Abstract
Background
In the Women's Health Initiative (WHI) randomized controlled trial, use of estrogen plus progestin increased lung cancer mortality. We conducted post hoc analyses in the WHI trial evaluating estrogen alone to determine whether use of conjugated equine estrogen without progestin had a similar adverse influence on lung cancer.
Methods
The WHI study is a randomized, double-blind, placebo-controlled trial conducted in 40 centers in the United States. A total of 10 739 postmenopausal women aged 50–79 years who had a previous hysterectomy were randomly assigned to receive a once-daily 0.625-mg tablet of conjugated equine estrogen (n = 5310) or matching placebo (n = 5429). Incidence and mortality rates for all lung cancers, small cell lung cancers, and non–small cell lung cancers in the two randomization groups were compared by use of hazard ratios (HRs) and 95% confidence intervals (CIs) that were estimated from Cox proportional hazards regression analyses. Analyses were by intention to treat, and all statistical tests were two-sided.
Results
After a mean of 7.9 years (standard deviation = 1.8 years) of follow-up, 61 women in the hormone therapy group were diagnosed with lung cancer compared with 54 in the placebo group (incidence of lung cancer per year = 0.15% vs 0.13%, respectively; HR of incidence = 1.17, 95% CI = 0.81 to 1.69, P = .39). Non–small cell lung cancers were of comparable number, stage, and grade in both groups. Deaths from lung cancer did not differ between the two groups (34 vs 33 deaths in estrogen and placebo groups, respectively; HR of death = 1.07, 95% CI = 0.66 to 1.72, P = .79).
Conclusion
Unlike use of estrogen plus progestin, which increased deaths from lung cancer, use of conjugated equine estrogen alone did not increase incidence or death from lung cancer.
CONTEXT AND CAVEATS
Prior knowledge
In a randomized controlled trial, use of estrogen plus progestin among postmenopausal women was found to statistically significantly increase lung cancer mortality but not incidence.
Study design
In another randomized controlled trial, postmenopausal women aged 50–79 years who had a previous hysterectomy were randomly assigned to conjugated equine estrogen or matching placebo in the previous trial. Lung cancer incidence and mortality were determined.
Contribution
After approximately 8 years of follow-up, the incidence of lung cancer and lung cancer–specific mortality were similar between women using estrogen and women using placebo. At diagnosis, the stage and grade of non–small cell lung cancers in both groups were similar.
Implications
Differences between combined estrogen plus progestin use and estrogen alone use on death from lung cancer require further investigation.
Limitations
Only a modest number of participants were diagnosed with lung cancer. No information was available regarding cancer therapy.
From the Editors
In the Women's Health Initiative (WHI) randomized controlled trial that evaluated estrogen alone in postmenopausal women who had had a previous hysterectomy, the intervention was stopped after a mean of 7.1 years (SD = 1.6 years) when no reduction in the risk of coronary heart disease was observed, whereas increased risk of stroke was identified (1). At that time, there was no difference in total incidence of malignancy between the estrogen-alone and placebo randomization groups.
In the WHI randomized trial evaluating combined estrogen plus progestin therapy in postmenopausal women with no previous hysterectomy, a statistically significant increase in lung cancer deaths, but not in incidence, emerged in the combined hormone therapy group (2). A hormonal influence on lung cancer has been indicated previously by the higher survival rate for women with lung cancer than for men (3–5) and by the presence of estrogen receptors (both α and β types) and progesterone receptors in a substantial proportion of lung cancers (6–8). Results of previous observational studies that investigated the association between menopausal hormone therapy and lung cancer incidence have been mixed, however, with hormone use being associated with increased risk (9,10), no effect (11–13), or reduced risk (8,14–17), although few reports included details of the hormone therapy regimens. Because the effect of estrogen alone on lung cancer was unsettled, we conducted post hoc analyses in the WHI trial evaluating conjugated equine estrogen alone to determine whether use of estrogen alone was associated with lung cancer incidence and, in particular, with increased lung cancer mortality as observed with combined hormone therapy (2,18).
Participants and Methods
The WHI study is a randomized, double-blind, placebo-controlled trial conducted in 40 centers in the United States evaluating estrogen alone that enrolled 10 739 predominantly healthy postmenopausal women with previous hysterectomy, as previously described (1,19). The study is registered with ClinicalTrials.gov, number NCT 00000611. Eligibility criteria included having a previous hysterectomy with or without oophorectomy, age between 50 and 79 years, and postmenopausal status defined as previous bilateral oophorectomy or absence of menstruation for 1 year. Women with previous breast cancer, anticipated survival less than 3 years, or any other previous cancers within the last 10 years, except for nonmelanoma skin cancer, were not eligible. Menopausal hormone therapy users had a required 3-month washout period that was free of any hormone therapy before entry. The trial was approved by institutional review boards at each clinical center, and participants provided written informed consent. Information on baseline characteristics including tobacco use was collected by use of standardized questionnaires, and history of menopausal hormone use was collected at entry by interview (19).
Women were randomly assigned (n = 5310 to hormone therapy and n = 5429 to placebo) to treatment by use of a computerized, stratified, permuted block algorithm to daily use of a 0.625-mg tablet of conjugated equine estrogen (Premarin; Wyeth, Collegeville, PA) or to an identical-appearing placebo between December 1, 1993, and October 11, 1998. The randomization was conducted at the Clinical Coordinating Center by staff who were not involved in the clinical protocol implementation. All participants and clinical center staff were blinded to group allocation, with unblinding only if needed to manage adverse events. A breast cancer diagnosis, venous thromboembolic events, development of specified endometrial pathologies, malignant melanoma, or nonstudy hormone use required discontinuation of study drugs (18,19).
Contacts for clinical outcomes were performed at 6-month intervals, and there were yearly clinic visits. Primary monitored outcomes included coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, and death. Reported clinical outcomes were confirmed by medical record and pathology report review by physician adjudicators at each clinic. All outcomes including lung cancers were then centrally adjudicated by reviewers blinded to randomization assignment. Cancers were coded for stage and grade by use of the Surveillance, Epidemiology, and End Results system (20). Attribution of cause of death was based on review of medical record (21). Methodology for determining age at menopause used date of bilateral oophorectomy or last age of any menstrual bleeding before hysterectomy. Women with hysterectomy without bilateral oophorectomy had age at menopause determined as previously described (Table 1) (1,22). Past smokers were defined as self-reported former smokers who had ever smoked more than 100 cigarettes in their lifetime.
Table 1.
Descriptive characteristics of participants by randomization group
| Characteristic | Estrogen alone group, No. (%) | Placebo group, No. (%) |
| No. of participants randomly assigned | 5310 (100.0) | 5429 (100.0) |
| Age at screening, y | ||
| 50–59 | 1639 (30.9) | 1674 (30.8) |
| 60–69 | 2386 (44.9) | 2465 (45.4) |
| 70–79 | 1285 (24.2) | 1290 (23.8) |
| Race or ethnicity | ||
| White | 4009 (75.5) | 4075 (75.1) |
| Black | 781 (14.7) | 835 (15.4) |
| Hispanic | 319 (6.0) | 332 (6.1) |
| American Indian | 41 (0.8) | 34 (0.6) |
| Asian/Pacific Islander | 86 (1.6) | 78 (1.4) |
| Unknown | 74 (1.4) | 75 (1.4) |
| Body mass index, kg/m2* | ||
| <25 | 1110 (21.0) | 1096 (20.3) |
| 25 to <30 | 1798 (34.0) | 1915 (35.5) |
| ≥30 | 2375 (45.0) | 2385 (44.2) |
| Hormone use | ||
| Never | 2769 (52.2) | 2769 (51.0) |
| Past | 1871 (35.2) | 1947 (35.9) |
| Current† | 669 (12.6) | 709 (13.1) |
| Duration of previous hormone use | ||
| None | 2769 (52.2) | 2769 (51.0) |
| <5 y | 1351 (25.4) | 1411 (26.0) |
| 5–10 y | 469 (8.8) | 514 (9.5) |
| ≥10 y | 720 (13.6) | 731 (13.5) |
| Oral contraceptive use ever | 2053 (38.7) | 2052 (37.8) |
| Tobacco exposure | ||
| Smoking status | ||
| Never | 2723 (51.9) | 2705 (50.4) |
| Past | 1986 (37.8) | 2090 (38.9) |
| Current | 542 (10.3) | 571 (10.6) |
| No. of cigarettes per d‡ | ||
| <25 | 1997 (81.5) | 2121 (82.0) |
| ≥25 | 452 (18.5) | 465 (18.0) |
| Years smoked | ||
| <30 | 1521 (61.8) | 1612 (62.0) |
| ≥30 | 940 (38.2) | 990 (38.0) |
| Pack-years of smoking | ||
| 0 (never-smoker) | 2723 (52.9) | 2705 (51.5) |
| <5 | 691 (13.4) | 686 (13.0) |
| 5 to <20 | 681 (13.2) | 750 (14.3) |
| ≥20 | 1049 (20.4) | 1116 (21.2) |
| General health status | ||
| Excellent | 550 (10.4) | 658 (12.2) |
| Very good | 2004 (38.1) | 1975 (36.7) |
| Good | 2042 (38.8) | 2095 (38.9) |
| Fair | 625 (11.9) | 609 (11.3) |
| Positive history of lung cancer§ | 2 (<0.1) | 7 (0.1) |
| Oophorectomy status | ||
| Ovarian preservation║ | 2973 (60.5) | 2917 (58.0) |
| Bilateral oophorectomy | 1938 (39.5) | 2111 (42.0) |
| Years since menopause¶ | ||
| <10 | 827 (19.0) | 817 (18.3) |
| 10–19 | 1292 (29.7) | 1333 (29.8) |
| ≥20 | 2230 (51.3) | 2319 (51.9) |
| Physical activity, metabolic equivalents per wk | ||
| 0 | 1081 (22.2) | 1043 (21.3) |
| >0–3.75 | 1089 (22.3) | 1142 (23.3) |
| >3.75–8.75 | 914 (18.7) | 895 (18.2) |
| >8.75–17.5 | 968 (19.8) | 959 (19.6) |
| >17.5 | 828 (17.0) | 866 (17.7) |
Because of rounding, percentages may not all total 100.
A 3-month “washout” was required before entry.
Current and previous smokers were combined when estimating the total number of cigarettes per day, years smoked, and past years of smoking.
Lung cancer that was diagnosed more than 10 years previously.
Preservation of any portion of any ovary.
Age at menopause was defined as date of bilateral oophorectomy, last age of any menstrual bleeding before hysterectomy, or age at which menopausal hormone therapy was started. For women with hysterectomy without bilateral oophorectomy, the age at menopause was the age when menopausal hormone therapy was started or when the first vasomotor symptoms appeared. Age at menopause could not be defined in 15% of participants (22).
The intervention ended on February 29, 2004, the date when the participants were instructed to stop their study pills after a mean of 7.1 years (SD = 1.6 years) of intervention. Analyses in the current report include events through March 31, 2005, the end of the originally planned intervention period for a mean follow-up of 7.9 years (SD = 1.8 years).
Lung cancer was not a prospectively defined study outcome, and no protocol-defined chest imaging at entry or serially was performed. Work-up of chest findings was directed by community physicians because the WHI clinical centers provided clinical trial oversight but not comprehensive health care. On the basis of results in the WHI trial evaluating use of estrogen plus progestin and a literature review, a prospective analysis plan was developed and subsequently reviewed and approved by the WHI Publication and Presentation Committee on September 4, 2008. The primary objective of the current analyses was to determine whether use of estrogen alone had an influence on lung cancer mortality.
Statistical Analysis
Comparisons of participant characteristics at baseline were based on χ2 tests of association. Participants with missing values for individual factors were excluded from corresponding analyses.
Lung cancer results were assessed with time-to-event methods and were based on the intention-to-treat principle. Lung cancer incidence in the two randomization groups was compared by use of hazard ratios (HRs), corresponding 95% confidence intervals (CIs), and P values from Wald χ2 statistics that were estimated from Cox proportional hazards models stratified by age at entry (50–59, 60–69, or 70–79 years), previous lung cancer (yes or no), and dietary modification trial participation (randomly assigned to intervention, control, or not randomly assigned) (yes or no) because women who entered this trial could also have entered the WHI dietary trial (23). The proportional hazards assumption was tested by incorporating an interaction term of conjugated equine estrogen arm with the logarithm of follow-up time in a Cox model with the main effect of conjugated equine estrogen arm and testing for deviation from unity. All P values were greater than .20 and did not show a violation of the assumption. Kaplan–Meier plots were used to examine lung cancer incidence and mortality as a function of time from randomization.
Subgroup analyses of whether the effects of conjugated equine estrogen alone on lung cancer outcomes (incidence and death from lung cancer) varied by level of several baseline factors, including smoking status, were examined in Cox proportional hazard models with P values from Wald χ2 statistics. Nine subgroup comparisons were examined, and so fewer than one statistically significant interaction might be expected by chance alone.
Differences in the incidence of and mortality from several cancers in the estrogen plus progestin group compared with the estrogen-alone group, as reported previously in WHI randomized controlled trials (2), prompted interest in formal cross-study comparisons of these events. Differences between use of estrogen plus progestin and use of estrogen alone on lung cancer incidence and mortality, breast cancer incidence, and colorectal cancer incidence were explored by use of Cox regression models stratified according to age, dietary modification trial randomization assignment, and previous lung cancer. The ratio of the hazard ratios for estrogen alone to estrogen plus progestin is reported for each endpoint.
A level of .05 was used for assessing the statistical significance of P values in all analyses. SAS for Windows, version 9.1.3 (SAS Institute, Inc, Cary, NC), and S-Plus for Windows, version 8.0 (Insightful Corp, Somerville, MA), were used for all analyses. All statistical tests were two-sided.
Results
Baseline clinical and demographic characteristics were comparable in the two randomization groups, including previous hormonal exposure, age, education, self-reported health, and race or ethnicity. Tobacco exposure was also closely comparable with approximately 51% never-smokers, 38% past smokers, and 10% current smokers in each group. Also balanced were the number of cigarettes smoked per day and the number of years smoked (Table 1). More women in the placebo group than in the estrogen-alone group had had a previous breast biopsy examination and bilateral oophorectomy.
Per protocol, all participants had had a previous hysterectomy. A full consort diagram for participant flow in this trial has been previously described (1).
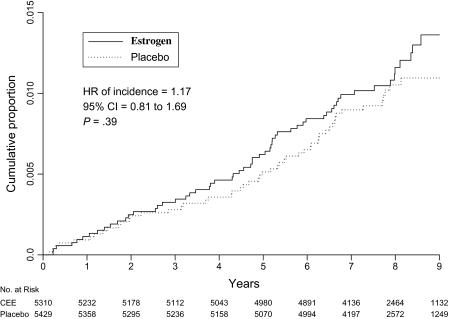
In intention-to-treat analyses, 61 women in the estrogen-alone group (incidence per year = 0.15%) were diagnosed with lung cancer compared with 54 in the placebo group (incidence per year = 0.13%; HR = 1.17, 95% CI = 0.81 to 1.69, P = .39) (Figure 1). The incidence of neither non–small cell lung cancer (51 diagnoses in the estrogen-alone treatment group and 48 in the placebo group; HR = 1.10, 95% CI = 0.74 to 1.64, P = .62) nor small cell lung cancer (nine diagnoses in the estrogen alone group and six in the placebo group; HR = 1.57, 95% CI = 0.56 to 4.41, P = .39) was associated with randomization assignment (Table 2). Results were unchanged in analyses in which women with lung cancer history were excluded. Lung cancer histology was similar in the two randomization groups. The non–small cell lung cancers in the estrogen-alone group and in the placebo group were of similar stage and grade (Table 2).
Figure 1.
Incidence of lung cancer. Kaplan–Meier cumulative hazards for incidence of lung cancer are presented by study group and time in the trial. The hazard ratio (HR), 95% confidence interval (CIs), and P values were from Cox proportional hazards regression models, stratified by age, previous lung cancer, and randomization assignment in the dietary modification trial. All statistical tests were two-sided. CEE = conjugated equine estrogen.
Table 2.
Lung cancer incidence and characteristics by randomization group*
| Lung cancer category | Estrogen-alone group, No. (annualized %) | Placebo group, No. (annualized %) | HR (95% CI)† | P† |
| Lung cancer incidence | 61 (0.15) | 54 (0.13) | 1.17 (0.81 to 1.69) | .39 |
| NSCLC | 51 (0.12) | 48 (0.11) | 1.10 (0.74 to 1.64) | .62 |
| SCLC‡ | 9 (0.02) | 6 (0.01) | 1.57 (0.56 to 4.41) | .39 |
| NSCLC histology§ | ||||
| Adenocarcinoma | 23 (0.06) | 26 (0.06) | 0.91 (0.52 to 1.60) | .74 |
| Squamous cell | 10 (0.02) | 4 (0.01) | 2.70 (0.85 to 8.64) | .09 |
| Large cell or neuroendocrine | 4 (0.01) | 3 (0.01) | 1.34 (0.30 to 5.99) | .70 |
| Unspecified | 14 (0.03) | 15 (0.04) | 0.98 (0.47 to 2.02) | .95 |
| NSCLC stage | ||||
| Local | 11 (0.03) | 11 (0.03) | 1.06 (0.46 to 2.45) | .89 |
| Regional | 11 (0.03) | 10 (0.02) | 1.12 (0.48 to 2.63) | .80 |
| Distant metastases | 22 (0.05) | 22 (0.05) | 1.04 (0.57 to 1.87) | .91 |
| NSCLC grade | ||||
| Well differentiated | 2 (<0.01) | 6 (0.01) | 0.34 (0.07 to 1.71) | .19 |
| Moderately differentiated | 10 (0.02) | 4 (0.01) | 2.56 (0.80 to 8.17) | .11 |
| Poorly differentiated | 14 (0.03) | 19 (0.04) | 0.77 (0.38 to 1.53) | .45 |
| Anaplastic | 1 (<0.01) | 0 (0.00) | — | — |
CI = confidence interval; HR = hazard ratio; NSCLC = non–small cell lung cancer; SCLC = small cell lung cancer.
Hazard ratios, 95% confidence intervals, and P values are from Cox proportional hazards models, stratified according to age, previous lung cancer, and dietary modification trial randomization. All statistical tests were two-sided.
There were insufficient numbers of SCLCs in both trials to permit subgroup comparisons.
One patient with biopsy-proven cancer had a missing value for histology.
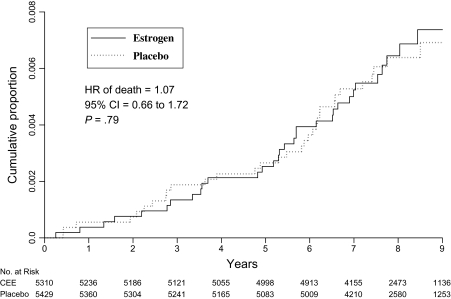
When all deaths after a lung cancer diagnosis were considered, the cause of death was attributed to lung cancer in 34 (56%) of the 61 deaths in the estrogen-alone group and in 33 (61%) of the 54 deaths in the placebo group. Death from lung cancer was closely comparable among women in the estrogen-alone group (34 deaths) and those in the placebo group (33 deaths; HR = 1.07, 95% CI = 0.66 to 1.72, P = .79) (Figure 2). Similar results were observed for deaths from any cause that occurred after a lung cancer diagnosis (Table 3). Deaths from non–small cell lung cancer and deaths after a non–small cell diagnosis did not differ between randomization groups. Few deaths from small cell lung cancer were observed (eight deaths in the estrogen-alone group and four in the placebo group), and all deaths were attributed to the lung cancer (Table 3).
Figure 2.
Deaths from lung cancer. Kaplan–Meier cumulative hazards for death from lung cancer are presented by study group and time in the trial. The hazard ratio (HR), 95% confidence interval (CI), and P values were from Cox proportional hazards regression models, stratified by age, previous lung cancer, and randomization assignment in the dietary modification trial. All statistical tests were two-sided. CEE = conjugated equine estrogen.
Table 3.
Lung cancer mortality by randomization group*
| Outcome category | Estrogen-alone group, No. (annualized %) | Placebo group, No. (annualized %) | HR (95% CI)† | P† |
| Death from lung cancer | 34 (0.08) | 33 (0.08) | 1.07 (0.66 to 1.72) | .79 |
| Death from NSCLC‡ | 25 (0.06) | 29 (0.07) | 0.89 (0.52 to 1.52) | .67 |
| Death from SCLC | 8 (0.02) | 4 (0.01) | 2.11 (0.62 to 7.01) | .22 |
| Death after lung cancer diagnosis§ | 39 (0.09) | 35 (0.08) | 1.15 (0.73 to 1.82) | .54 |
| Death after NSCLC | 30 (0.07) | 31 (0.07) | 1.00 (0.60 to 1.65) | 1.00 |
| Death after SCLC | 8 (0.02) | 4 (0.01) | 2.11 (0.64 to 7.01) | .22 |
CI = confidence interval; HR = hazard ratio; NSCLC = non–small cell lung cancer; SCLC = small cell lung cancer.
Hazard ratios, 95% confidence intervals, and P values were from Cox proportional hazards models, stratified according to age, previous lung cancer, and dietary modification trial randomization assignment. All statistical tests were two-sided.
Follow-up started at randomization, and the denominator includes all participants. Deaths were directly attributed to lung cancer.
Follow-up started at randomization, and denominator includes all participants. All deaths after lung cancer diagnosis are included, regardless of attributed etiology.
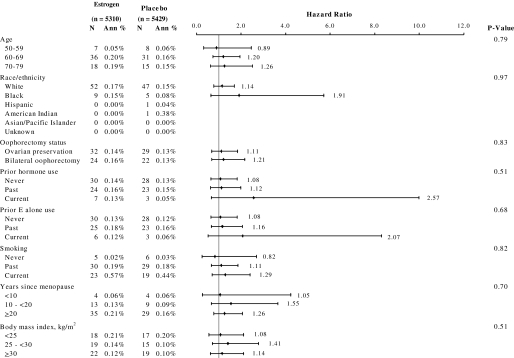
Of subgroup analyses, no statistically significant treatment interactions were observed between lung cancer incidence (Figure 3) or death from lung cancer (data not shown) and the following variables: age at screening, race or ethnicity, years since menopause, oophorectomy status, previous hormone therapy use, previous estrogen-alone use, smoking status, or body mass index. Current smokers were at substantially greater risk of being diagnosed with or dying from lung cancer than past smokers and especially never-smokers.
Figure 3.
Cumulative risk of lung cancer incidence by study group and selected baseline characteristics. Hazard ratios, 95% confidence intervals (whiskers), and P values were from Cox proportional hazards models stratified by age, previous lung cancer, and randomization assignment in the dietary modification trial. P values were from a Wald χ2 test for the interaction between the given characteristic and treatment group. Because of missing or equivocal information on smoking, years since menopause, and oophorectomy status, the numbers in the subsets are less than the total number of lung cancers. N = number of participants with lung cancer. Ann % = annualized percent; E = estrogen. All statistical tests were two-sided.
Differences between use of conjugated equine estrogen plus medroxyprogesterone acetate (24–27) and use of estrogen alone (1,28,29) and results on selected cancers from the two separate WHI randomized trials are outlined in Table 4. In cross-study comparisons, a statistically significant difference was observed between hormone therapy use and breast cancer incidence (P = .01) and between hormone therapy use and colorectal cancer incidence (P = .004) in the two trials (29). There was a greater influence of combined hormone therapy compared with estrogen alone on death from lung cancers, but the difference between trials was not statistically significant (HR = 0.62, 95% CI = 0.34 to 1.15, P = .13). For deaths from non–small cell lung cancer, the difference between estrogen plus progestin and estrogen-alone trials was statistically significant (P = .03 comparing HR = 1.87, 95% CI = 1.22 to 2.88 with HR = 0.89, 95% CI = 0.52 to 1.52) (Table 4).
Table 4.
Selected cancer outcomes in the Women's Health Initiative (WHI) randomized clinical trials of estrogen plus progestin in women with a uterus and estrogen alone in women with previous hysterectomy*
| Cancer category | Estrogen-alone group† |
Estrogen plus progestin group‡ |
HR comparison of estrogen alone vs estrogen plus progestin§ |
|||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Invasive breast cancer incidence | 0.80 (0.62 to 1.04) | .09 | 1.24 (1.01 to 1.54) | .003 | 0.65 (0.46 to 0.90) | .01 |
| Colorectal cancer incidence | 1.12 (0.77 to 1.63) | .55 | 0.56 (0.38 to 0.81) | .003 | 2.21 (1.29 to 3.81) | .004 |
| Endometrial cancer incidence | No study | 1.04 (0.71 to 1.53) | — | — | ||
| Lung cancer║ | ||||||
| Incidence | 1.17 (0.81 to 1.69) | .39 | 1.23 (0.92 to 1.63) | .16 | 0.96 (0.60 to 1.52) | .85 |
| Death | 1.07 (0.66 to 1.72) | .79 | 1.71 (1.16 to 2.52) | .01 | 0.62 (0.34 to 1.15) | .13 |
| NSCLC | ||||||
| Incidence | 1.10 (0.74 to 1.64) | .62 | 1.23 (0.92 to 1.63) | .16 | 0.86 (0.52 to 1.42) | .57 |
| Death | 0.89 (0.52 to 1.52) | .67 | 1.87 (1.22 to 2.88) | .004 | 0.48 (0.24 to 0.95) | .03 |
Except for lung cancer results from the estrogen-alone group, other results from the individual trials have been previously published (2,24,25,28,29). There were insufficient numbers of small cell lung cancers in both trials to permit subgroup comparisons. Differences in outcomes between trials were explored using Cox regression models stratified according to age, previous lung cancer, and dietary modification trial randomization. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio; NSCLC = non–small cell lung cancer.
Estrogen alone = conjugated equine estrogen.
Estrogen plus progestin = conjugated equine estrogens plus medroxyprogesterone acetate.
The ratio of the hazard ratios for the effects of estrogen alone on the indicated cancer outcomes was compared with the hazard ratios for the effects of estrogen plus progestin on the same outcomes. The WHI trial evaluating estrogen plus progestin entered 16 608 postmenopausal women with an intact uterus. The WHI trial evaluating estrogen alone entered 10 739 postmenopausal women with previous hysterectomy. There were differences in the characteristics of women entering these two trials.
The lung cancer annualized incidence in the placebo group of the estrogen-alone and estrogen plus progestin trials was the same (0.13% per year in both placebo groups).
Discussion
In post hoc analyses of the WHI randomized placebo-controlled clinical trial, use of conjugated equine estrogen alone was not associated with lung cancer incidence or death from lung cancer in women with previous hysterectomy. Use of estrogen alone also was not associated with non–small cell lung cancer incidence or mortality; however, the limited numbers of small cell lung cancers observed in this trial preclude firm conclusion regarding hormone influence. These findings differ from those reported in the WHI randomized clinical trial evaluating use of combined estrogen plus progestin in women with no previous hysterectomy, in which a statistically significant increased number of deaths from lung cancer, including deaths from non–small cell lung cancer, was observed among women in the combined hormone group (2).
In this analysis of the trial evaluating conjugated equine estrogen, although the number of deaths from lung cancer was modest in the estrogen-alone group (34 deaths) compared with the placebo group (33 deaths), other findings suggest a real difference in results between use of conjugated equine estrogen and use of combined hormone therapy (2). The WHI randomized trial evaluating use of combined estrogen plus progestin during a mean intervention of 5.6 years identified an adverse effect on lung cancer mortality, likely mediated by increased diagnoses of poorly differentiated non–small cell lung cancers and metastatic non–small cell lung cancers (2). With use of estrogen alone for a longer mean intervention of 7.1 years and a total follow-up of 7.9 years in this trial evaluating conjugated equine estrogen, fewer deaths from non–small cell lung cancer in the estrogen-alone group (25 deaths) than in the placebo group (29 deaths), fewer poorly differentiated non–small cell lung cancers (14 vs 19 cancers, respectively), and the same number of cancers diagnosed with distant metastases (22 vs 22 metastatic cancers, respectively) were observed. Thus, no association between use of estrogen alone and lung cancer mortality has emerged.
Although the effects of combined estrogen plus progestin therapy and estrogen-alone therapy on coronary heart disease and vascular processes are generally comparable (22), differences in the association between these two therapies with various cancers were found. There were dissimilar findings on cancer incidence and outcome in the WHI randomized trial evaluating combined hormone therapy in women with a uterus and in the WHI randomized trial evaluating estrogen alone in women with previous hysterectomy (Table 4). Interpretation of these cross-study comparisons requires caution because characteristics of women entering these two trials were different (1,26).
Use of combined estrogen plus progestin statistically significantly increased breast cancer incidence (24,27), whereas use of estrogen alone resulted in a trend for a reduced incidence (28). Use of combined estrogen plus progestin statistically significantly reduced colorectal cancer incidence (25), but the use of estrogen alone was not associated with a reduction in colorectal cancer (29). In the absence of full-scale randomized clinical trial, evidence on use of estrogen alone in women with a uterus and information regarding effects of use of estrogen alone compared with use of estrogen plus progestin on endometrial cancer are somewhat mixed. In the WHI randomized clinical trial, endometrial cancers were not increased with use of combined hormone therapy (26,30). In the Postmenopausal Estrogen/Progestin Interventions trial, relatively short-term use of estrogen alone substantially increased endometrial hyperplasia, but use of combined estrogen plus progestin protected the endometrium from hyperplastic change (31). Although the Million Women Study (32) and the preponderance of observational studies, including the meta-analyses incorporated in that report (32), suggest that the addition of progestin reduces estrogen-related risk of endometrial cancer, full protection may be dependent on the dose and particular hormone therapy regimen used (33–35).
With respect to lung cancer, the WHI clinical trial evaluating conjugated equine estrogen plus medroxyprogesterone acetate reported (2) a statistically significant increase in deaths from lung cancer apparently restricted to non–small cell lung cancer with combined hormone use. In the analysis of the WHI clinical trial evaluating conjugated equine estrogen alone, estrogen use was not associated with lung cancer mortality (Table 4). Although a single unifying hypothesis or mechanism does not emerge from these findings, progestins appear to modulate the association between estrogen and the malignant process in an organ-specific manner.
In observational studies (36–39), lung cancers that express estrogen receptor β have been associated with favorable prognosis. In one report (40), better prognosis for non–small cell lung cancers was associated with a positive progesterone receptor status than with a negative progesterone receptor status. However, relationships among hormone receptor status, cancer prognosis, and response to hormonal manipulation are complex. Although breast cancers that are positive for the routinely measured estrogen receptor α, and especially the progesterone receptor, have favorable prognosis (41,42), use of exogenous estrogen plus progestin, which stimulates receptor activity, increased breast cancer growth (24,27). In contrast, positive estrogen receptor status is predictive of clinical benefit to the estrogen receptor antagonist, tamoxifen (41). One explanation of these observations could be that stimulation of the progesterone receptor, either alone or with stimulation of the estrogen receptor, is needed to increase lung cancer growth, but that stimulation of the estrogen receptor alone is not sufficient. Although the influence of reproductive hormones on lung cancer has been assessed previously in pre-clinical models (7,43), because most studies evaluated only use of estrogen alone, they provide no basis for explicating divergent findings on clinical lung cancer observed with use of estrogen alone and use of combined estrogen plus progestin.
Several decades ago, the Coronary Drug Project identified men who had had a previous myocardial infarction and randomly assigned them, as part of a multicomponent clinical trial, to conjugated equine estrogen at 2.5 mg/day (n = 1119) or to placebo (n = 2788), anticipating a reduction in future cardiac events in the conjugated equine estrogen arm. However, this intervention was stopped for primary endpoint futility when increased lung cancer mortality was observed in the conjugated equine estrogen group (0.54% annualized risk) compared with the placebo group (0.14% annualized risk) (P = .026) (44). This result was based on only 10 participants with lung cancer and so a chance finding cannot be ruled out. Additionally, because the dosage given to men was about four times that used in the WHI trial, sex and dose–response differences also could be considered.
A recent systematic review (45) found mixed results in 16 observational studies that examined associations between menopausal hormone therapy and lung cancer incidence. Unfortunately, most of these 16 studies did not report the type of hormone therapy used, smoking status, or outcomes by histological subtype of lung cancer. In the two studies (15,17) providing separate results for both estrogen alone and combined estrogen plus progestin use, lower incidence of lung cancer was associated with both estrogen alone and combined hormone therapy users compared with nonusers. However, a more recent study (46) reported higher risk of lung cancer with combined estrogen plus progestin users compared with nonusers.
Few observational studies have reported on menopausal hormone therapy use and lung cancer mortality. One study (47) reported that hormone therapy was associated with decreased lung cancer survival; two (48,49) reported no association; and one (50) reported, on the basis of a few patients, increased lung cancer survival. Given the results from the WHI randomized trials, future studies should include lung cancer disease category analyses by separate hormone therapy regimens.
Study strengths include the randomized double-blind study design and the large and ethnically diverse study population. The randomization group had comparable smoking histories, and central adjudication of lung cancers was performed.
This study had several limitations. There was a modest number of patients with lung cancer and an absence of information regarding cancer therapy. In addition, the possibility of a chance finding cannot be ruled out because of the nature of post hoc analyses.
In summary, in post hoc analyses in a randomized clinical trial setting, use of conjugated equine estrogen alone was not associated with lung cancer incidence or mortality. The evidence for a null effect was strongest for non–small cell lung cancer. These findings should be reassuring for women with previous hysterectomy, who use estrogen alone for climacteric symptom management. The difference between combined estrogen plus progestin use and estrogen alone use on death from lung cancer requires further investigation.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–19, 32122, 42107–26, 42129–32, and 44221.
Footnotes
The funding organization had representation on the steering committee, which governed the design and conduct of the study, the interpretation of the data, and approval of the article but did not participate in the preparation of the article. The corresponding author has full access to the data and made the final decision when and where to submit the article for publication.
R. T. Chlebowski has received speaker's fee and honorarium for advisory boards and consulting from AstraZeneca and Novartis; honorarium for advisory boards and consulting for Lilly, Amgen, and Pfizer; and grant support from Amgen. R. T. Chlebowski, G. L. Anderson, M. L. Stefanick, J. E. Manson, J. Wactawski-Wende, M. Gass, J. M. Kotchen, K. C. Johnson, M. J. O’Sullivan, J. K. Ockene, and F. A. Hubbell have received grant support from National Institutes of Health; K. C. Johnson and R. T. Chlebowski additionally have received grant support from the National Cancer Institute of Canada. M. Gass has received grant support from Wyeth. A. G. Schwartz, H. Wakelee, R. J. Rodabough, J. W. Chien, and C. Chen have no conflicts of interest.
We gratefully acknowledge the dedicated efforts of investigators and staff at the WHI clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung, and Blood Institute program office (listing available at http://www.whi.org). Most importantly, we recognize the WHI participants for their extraordinary commitment to the WHI program.
References
- 1.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Schwartz AG, Wakelee H, et al. Estrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomized controlled trial. Lancet. 2009;374(9697):1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Palomares MR, Lillington L, Grosvenor M. Recent implications of weight loss on lung cancer management. Nutrition. 1996;12(suppl 1):S42–S47. doi: 10.1016/0899-9007(96)90018-0. [DOI] [PubMed] [Google Scholar]
- 4.Wakelee HA, Wang W, Schiller JH, et al. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 2006;1(5):441–446. [PubMed] [Google Scholar]
- 5.International Early Lung Cancer Action Program Investigators. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296(2):180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 6.Cagle PT, Mody DR, Schwartz MR. Estrogen and progesterone receptors in bronchogenic carcinoma. Cancer Res. 1990;50(20):6632–6635. [PubMed] [Google Scholar]
- 7.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62(7):2141–2150. [PubMed] [Google Scholar]
- 8.Schwartz AG, Wenzlaff AS, Prysak GM, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol. 2007;25(36):5785–5792. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 9.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86(11):869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Inoue M, Sobue T, et al. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int J Cancer. 2005;117(4):662–666. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 11.Kruezer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol. 2003;32(2):263–271. doi: 10.1093/ije/dyg064. [DOI] [PubMed] [Google Scholar]
- 12.Elliott AM, Hannaford PC. Use of exogenous hormones by women and lung cancer: evidence from the Royal College of General Practitioners’ Oral Contraception Study. Contraception. 2006;73(4):331–335. doi: 10.1016/j.contraception.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Olsson H, Bladstrom A, Ingvar C. Are smoking-associated cancers prevented or postponed in women using hormone replacement therapy? Obstet Gynecol. 2003;102(3):565–570. doi: 10.1016/s0029-7844(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 15.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10(1 pt 1):113–123. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 16.Ramnath N, Menezes RJ, Loewen G, et al. Hormone replacement therapy as a risk factor for non-small cell lung cancer: results of a case-control study. Oncology. 2007;73(5–6):305–310. doi: 10.1159/000134238. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez C, Spencer Feigelson H, Deka A, et al. Postmenopausal hormone therapy and lung cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2008;17(3):655–660. doi: 10.1158/1055-9965.EPI-07-2683. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski RT. Menopause hormone therapy, hormone receptor status, and lung cancer in women. Semin Oncol. 2009;36(6):566–571. doi: 10.1053/j.seminoncol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;(suppl 9):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Surveillance, epidemiology and end results. 2006 http://seer.cancer.gov/. Accessed July 22, 2009. [Google Scholar]
- 21.Curb D, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(suppl 9):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 22.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 23.Cox DR. Regression analysis and life tables. JR Stat Soc. 1972;34(2):187–220. [Google Scholar]
- 24.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative randomized trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 25.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 26.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Chlebowski RT, Kuller L, Prentice RL, et al. Breast cancer after estrogen plus progestin use in postmenopausal women. N Engl J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanick M, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography in postmenopausal women with hysterectomy: the Women's Health Initiative Randomized Trial. JAMA. 2006;295(14):1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 29.Ritenbaugh C, Stanford JL, Wu L, et al. Conjugated equine estrogens and colorectal cancer incidence and survival: the Women's Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2609–2618. doi: 10.1158/1055-9965.EPI-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson GL, Judd HL, Kaunitz AM, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures. JAMA. 2003;290(13):1739–1748. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- 31.The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA. 1996;275(5):370–375. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 32.Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–1551. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 33.Lacey JV, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1724–1731. doi: 10.1158/1055-9965.EPI-05-0111. [DOI] [PubMed] [Google Scholar]
- 34.Pike MC, Peters RK, Cozen W, et al. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997;89(15):1110–1116. doi: 10.1093/jnci/89.15.1110. [DOI] [PubMed] [Google Scholar]
- 35.Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the nurses’ health study cohort 1976–2004. Int J Cancer. 2010;126(1):208–216. doi: 10.1002/ijc.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CY, Change YL, Shih JY, Lee YC. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130(4):979–986. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Kawai H, Ishii A, Washiya K, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res. 2005;11(14):5084–5089. doi: 10.1158/1078-0432.CCR-05-0200. [DOI] [PubMed] [Google Scholar]
- 38.Skov BG, Fischer BM, Pappot H. Estrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59(1):88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27(3):411–417. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 40.Ishibashi H, Suzuki T, Suzuki S, et al. Progesterone receptor in non-small cell lung cancer—a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65(14):6450–6458. doi: 10.1158/0008-5472.CAN-04-3087. [DOI] [PubMed] [Google Scholar]
- 41.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 42.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 43.Hammoud Z, Tan B, Badve S, Bigsby RM. Estrogen promotes tumor progression in a genetically defined mouse model of lung adenocarcinoma. Endocr Relat Cancer. 2008;15(2):475–483. doi: 10.1677/ERC-08-0002. [DOI] [PubMed] [Google Scholar]
- 44.The Coronary Drug Project Research Group. The Coronary Drug Project: findings leading to discontinuation of the 2.5 mg/day estrogen group. JAMA. 1973;226(6):652–657. [PubMed] [Google Scholar]
- 45.Greiser EM, Doser M. Menopausal hormone therapy and risk of lung cancer—systematic review and meta-analysis. Maturitas. 2010;65(3):198–204. doi: 10.1016/j.maturitas.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 46.Slatore CG, Chien JW, Au DH, Satia JA, White E. Lung cancer and hormone replacement therapy: association in the Vitamins and Lifestyle Study. J Clin Oncol. 2010;28(9):1–7. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24(1):59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 48.Huang B, Carloss H, Wyatt SW, Riley E. Hormone replacement therapy and survival in lung cancer in postmenopausal women in a rural population. Cancer. 2009;115(18):4167–4175. doi: 10.1002/cncr.24475. [DOI] [PubMed] [Google Scholar]
- 49.Ayeni O, Robinson A. Hormone replacement therapy and outcomes for women with non-small cell lung cancer: can an association be confirmed? Curr Oncol. 2009;16(3):21–25. doi: 10.3747/co.v16i3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ettinger B, Friedman GD, Bush T, Quesenberry CP., Jr Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet Gynecol. 1996;87(1):6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]