Abstract
Several lines of evidence indicate that calcium deficiency is associated with cellular defects in many tissues and organs. Owing to the large in vivo gradient between ionized extra- and intracellular Ca2+ concentrations ([Ca2+]i), it is generally recognized that the prevailing circulating Ca2+ does not significantly affect resting cytosolic Ca2+. To probe the consequences of hypocalcemia on [Ca2+]i, a model of chronic hypocalcemia secondary to vitamin D (D) deficiency was used. Hepatocytes were isolated from livers of hypocalcemic D-deficient, of normocalcemic D3-repleted, or of normal control rats presenting serum Ca2+ of 0.78 +/- 0.02, 1.24 +/- 0.03, or 1.25 +/- 0.01 mM, respectively (P < 0.0001). [Ca2+]i was measured in cell couplets using the fluorescent probe Fura-2. Hepatocytes of normocalcemic D3-repleted and of normal controls exhibited similar [Ca2+]i of 227 +/- 10 and 242 +/- 9 nM, respectively (NS), whereas those of hypocalcemic rats had significantly lower resting [Ca2+]i (172 +/- 10 nM; P < 0.0003). Stimulation of hepatocytes with the alpha 1-adrenoreceptor agonist phenylephrine illicited increases in cytosolic Ca2+ leading to similar [Ca2+]i and phosphorylase a (a Ca(2+)-dependent enzyme) activity in all groups but in contrast to normocalcemia, low extracellular Ca2+ was often accompanied by a rapid decay in the sustained phase of the [Ca2+]i response. When stimulated with the powerful hepatic mitogen epidermal growth factor (EGF), hepatocytes isolated from hypocalcemic rat livers responded with a blunted maximal [Ca2+]i of 237.6 +/- 18.7 compared with 605.2 +/- 89.9 nM (P < 0.0001) for their normal counterparts, while the EGF-mediated DNA synthesis response was reduced by 50% by the hypocalcemic condition (P < 0.03). Further studies on the possible mechanisms involved in the perturbed [Ca2+]i homeostasis associated with chronic hypocalcemia revealed the presence of an unchanged plasma membrane Ca2+ ATPase but of a significant decrease in agonist-stimulated Ca2+ entry as indicated using Mn2+ as surrogate ion (P < 0.03). Our data, thus indicate that, in rat hepatocytes, the in vivo calcium status significantly affects resting [Ca2+]i, and from this we raise the hypothesis that this lower than normal [Ca2+]i may be linked, in calcium disorders, to inappropriate cell responses mediated through the calcium signaling pathway as illustrated by the response to phenylephrine and EGF.
Full text
PDF
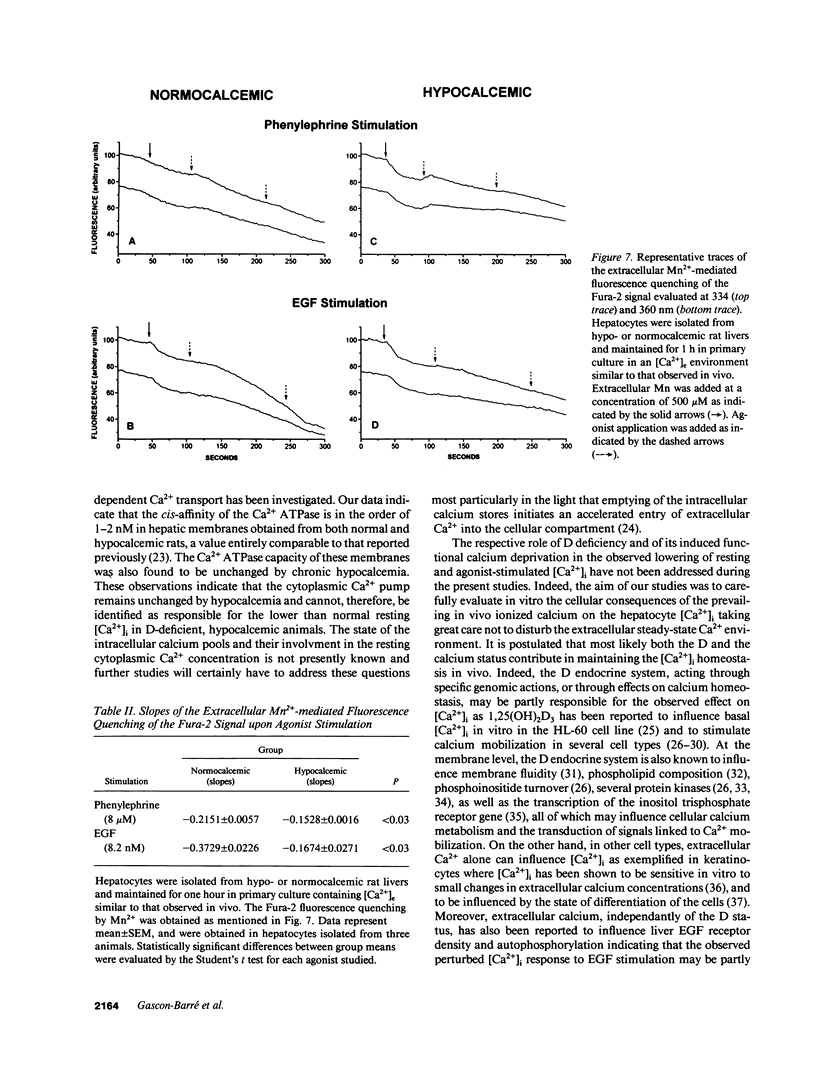
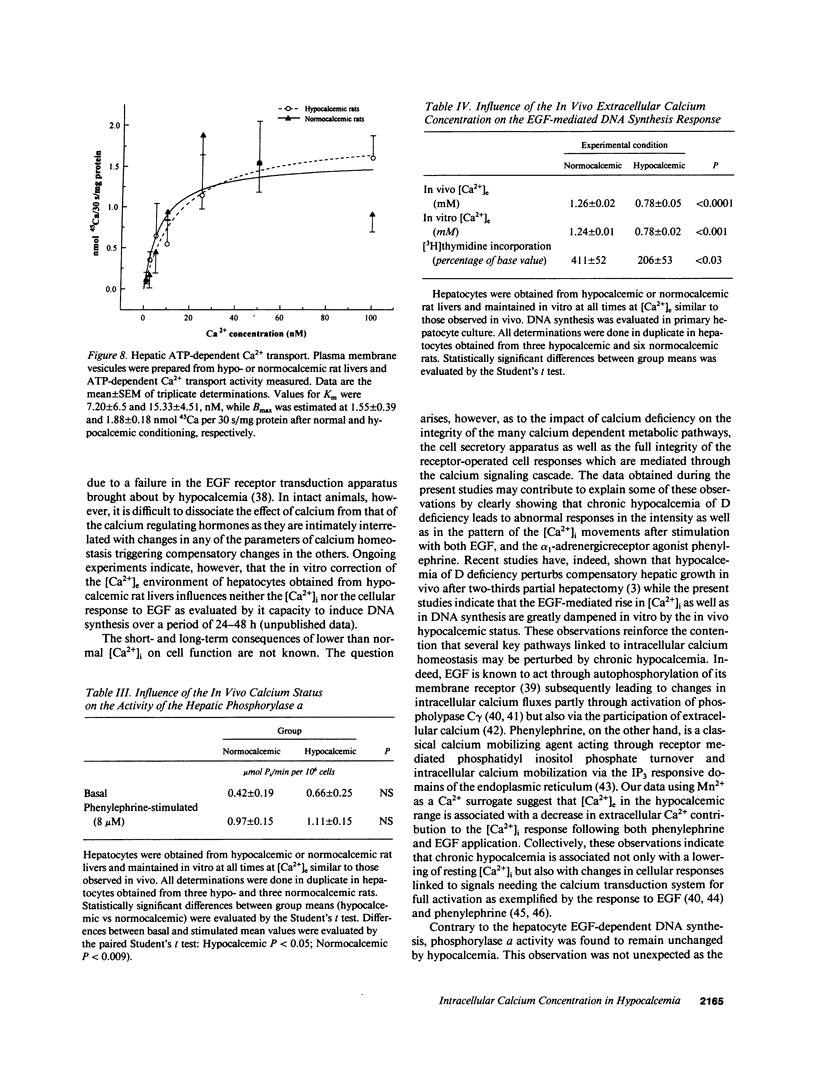


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baran D. T., Kelly A. M. Lysophosphatidylinositol: a potential mediator of 1,25-dihydroxyvitamin D-induced increments in hepatocyte cytosolic calcium. Endocrinology. 1988 Mar;122(3):930–934. doi: 10.1210/endo-122-3-930. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Jin Y., Hui P. 1,25-dihydroxyvitamin D3 enhances the transcription and expression of the inositol trisphosphate receptor gene in HL-60 cells. Mol Pharmacol. 1993 Aug;44(2):292–297. [PubMed] [Google Scholar]
- Burgess G. M., Bird G. S., Obie J. F., Putney J. W., Jr The mechanism for synergism between phospholipase C- and adenylylcyclase-linked hormones in liver. Cyclic AMP-dependent kinase augments inositol trisphosphate-mediated Ca2+ mobilization without increasing the cellular levels of inositol polyphosphates. J Biol Chem. 1991 Mar 15;266(8):4772–4781. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Chisholm J. C., Kim S., Tashjian A. H., Jr Modulation by 1,25-dihydroxycholecalciferol of the acute change in cytosolic free calcium induced by thyrotropin-releasing hormone in GH4C1 pituitary cells. J Clin Invest. 1988 Mar;81(3):661–668. doi: 10.1172/JCI113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. R., Johnson L., Fraser D. R. A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature. 1987 Jan 1;325(6099):62–65. doi: 10.1038/325062a0. [DOI] [PubMed] [Google Scholar]
- Dubé C., Vallières S., Ethier C., Benbrahim N., Tremblay C., Gascon-Barré M. In micronodular cirrhosis, hepatocytes retain a normal C-25 hydroxylation capacity toward vitamin D3: a study using the rat carbon tetrachloride-induced cirrhotic model. Hepatology. 1991 Mar;13(3):489–499. doi: 10.1002/hep.1840130317. [DOI] [PubMed] [Google Scholar]
- Ethier C., Goupil D., Demers C., Hendy G. N., Gascon-Barré M. Hypocalcemia, regardless of the vitamin D status, decreases epidermal growth factor receptor density and autophosphorylation in rat livers. Endocrinology. 1993 Aug;133(2):780–792. doi: 10.1210/endo.133.2.8393775. [DOI] [PubMed] [Google Scholar]
- Ethier C., Kestekian R., Beaulieu C., Dubé C., Havrankova J., Gascon-Barré M. Vitamin D depletion retards the normal regeneration process after partial hepatectomy in the rat. Endocrinology. 1990 Jun;126(6):2947–2959. doi: 10.1210/endo-126-6-2947. [DOI] [PubMed] [Google Scholar]
- Fernandez L. M., Massheimer V., de Boland A. R. Cyclic AMP-dependent membrane protein phosphorylation and calmodulin binding are involved in the rapid stimulation of muscle calcium uptake by 1,25-dihydroxyvitamin D3. Calcif Tissue Int. 1990 Nov;47(5):314–319. doi: 10.1007/BF02555915. [DOI] [PubMed] [Google Scholar]
- GUROFF G., DELUCA H. F., STEENBOCK H. Citrate and action of vitamin D on calcium and phosphorus metabolism. Am J Physiol. 1963 May;204:833–836. doi: 10.1152/ajplegacy.1963.204.5.833. [DOI] [PubMed] [Google Scholar]
- Gascon-Barré M., Gamache M. Contribution of the biliary pathway to the homeostasis of vitamin D3 and of 1,25-dihydroxyvitamin D3. Endocrinology. 1991 Nov;129(5):2335–2344. doi: 10.1210/endo-129-5-2335. [DOI] [PubMed] [Google Scholar]
- Glick A. B., McCune B. K., Abdulkarem N., Flanders K. C., Lumadue J. A., Smith J. M., Sporn M. B. Complex regulation of TGF beta expression by retinoic acid in the vitamin A-deficient rat. Development. 1991 Apr;111(4):1081–1086. doi: 10.1242/dev.111.4.1081. [DOI] [PubMed] [Google Scholar]
- Graf J., Gautam A., Boyer J. L. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouneaux C., Audigier Y., Goldsmith P., Pecker F., Lotersztajn S. Gs mediates hormonal inhibition of the calcium pump in liver plasma membranes. J Biol Chem. 1993 Feb 5;268(4):2368–2372. [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Kawanishi T., Blank L. M., Harootunian A. T., Smith M. T., Tsien R. Y. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989 Aug 5;264(22):12859–12866. [PubMed] [Google Scholar]
- Kruszewski F. H., Hennings H., Yuspa S. H., Tucker R. W. Regulation of intracellular free calcium in normal murine keratinocytes. Am J Physiol. 1991 Nov;261(5 Pt 1):C767–C773. doi: 10.1152/ajpcell.1991.261.5.C767. [DOI] [PubMed] [Google Scholar]
- Lemay J., Gascon-Barré M. Responsiveness of the intestinal 1,25-dihydroxyvitamin D3 receptor to magnesium depletion in the rat. Endocrinology. 1992 May;130(5):2767–2777. doi: 10.1210/endo.130.5.1315257. [DOI] [PubMed] [Google Scholar]
- Levy R., Nathan I., Shany S. 1,25-Dihydroxyvitamin D-3 alters membrane phospholipid composition and enhances calcium efflux in HL-60 cells. Biochim Biophys Acta. 1987 Aug 20;902(2):178–182. doi: 10.1016/0005-2736(87)90293-8. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S., Epand R. M., Mallat A., Pecker F. Inhibition by glucagon of the calcium pump in liver plasma membranes. J Biol Chem. 1984 Jul 10;259(13):8195–8201. [PubMed] [Google Scholar]
- Marceau N., Noël M., Deschênes J. Growth and functional activities of neonatal and adult rat hepatocytes cultured on fibronectin coated substratum in serum-free medium. In Vitro. 1982 Jan;18(1):1–11. doi: 10.1007/BF02796379. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Naveh-Many T., Marx R., Keshet E., Pike J. W., Silver J. Regulation of 1,25-dihydroxyvitamin D3 receptor gene expression by 1,25-dihydroxyvitamin D3 in the parathyroid in vivo. J Clin Invest. 1990 Dec;86(6):1968–1975. doi: 10.1172/JCI114931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Many T., Silver J. Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. J Clin Invest. 1990 Oct;86(4):1313–1319. doi: 10.1172/JCI114840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemere I., Yoshimoto Y., Norman A. W. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology. 1984 Oct;115(4):1476–1483. doi: 10.1210/endo-115-4-1476. [DOI] [PubMed] [Google Scholar]
- Pillai S., Menon G. K., Bikle D. D., Elias P. M. Localization and quantitation of calcium pools and calcium binding sites in cultured human keratinocytes. J Cell Physiol. 1993 Jan;154(1):101–112. doi: 10.1002/jcp.1041540113. [DOI] [PubMed] [Google Scholar]
- Prpić V., Green K. C., Blackmore P. F., Exton J. H. Vasopressin-, angiotensin II-, and alpha 1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem. 1984 Feb 10;259(3):1382–1385. [PubMed] [Google Scholar]
- Putney J. W., Jr Receptor-regulated calcium entry. Pharmacol Ther. 1990;48(3):427–434. doi: 10.1016/0163-7258(90)90059-b. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rashed S. M., Patel T. B. Regulation of hepatic energy metabolism by epidermal growth factor. Eur J Biochem. 1991 May 8;197(3):805–813. doi: 10.1111/j.1432-1033.1991.tb15975.x. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. The calcium pump of the surface membrane and of the sarcoplasmic reticulum. Annu Rev Physiol. 1989;51:473–485. doi: 10.1146/annurev.ph.51.030189.002353. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Kurihara S., Kawaguchi Y., Sakai O. Vitamin D3 metabolites increase [Ca2+]i in rabbit renal proximal straight tubule cells. Am J Physiol. 1991 May;260(5 Pt 2):F757–F763. doi: 10.1152/ajprenal.1991.260.5.F757. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Hruska K. A., Seino Y., Malone J. D., Nishii Y., Teitelbaum S. L. Disassociation of the macrophage-maturational effects of vitamin D from respiratory burst priming. J Biol Chem. 1991 Jun 15;266(17):10888–10892. [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Wali R. K., Baum C. L., Sitrin M. D., Brasitus T. A. 1,25(OH)2 vitamin D3 stimulates membrane phosphoinositide turnover, activates protein kinase C, and increases cytosolic calcium in rat colonic epithelium. J Clin Invest. 1990 Apr;85(4):1296–1303. doi: 10.1172/JCI114567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways D. K., Dodd R. C., Bennett T. E., Gray T. K., Earp H. S. 1,25-Dihydroxyvitamin D3 enhances phorbol ester-stimulated differentiation and protein kinase C-dependent substrate phosphorylation activity in the U937 human monoblastoid cell. Endocrinology. 1987 Nov;121(5):1654–1661. doi: 10.1210/endo-121-5-1654. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Yang L. J., Baffy G., Rhee S. G., Manning D., Hansen C. A., Williamson J. R. Pertussis toxin-sensitive Gi protein involvement in epidermal growth factor-induced activation of phospholipase C-gamma in rat hepatocytes. J Biol Chem. 1991 Nov 25;266(33):22451–22458. [PubMed] [Google Scholar]


