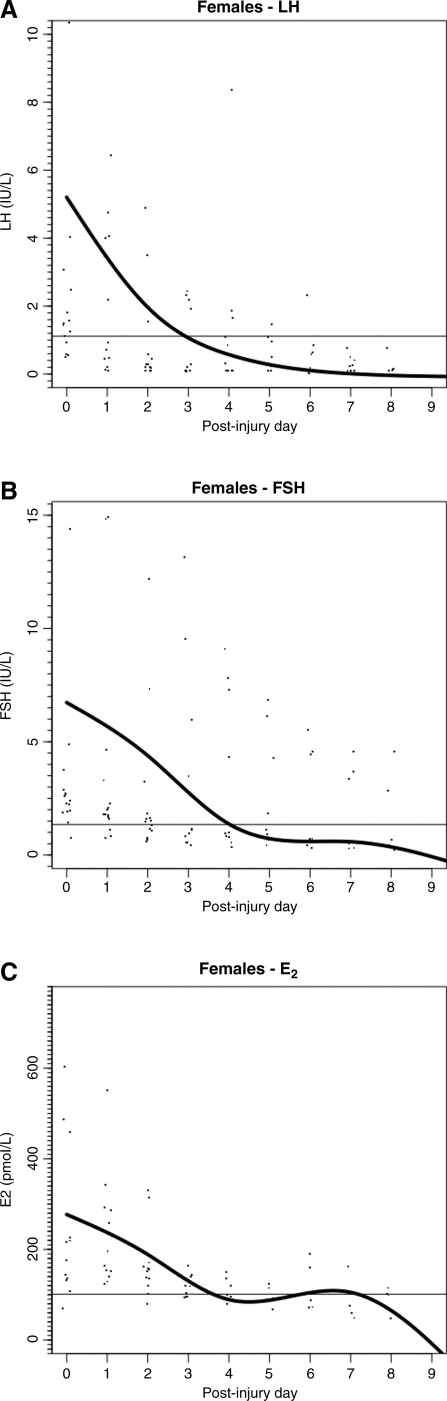
For the TBI cohort, LH, FSH, testosterone in men, and estradiol in women, all had a similar pattern of decline over the 10-day post-injury period (Figs. 1 and 2). Similar patterns of decline (data not shown) of LH, FSH, and testosterone levels were seen for the ECT cohort in male patients. For male TBI patients, the percentage of values below the fifth percentile for LH was 50%, for FSH 46%, and for testosterone 91%. The percentage of male patients with at least one value below the fifth percentile for LH was 82%, for FSH 66%, and for testosterone was 100%. For female TBI patients, the overall percentage of all values below the fifth percentile for LH was 66%, and for FSH 40%. The percentage of female patients with at least one value below the fifth percentile for LH was 89%, and 53% for FSH. In pre-menopausal women, 39% of estradiol values were acutely low, and 43% of subjects had at least one acutely low value (Tables 3A and 3B). Similar rates of low LH, FSH, and testosterone were seen in male ECT patients.
FIG. 1.
Daily mean (± standard deviation) gonadotropin and testosterone levels in male traumatic brain injury patients (n = 82). (A) Luteinizing hormone (LH). (B) Follicle-stimulating hormone (FSH). (C) Total testosterone (T).
FIG. 2.
Daily mean (± standard deviation) gonadotropin and estradiol levels in female traumatic brain injury patients (n = 19). (A) Luteinizing hormone (LH). (B) Follicle-stimulating hormone (FSH). (C) Estradiol (E2).
Table 3A.
Low (<Fifth Percentile) Somatotroph and Gonadotroph Hormone Levels by Collection (Post-Injury Days 0–10)
| |
Acutely low collections |
||
|---|---|---|---|
| Fifth percentile cut-off | Total n | Percentage of acutely low collections (n) | |
| Luteinizing hormone (LH) | 606 | 53% (319) | |
| Male LH | 1.4 IU/L | 508 | 50% (254) |
| Female LH | 1.1 IU/L | 98 | 66% (65) |
| Follicle-stimulating hormone (FSH) | 611 | 46% (278) | |
| Male FSH | 1.1 IU/L | 512 | 46% (238) |
| Female FSH | 1.3 IU/L | 99 | 40% (40) |
| Testosterone (males) | 10.3 nmol/L | 521 | 91% (474) |
| Estradiol (females) | 101.2 pmol/L | 61 | 39% (24) |
| Growth hormone | Non-detectable (≤0.2 μg/L) | 611 | 13% (77) |
| Males | 505 | 13% (67) | |
| Females | 106 | 9% (10) | |
| Insulin-like growth factor-1 | 619 | 55% (342) | |
| Age 16–24 | 26.8 nmol/L | 196 | 67% (131) |
| Age 25–39 | 16.8 nmol/L | 186 | 66% (123) |
| Age 40–54 | 13.2 nmol/L | 124 | 68% (84) |
| Age 55+ | 10.4 nmol/L | 113 | 4% (4) |
Table 3B.
Patients with Any Low (<Fifth Percentile) Somatotroph and Gonadotroph Hormone Levels (Post-Injury Days 0–10)
| |
Acutely low subjects |
||
|---|---|---|---|
| Fifth percentile cut-off | Total n | Percentage of subjects (n) | |
| Luteinizing hormone (LH) | 101 | 83% (84) | |
| Male LH | 1.4 IU/L | 82 | 82% (67) |
| Female LH | 1.1 IU/L | 19 | 89% (17) |
| Follicle-stimulating hormone (FSH) | 101 | 63% (64) | |
| Male FSH | 1.1 IU/L | 82 | 66% (54) |
| Female FSH | 1.3 IU/L | 19 | 53% (10) |
| Testosterone (males) | 10.3 nmol/L | 82 | 100% (82) |
| Estradiol (females) | 101.2 pmol/L | 14 | 43% (6) |
| Growth hormone | Non-detectable (≤0.2 μg/L) | 101 | 38% (38) |
| Males | 82 | 40% (33) | |
| Females | 19 | 26% (5) | |
| Insulin-like growth factor-1 | 101 | 77% (78) | |
| Age 16–24 | 26.8 nmol/L | 34 | 82% (28) |
| Age 25–39 | 16.8 nmol/L | 32 | 84% (27) |
| Age 40–54 | 13.2 nmol/L | 19 | 84% (16) |
| Age 55+ | 10.4 nmol/L | 16 | 6% (1) |
Somatotroph axis
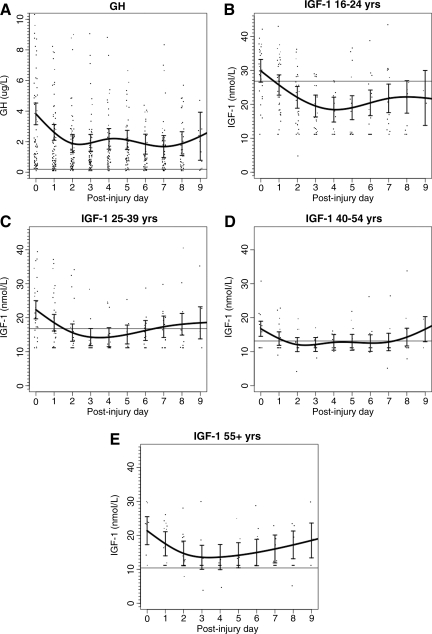
For the TBI patients, GH and IGF-1 had a general pattern of decline and recovery over the 10-day post-injury period, including similar patterns across age groups for IGF-1 (Fig. 3A–E). However, GH levels overall remained relatively normal or slightly elevated throughout the observation period. A similar pattern of decline and recovery (data not shown) was seen for the ECT cohort across age groups for IGF-1.
FIG. 3.
Daily mean (± standard deviation) growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels in male and female traumatic brain injury patients (n = 101). (A) GH (n = 101). (B) IGF-1 (age 16–24 years; n = 34). (C) IGF-1 (age 25–39 years; n = 32). (D) IGF-1 (age 40–54 years; n = 19). (E) IGF-1 (age > 55 years; n = 16).
For the TBI cohort, the percentage of non-detectable GH values (≤0.2 μg/L) was 13%; and simultaneous IGF-1 values were below the age-adjusted fifth percentile in 55%. The percentage of TBI patients with at least one non-measurable GH value was 38%, and with an IGF-1 value below the fifth percentile was 77%. For the ECT cohort, 61% of patients had at least one IGF-1 value below the fifth percentile.
Multiple hormonal deficits
When patients were categorized by how many measured hormones were below the fifth percentile cut-off for at least 1 day, 1% of the 101 TBI patients had no hormonal deficits, 8% had one hormonal deficit, 17% had two deficits, 20% had three deficits, 49% had four deficits, and 6% had deficits in all five measured hormones. Overall, 74% of the TBI cohort expressed low levels in at least three of the five measured hormones.
Factors associated with gonadotroph and somatotroph suppression
Multivariate analysis revealed no factors predictive of LH suppression, and only younger age was associated with FSH suppression. In patients under 40 years of age, 58% had low FSH levels, compared to only 26% in male and pre-menopausal female patients ≥40 years of age (p = 0.0001); however, this result may reflect the fact that serum FSH levels are lower in younger patients. Regarding testosterone in male patients, acute anemia and vehicular mechanism of injury were both associated with low values. Testosterone was low in 94%, 93%, and 73%, of studies in patients with severe, moderate, or no anemia, respectively. However, multivariate analysis did not reveal any independent predictors of low values (including age and BMI), largely due to the high percentage of abnormally low serum testosterone concentrations overall. Because of the small sample size, predictive factors for low estradiol in female patients were difficult to determine; however, there appear to be at least weak associations with hypoxia, CT score, and the use of propofol and pentobarbital. Regarding somatotroph function, younger age, higher ISS, and any risk factors for ischemia (anemia, hypoxia, and hypotension) were all predictive of low age-adjusted IGF-1 values. On multivariate analysis, only age and anemia remained independently predictive. In patients <40 years of age, 66% of studies were low, versus 37% in older patients (p = 0.0001). Low IGF-1 values occurred in 32%, 52%, and 67% of studies, in patients with no, moderate, or severe anemia (p = 0.003). Older patients were more likely to have acutely low GH, as were patients with higher BMI. Both factors remained independently predictive in multivariate analysis. In patients <40 years of age, 11% of GH values were low, versus 16% in older patients (p = 0.01). In patients that had BMI <25 kg/m2, 5% of studies were low, versus 20% in overweight patients (p = 0.004).
Outcome analysis by Extended Glasgow Outcome Scale score and severity of hormonal deficits
The GOS-E scores of 83 patients with long-term follow-up were analyzed as a function of the number of acute hormonal deficits, and specifically as a function of the frequency of low testosterone (in men), low estradiol (in women), low IGF-1 and/or non-measurable GH, and moderate or severe adrenal insufficiency. This analysis showed no significant correlation between outcome and acute hormonal suppression. However, poor outcome was strongly associated with other factors that have been identified previously: lower initial GCS score (p < 0.001), abnormal pupillary status (p = 0.001), hypotension and/or hypoxia (p < 0.0001), poorer CT findings (p < 0.0001), increased ICP (p = 0.0004), and decreased CPP (p = 0.001). Multivariate analysis of these factors showed that the best three-predictor model for outcome is based upon patient age, pupillary status, and ICP course.
Discussion
Summary of findings
In this prospective study of 101 TBI patients, LH suppression was observed in 83% of patients, FSH suppression in 63%, low estradiol in 43% of pre-menopausal women, and low testosterone in 100% of men; these low levels persisted over the observation period. The acute somatotroph response to injury was more variable, with daily morning GH values generally elevated above the mean. However, non-detectable GH values were observed in 38% of patients, and a low age-adjusted IGF-1 level was observed at least once in 77% of patients. There was a trend of recovery of IGF-1 levels from days 7–9 post-injury. Overall, 74% of the cohort had suppression of at least three hormone levels. There were no strong predictors of impaired gonadotroph function on multivariate analysis other than younger age for FSH. In contrast, both younger age and anemia were associated with suppressed IGF-1 levels, while older age and higher BMI were associated with acutely low GH. Similar rates of somatotroph and gonadotroph suppression were observed in a smaller cohort of extracranial trauma patients who did not sustain significant TBI, indicating that the observed hormonal suppression may not be TBI-specific, but may be more related to the generalized stress of critical illness. Below we discuss these findings relative to prior studies, as well possible future TBI treatments.
Acute hormonal suppression after TBI and critical illness
Prior studies have shown varying rates of acute somatotroph and gonadotroph dysfunction after TBI, multiple trauma, and other types of critical illness. Several studies specific to TBI are noteworthy. In 1988 Chiolero and associates measured ACTH, GH, prolactin, and TSH daily for 5 days after injury in 36 patients with isolated severe TBI, multiple trauma with severe TBI, or multiple trauma without TBI (Chiolero et al., 1988a). GH was elevated in all three groups, and prolactin was measured in patients with combined multiple trauma and TBI. Levels of IGF-1 and gonadotropins were not measured. In 1991 Hackl and colleagues studied anterior pituitary function in 21 severe TBI patients with one blood draw in 11 patients, and 2–5 blood draws in 10 patients. Relevant to our study, testosterone levels were low in all men, and basal GH levels were elevated in severely injured patients with raised ICP (Hackl et al., 1991). Della Corte and co-workers in 1998 also showed, in 22 intubated TBI patients, relatively normal GH levels acutely, and improving IGF-1 levels at 7 and 15 days post-injury compared to day 2 post-injury (Della Corte et al., 1998). Patients with poor outcomes had a higher peak GH in response to thyrotropin-releasing hormone. Agha and associates in 2004 studied 50 TBI patients with both basal hormone levels and stimulation testing performed once, on average 12 days post-injury. Based on a glucagon stimulation test, 18% of patients had GH deficiency and 16% had adrenal insufficiency. Low testosterone was seen in 79% of males, and low estradiol was seen in 90% of pre-menopausal females (Agha et al., 2004).
More recently, in 2007 Klose and associates studied 46 TBI patients longitudinally over 1 year, including a one-time assessment in the acute injury period (between days 0 and 12 post-injury). Relevant to the current study, they also found low gonadotropins acutely after injury in both men and women. They also found more suppression with increased injury severity (Klose et al., 2007b). Kleindienst and colleagues, in a 2009 study of 71 TBI patients (78% mild or moderate TBI; 22% severe TBI), also showed acute suppression of the gonadotroph and somatotroph axes, but in lower overall percentages of patients than in the present study (Kleindienst et al., 2009). As in the current study, clinical parameters such as imaging findings, post-injury GCS scores, and outcomes were not found to be independently predictive of acute pituitary dysfunction. Accordingly, these recent studies suggest that acute dysfunction of the hypothalamic-pituitary axis may manifest in response to critical illness, independent of TBI. Several other prior reports of multiple trauma patients without TBI, and more general ICU populations with critical illness, indicate that suppression of the gonadal axis and suppression of IGF-1 may not be TBI-specific, and instead can be a general response to critical illness (Chiolero et al., 1988a; Van den Berghe, 2003; Van den Berghe et al., 1994, 1997). Additionally, dopamine infusion in critically ill patients is also associated with attenuated pulsatile GH and LH secretion, and is associated with low testosterone (Van den Berghe and De Zegher, 1996).
Prior studies have shown hypogonadism with low testosterone after acute myocardial infarction, elective surgery, multiple trauma without TBI, and hemorrhagic and ischemic stroke (Dimopoulou et al., 2005; Woolf et al., 1985). Another study indicates that low testosterone may persist for several weeks after injury (Lee et al., 1994). In the present study, no clinical factors were strongly predictive of gonadotroph function for either men or women. Given that 100% of men in both the TBI and ECT cohorts had low testosterone, the cause of this suppression is likely not directly related to head injury per se, but instead to critical illness in general. Because of the severe suppression of gonadal function in all male patients, serum testosterone showed no decrease with age or BMI, as has been demonstrated in healthy men (Allen et al., 2002; Gapstur et al., 2002; Jensen et al., 2004). Regarding somatotroph function, the association of non-measurable GH levels with higher BMI and older age is consistent with prior studies of GH levels in the non-acute care setting (Qu et al., 2005).
Recent studies have shown conflicting evidence about other independent predictors of pituitary dysfunction in the acute setting. Klose and associates in 2007 suggested that acutely elevated ICP predicted pituitary dysfunction at 13 months post-injury in 104 TBI patients (Klose et al., 2007a). Dimopoulou and colleagues in 2004 reported an association between severity of CT findings and endocrine hyporesponsiveness to stimulation testing in 34 TBI patients, with no predictive value attributable to increased ICP (Dimopoulou et al., 2004). In the current study, neither ICP nor specific CT findings were found to predict pituitary dysfunction in multivariate analyses. Nevertheless, the relatively low yield of CT findings and ICP in the current study may indicate the utility of higher-resolution neuroimaging to more effectively predict pituitary dysfunction. Maiya and co-workers in 2008 discovered specific focal changes in pituitary morphology, pituitary hemorrhage, infarction, and edema using magnetic resonance imaging in 41 TBI patients (Maiya et al., 2008). The present study found that global ischemia is inconsistently associated with both somatotroph and gonadotroph dysfunction, and that CT findings are only weakly associated with estradiol suppression in women; hence there may be a role for MRI in identifying the pathophysiological processes that underlie subsequent endocrine disturbances.
Study limitations
This study is limited by the fact that basal hormone levels were assessed only once daily. Given the known pulsatile secretory pattern of GH, LH, and FSH, the true pattern of their release in the acute setting may not be well delineated by once-daily measurements. Additionally, stimulation tests were not performed, which better assess the responsiveness of the hypothalamic-pituitary axis. Although such stimulation tests were done in some prior studies, they are challenging to do in the ICU, and we elected not to do so here. Patients in this acute study that were available for follow-up did undergo stimulation testing within 6–9 months of injury (Bavisetty et al., 2008). Further complicating interpretation of these results is that hepatic production of IGF-1 depends not only on pituitary GH secretion, but also on nutritional, glycemic, and thyroid factors, all of which may be altered in the context of critical illness. Although FSH analysis excluded post-menopausal females, age itself was not accounted for in defining the lower limit of normal FSH values. We also did not test all pituitary hormones in this study, although all patients had daily ACTH and cortisol values obtained. These data were presented previously, showing that acute adrenal insufficiency occurs transiently in approximately 50% of moderate and severe TBI subjects, and is associated with lower blood pressure and increased vasopressor requirements (Cohan et al., 2005). We also did not assess thyroid function in this cohort, given the substantial literature on this topic in critical illness and in TBI (Agha et al., 2004; Della Corte et al., 1998; Dimopoulou et al., 2004; Hackl et al., 1991; Klose et al., 2007a; Woolf et al., 1988).
Potential significance of gonadotroph and somatotroph suppression
The clinical impact of acutely suppressed testosterone, estradiol, GH, and IGF-1 in the setting of brain injury is unknown. However, multiple studies indicate that there may be consequences of these acute hormonal deficiencies in the short term, in the subacute recovery phase (2 weeks to 3 months post-injury), and potentially long-term. Testosterone and estrogen have both been shown to be neuroprotective in numerous reports (Chisu et al., 2006; Garcia-Estrada et al., 1993; Hammond et al., 2001; Huppenbauer et al., 2005; Nguyen et al., 2005; Ramsden et al., 2003; Roof and Hall, 2000; Sawada et al., 1998; Sierra et al., 2003; Soustiel et al., 2005; Stein, 2001; Stein and Hoffman, 2003; Toung et al., 1998; Zhang et al., 2004). Testosterone is also a key anabolic factor, and androgen deficiency leads to loss of muscle mass and decreased muscle strength (Bhasin et al., 2006). Testosterone treatment reverses muscle loss (Page et al., 2005; Snyder et al., 1999; Wang et al., 2004). GH deficiency also results in decreased muscle mass, and treatment with GH has positive effects. The combination of GH and testosterone has additive or synergistic positive effects on muscle strength and mass (Harman and Blackman, 2003; Sattler et al., 2009). Thus, the post-trauma decrease in testosterone and GH levels may lead to delayed recovery and reduced rehabilitation in patients who suffer trauma. The profound lowering of serum testosterone in 100% of men, and suppressed estradiol observed in nearly 50% of women acutely after TBI, may adversely impact recovery in the acute phase of injury and in the initial months of recovery. Regarding somatotroph function, there are abundant GH and IGF-1 receptors in the brain. Growth hormone plays an important role in vascular reactivity, vascular tone, and CNS repair processes, while IGF-1 is critical in re-myelination and prevention of demyelination (D'Ercole et al., 1996; Hana et al., 2002; Napoli et al., 2003; Scheepens et al., 2001, 2000; Silha et al., 2005; Ye and D'Ercole, 1999; Ye et al., 2002; Zhong et al., 2005). Non-detectable GH levels were identified in 13% of blood samples and in 38% of subjects overall, and low IGF-1 levels were detected in 77% of subjects. Whether these low levels of IGF-1 preclude or limit early and subacute re-myelination of axonal shearing injuries is unclear. The acute and subacute clinical benefit of replacing GH or IGF-1 to normal physiological levels is also unclear. A study by Takala and associates of critically ill adults found that GH therapy at high doses was associated with increased mortality (Takala et al., 1999).
Conclusions
During the first 10 days after complicated mild, moderate, or severe TBI, circulating levels of LH, FSH, testosterone, and IGF-1 declined in the majority of patients, while GH levels typically remained normal to mildly elevated. That similar patterns of decline in LH, FSH, testosterone, and IGF-1 were seen in non-head injured extracranial trauma patients suggests that these hormonal declines are not head-injury-specific, and instead are more likely related to the general stress of critical illness. The short- and long-term impact of acutely low testosterone, estradiol, GH, and IGF-1 levels warrants further investigation, given the potential neuroprotective roles of these hormones in the setting of TBI. The potential clinical benefits of providing physiological hormone replacement therapy to hormonally-deficient TBI patients during the acute and subacute post-injury period warrant further study.
Acknowledgments
We thank Diana Nikas, R.N., N.P., Charlene Chaloner, R.N., Nancy Rudisill, R.N., Janice Wang-Polagruto, Ph.D., Lori Madden, R.N., N.P., Sylvia Lopez, R.N., and Dioni Rovello-Freking, R.N., N.P., for their invaluable assistance as coordinators of this study, and the GCRC nurses of the three clinical sites. Pauruchisty Buxey's dedicated support as manager of the database is greatly appreciated. The assistance of Noreen Mirza, James Wiseman, Stephanie Griffiths, Victor Goh, and the Core Laboratory of the Harbor-UCLA Medical Center GCRC, as well as Anthony Butch, Ph.D. of the UCLA Clinical Laboratory is gratefully acknowledged.
This work was supported by National Institutes of Health grants R01 NS 40777 to D.F.K.; K23 RR 1729801 to P.C.; and MO1 RR 00425, M01 RR 00865, and M01 RR 19975 to the GCRCs at Harbor-UCLA, UCLA, and UC Davis Medical Centers, respectively.
Author Disclosure Statement
No competing financial interests exist.
References
- Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. JAMA. 1999;282:974–983. [PubMed] [Google Scholar]
- Adekoya N. Thurman D.J. White D.D. Webb K.W. Surveillance for traumatic brain injury deaths—United States, 1989–1998. MMWR Surveill. Summ. 2002;51:1–14. [PubMed] [Google Scholar]
- Agha A. Rogers B. Mylotte D. Taleb F. Tormey W. Phillips J. Thompson C.J. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin. Endocrinol. (Oxf.) 2004;60:584–591. doi: 10.1111/j.1365-2265.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- Albanese J. Martin C. Lacarelle B. Saux P. Durand A. Gouin F. Pharmacokinetics of long-term propofol infusion used for sedation in ICU patients. Anesthesiology. 1990;73:214–217. doi: 10.1097/00000542-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Allen N.E. Appleby P.N. Davey G.K. Key T.J. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13:353–363. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- Ariza M. Mataro M. Poca M.A. Junque C. Garnacho A. Amoros S. Sahuquillo J. Influence of extraneurological insults on ventricular enlargement and neuropsychological functioning after moderate and severe traumatic brain injury. J. Neurotrauma. 2004;21:864–876. doi: 10.1089/0897715041526203. [DOI] [PubMed] [Google Scholar]
- Bailie G.R. Cockshott I.D. Douglas E.J. Bowles B.J. Pharmacokinetics of propofol during and after long-term continuous infusion for maintenance of sedation in ICU patients. Br. J. Anaesth. 1992;68:486–491. doi: 10.1093/bja/68.5.486. [DOI] [PubMed] [Google Scholar]
- Bavisetty S. Bavisetty S. McArthur D.L. Dusick J.R. Wang C. Cohan P. Boscardin W.J. Swerdloff R. Levin H. Chang D.J. Muizelaar J.P. Kelly D.F. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery. 2008;62:1080–1093. doi: 10.1227/01.neu.0000325870.60129.6a. discussion 1093–1094. [DOI] [PubMed] [Google Scholar]
- Bhasin S. Cunningham G.R. Hayes F.J. Matsumoto A.M. Snyder P.J. Swerdloff R.S. Montori V.M. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- Borgaro S.R. Prigatano G.P. Kwasnica C. Rexer J.L. Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Inj. 2003;17:189–198. doi: 10.1080/0269905021000013183. [DOI] [PubMed] [Google Scholar]
- Bullock R. Chesnut R.M. Clifton G. Ghajar J. Marion D.W. Narayan R.K. Newell D.W. Pitts L.H. Rosner M.J. Wilberger J.W. Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur. J. Emerg. Med. 1996;3:109–127. doi: 10.1097/00063110-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Cernak I. Savic J. Lazarov A. Relations among plasma prolactin, testosterone, and injury severity in war casualties. World J. Surg. 1997;21:240–245. doi: 10.1007/s002689900223. discussion 246. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chiolero R. Lemarchand T. Schutz Y. De Tribolet N. Felber J.P. Freeman J. Jequier E. Plasma pituitary hormone levels in severe trauma with or without head injury. J. Trauma. 1988a;28:1368–1374. doi: 10.1097/00005373-198809000-00011. [DOI] [PubMed] [Google Scholar]
- Chiolero R.L. Lemarchand-Beraud T. Schutz Y. De Tribolet N. Bayer-Berger M. Freeman J. Thyroid function in severely traumatized patients with or without head injury. Acta Endocrinol. (Copenh.) 1988b;117:80–86. doi: 10.1530/acta.0.1170080. [DOI] [PubMed] [Google Scholar]
- Chisu V. Manca P. Lepore G. Gadau S. Zedda M. Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Arch. Ital. Biol. 2006;144:63–73. [PubMed] [Google Scholar]
- Choi S.C. Marmarou A. Bullock R. Nichols J.S. Wei X. Pitts L.H. Primary end points in phase III clinical trials of severe head trauma: DRS versus GOS. The American Brain Injury Consortium Study Group. J. Neurotrauma. 1998;15:771–776. doi: 10.1089/neu.1998.15.771. [DOI] [PubMed] [Google Scholar]
- Cohan P. Wang C. McArthur D.L. Cook S.W. Dusick J.R. Armin B. Swerdloff R. Vespa P. Muizelaar J.P. Cryer H.G. Christenson P.D. Kelly D.F. Acute secondary adrenal insufficiency after traumatic brain injury: A prospective study. Crit. Care Med. 2005;33:2358–2366. doi: 10.1097/01.ccm.0000181735.51183.a7. [DOI] [PubMed] [Google Scholar]
- Cormio M. Gopinath S.P. Valadka A. Robertson C.S. Cerebral hemodynamic effects of pentobarbital coma in head-injured patients. J. Neurotrauma. 1999;16:927–936. doi: 10.1089/neu.1999.16.927. [DOI] [PubMed] [Google Scholar]
- D'Ercole A.J. Ye P. Calikoglu A.S. Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol. Neurobiol. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- Della Corte F. Mancini A. Valle D. Gallizzi F. Carducci P. Mignani V. De Marinis L. Provocative hypothalamopituitary axis tests in severe head injury: correlations with severity and prognosis. Crit. Care Med. 1998;26:1419–1426. doi: 10.1097/00003246-199808000-00030. [DOI] [PubMed] [Google Scholar]
- Dimopoulou I. Kouyialis A.T. Orfanos S. Armaganidis A. Tzanela M. Thalassinos N. Tsagarakis S. Endocrine alterations in critically ill patients with stroke during the early recovery period. Neurocrit. Care. 2005;3:224–229. doi: 10.1385/ncc:3:3:224. [DOI] [PubMed] [Google Scholar]
- Dimopoulou I. Tsagarakis S. Theodorakopoulou M. Douka E. Zervou M. Kouyialis A.T. Thalassinos N. Roussos C. Endocrine abnormalities in critical care patients with moderate-to-severe head trauma: incidence, pattern and predisposing factors. Intensive Care Med. 2004;30:1051–1057. doi: 10.1007/s00134-004-2257-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg H.M. Gary H.E., Jr. Aldrich E.F. Saydjari C. Turner B. Foulkes M.A. Jane J.A. Marmarou A. Marshall L.F. Young H.F. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J. Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- Fleischer A.S. Rudman D.R. Payne N.S. Tindall G.T. Hypothalamic hypothyroidism and hypogonadism in prolonged traumatic coma. J. Neurosurg. 1978;49:650–657. doi: 10.3171/jns.1978.49.5.0650. [DOI] [PubMed] [Google Scholar]
- Gapstur S.M. Gann P.H. Kopp P. Colangelo L. Longcope C. Liu K. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol. Biomarkers Prev. 2002;11:1041–1047. [PubMed] [Google Scholar]
- Garcia-Estrada J. Del Rio J.A. Luquin S. Soriano E. Garcia-Segura L.M. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- Gilman A. Goodman L. Gilman A. Goodman and Gilman's The Pharmacological Basis of Therapeutics. Macmillan; New York: 1980. [Google Scholar]
- Glenn T.C. Kelly D.F. Boscardin W.J. McArthur D.L. Vespa P. Oertel M. Hovda D.A. Bergsneider M. Hillered L. Martin N.A. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Hackl J.M. Gottardis M. Wieser C. Rumpl E. Stadler C. Schwarz S. Monkayo R. Endocrine abnormalities in severe traumatic brain injury—a cue to prognosis in severe craniocerebral trauma? Intensive Care Med. 1991;17:25–29. doi: 10.1007/BF01708405. [DOI] [PubMed] [Google Scholar]
- Hammond J. Le Q. Goodyer C. Gelfand M. Trifiro M. LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hana V. Prazny M. Marek J. Skrha J. Justova V. Reduced microvascular perfusion and reactivity in adult GH deficient patients is restored by GH replacement. Eur. J. Endocrinol. 2002;147:333–337. doi: 10.1530/eje.0.1470333. [DOI] [PubMed] [Google Scholar]
- Harman S.M. Blackman M.R. The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Horm. Res. 2003;60:121–124. doi: 10.1159/000071236. [DOI] [PubMed] [Google Scholar]
- Harrell F.E., Jr. Lee K.L. Pollock B.G. Regression models in clinical studies: determining relationships between predictors and response. J. Natl. Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- Heinen E. Herrmann J. Konigshausen T. Kruskemper H.L. Secondary hypothyroidism in severe non thyroidal illness? Horm. Metab. Res. 1981;13:284–288. doi: 10.1055/s-2007-1019245. [DOI] [PubMed] [Google Scholar]
- Huppenbauer C.B. Tanzer L. Doncarlos L.L. Jones K.J. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J. Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illievich U.M. Petricek W. Schramm W. Weindlmayr-Goettel M. Czech T. Spiss C.K. Electroencephalographic burst suppression by propofol infusion in humans: hemodynamic consequences. Anesth. Analg. 1993;77:155–160. [PubMed] [Google Scholar]
- Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Jensen T.K. Andersson A.M. Jorgensen N. Andersen A.G. Carlsen E. Petersen J.H. Skakkebaek N.E. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil. Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Gao G.Y. Li W.P. Yu M.K. Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Kelly D.F. Goodale D.B. Williams J. Herr D.L. Chappell E.T. Rosner M.J. Jacobson J. Levy M.L. Croce M.A. Maniker A.H. Fulda G.J. Lovett J.V. Mohan O. Narayan R.K. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. J. Neurosurg. 1999;90:1042–1052. doi: 10.3171/jns.1999.90.6.1042. [DOI] [PubMed] [Google Scholar]
- Kelly D.F. McArthur D.L. Levin H. Swimmer S. Dusick J.R. Cohan P. Wang C. Swerdloff R. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J. Neurotrauma. 2006;23:928–942. doi: 10.1089/neu.2006.23.928. [DOI] [PubMed] [Google Scholar]
- Kleindienst A. Brabant G. Bock C. Maser-Gluth C. Buchfelder M. Neuroendocrine function following traumatic brain injury and subsequent intensive care treatment: a prospective longitudinal evaluation. J. Neurotrauma. 2009;26:1435–1446. doi: 10.1089/neu.2008.0601. [DOI] [PubMed] [Google Scholar]
- Klose M. Juul A. Poulsgaard L. Kosteljanetz M. Brennum J. Feldt-Rasmussen U. Prevalence and predictive factors of post-traumatic hypopituitarism. Clin. Endocrinol. (Oxf.) 2007a;67:193–201. doi: 10.1111/j.1365-2265.2007.02860.x. [DOI] [PubMed] [Google Scholar]
- Klose M. Juul A. Struck J. Morgenthaler N.G. Kosteljanetz M. Feldt-Rasmussen U. Acute and long-term pituitary insufficiency in traumatic brain injury: a prospective single-centre study. Clin. Endocrinol. (Oxf.) 2007b;67:598–606. doi: 10.1111/j.1365-2265.2007.02931.x. [DOI] [PubMed] [Google Scholar]
- Kraus J.F. Rice T.M. Peek-Asa C. McArthur D.L. Facial trauma and the risk of intracranial injury in motorcycle riders. Ann. Emerg. Med. 2003;41:18–26. doi: 10.1067/mem.2003.1. [DOI] [PubMed] [Google Scholar]
- Lee S.C. Zasler N.D. Kreutzer J.S. Male pituitary-gonadal dysfunction following severe traumatic brain injury. Brain Inj. 1994;8:571–577. doi: 10.3109/02699059409151009. [DOI] [PubMed] [Google Scholar]
- Leon-Sanz M. Lorente J.A. Larrodera L. Ros P. Alvarez J. Esteban A.E. Landin L. Pituitary-thyroid function in patients with septic shock and its relation with outcome. Eur. J. Med. Res. 1997;2:477–482. [PubMed] [Google Scholar]
- Liang K.Y. Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Maas A. I. Marmarou A. Murray G.D. Steyerberg E.W. Clinical trials in traumatic brain injury: current problems and future solutions. Acta Neurochir. 2004;(Suppl. 89):113–118. doi: 10.1007/978-3-7091-0603-7_16. [DOI] [PubMed] [Google Scholar]
- Maiya B. Newcombe V. Nortje J. Bradley P. Bernard F. Chatfield D. Outtrim J. Hutchinson P. Matta B. Antoun N. Menon D. Magnetic resonance imaging changes in the pituitary gland following acute traumatic brain injury. Intensive Care Med. 2008;34:468–475. doi: 10.1007/s00134-007-0902-x. [DOI] [PubMed] [Google Scholar]
- Marmarou A. Anderson R.L. Ward J.D. Choi S.C. Young H.F. Eisenberg H.M. Foulkes M.A. Marshall L.F. Jane J.A. Impact of ICP instability and hypotension on outcome inpatients with severe head trauma. J. Neurosurg. 1991;75:S59–S66. [Google Scholar]
- Marshall L.F. Gautille T. Klauber M.R. Eisenberg H.M. Jane J.A. Luerssen T.G. Marmarou A. Foulkes M.A. The outcome of severe closed head injury. J. Neurosurg. 1991a;75:S28–S36. [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. Clark M.V.B. Eisenberg H.M. Jane J.A. Luerssen T.G. Marmarou A. Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991b;75:S14–S20. [Google Scholar]
- Miller J.D. Sweet R.C. Narayan R. Becker D.P. Early insults to the injured brain. JAMA. 1978;240:439–442. [PubMed] [Google Scholar]
- Napoli R. Guardasole V. Angelini V. D'Amico F. Zarra E. Matarazzo M. Sacca L. Acute effects of growth hormone on vascular function in human subjects. J. Clin. Endocrinol. Metab. 2003;88:2817–2820. doi: 10.1210/jc.2003-030144. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J.M. Statistical methods for longitudinal and clustered designs with binary responses. Stat. Methods Med. Res. 1992;1:249–273. doi: 10.1177/096228029200100303. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V. Yao M. Pike C.J. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J. Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Oertel M. Boscardin W.J. Obrist W.D. Glenn T.C. McArthur D.L. Gravori T. Lee J.H. Martin N.A. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J. Neurosurg. 2005;103:812–824. doi: 10.3171/jns.2005.103.5.0812. [DOI] [PubMed] [Google Scholar]
- Page S.T. Amory J.K. Bowman F.D. Anawalt B.D. Matsumoto A.M. Bremner W.J. Tenover J.L. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J. Clin. Endocrinol. Metab. 2005;90:1502–1151. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Qoubaitary A. Meriggiola C. Ng C.M. Lumbreras L. Cerpolini S. Pelusi G. Christensen P.D. Hull L. Swerdloff R.S. Wang C. Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men. J. Androl. 2006;27:853–867. doi: 10.2164/jandrol.106.000281. [DOI] [PubMed] [Google Scholar]
- Qu X.D. Gaw Gonzalo I.T. Al Sayed M.Y. Cohan P. Christenson P.D. Swerdloff R.S. Kelly D.F. Wang C. Influence of body mass index and gender on growth hormone (GH) responses to GH-releasing hormone plus arginine and insulin tolerance tests. J. Clin. Endocrinol. Metab. 2005;90:1563–1569. doi: 10.1210/jc.2004-1450. [DOI] [PubMed] [Google Scholar]
- Ragnarsson K.T. Results of the NIH consensus conference on “rehabilitation of persons with traumatic brain injury.”. Restor. Neurol. Neurosci. 2002;20:103–108. [PubMed] [Google Scholar]
- Ramsden M. Shin T.M. Pike C.J. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Rice J.A. Wu C.O. Nonparametric mixed effects models for unequally sampled noisy curves. Biometrics. 2001;57:253–259. doi: 10.1111/j.0006-341x.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Hall E.D. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J. Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A.S. Peltonen E.E. Kaisers U. Kuchler I. Lanksch W.R. Unterberg A.W. Secondary insults in severe head injury—do multiply injured patients do worse? Crit. Care Med. 2001;29:1116–1123. doi: 10.1097/00003246-200106000-00004. [DOI] [PubMed] [Google Scholar]
- Sattler F.R. Castaneda-Sceppa C. Binder E.F. Schroeder E.T. Wang Y. Bhasin S. Kawakubo M. Stewart Y. Yarasheski K.E. Ulloor J. Colletti P. Roubenoff R. Azen S.P. Testosterone and growth hormone improve body composition and muscle performance in older men. J. Clin. Endocrinol. Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H. Ibi M. Kihara T. Urushitani M. Akaike A. Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J. Neurosci. Res. 1998;54:707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Schafer J.L. Multiple imputation: a primer. Stat. Methods Med. Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- Scheepens A. Sirimanne E.S. Breier B.H. Clark R.G. Gluckman P.D. Williams C.E. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience. 2001;104:677–687. doi: 10.1016/s0306-4522(01)00109-9. [DOI] [PubMed] [Google Scholar]
- Scheepens A. Williams C.E. Breier B.H. Guan J. Gluckman P.D. A role for the somatotropic axis in neural development, injury and disease. J. Pediatr. Endocrinol. Metab. 2000;13(Suppl. 6):1483–1491. doi: 10.1515/jpem-2000-s623. [DOI] [PubMed] [Google Scholar]
- Servadei F. Nasi M.T. Giuliani G. Cremonini A.M. Cenni P. Zappi D. Taylor G.S. CT prognostic factors in acute subdural haematomas: the value of the ‘worst’ CT scan. Br. J. Neurosurg. 2000;14:110–116. doi: 10.1080/02688690050004525. [DOI] [PubMed] [Google Scholar]
- Sierra A. Azcoitia I. Garcia-Segura L. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocrine. 2003;21:43–51. doi: 10.1385/endo:21:1:43. [DOI] [PubMed] [Google Scholar]
- Silha J.V. Krsek M. Hana V. Marek J. Weiss V. Jezkova J. Rosicka M. Jarkovska Z. Murphy L.J. The effects of growth hormone status on circulating levels of vascular growth factors. Clin. Endocrinol. (Oxf.) 2005;63:79–86. doi: 10.1111/j.1365-2265.2005.02303.x. [DOI] [PubMed] [Google Scholar]
- Slag M.F. Morley J.E. Elson M.K. Crowson T.W. Nuttall F.Q. Shafer R.B. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA. 1981;245:43–45. [PubMed] [Google Scholar]
- Snyder P.J. Peachey H. Hannoush P. Berlin J.A. Loh L. Lenrow D.A. Holmes J.H. Dlewati A. Santanna J. Rosen C.J. Strom B.L. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J. Clin. Endocrinol. Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- Soustiel J.F. Palzur E. Nevo O. Thaler I. Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J. Neurotrauma. 2005;22:345–352. doi: 10.1089/neu.2005.22.345. [DOI] [PubMed] [Google Scholar]
- Stein D.G. Hoffman S.W. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr. Rehabil. 2003;6:13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- Stein D.G. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Takala J. Ruokonen E. Webster N.R. Nielsen M.S. Zandstra D.F. Vundelinckx G. Hinds C.J. Increased mortality associated with growth hormone treatment in critically ill adults. N. Engl. J. Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Teasdale G.M. Pettigrew L.E. Wilson J.T. Murray G. Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma. 1998;15:587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- Tomei G. Sganzerla E. Spagnoli D. Guerra P. Lucarini C. Gaini S.M. Villani R. Posttraumatic diffuse cerebral lesions. Relationship between clinical course, CT findings and ICP. J. Neurosurg. Sci. 1991;35:61–75. [PubMed] [Google Scholar]
- Toung T.J. Traystman R.J. Hurn P.D. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. De Zegher F. Anterior pituitary function during critical illness and dopamine treatment. Crit. Care Med. 1996;24:1580–1590. doi: 10.1097/00003246-199609000-00024. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. De Zegher F. Lauwers P. Veldhuis J.D. Growth hormone secretion in critical illness: effect of dopamine. J. Clin. Endocrinol. Metab. 1994;79:1141–1146. doi: 10.1210/jcem.79.4.7962286. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. De Zegher F. Veldhuis J.D. Wouters P. Awouters M. Verbruggen W. Schetz M. Verwaest C. Lauwers P. Bouillon R. Bowers C.Y. The somatotropic axis in critical illness: effect of continuous growth hormone (GH)-releasing hormone and GH-releasing peptide-2 infusion. J. Clin. Endocrinol. Metab. 1997;82:590–599. doi: 10.1210/jcem.82.2.3736. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. Endocrine evaluation of patients with critical illness. Endocrinol. Metab. Clin. North Am. 2003;32:385–410. doi: 10.1016/s0889-8529(03)00005-7. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. Weekers F. Baxter R.C. Wouters P. Iranmanesh A. Bouillon R. Veldhuis J.D. Five-day pulsatile gonadotropin-releasing hormone administration unveils combined hypothalamic-pituitary-gonadal defects underlying profound hypoandrogenism in men with prolonged critical illness. J. Clin. Endocrinol. Metab. 2001;86:3217–3226. doi: 10.1210/jcem.86.7.7680. [DOI] [PubMed] [Google Scholar]
- Vandesteene A. Trempont V. Engelman E. Deloof T. Focroul M. Schoutens A. De Rood M. Effect of propofol on cerebral blood flow and metabolism in man. Anaesthesia. 1988;43(Suppl):42–43. doi: 10.1111/j.1365-2044.1988.tb09067.x. [DOI] [PubMed] [Google Scholar]
- Vollmer D.G. Torner J.C. Jane J.A. Sadovnic B. Charlebois D. Eisenberg H.M. Foulkes M.A. Marmarou A. Marshall L.F. Age and outcome following traumatic coma: why do older patients fare worse? J. Neurosurg. 1991;75:S37–S49. [Google Scholar]
- Wang C. Cunningham G. Dobs A. Iranmanesh A. Matsumoto A.M. Snyder P.J. Weber T. Berman N. Hull L. Swerdloff R.S. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J. Clin. Endocrinol. Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- Woolf P.D. Hamill R.W. McDonald J.V. Lee L.A. Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. J. Clin. Endocrinol. Metab. 1985;60:444–450. doi: 10.1210/jcem-60-3-444. [DOI] [PubMed] [Google Scholar]
- Woolf P.D. Hamill R.W. McDonald J.V. Lee L.A. Kelly M. Transient hypogonadotrophic hypogonadism after head trauma: effects on steroid precursors and correlation with sympathetic nervous system activity. Clin. Endocrinol. (Oxf.) 1986;25:265–274. doi: 10.1111/j.1365-2265.1986.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Woolf P.D. Lee L.A. Hamill R.W. McDonald J.V. Thyroid test abnormalities in traumatic brain injury: correlation with neurologic impairment and sympathetic nervous system activation. Am. J. Med. 1988;84:201–208. doi: 10.1016/0002-9343(88)90414-7. [DOI] [PubMed] [Google Scholar]
- Ye P. D'Ercole A.J. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology. 1999;140:3063–3072. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- Ye P. Li L. Richards R.G. DiAugustine R.P. D'Ercole A.J. Myelination is altered in insulin-like growth factor-I null mutant mice. J. Neurosci. 2002;22:6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Champagne N. Beitel L.K. Goodyer C.G. Trifiro M. LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J. Neurosci. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J. Deng J. Phan J. Dlouhy S. Wu H. Yao W. Ye P. D'Ercole A.J. Lee W.H. Insulin-like growth factor-I protects granule neurons from apoptosis and improves ataxia in weaver mice. J. Neurosci. Res. 2005;80:481–490. doi: 10.1002/jnr.20490. [DOI] [PubMed] [Google Scholar]