Abstract
IL-1α and IL-1β were evaluated for their ability to provide adjuvant activity for the induction of serum antibody responses when nasally-administered with protein antigens in mice and rabbits. In mice, intranasal (i.n.) immunization with pneumococcal surface protein A (PspA) or tetanus toxoid (TT) combined with IL-1β induced protective immunity that was equivalent to that induced by parenteral immunization. Nasal immunization of awake (i.e., not anesthetized) rabbits with IL-1-adjuvanted vaccines induced highly variable serum antibody responses and was not as effective as parenteral immunization for the induction of antigen-specific serum IgG. However, i.n. immunization of deeply anesthetized rabbits with rPA + IL-1α consistently induced rPA-specific serum IgG ELISA titers that were not significantly different than those induced by intramuscular (IM) immunization with rPA + alum although lethal toxin neutralizing titers induced by nasal immunization were lower than those induced by IM immunization. Gamma scintigraphy demonstrated that the enhanced immunogenicity of nasal immunization in anesthetized rabbits correlated with an increased nasal retention of i.n. delivered non-permeable radio-labeled colloidal particles. Our results demonstrate that, in mice, IL-1 is an effective adjuvant for nasally-administered vaccines for the induction of protective systemic immunity and that in non-rodent species, effective induction of systemic immunity with nasally-administered vaccines may require formulations that ensure adequate retention of the vaccine within the nasal cavity.
Keywords: adjuvant, interleukin 1, nasal
INTRODUCTION
There is a growing interest in needle-free, non-invasive methods of vaccination, especially in developing countries, since the re-use of consumable non-sterile supplies is often responsible for transmission of infectious agents [1–6]. One modality for immunization that is needle-free is nasal (mucosal) immunization. The development of safe and effective nasal immunization regimens would provide a relatively non-invasive approach for vaccination that may be useful for mass vaccination campaigns [1, 4, 7–9] as well as for use in people who desire an alternative to injected vaccines due to fear of needles [10–14]. The FDA approval and efficacy of the nasally-administered influenza vaccine Flumist (an unadjuvanted, attenuated replicating virus) [15–17], demonstrates that nasal immunization has the potential to be safe and effective for use in humans. The ability to induce both systemic and mucosal immunity (i.e., S-IgA) is a benefit of mucosal immunization[18, 19]. However, even if mucosal immunity is not required to protect the host against the pathogen of interest, nasal immunization should be considered as a non-invasive method of immunization that provides an alternative to delivery of vaccines via a needle injection.
When non-replicating antigens (proteins, peptides, polysaccharides) are used for nasal immunization, adjuvants must be used to maximize the induction of antigen-specific immune responses since nasal immunization in the absence of adjuvant may not induce the desired immune response and may induce antigen-specific tolerance [20–24]. The balance between the induction of tolerance and adjuvant-induced immunity is thought to be associated with the ability of adjuvants to prevent the induction of regulatory T cells (T regs[25]) secondary to adjuvant-dependent enhanced antigen uptake by dendritic cells (DC), altered DC migration and enhanced DC co-stimulatory activity [26–29]. Although cholera toxin (CTx) and related toxins have been used as mucosal vaccine adjuvants, the list of observed adverse effects (induction of IgE, lethal anaphylaxis, pulmonary inflammation, diarrhea, accumulation in olfactory tissues and Bell’s Palsy[30–36]) associated with their use as mucosal vaccine adjuvants will likely prevent their use in humans. To identify non-toxin nasal vaccine adjuvants, we evaluated recombinant cytokines for their ability to provide adjuvant activity comparable to that provided by CTx. Our previous studies in mice demonstrated that interleukin-1-alpha (IL-1α) and beta (IL-1β) provided adjuvant activity when nasally administered with protein antigens and induced both antigen-specific serum IgG and mucosal IgA comparable to that induced by CTx [37, 38].
Effective nasal immunization in mice models may not translate into efficient immunization in humans, or other animal species with an upper respiratory tract anatomy similar to humans. Unlike rodents which have organized nasal-associated lymphoid tissue (NALT) in the floor of the nasal cavity [39–43], the nasal inductive (NALT-like) tissues in larger animals such as rabbits, non-human primates and humans, likely include tonsils, adenoids and Waldeyer’s ring[44, 45] which anatomically are less accessible to nasally-delivered antigen/adjuvant compared to NALT in rodents. Indeed, we have previously demonstrated in mice that the nasal route of immunization was able to induce serum IgG comparable to that induced by an injected vaccine [46]. However, our past experience with nasal immunization of non-human primates demonstrated that although nasal immunization (antigen + adjuvant) was able to induce statistically increased serum IgG responses, the antibody responses induced by adjuvanted nasal immunization were approximately 10-fold lower than those induced by adjuvanted intramuscular immunization [47]. Accordingly, for non-rodent species, more research is needed to optimize nasal immunization regimens in non-rodent species to achieve and maximize the induction of antigen-specific immunity, in both the systemic and mucosal compartments.
The use of anesthesia with nasal immunization has been reported to augment antigen-specific immune responses in mice, including anti-anthrax protective antigen (PA) antibody responses as well as anthrax lethal toxin neutralizing activity [48, 49]. The anesthetic agents that augmented the vaccine-induced immune responses were agents such as propofol and pentobarbital and were delivered by needle injection. The ability of the anesthetics to increase the efficacy of nasal immunization may be attributed to an increased delivery of vaccine to the lower respiratory tract[50] and/or delayed mucociliary clearance due to decreased epithelial ciliary beat frequency [51]. Although the use of injectable anesthetics with nasal immunization regimens applied to humans is not realistic since it would negate our goal of developing needle-free methods of vaccines, the use of anesthetics with laboratory animals is needed to allow safe animal handling and effective vaccine delivery. Therefore, anesthetized laboratory models will continue to be used in nasal immunization studies and it is uncertain whether the use of anesthesia influences the efficacy of nasal immunization in larger research animals (rabbits, non-human primates). A clearer understanding of technical issues that influence the efficiency of nasal immunization in larger non-rodent species will provide valuable insight to guide the fruitful development of effective nasally-administered vaccines for use in humans.
In the present study we evaluated the ability of IL-1, when used as a nasal vaccine adjuvant in mice, to induce protection against a systemic lethal challenge with Streptococcus pneumoniae and tetanus toxin. In addition, we assessed the adjuvant activity of IL-1 by the nasal immunization route in a rabbit model and monitored core body temperature as an index of pyrogenicity. For this rabbit model, systemic anesthesia was also investigated to evaluate its influence upon efficacy of nasal immunization adjuvanted with IL-1.
MATERIALS AND METHODS
Animals
Female BALB/c mice, 16–18 grams, were purchased from Charles River. Female New Zealand White (NZW) rabbits, 2.5–3 kg, were purchased from Robinson Services (Mocksville, NC). Animals were housed in cages and provided food and water ad libitum. Mice were housed in groups of 5 mice per cage (all mice within a cage received the same vaccine) and rabbits were housed individually. Procedures for use and care of animals were approved by Duke University’s Institutional Animal Care and Use Committee.
Reagents
Recombinant protective antigen (rPA), recombinant lethal factor (rLF), cholera toxin (CT) and tetanus toxin were purchased from List Biologicals (Campbell, CA). rPA and rLF were also obtained from BEI Resources. PspA protein was graciously obtained from Dr. Gary Nabors, Aventis, Lot # 589A-11, Rx1M1 PspA. Imject Alum was obtained from PIERCE (Cat # 77160; aluminum hydroxide). Tetanus toxoid was a kind gift from Pasteur Merrieux Connaught. ISA-51[52–54] was obtained from Seppic (Fairfield, NJ) and was used to make water in oil emulsion vaccines. We have previously reported that both murine IL-1α and IL-1β exhibit nasal adjuvant activity[37]. Recombinant human (rhu) IL-1α was purchased from R&D Systems (Minneapolis, MN) and rhuIL-1β was provided by CISTRON Biotechnology (Pine Brook, NJ). Others have demonstrated that human IL-1α and IL-1β exhibit biological activity across species barriers [55–58] and that recombinant human IL-1α exhibits mucosal adjuvant activity in rabbits[59]. Therefore, to allow these preclinical studies to utilize the form of IL-1α or IL-1β that may be used in human studies, rhuIL-1α or rhuIL-1β was used in our murine and rabbit studies. Sterile and non-pyrogenic technetium-99m (Tc99m) was acquired from Duke University radio-pharmacy and added to sodium thiosulfate according to the manufacturer’s (Pharmalucence, Bedford, MA) instructions to generate Tc99m-labeled sulfur colloid particles, as previously described[60]. The final labeled sulfur colloid preparation is isotonic and at neutral pH.
The adjuvant activity mediated by IL-1α/IL-1β is not due to contaminating bacterial components (i.e., endotoxin) since IL-1-containing vaccine formulations did not exhibit adjuvant activity in IL-1 receptor deficient mice while control vaccine formulations utilizing cholera toxin as the adjuvant exhibited adjuvant activity in IL-1 receptor deficient mice comparable to that observed in wild-type mice (data not shown). Vaccine formulations were prepared in endotoxin-free Dulbecco’s phosphate buffered saline with calcium and magnesium (Mediatech Inc., Manassas, VA).
Mouse immunization, DTH and challenge
Nasal and subcutaneous immunization of mice with tetanus toxoid or PspA was performed as indicated in the legends for Tables 1 and 2. For intranasal immunization, mice were briefly anesthetized with isoflurane. DTH ear swelling measurements were performed as previously described [46, 61]. Tetanus toxin challenge was performed by subcutaneous injection of 10 LD50 tetanus toxin diluted in gelatin/PBS with subsequent monitoring of mice for signs of morbidity [62]. Streptococcus pneumoniae (strain A66.1) challenge was performed by intravenous injection of 1.125 × 105 CFU diluted in HBSS with subsequent monitoring of mice for 14 days for signs of morbidity [63–65].
Table 1. IL-1β is an effective nasal vaccine adjuvant in mice and induces protective immunity against a systemic Streptococcus pneumoniae challenge.
Female BALB/c mice (17–19 mice per group) were immunized as indicated in the table above. Vaginal lavage samples were collected on day +56 and tested for the presence of PspA-specific IgA. Serum samples were collected on day +71 and tested for the presence of PspA-specific IgG. On day +72, mice (9–10 per group) were challenged by an intravenous injection of 1.125 × 105 CFU Streptococcus pneumoniae strain A66.1 (in 100 μl HBSS) and monitored for signs of morbidity for 14 days; mice were euthanized when moribund.
| Group | PspA dose | Route of Immunization | Adjuvant, dose | Immunization Schedule | Day 71 Serum Anti-PspA IgG | Day 56 Vaginal Anti-PspA IgA | Day 72 % Survival | Median Time to Morbidity |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 μg | Subcutaneous | alum | Day 0, 21, 42 | 1:135,942A | <1:16 | 68C | >14D |
| 2 | 1 μg | Intranasal | None | <1:32 | <1:16 | 0 | 3 | |
| 3 | 1 μg | Intranasal | IL-1β, 5 μg | 1:50,766A | 1:37B | 68C | >14D |
Serum anti-PspA IgG titers significantly greater than those in mice immunized with PspA alone (ANOVA and Tukey’s multiple comparison test, p<0.05).
Vaginal anti-PspA IgA titers significantly greater than those in mice immunized with PspA + alum subcutaneously or mice immunized nasally with PspA alone (ANOVA and Tukey’s multiple comparison test, p<0.05).
survival significantly greater than that in mice immunized with PspA alone (Fisher’s Exact test, p<0.0001).
The median time to morbidity for mice vaccinated with PspA + alum or PspA + IL-1 was significantly greater than mice immunized with PspA alone (Gehan-Breslow-Wilcoxon Test, p<0.0001).
Table 2. IL-1β is an effective nasal vaccine adjuvant in mice and induces protective immunity against a systemic tetanus toxin challenge.
Female BALB/c mice (9–17 mice per group) were immunized as indicated in the table above. Serum and vaginal lavage samples were collected on day +56 and tested for the presence of TT-specific IgG and IgA, respectively. DTH ear swelling responses (8–10 per group) were measured on day +58, 48 hours after injection of TT into the ear pinnae on day +56. On day +60, mice (9–10 per group) were challenge by a subcutaneous injection of 10 LD50 tetanus toxin; mice were euthanized when moribund.
| Group | Tetanus Toxoid dose | Route of Immunization | Immunization Schedule | Adjuvant, dose | Adjuvant Use Schedule | Day 56 Serum Anti-TT IgG | Day 56 Vaginal Anti-TT IgA | Day 56 DTH | Day 60 % Survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 μg | Subcutaneous | Day 0, 21, 42 | ISA-51 | Day 0, 21, 42 | 1:984,540A | <1:16 | 120.3F | 100J |
| 2 | 50 μg | Intranasal | None | None | 1:1,176 | <1:16 | 9.6 | 10 | |
| 3 | 50 μg | CT, 1 μg | Day 0, 21, 42 | 1:671,553A | 1:1,024E | 111.6F | 100J | ||
| 4 | 50 μg | IL-1β, 5 μg | 1:356,722A | 1:1,448E | 69.1F,G,I | 100J | |||
| 5 | 50 μg | IL-1β, 1 μg | 1:297,353A | 1:512E | 62.2F,G,I | 100J | |||
| 6 | 50 μg | IL-1β, 0.2 μg | 1:138,250A,B,D | 1:645E | 56.0F,G,I | 100J | |||
| 7 | 50 μg | CT, 1 μg | Day 0 | 1:446,787A | 1:861E | 99.6F | 100J | ||
| 8 | 50 μg | IL-1β, 5 μg | 1:94,848A,C,D | 1:256E | 50.1F,H,I | 100J | |||
| 9 | 50 μg | IL-1β, 1 μg | 1:98,527A,C,D | 1:362E | 38.3H,I | 100J | |||
| 10 | 50 μg | IL-1β, 0.2 μg | 1:65,536A,C,D | 1:203E | 32.8H,I | 100J |
serum anti-TT IgG endpoint titers significantly greater than the titer in mice immunized with TT alone (ANOVA and Tukey’s multiple comparison test, p<0.05).
serum anti-TT IgG endpoint titer significantly lower than the titer in mice immunized nasally with TT + CT with CT used for each immunization (ANOVA and Tukey’s multiple comparison test, p<0.05).
serum anti-TT IgG endpoint titer significantly lower than the titer in mice immunized nasally with TT + CT with CT used for the first immunization only (ANOVA and Tukey’s multiple comparison test, p<0.05).
serum anti-TT IgG endpoint titer significantly lower than the titer in mice immunized subcutaneously with TT + ISA-51.
vaginal anti-TT IgA endpoint titers significantly greater than those in mice immunized nasally with TT alone (ANOVA and Tukey’s multiple comparison test, p<0.05).
antigen-specific ear swelling (inches × 10−4) significantly greater than TT-specific ear swelling in mice immunized nasally with TT alone (ANOVA and Tukey’s multiple comparison test, p<0.05).
TT-specific DTH responses significantly lower than those in mice immunized nasally with TT + CT with CT used for each immunization (ANOVA and Tukey’s multiple comparison test, p<0.05).
TT-specific DTH responses significantly lower than those in mice immunized nasally with TT + CT with CT used for the first immunization only (ANOVA and Tukey’s multiple comparison test, p<0.05).
TT-specific DTH responses significantly lower than those in mice immunized subcutaneously with TT + ISA-51 (ANOVA and Tukey’s multiple comparison test, p<0.05).
survival significantly greater than that observed in mice immunized with TT alone (two-sided Fisher’s Exact Test, p=0.048).
Rabbit immunization, temperature monitoring, sample collection and anesthesia
For experiments in rabbits using tetanus toxoid, NZW rabbits (3 rabbits per group) were intranasally (i.n.) or intramuscularly (IM) immunized on days 0, 28 and 56 with TT (100 μg) and the indicated dose of rhuIL-1β. The vaccine was prepared in a total volume of 200 μL sterile PBS and delivered in the hind leg (200 μl in a single injection) for the IM groups (brief physical restraint). For nasal immunization, awake rabbits (brief physical restraint), received 100 μl of the vaccine formulation per nostril delivered using a laboratory pipette. Blood was collected into vacutainers from the central ear artery of sedated rabbits using butterfly needles on days 21, 49 and 77 and then centrifuged at 3000 xg for 20 minutes to obtain serum. Body temperature was monitored using a temperature transmitter surgically-implanted in the rabbit abdomen prior to the study (Mini Mitter Co., Bend, OR).
For experiments in rabbits using recombinant protective antigen (rPA), NZW rabbits (4 – 8 rabbits per group) were intramuscularly immunized by injecting 10 μg rPA + alum (350 μg) in 100 μl PBS on days 0, 21 and 42 using alternate thighs for vaccination. A dose of 10 μg rPA was selected for the intramuscular immunization since this dose of rPA is known to provide complete protection in rabbits against an anthrax spore challenge when delivered intramuscularly with alum on days 0 and 28[66]. For nasal immunization, a 2-fold higher dose of rPA was selected since we expected a higher dose of rPA would be needed to allow nasal immunization to be as effective as intramuscular immunization due to differences between parenteral injection and topical nasal delivery of antigen[67]. Rabbits were intranasally immunized on days 0, 21 and 42 [68] with rPA (20 μg) and the indicated dose of rhuIL-1α in a total volume of 200 μL sterile PBS (100 μL per nostril) using a laboratory pipette. Blood was collected and prepared as previously described on days 21, 42 and 56. Depending on the experiment, rabbits were held on their backs and nasally immunized while awake and under brief physical restraint or under sedation/anesthesia with: a) intramuscular acepromazine (1 mg/kg) + butorphanol (1 mg/kg), b) intramuscular acepromazine (1 mg/kg) + inhalation isoflurane (4% at 4 mLs per min until there was no response to toe pinch reflex test), or c) intramuscular ketamine (17.5 mg/kg) + xylazine (2.5 mg/kg). Acepromazine + butorphanol sedated the rabbit but the rabbit remained conscious, upright and mobile in its cage. Acepromazine + isoflurane provided sedation due to the use of acepromazine with isoflurane providing deep anesthesia that was of short duration with the rabbit conscious, upright and mobile in its cage within 5 minutes after receiving the nasal vaccine. Ketamine + xylazine provided deep anesthesia with the rabbits remaining deeply anesthetized for at least 30 minutes after receiving the nasal vaccine. All rabbits were held on their backs for an additional 30 sec after nasal immunization before being returned to an upright position and placed in their cage, except for the ketamine + xylazine-anesthetized rabbits which were placed on their sides and kept warm until they regained consciousness.
ELISA
A standard ELISA using alkaline phosphatase-conjugated anti-mouse detection antibodies (Southern Biotech, Birmingham, AL) and PNPP substrate was used to measure tetanus toxoid and PspA-specific IgG ands IgA antibodies in serum and vaginal lavage (used to evaluate antigen-specific mucosal immunity at sites anatomically distant from the site of immunization) as previously described except that plates were coated with tetanus toxoid or PspA, respectively, at 2 μg/ml[46, 69]. This same technique was used to measure tetanus toxoid-specific antibody responses in rabbits after nasal or intramuscular immunization with tetanus toxoid except that alkaline phosphatase-conjugated anti-rabbit IgG and IgA detection reagents were used (Bethyl Laboratories, Montgomery, TX).
Anthrax protective antigen-specific serum responses were determined using ELISA performed as previously described [70, 71] using 2 μg/mL of rPA diluted in carbonate/bicarbonate buffer as coating antigen, alkaline phosphatase-anti-rabbit IgG (Bethyl Laboratories, Montgomery, TX) detection antibody and AttoPhos substrate (Promega; Madison, WI).
Serum samples were diluted 1:32 or 1:64 (log2 1:25 or 1:26 ) followed by additional 2-fold dilutions to allow determination of the end-point titer. Regardless of the ELISA method used, the endpoint titer represents the last immune sample dilution that provided an ELISA raw data value at least 3-fold higher than the value of the corresponding naïve sample at the same dilution. The ELISA titers presented in the manuscript represent geometric mean titers with log2 titers used for statistical analysis. Serum samples with undetectable ELISA titers were assigned a log2 value one dilution lower than the starting dilution for calculation of geometric mean titers and performance of statistical comparisons.
In vitro LeTx neutralization assay
A macrophage toxicity assay using J774A.1 mouse macrophage cells (ATCC #TIB-67; Manassas, VA) was used to assess the ability of anti-PA antibody present in rabbit serum samples to neutralize lethal toxin. The standard assay was performed as previously described [71] in which serum (starting dilution of 1:16 or 1:24) was pre-incubated with LeTx except that the final concentration of rPA and rLF in the serum-LeTx mixture applied to the J774A.1 cells was 93.8 ng/mL of rPA and 93.8 ng/mL of rLF. Percent neutralization was calculated using the following formulation: (Sample OD value–LeTx Standard OD value) ÷ (Cells only OD value–LeTx Standard OD value) × 100. The optical density of a media only well (i.e. no cells) was subtracted from all values before calculating percent neutralization. The percentage cell viability at each serum dilution was plotted versus serum log2 dilutions using a second order polynomial curve and the serum dilution able to neutralize 50% of LeTx (NT50) was calculated. Samples that had no detectable lethal toxin neutralizing activity were assigned a value of 3 (one log2 dilution below the starting serum dilution) for statistical analysis.
2-Dimensional Gamma scintigraphy
Rabbits were sedated/anesthetized using methods described above. The radio-labeled sulfur colloid particles (Tc99m-SC ) were delivered in 100 μl PBS to the left nostril of the rabbits. Animals were positioned upright, and then imaged 2-dimensionally, laterally from the left side using an Anger camera with a medium energy, parallel hole collimation. Imaging commenced immediately (time 0) after nasal delivery of the Tc99m-SC and animals were subsequently re-imaged at time points: 30, 60, 90, 120 and 180 minutes post-delivery. Collected images were background subtracted and corrected for isotopic decay of the Tc99m-SC. For assessment of nasal deposition and kinetics of clearance, regions of interest were generated by computer program processing (Nuclear Mac, Scientific Imaging, Inc.) to overlie the nasal cavity, and relied upon to estimate the initial deposition of Tc99m-SC and its subsequent removal over time from the nasal epithelium. At neutral pH, Tc99m-SC is non permeable to respiratory epithelial surfaces and thus removed by mucociliary clearance mechanisms. The Tc99m binds to sulfur colloid with high efficiency (> 99.9%) when performed aseptically using the manufacturer’s guidelines (CIS-US, Inc, Bedford, MA) and remains stable for a 6 h period, post-preparation.
Statistical analysis
ANOVA with Tukey’s multiple comparison test was used to compare vaccine-induced antibody responses (ELISA titers, lethal toxin neutralizing antibody titers, DTH) between multiple groups (Tables 1, 2 and 3 and Figure 4). Survival after challenge was compared between vaccinated and control groups using the Fisher’s Exact Test (Tables 1 and 2). Survival curves (for Streptococcus pneumoniae challenge) were compared using a Gehan-Breslow-Wilcoxon Test. ANOVA followed by Dunnett’s test to compare the post-vaccination temperatures to the time 0 temperature was used to evaluate change in body temperature after vaccination of rabbits with IL-1 adjuvanted vaccines. Due to the non-parametric distribution of the data points, the Mann Whitney test was used to compare serum anti-TT IgG titers between the rabbit nasal and IM groups (Figure 3) and total and nasal deposition of sulfur colloid (Figures 5B, 5C). An unpaired Student’s T-test was used to compare nasal clearance of the colloidal particles at each time point in rabbits (Figure 5E) and the area under the curve (Figure 5F). All analysis was performed using PRISM software (GraphPad Software, La Jolla, CA). p≤0.05 was considered significant.
Table 3. Serum lethal toxin neutralizing antibody titers induced by nasal immunization are less potent as compared to those induced by intramuscular immunization.
Day 56 serum from rabbits nasally or intramuscularly immunized with rPA were tested for the presence of anthrax lethal toxin neutralizing antibodies using a macrophage toxicity assay. Lethal toxin neutralizing titers represent the titer calculated to neutralize 50% of the cell death induced by the lethal toxin. 50% neutralization titers were compared by ANOVA followed by Tukey’s multiple comparison test.
| Immunization Route | Anesthesia | Rabbit ID # | LeTx 50% Neutralizing Antibody Titer | LeTx neutralizing GMT (95% CI) |
|---|---|---|---|---|
| Nasal | None | 3-353 | 1:22 | 1:51.24 (1:2.2–1:1,154) |
| 3-355 | 1:781 | |||
| 3-357 | 1:16 | |||
| 3-352 | 1:45 | |||
| Nasal | Acepromazine + Butorphanol | 3-54 | 1:283 | 1:26.4 (1:1.8–1:384) |
| 3-356 | 1:27 | |||
| 3-347 | 1:16 | |||
| 3-349 | 1:16 | |||
| Nasal | Acepromazine + Isoflurane | 3-351 | 1:26 | 1:112.9 (1:34.6–1:368) |
| 3-346 | 1:128 | |||
| 3-348 | 1:64 | |||
| 3-350 | 1:232 | |||
| 4-354 | 1:318 | |||
| 4-350 | 1:32 | |||
| 4-356 | 1:60 | |||
| 4-353 | 1:1,768 | |||
| Nasal | Ketamine + xylazine | 3-359 | 1:1,527 | 1:539.6B (1:196–1:1,485) |
| 3-361 | 1:1,762 | |||
| 3-358 | 1:1,346 | |||
| 3-360 | 1:180 | |||
| 4-359 | 1:2,119 | |||
| 4-351 | 1:176 | |||
| 4-357 | 1:168 | |||
| 4-360 | 1:176 | |||
| Intramuscular | None | 1-338 | 1:20,951 | 1:29,303A (1:19,437–1:44,177) |
| 1-403 | 1:36,415 | |||
| 1-334 | 1:35,346 | |||
| 1-420 | 1:27,340 | |||
neutralization 50% titers significantly greater than all nasal immunization groups.
neutralization titer significantly greater than in acepromazine + butorphanol treated rabbits.
Figure 4. The state of anesthesia influences the efficacy of nasal immunization in rabbits.
Female NZW rabbits were nasally immunized with 20 μg rPA combined with rhIL-1α (5–15 μg) on day 0, 21 and 42. There were 4 - 8 rabbits per group Control rabbits were immunized intramuscularly with 10 μg rPA + alum on day 0, 21 and 42. Nasally immunized rabbits were awake, sedated with acepromazine/butorphanol (sedated but upright and mobile in their cage), sedated with acepromazine and anesthetized with isoflurane immediately before immunization (sedated with anesthesia lasting less than 5 minutes after vaccination) or anesthetized with ketamine/xylazine before immunization (deeply anesthetized for at least 30 minutes following nasal immunization). Serum was collected on day 42 and 56 and tested for the presence of anti-PA IgG by ELISA. Serum anti-PA IgG were compared between groups using a one way ANOVA and Tukey’s Multiple Comparison Test (PRISM). a. serum anti-PA IgG titers significantly greater than in intranasally immunized awake rabbits, p<0.05. b. serum anti-PA IgG titers significantly greater than in intranasally immunized rabbits treated with acepromazine/butorphanol p<0.05. c. serum anti-PA IgG titers significantly greater than in intranasally immunized rabbits treated with acepromazine/isoflurane p<0.05. d. serum anti-PA IgG titers significantly greater than in intranasally immunized rabbits treated with ketamine/xylazine p<0.05.
Figure 3. Nasal immunization of rabbits induces variable antigen-specific IgG responses that are significantly lower than IgG responses induced by intramuscular immunization.
Female NZW rabbits (3 per group for each IL-1β adjuvant group) were immunized by the nasal or intramuscular route while awake with 100 μg tetanus toxoid combined with rhIL-1β (0.5, 12.5, 315.5 or 7812.5 ng/kg) on day 0, 28 and 56. Serum was collected on day 77 and tested for the presence of anti-TT IgG by ELISA. The serum anti-TT IgG titer in nasally immunized rabbits was 1:21,081 (geometric mean titer) while the serum anti-TT IgG titer in intramuscularly immunized rabbits was 1:4,194,304 (geometric mean titer; p=0.0001 vs nasal immunization, Mann Whitney).
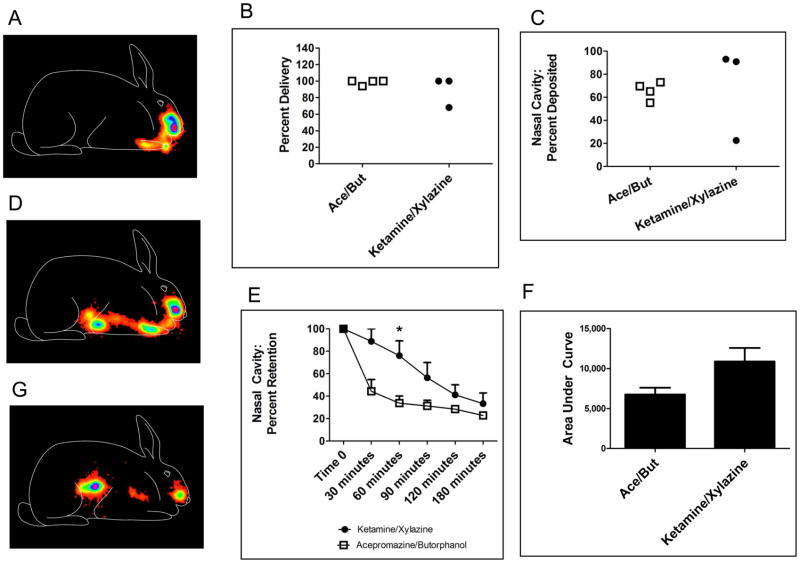
Figure 5. Anesthetic state influences nasal retention in rabbits.
Female NZW rabbits (3–4 per group) were used to monitor the nasal retention of non-permeable Tc99m-sulfur colloid (Tc99m-SC) after delivery to nasal mucosal surfaces in acepromazine/butorphanol-sedated, or ketamine/xylazine-anesthetized, rabbits. A. Typical scintigraphy image of Tc99m-SC acquired from the lateral aspect immediately after delivery with activity deposited within the nasal cavity (rabbit outline drawn for orientation purposes only). B. Efficacy of delivery to rabbits. Total radioactivity delivered to rabbits at time zero (includes nasal cavity, lungs, and swallowed activity within the digestive tract) as percentage of expected delivery. No difference between groups; p=0.8571, Mann Whitney. C. Efficacy of nasal cavity delivery. Radioactivity deposited regionally within the nasal cavity as percentage of total activity administered to rabbits. No difference between groups; p=0.6286, Mann Whitney. D. One of seven rabbits aspirated the Tc99m-sulfur colloid at the time of delivery resulting in immediate accumulation of activity in the lungs and digestive tract (the ketamine/xylazine-anesthetized rabbit with a low percentage of nasal cavity delivery in Figure 5C). E. Retention of radioactivity initially deposited within nasal cavity. Percent of delivered activity remaining in nasal cavity region of interest, compared between groups at each time point using two-tailed unpaired Student’s T-test; *: p=0.0245. F. Nasal retention: area under the retention versus time curve. The retention data of Figure 5E were used to calculate the total area under the curve for the acepromazine/butorphanol and ketamine/xylazine groups, respectively:* p =0.063 two-tailed unpaired Student’s T-test. G. Typical scintigraphy image of Tc99m-sulfur colloid 180 minutes after delivery with regional retention of radioactivity within the nasal cavity as well as clearance to the stomach.
RESULTS
IL-1β is an effective nasal vaccine adjuvant in mice for the induction of protective immunity against a systemic Streptococcus pneumoniae challenge
An important aspect of mucosal immunization is that it elicits mucosal immunity in addition to systemic protective immunity. However, because of recent interest in needle-free methods of immunization [1, 4, 9] we have looked to mucosal immunization because it can be used to elicit systemic protection against pathogens and toxins, without the necessity of an injection. Studies were performed to evaluate the nasal adjuvant activity of IL-1β in mice using pneumococcal surface protein A (PspA) as the antigen to determine if nasal immunization was as effective as parenteral immunization using the FDA-approved adjuvant alum for the induction of antigen-specific serum IgG and protection against a lethal bacterial challenge. Female BALB/c mice (17–19 per group) were nasally immunized with 1 μg PspA alone or combined with 5 μg IL-1β on days 0, 21 and 42 (Table 1). A systemic immunization control consisted of mice immunized subcutaneously with 1 μg PspA + alum on days 0, 21 and 42. Serum samples collected on day 71 demonstrated that needle-free nasal immunization with PspA + IL-1β induced serum anti-PspA IgG titers (1:50,766) that were significantly greater than serum anti-PspA IgG titers in mice immunized with antigen alone (<1:32, p<0.05). Although the serum anti-PspA IgG titers induced by nasal immunization with PspA + IL-1β were 2.7 fold lower than those induced in mice immunized subcutaneously (1:135,942), the difference was not significant. Only mice nasally immunized with PspA + IL-1β developed significantly increased mucosal IgA responses (1:37; p<0.05 vs intranasal PspA alone or subcutaneous PspA + alum). On day 72, all mice were challenged by the intravenous route with 1.125 × 105 CFU Streptococcus pneumoniae strain A66.1 and monitored for signs of morbidity for 14 days. Of the mice nasally immunized with PspA + IL- 1β, 68% were protected against morbidity (p<0.0001 vs mice immunized with PspA alone). Protection against death induced by nasal immunization with PspA + IL-1β was identical to that observed in mice immunized subcutaneously with PspA + alum (68%). Both the nasal vaccine using IL-1β as an adjuvant and the subcutaneously-administered positive control vaccine provided a significantly increased median time to morbidity (greater than 14 days) as compared to mice immunized nasally with PspA alone (median time to morbidity of 3 days; p<0.0001 vs mice immunized with PspA + adjuvant). Thus, needle-free nasal immunization of mice utilizing IL-1β as a vaccine adjuvant is as effective as subcutaneous immunization for the induction of antigen-specific serum IgG antibody titers and protection against a lethal intravenous challenge with Streptococcus pneumoniae while also inducing mucosal IgA responses.
IL-1β is an effective nasal vaccine adjuvant in mice for the induction of protective immunity against a systemic tetanus toxin challenge
To confirm the efficacy of nasal immunization using IL-1β as the adjuvant with a different protein antigen, female BALB/c mice (9–17 per group) were immunized with 50 μg tetanus toxoid (TT) by the subcutaneous or nasal routes on days 0, 21 and 42. ISA-51[54], a water-in-oil emulsion adjuvant that has been used in human clinical studies[72, 73], was used as the adjuvant for the subcutaneous immunization control while mice nasally immunized with TT alone served as the antigen alone control. The remaining mice were divided into two large subgroups; one subgroup was nasally immunized on days 0, 21 and 42 with TT combined with adjuvant (CTx or IL-1β at 0.2, 1 or 5 μg) while another subgroup was immunized on day 0 with TT combined with adjuvant while mice were boosted on days 21 and 42 with antigen alone (see the legend for Table 2 for more details). This experiment was designed to determine if the use of adjuvant with only the priming immunization was sufficient for the induction of protective immunity.
As shown in Table 2, nasal immunization of mice with 50 μg TT alone was minimally immunogenic and induced a day 56 anti-TT IgG titer of 1:1,176. In contrast, nasal immunization with TT + IL-1β (0.2, 1 or 5 μg) on days 0, 21 and 42 induced, in a dose dependent fashion, significantly higher levels of serum anti-TT IgG on day 56 when compared to mice nasally immunized with antigen alone (p<0.05). Day 56 serum geometric mean antibody titers for the 0.2, 1 and 5 μg IL-1β groups were 1:138,250, 1:297,353 and 1:356,722, respectively. CTx used as a control mucosal adjuvant induced a day 56 serum anti-TT IgG titer of 1:671,553. IL-1β at 1 and 5 μg doses induced serum IgG titers that were not significantly different than those induced by CTx. Of the nasally immunized groups that used adjuvant with each immunization, only the TT + 0.2 μg IL-1β group had a serum anti-TT IgG titer significantly lower than that induced by subcutaneous immunization with TT + ISA-51 (Table 2, p<0.05).
The use of adjuvants only with the day 0 immunization also induced serum anti-TT IgG titers that were significantly increased above titers in mice immunized with antigen alone (Table 2, p<0.05). Day 56 serum geometric mean antibody titers for the day 0 IL-1β groups 0.2, 1 and 5 μg were 1:65,536, 1:98,527 and 1:94,848, respectively. CTx used as an adjuvant at day 0 only induced a day 56 serum anti-TT IgG titer of 1:446,787. Despite significant adjuvant activity for all IL-1β groups, all IL-1β groups had serum anti-TT IgG titers significantly lower than those induced when CTx was used as an adjuvant with only the day 0 vaccination (p<0.05). All groups that utilized IL-1β as an adjuvant at day 0 only had serum anti-TT IgG titers significantly lower than that induced by subcutaneous immunization with TT + ISA-51 (Table 2, p<0.05).
Although mucosal immunity is not required for protection against tetanus toxin, vaginal anti-TT IgA titers were measured as an indicator of induction of mucosal immune responses in the female reproductive tract since mucosal IgA at this site demonstrates the ability of nasal immunization to induce IgA responses at distant mucosal tissues and IgA at this site may be important in protection against sexually-transmitted diseases(Table 2). Only mice immunized by the nasal route with antigen combined with adjuvant (CTx or IL-1β) had vaginal IgA responses that were significantly increased compared to mice nasally immunized with TT alone (i.e., no adjuvant; p<0.05) or mice subcutaneously immunized with TT + ISA-51 (p<0.05). Mice immunized with 50 μg TT + 5 μg IL-1β on days 0, 21 and 42 had the absolute highest vaginal anti-TT IgA response with a titer of 1:1,448. However, there were no significant differences for the vaginal anti-TT IgA between any of the nasal immunization groups that utilized adjuvant (CTx or IL-1β).
Delayed type hypersensitivity (DTH) responses were measured using an ear swelling assay at day 56 as a method to measure vaccine-induced T cell-mediated immunity in vivo (Table 2). Mice immunized with TT + ISA-51 subcutaneously and mice immunized nasally with TT + adjuvants for all three immunizations (day 0, 21 and 42) or TT + CTx or IL-1β (5 μg) administered with the day 0 immunization only had TT-specific DTH responses that were significantly greater than TT-specific DTH responses measured in mice immunized nasally with TT alone (p<0.05). TT-specific DTH responses induced by IL-1β were significantly less than DTH responses induced by nasal immunization with TT + CTx using the same adjuvant schedule (p<0.05) or subcutaneous immunization using TT + ISA-51 (p<0.05).
Mice were challenged at day 60 by the intraperitoneal route with 10 LD50 of tetanus toxin and monitored for signs of morbidity to determine the protective capacity of the various vaccine groups. Mice nasally immunized with TT alone exhibited 10% survival while all adjuvant groups demonstrated 100% protection against morbidity (p=0.048, Fisher’s Exact test). Our results demonstrate that IL-1β was an effective adjuvant for needle-free nasal immunization that induced complete protection against a parenteral toxin challenge. Despite significant differences in the serum anti-TT IgG titers between some of the nasal groups as compared to the IM control group (Table 2), adjuvanted nasal immunization was as effective as parenteral immunization for the induction of protective immunity. Additionally, use of adjuvant with the priming immunization only (in a three dose vaccination regimen) was sufficient for the induction of significantly elevated serum IgG, mucosal IgA, DTH and protective immunity.
Evaluation of the safety and efficacy of IL-1β used as a nasal vaccine adjuvant in rabbits when coadministered with tetanus toxoid
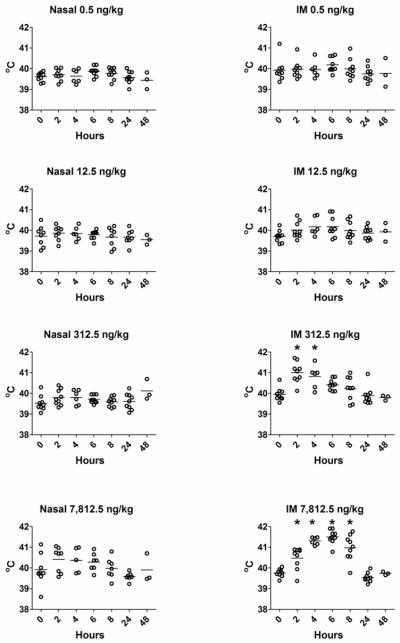
Studies are needed to assess the efficacy of IL-1 as a nasal vaccine adjuvant in larger animals, such as rabbits, that contain a nasal cavity anatomy more closely related to humans[74, 75]. Rabbits are also used to evaluate the pyrogenic nature of pharmaceuticals for use in humans[76–78]. Since IL-1 has both vaccine adjuvant and pyrogenic activities[79–81] when delivered parenterally, this experiment was performed to evaluate both the adjuvant and pyrogenic activity of IL-1 when used as an adjuvant for nasally-administered vaccines and to compare the efficacy of nasal immunization to parenteral immunization. In this experiment, awake rabbits were nasally or intramuscularly immunized with 100 μg of tetanus toxoid (TT) combined with increasing doses of rhuIL-1β (0.5, 12.5, 312.5 or 7812.5 ng/kg; approximately 1.25 ng, 31.25 ng, 0.781 μg or 19.5 μg per 2.5 kg rabbit, respectively) on days 0, 28 and 56. Since IL-1 could serve as a positive control adjuvant and pyrogen [79–81], rhIL- 1β was used as the adjuvant for the intramuscularly immunized rabbits. Changes in core body temperature were monitored immediately before and at 2, 4, 6, 8, 24 and 48 hours after each vaccination. As shown in Figure 1, rabbit core body temperature significantly increased at 2 and 4 hours after intramuscular immunization with TT + 312.5 ng/kg of IL-1β (p<0.05) and at 2, 4, 6 and 8 hours after IM immunization with 7812.5 ng/kg of IL-1β (p<0.05). In contrast, there were no significant changes in core body temperature at any time point after nasal immunization with TT + IL-1β (0.5, 12.5, 312.5 or 7812.5 ng/kg). Thus, although intramuscular administration of IL-1β at 312.5 and 7,812.5 ng/kg induced fever, nasally-delivered IL-1β in rabbits did not.
Figure 1. Intramuscular but not nasal delivery of IL-1β as a vaccine adjuvant induces increased core body temperature in rabbits.
NZW female rabbits were immunized with 100 μg tetanus toxoid combined with rhIL-1β as adjuvant by the nasal or intramuscular route on day 0, 28 and 56. Rabbits were not sedated or anesthetized for performance of the immunizations. Body temperature was monitored immediately before (time 0) and at 2, 4, 6, 8 and 24 hours after immunization and temperatures were compared within each vaccine group using ANOVA followed by Dunnett’s test to compare the post-vaccination temperatures to the time 0 temperature. There were three rabbits per adjuvant group and temperature readings from each day (day 0, 28 and 56) were combined. Rabbits immunized by the intramuscular route with TT + IL-1β at 312.5 ng/kg had significantly increased core body temperatures at 2 and 4 hours after immunization (ANOVA, Dunnett’s test, p<0.05). Rabbits immunized with TT + IL-1β at 7,812.5 ng/kg had significantly increased core body temperatures at 2, 4, 6 and 8 hours after immunization (ANOVA, Dunnett’s, p<0.05). All other comparisons within groups between time 0 and post-vaccination body temperatures did not identify any significant change in core body temperature (ANOVA, Dunnett’s test).
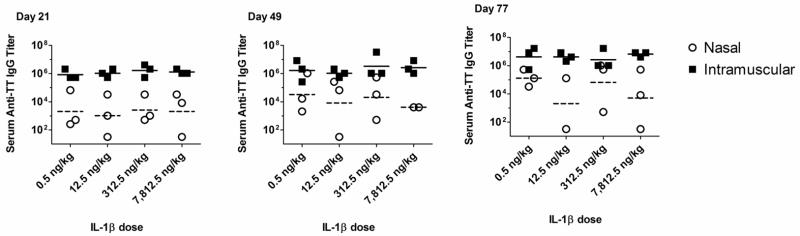
Serum anti-TT IgG was measured by ELISA on days 21, 49 and 77 to compare the efficacy of intranasal immunization to intramuscular immunization. Day 21 serum anti-TT IgG titers in rabbits nasally immunized with TT + IL-1β were highly variable and did not demonstrate a dose response to IL-1β. Serum anti-TT IgG responses at day 21 in rabbits nasally immunized with TT + IL-1β ranged from undetectable (one rabbit in the 12.5 ng/kg and one rabbit in the 7,812.5 ng/kg) to a peak titer of 1:65,536 in a rabbit nasally immunized with TT + 0.5 ng/kg IL-1β (Figure 2). Serum anti-TT IgG titers at day 21 in rabbits intramuscularly immunized with TT + IL-1β also did not demonstrate a dose response to IL- 1β although serum anti-TT titers were consistently elevated, ranging from 1:524,288–1:2,097,152 (Figure 2). By day 49, three weeks after the day 28 boost, serum anti-TT IgG titers remained highly variable in animals immunized by the nasal route while serum anti-TT IgG titers in rabbits intramuscularly immunized remained consistently elevated with titers ranging from 1:262,144 to 1:33,554,432 (Figure 2). In some nasally immunized rabbits, serum anti-TT IgG titers were comparable to anti-TT IgG titers induced by intramuscular immunization. For example, one rabbit nasally immunized with TT + 0.5 ng/kg IL-1β had a day 49 serum anti-TT IgG titer of 1:1,048,576 while titers of 1:262,144 and 1:524,288 were detected in rabbits nasally immunized using TT + IL-1β at 12.5 or 312.5 ng/kg, respectively (Day 49, Figure 2). Even after three immunizations, serum anti-TT IgG titers at day 77 in rabbits nasally immunized with TT + IL-1β remained highly variable with titers ranging from undetectable to 1:1,048,576 while serum anti-TT IgG titers in rabbits intramuscularly immunized were consistently elevated with titers as high as 1:16,777,216 (Day 77, Figure 2). Our results suggest that intranasal immunization with TT + IL-1β has the potential to induce high-titered serum anti-TT IgG responses comparable to that induced by intramuscular immunization although the serum anti-TT IgG responses induced by intranasal immunization are unacceptable due to the extensive animal-to-animal variation.
Figure 2. Serum anti-TT IgG titers after nasal or intramuscular immunization with TT and IL-1β in rabbits.
Female NZW rabbits were immunized by the nasal or intramuscular route while awake with 100 μg tetanus toxoid combined with rhIL-1β on day 0, 28 and 56. Rabbits were not sedated or anesthetized for performance of the immunizations. There were 3 rabbits in all groups. Serum was collected on day 21, 49 and 77 and tested for the presence of anti-TT IgG by ELISA (day 49 intranasal 7,812.5 ng/kg IL-1β and day 77 intranasal 12.5 ng/kg IL-1β had only two rabbits/group due to the inability to collect blood from one rabbit). The horizontal bars in each scatter plot represent the geometric mean titer (the solid bar for intramuscularly immunized rabbits and the dashed bar for intranasally immunized rabbits). No statistical comparisons were performed between groups due to the lack of power resulting from the low numbers of animals per group. Undetectable serum antibody responses are outside the range of the Y axis and are therefore not represented in the figure.
To evaluate the overall performance of nasal immunization as compared to intramuscular immunization, we combined the day 77 serum anti-TT IgG titers from all rabbits immunized nasally with TT + IL-1β (all doses) and compared them to the serum anti-TT IgG titers of all rabbits immunized intramuscularly with TT + IL-1β (all doses). The serum anti-TT IgG geometric mean titer (GMT) in nasally immunized rabbits was 1:21,081 (range 1:32 to 1: 1,048,576.) while the serum anti-TT IgG GMT in intramuscularly immunized rabbits was 1:4,194,304 (range 1:524,288 to 1:16,777,216) (Figure 3), a 198-fold difference in GMT between IN and IM immunization (p=0.0001). If we compare the maximum day 77 serum IgG titer in a nasally immunized rabbit (1:1,048,576; TT + 312.5 ng/kg IL-1β) with the maximum day 77 serum IgG titer in an intramuscularly immunized rabbit (1:16,777,216; TT + 312.5 ng/kg IL-1β), the maximum serum anti-TT IgG titer after nasal immunization is 16-fold less than the maximum serum anti-TT IgG titer induced after intramuscular immunization. Regardless of the comparison to the anti-TT IgG titers induced by intramuscular immunization, the serum anti-IgG titers induced by nasal immunization were extremely variable. This lack of reproducible induction of serum IgG responses after nasal immunization in rabbits using IL-1β as the adjuvant is in contrast to the efficacy of nasal immunization observed in mouse models where the antigen-specific serum IgG titers induced by nasal immunization using IL-1β as an adjuvant were highly reproducible and within 3-fold of the antigen-specific serum IgG titers induced by subcutaneous immunization (Tables 1 and 2).
Nasal immunization with TT + IL-1β failed to induce detectable fecal anti-TT IgA responses although fecal anti-TT IgG responses were detectable in some groups (data not shown). Thus, nasally delivered IL-1β lacked the adverse effects (pyrogenicity) observed when IL-1β was delivered intramuscularly although nasal immunization of rabbits was not as effective as intramuscular immunization for the consistent induction of high-titered, antigen-specific serum IgG.
Evaluation of the efficacy of IL-1α used as a nasal vaccine adjuvant in rabbits when coadministered with anthrax protective antigen. The state of anesthesia influences the efficacy of nasal immunization in rabbits
The rabbit nasal immunization experiments described above in Figures 1 – 3 using TT + IL-1β were performed in rabbits that were awake. The use of anesthesia is known to enhance the immunogenicity of nasally delivered vaccines in mice [48, 49], including studies that have utilized anthrax protective antigen as the immunogen. However, the impact the use of anesthetics has on the efficacy of nasal immunization in rabbits is not known.
To determine if the use of anesthesia influenced the immunogenicity of nasal immunization in rabbits, rabbits were nasally immunized while awake or anesthetized with agents providing various depths of anesthesia. Acepromazine combined with butorphanol was utilized to sedate the rabbits. With this treatment, rabbits were sedated but remained conscious, upright and mobile within the cage. Acepromazine sedation followed by isoflurane anesthesia was used to provide a temporary deep anesthesia that was of short duration with the rabbit conscious, upright and mobile in its cage within 5 minutes after receiving the nasal vaccine. Ketamine + xylazine provided deep anesthesia with the rabbits remaining deeply anesthetized for at least 30 minutes after receiving the nasal vaccine.
Anthrax recombinant protective antigen (rPA) was used as the vaccine immunogen to allow a comparison between the results from this current study and previously published work that utilized rPA as an immunogen for nasal delivery to awake or ketamine-anesthetized rabbits[68, 82]. IL-1α was used as the adjuvant to evaluate the adjuvant activity of IL-1α when nasally delivered to rabbits [37, 55]. rPA (20 μg) was adjuvanted with rhuIL-1α (5 or 15 μg) and delivered nasally to female NZW rabbits (4–8 per group) on days 0, 21 and 42 while awake, sedated with acepromazine + butorphanol, sedated with acepromazine and anesthetized with isoflurane or deeply anesthetized with ketamine + xylazine. Intramuscular immunization with rPA (10 μg) + alum on days 0, 21 and 42 was used to represent a parenteral immunization regimen that may be used in humans to induce protective immunity against anthrax and therefore anesthetics were not used in this group[66]. Serum collected on days 42 and 56 was tested for the presence of anti-PA IgG titers by ELISA. As shown in Figure 4, day 42 serum anti-PA IgG titers from rabbits nasally immunized while under deep anesthesia with ketamine + xylazine were significantly higher (p<0.05) compared to rabbits nasally immunized while awake, acepromazine + butorphanol-sedated or acepromazine + isoflurane anesthetized and comparable to serum anti-PA IgG titers in rabbits immunized intramuscularly with rPA (10 μg) + alum on days 0, 21 and 42. On day 56, serum anti-PA IgG geometric mean titers in the ketamine + xylazine group (1:4.98 × 106) were significantly greater (p<0.05) than anti-PA IgG titers in rabbits that were awake (1:32,768) or sedated with acepromazine + butorphanol (geometric mean 1:32,768) but comparable to anti-PA IgG titers in rabbits nasally-immunized while treated with aceopromazine/isoflurane (1:623,487) or immunized intramuscularly (geometric mean 1:1.995 × 107; Figure 4). Our results demonstrate that deep anesthesia induced by ketamine + xylazine enhanced the induction of more consistent and elevated serum IgG responses with less variation in serum PA-specific IgG titers on days 42 and 56 as compared to other nasally vaccinated groups.
Fecal anti-PA IgA and IgG were also measured at day 56 with geometric mean anti-PA IgG and IgA titers less than 1:6 in all of the groups of rabbits nasally immunized with rPA + IL- 1α (data not shown).
Serum lethal toxin neutralizing antibodies were also measured to determine if the use of anesthetics influenced the induction of protective serum antibodies after nasal immunization. Of the nasally immunized rabbits, serum LeTx neutralizing activity (NT50 titer) was highest in animals that were anesthetized with ketamine + xylazine (LeTx NT50 of 1:539.6, Table 3) and significantly higher compared to rabbits treated with acepromazine + butorphanol (LeTx NT50 of 1:26.4, Table 3, p<0.05). Although the use of anesthetics with nasal immunization enhanced the induction of serum LeTx neutralizing antibody responses, IM immunization with rPA + alum induced serum LeTx NT50 titers (1:29,303) significantly greater than NT50 titers induced by any nasal immunization group (p<0.05).
Anesthesia influences nasal clearance rates in rabbits
We hypothesized that nasal vaccination was more effective in deeply anesthetized rabbits due to enhanced vaccine delivery or altered nasal clearance. To test these hypotheses, we utilized gamma scintigraphy to monitor nasal delivery and clearance[83, 84] in sedated (acepromazine + butorphanol) or anesthetized (ketamine + xylazine) rabbits. Rabbits were administered Tc99m-sulfur colloid intranasally (in a volume of 100 μl to a single nostril) while sedated with acepromazine + butorphanol or while deeply anesthetized with ketamine + xylazine. Rabbits were imaged immediately after nasal delivery to evaluate the efficacy of nasal delivery to sedated or deeply anesthetized rabbits. A typical scintigraphy image immediately after nasal delivery is presented in Figure 5A. Total activity delivered per rabbit (3–4 per group) was calculated as a percentage of the calculated stoichiometric delivery and the state of anesthesia did not influence total activity delivered (Figure 5B; p=0.8571, Mann Whitney). We used computer programming to quantify regional activity delivered only within the target site of the nasal cavity, immediately after delivery. One rabbit in the ketamine + xylazine group aspirated the radiolabeled sulfur colloid to the lungs and stomach at the time of delivery resulting in one rabbit with a greatly reduced amount of sulfur colloid in the nasal cavity (22.5% of expected; Figure 5C). However, the state of anesthesia did not significantly influence nasal cavity deposition (at time zero) of the sulfur colloid (p=0.6286, Mann Whitney, Figure 5C). Figure 5D contains the scintigraphy image of the rabbit that aspirated sulfur colloid at the time of nasal delivery with sulfur colloid immediately appearing in the lungs and stomach (verified by removal of organs after euthanasia). Rabbits were monitored over time (30, 60, 90, 120 and 180 minutes) to determine if the state of anesthesia influenced clearance of the sulfur colloid through the nasal cavity. As shown in Figure 5E, nasal clearance of Tc99m-sulfur colloid was decreased in rabbits anesthetized with ketamine + xylazine as compared to rabbits sedated with acepromazine + butorphanol. At 30 minutes after nasal delivery, 88.9 ± 15.7% of sulfur colloid measured in the nasal cavity at time 0 (calculated from Figure 5C) remained in the nasal cavity of ketamine + xylazine anesthetized rabbits while only 44.38 ± 20.9% of delivered sulfur colloid remained in the nasal cavity of acepromazine + butorphanol sedated rabbits (p=0.0597). Sulfur colloid could be detected in the bedding of acepromazine ± butorphanol sedated rabbits at 30 minutes post nasal delivery indicating a large fraction of the labeled sulfur colloid that had been initially delivered to the nasal region, was lost from the nasal cavity by non-physiologic means, within 30 minutes of delivery (data not shown). Sulfur colloid could not be detected in the bedding of ketamine + xylazine anesthetized rabbits. The amount of radiolabeled sulfur colloid remaining in the nasal cavity at 60 minutes after nasal delivery was significantly greater in ketamine + xylazine anesthetized rabbits (76.17 ± 22.75% of expected) than in the acepromazine + butorphanol rabbits (33.75 ± 12.76% of expected; p=0.0245)). There was no difference between the amount of radiolabeled sulfur colloid in the nasal cavity of ketamine + xylazine and the acepromazine + butorphanol rabbits at 90, 120 or 180 minutes after nasal delivery. Using the data from Figure 5E, the calculated 50% clearance time in acepromazine + butorphanol sedated rabbits was 24 minutes while the 50% clearance time in ketamine + xylazine anesthetized rabbits was 100 minutes. The total area under the curve (AUC) was calculated for the nasal clearance curves in Figure 5E, and the ketamine + xylazine anesthetized rabbits produced a total AUC of 10,881 ± 2,927 (percent minutes) while the AUC for the acepromazine + butorphanol rabbits was 6,744 ± 1,704, (p=0.063; Figure 5F). Figure 5G shows a typical scintigraphy image of Tc99m-sulfur colloid 180 minutes after nasal delivery with activity divided between the nasal cavity and stomach. Mucociliary clearance moved a portion of the sulfur colloid through the nasal cavity and it was then swallowed with no activity detected in the lungs. Of a total of 7 rabbits tested using gamma scintigraphy, 6 of 7 rabbits had results similar to Figure 5G; only one rabbit had activity detectable in the lung after nasal delivery. Our results suggest that the depth of anesthesia influences the immunogenicity of nasally-delivered vaccines secondary to retention within the nasal cavity with a deeply anesthetized state providing increased nasal retention.
DISCUSSION
In this study we have demonstrated that recombinant interleukin 1α and β are effective vaccine adjuvants when nasally delivered to mice or rabbits. IL-1 was an effective nasal vaccine adjuvant in mice for the induction of antigen-specific serum IgG and mucosal IgA and protection against systemic challenge with tetanus toxin or Streptococcus pneumoniae. Although IL-1 was pyrogenic when delivered intramuscularly to rabbits, IL-1 did not exhibit pyrogenic activity when used as a nasal vaccine adjuvant. The efficacy of nasal immunization in rabbits was significantly influenced by the use of anesthesia at the time of immunization with deeper anesthesia correlating with increased immunogenicity of the nasally delivered vaccine. Deep anesthesia (ketamine + xylazine) resulted in improved nasal retention of Tc99m-sulfur colloid and suggested that nasal immunization techniques that improve nasal retention of the vaccine should increase the immunogenicity of the vaccine.
Our results in mice demonstrated that effective nasal immunization induced antigen-specific serum IgG responses comparable to those induced by parenteral immunization. Additionally, protection against systemic toxin or bacterial challenges induced by nasal immunization was comparable to protection induced by parenteral immunization. The protection observed against lethal challenge with Streptococcus pneumoniae A66.1 provided by nasal immunization of mice with PspA + IL-1 was comparable to protection observed by other groups that have utilized nasal immunization of mice with PspA (100 ng) + native or mutant CT (5 μg)[63]. Our murine studies support the conclusions that 1) IL-1 is an effective nasal vaccine adjuvant and 2) nasal immunization provides a needle-free method of immunization able to induce protective systemic immunity comparable to protection provided by a vaccine delivered with a needle.
Nasal immunization of mice with antigen combined with IL-1 supported the induction of antigen-specific IgA in mucosal secretions. The use of IL-1 as an adjuvant was required for the induction of IgA responses since immunization with antigen alone failed to induce detectable antigen-specific IgA. Although mucosal IgA responses were not required for protection in our murine challenge studies, the use of a mucosal route of immunization able to induce mucosal IgA responses may be beneficial for protection against pathogens that infect via the mucosal surfaces of the host [85]. Despite the expected mucosal IgA results in nasally-immunized mice, nasal immunization of rabbits did not induce detectable antigen-specific IgA responses. Our results agree with results from others that have reported nasal immunization of rabbits with rPA combined with adjuvant induced minimal or undetectable antigen-specific IgA responses in nasal secretions [86]. Nasal immunization of rabbits with influenza antigen also failed to induce detectable antigen-specific mucosal IgA [87]. Although rabbits provide a convenient non-rodent species for the performance of vaccine studies, the lack of induction of mucosal IgA responses after nasal immunization of rabbits is puzzling. It is not clear if the lack of detectable mucosal IgA responses in rabbits after nasal immunization is due to vaccine formulation issues such as antigen dose (since induction of mucosal IgA may be antigen dose-dependent [88]) or if rabbits, in contrast to mice, do not develop significant mucosal IgA responses after nasal immunization. Additional studies in rabbits are needed to identify the vaccine/vaccination parameters that influence the induction of antigen-specific mucosal IgA responses after nasal immunization.
Safety must always be evaluated when a new vaccine formulation is developed. IL-1 is known to exhibit pyrogenic activity[81] and human clinical studies that evaluated IL-1 for cancer treatment reported a variety of adverse events for parenterally delivered IL-1 including fever, chills and hypotension[89]. We selected rabbits as an animal model in our studies since rabbits have a nasal cavity anatomy that is more similar to the human nasal cavity (as compared to mice[74]) and rabbits are often used to test the pyrogenicity of substances before their use in humans[76–78]. IL-1 has been used in human clinical studies at up to 1.0 μg/kg [89]. We tested IL-1β in rabbits at doses of 0.5, 12.5, 312.5 and 7,812.5 ng/kg (0.0005 μg/kg - 7.8 μg/kg) to provide an extensive range of IL-1 doses to allow us to effectively evaluate the pyrogenicity of IL-1β when delivered nasally and to use an IL-1 dose greater than that used in human studies when delivered parenterally to determine if a higher IL-1β dose could be used nasally as a vaccine adjuvant without exhibiting pyrogenic effects. The doses of IL-1β we evaluated in rabbits were comparable to those used by others to evaluate the pyrogenic activity of IL-1β in rabbits[90]. Others have reported that 100 μg of recombinant ovine IL-1β provided both adjuvant and pyrogenic activity when delivered parenterally to sheep[79]. Confirming the pyrogenic activity reported for IL-1 in human, rabbit and sheep studies, we observed significant pyrogenic activity when IL-1β was delivered intramuscularly to rabbits at 312.5 or 7,812.5 ng/kg. However, IL-1 delivered nasally induced no significant change in core body temperature suggesting that topical mucosal delivery of IL-1 as a mucosal vaccine adjuvant attenuates systemic adverse effects observed when the same dose of IL-1 is delivered parenterally. Despite the variability of anti-TT serum IgG responses induced in rabbits by nasal immunization with TT + IL-1β, rabbits nasally immunized with TT + 0.5 ng/kg IL-1β had day 77 serum anti-TT IgG titers of 1:32,768, 1:131,072 and 1:524,288 and 2 rabbits nasally immunized with TT + 312.5 ng/kg IL-1β had day 77 anti-TT IgG titers of 1:524,288 and 1:1,048,576 with no measurable fever suggesting that vaccine formulations containing IL-1β may safely induce vaccine-specific antibody responses after nasal delivery while not inducing systemic adverse effects such as fever. Since IL-1 is able to cause fever when injected directly into the brain of rabbits at doses as low as 2.0 ng/kg IL-1β[91], our results suggest that despite the close proximity of the nasal cavity to neuronal tissues of the central nervous system, nasally delivered IL-1 did not directly access the central nervous system. In a separate study, we also observed that nasal delivery of human GM-CSF and IL-1 as a vaccine adjuvant in non-human primates did not induce the production of primate anti-human GM-CSF neutralizing antibodies while intramuscular immunization with human GM-CSF formulated in a stable oil in water emulsion in the presence of a TLR4 ligand as a vaccine adjuvant induced significant anti-human GM-CSF neutralizing antibodies[47]. The use of the stable emulsion and TLR4 ligand likely contributed to the induction of anti-GM-CSF antibodies by providing a depot effect in the context of TLR4-dependent signaling. In contrast, nasal immunization with antigen combined with IL-1 and GM-CSF in saline was likely cleared from the nasal cavity within a few hours (expected results similar to Figure 5E) reducing the likelihood of induction of anti-cytokine antibodies that would reduce their adjuvant activity. This observation suggests that nasal immunization with IL-1 may not induce neutralizing anti-human IL-1 antibody responses. Collectively, our results suggest that topical nasal delivery has the potential to minimize or eliminate adverse effects (fever, anti-adjuvant immune responses) that may be observed when the adjuvants are delivered parenterally.
Our results are the first direct demonstration that the state of anesthesia influences the immunogenicity of nasally-delivered vaccines in rabbits. Despite the variability in serum antibody responses observed after nasal immunization of awake rabbits (Figure 2), nasal immunization of rabbits anesthetized with ketamine + xylazine with rPA + IL-1α induced serum anti-PA IgG titers that were significantly greater than the responses induced in rabbits that were nasally immunized while awake or sedated. More importantly, nasal immunization of rabbits anesthetized with ketamine + xylazine induced reproducible serum anti-PA IgG and elevated lethal toxin neutralizing titers. Our rabbit results agree with results reported for mice since the use of deep anesthesia enhanced the immunogenicity of nasally delivered vaccines in mice[48, 49]. A major issue that we currently face is how to translate our observations using nasal immunization of anesthetized rabbits into the development and optimization of effective nasal vaccines and nasal immunization strategies for use in humans. Since our goal is to develop a needle-free method of immunization, the use of injectable anesthetic agents is not practical for use in humans for the purpose of nasal immunization. However, as discussed below, the use of optimized nasal immunization methods should provide a nasal immunization method that effectively and reproducibly induces the desired immune responses.
The induction of serum anti-PA IgG and LeTx neutralizing titers and the influence of the anesthetic state after nasal immunization of rabbits observed in our study agree with results reported by others that have evaluated nasal immunization of rabbits with rPA[68, 82]. In our study, nasal immunization of ketamine + xylazine anesthetized rabbits with 20 μg rPA + IL-1α induced a day 56 serum anti-PA IgG geometric mean titer of 1:4.98 × 106 and serum LeTx neutralization titer 50 (NT50) of 1:539. Others that performed nasal immunization of rabbits anesthetized with ketamine/xylazine (personal communication, Vincent Sullivan[68]) with a liquid formulation containing 50 μg of rPA + 50 μg CpG on days 0, 21 and 42 induced a day 56 serum anti-PA IgG titer of ~1:200,000 and consistently elevated serum lethal toxin neutralizing antibody responses [68]. However, our study also determined that nasal immunization of awake rabbits with 20 μg rPA + IL-1α induced low day 56 serum anti-PA IgG and lethal toxin neutralizing antibody. This observation is confirmed in a study by Wimer-Mackin[82] that reported very low (i.e., not significantly different from negative control rabbits) serum anti-PA IgG ELISA responses and serum lethal toxin neutralizing titers after nasal immunization of awake rabbits with a liquid vaccine formulation containing 90 μg of rPA + 25 μg of MPL as adjuvant on days 0 and 28. However, when they formulated the vaccine as a dry powder containing the bioadhesive chitosan its immunogenicity for the induction of serum anti-PA ELISA and toxin neutralizing antibody responses was significantly increased[82]. It is tempting to speculate that the use of the dry powder vaccine formulation containing the bioadhesive chitosan increased the nasal retention of the vaccine (see below) to provide increased immunogenicity of nasally-delivered vaccines in animals that were not anesthetized. Additional studies are needed to optimize vaccine formulations so that nasal immunization in the absence of anesthesia consistently induces protective serum antibody responses.
Gamma scintigraphy determined that the superior immunogenicity of the nasal vaccine in ketamine + xylazine anesthetized rabbits correlated with enhanced vaccine retention within the nasal cavity of rabbits. The 50% clearance time in sedated rabbits was 24 minutes while the 50% clearance time in ketamine + xylazine anesthetized rabbits was 100 minutes. Others have utilized gamma scintigraphy in rabbits to evaluate the clearance of nasal vaccine formulations [92] and determined that unmodified PLGA microspheres had a 50% clearance time of 30 minutes while a chitosan modified PLGA microsphere formulation had a 50% clearance time of 180 minutes. Immunogenicity studies in mice using the two PLGA microsphere formulations determined that the chitosan modified PLGA microsphere with a prolonged nasal clearance time was more immunogenic than the unmodified PLGA microsphere and induced serum antibody responses comparable to those induced by parenteral immunization[92]. Gamma scintigraphy studies in sheep determined that the 50% clearance time for a control formulation was about 15 minutes while two chitosan formulations had 50% clearance times of 43 and 115 minutes[93]. As mentioned above, a dry powder vaccine formulation containing chitosan was significantly more immunogenic when delivered to awake rabbits than a liquid vaccine lacking chitosan [82]. Although nasal clearance studies were not performed in the study by Wimer-Mackin[82], the improved immunogenicity of the vaccine formulation containing chitosan is consistent with increased immunogenicity of vaccines due to increases in nasal clearance time. These observations support our conclusion that increasing the nasal residence time of nasally-administered vaccines may be required for optimal vaccine immunogenicity and that the use of bioadhesives such as chitosan may represent a strategy to develop nasal vaccine formulations that provide increased nasal residence time without the use of anesthetics.
The direct comparison of vaccine-induced immune responses between subjects nasally immunized and those vaccinated with a needle (intramuscular, subcutaneous) suggests that while nasal immunization may induce protective immunity comparable to that induced by needle immunization, the quality and quantity of immune responses induced will vary between those vaccinated intranasally or with a needle. In this study, nasal immunization of mice with protein antigen + adjuvant (CT or IL-1) induced protective immunity and serum IgG titers not significantly different than those induced by subcutaneous immunization. However, serum IgG titers induced by nasal immunization were always lower than those induced by parenteral immunization. The advantage of adjuvanted nasal immunization in the mouse was the induction of mucosal IgA responses that were not induced by needle immunization. In rabbits, even when using ketamine/xylazine anesthesia and a 2-fold greater antigen dose, nasal immunization was not as effective as intramuscular immunization for the induction of vaccine-induced toxin-neutralizing antibody responses, despite the induction of serum anti-PA IgG ELISA titers that were statistically similar between the two routes of immunization. Our results in mice are in agreement with comparisons made between the injected inactivated influenza vaccine and the intranasal live attenuated influenza vaccine where the nasal vaccine induced serum antibody responses lower than those induced by the injected vaccine, despite similar efficacies[94, 95]. The use of different vaccine formulations (i.e., live attenuated influenza vs inactivated influenza virus) complicated the interpretation of these results. However, when immune responses induced by nasal immunization of humans with the inactivated influenza virus vaccine were compared to those induced by the same vaccine delivered intramuscularly, nasal immunization was less effective than intramuscular immunization for the induction of serum hemagglutination inhibiting (HAI) antibodies[67]. Nasal immunization with a 4-fold greater dose of inactivated influenza vaccine than that used for IM immunization was required to allow nasal immunization to approach the efficacy of IM immunization based on the induction of serum HAI and influenza virus neutralizing antibodies[67]. It is possible that if our rabbit rPA nasal immunization studies had used 4-fold higher antigen doses than we used in the IM group, the anti-PA IgG ELISA titers and serum LeTx NT50 titers induced by nasal immunization may have been more like those induced by IM immunization. It is important to mention that the human influenza vaccine study was performed without the use of adjuvants for either the nasal or intramuscular routes of immunization[67]. While adjuvants and vaccine formulations (i.e., bioadhesives) are expected to increase the immunogenicity and efficacy of nasally-delivered vaccines, more work is needed to maximize the immunogenicity of nasally-delivered vaccines to allow minimal antigen doses to induce the desired immune response.
In conclusion, our results demonstrated that recombinant interleukin 1α and β provided effective adjuvant activity for nasally-administered vaccines and supported the induction of protective serum antibody responses. Studies in rabbits using varied anesthetic states demonstrated that the anesthetic state influenced the immunogenicity of nasally-delivered vaccines and suggested that the use of ketamine/xylazine anesthesia increased the retention of the vaccine in the nasal cavity to provide maximal immunogenicity for the nasally-delivered vaccine. Additional studies are required to develop nasal vaccine formulations that provide the required immunogenicity without the need for anesthesia. Strategies that may provide increased nasal retention include the use of bioadhesives and/or dry powder vaccine formulations. Due to the difficulties and potential dangers associated with handling larger animals such as non-human primates that are not sedated or anesthetized, studies to evaluate the safety and efficacy of nasally-administered vaccines when delivered to awake hosts may be performed in rabbits since the rabbit has nasal cavity lymphoid tissue similar to humans[75] and can be safely handled without the use of anesthetics. Despite the usefulness of the rabbit model for nasal immunization, human studies must be performed to optimize nasal immunization strategies for use in humans.
Acknowledgments
We would like to thank Alice Tripp, Afton Thompson and Nicole Paraggio for assistance with rabbit care and immunizations. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Anthrax Protective Antigen (PA), Recombinant from Bacillus anthracis, NR-140 and Anthrax Lethal Factor (LF), Recombinant from Bacillus anthracis, NR-570. This work was supported by NIH grants R01AI064879 and ES011961.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Giudice EL, Campbell JD. Needle-free vaccine delivery. Advanced Drug Delivery Reviews. 2006 Apr 20;58(1):68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Clements CJ, Larsen G, Jodar L. Technologies that make administration of vaccines safer. Vaccine. 2004 May 7;22(15–16):2054–8. doi: 10.1016/j.vaccine.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Clements CJ, Aguado MT, Jodar L. Technologies to improve immunisation safety. Drug Saf. 2001;24(14):1019–26. doi: 10.2165/00002018-200124140-00001. [DOI] [PubMed] [Google Scholar]
- 4.Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9(1):99. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- 5.Brody S. Declining HIV rates in Uganda: due to cleaner needles, not abstinence or condoms. Int J STD AIDS. 2004 Jul;15(7):440–1. doi: 10.1258/0956462041211324. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77(10):789–800. [PMC free article] [PubMed] [Google Scholar]
- 7.Baillie LW. Past, imminent and future human medical countermeasures for anthrax. J Appl Microbiol. 2006 Sep;101(3):594–606. doi: 10.1111/j.1365-2672.2006.03112.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Hagan DT, Rappuoli R. Novel approaches to vaccine delivery. Pharm Res. 2004 Sep;21(9):1519–30. doi: 10.1023/B:PHAM.0000041443.17935.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kersten G, Hirschberg H. Needle-free vaccine delivery. Expert Opin Drug Deliv. 2007 Sep;4(5):459–74. doi: 10.1517/17425247.4.5.459. [DOI] [PubMed] [Google Scholar]
- 10.Boivin JM, Poupon-Lemarquis L, Iraqi W, Fay R, Schmitt C, Rossignol P. A multifactorial strategy of pain management is associated with less pain in scheduled vaccination of children. A study realized by family practitioners in 239 children aged 4–12 years old. Fam Pract. 2008 Dec;25(6):423–9. doi: 10.1093/fampra/cmn069. [DOI] [PubMed] [Google Scholar]
- 11.Williams JP, Lednar W. New developments in influenza vaccine technology: a potential new prevention strategy for employers and managed care organizations. Am J Manag Care. 2002 Apr;8(5 Suppl):S143–54. [PubMed] [Google Scholar]
- 12.Aggerbeck H, Gizurarson S, Wantzin J, Heron I. Intranasal Booster Vaccination Against Diphtheria and Tetanus in Man. Vaccine. 1997;15(3):307–16. doi: 10.1016/s0264-410x(96)00175-2. [DOI] [PubMed] [Google Scholar]
- 13.McSherry J. Perspectives on needle phobia. Journal of Family Practice. 1995;41(5):437–512. [PubMed] [Google Scholar]
- 14.Hamilton JG. Needle phobia: a neglected diagnosis. Journal of Family Practice. 1995;41(2):169–75. [PubMed] [Google Scholar]
- 15.FDA News. First Nasal Mist Flu Vaccine Approved. 2003 [cited June 17, 2003]; Available from: http://www.fda.gov/bbs/topics/NEWS/2003/NEW00913.html.
- 16.McCarthy MW, Kockler DR. Trivalent intranasal influenza vaccine, live. Ann Pharmacother. 2004 Dec;38(12):2086–93. doi: 10.1345/aph.1E191. [DOI] [PubMed] [Google Scholar]
- 17.Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, et al. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004 Oct 1;39(7):920–7. doi: 10.1086/423001. [DOI] [PubMed] [Google Scholar]
- 18.Staats HF, Jackson RJ, Marinaro M, Takahashi I, Kiyono H, McGhee JR. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol. 1994 Aug;6(4):572–83. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 19.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 20.Prakken BJ, van der Zee R, Anderton SM, van Kooten PJS, Kuis W, Eden WV. Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis. Proc Natl AcadSci (USA) 1997;94:3284–9. doi: 10.1073/pnas.94.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staines NA, Harper N, Ward FJ, Malmstrom V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal administration of synthetic peptide 184–198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368–75. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao BG, Link H. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85(2):119–28. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 23.Bai XF, Shi FD, Xiao BG, Li HL, van der Meide PH, Link H. Nasal administration of myelin basic protein prevents relapsing experimental autoimmune encephalomyelitis in DA rats by activating regulatory cells expressing IL-4 and TGF-beta mRNA. Journal of Neuroimmunology. 1997;80(1–2):65–75. doi: 10.1016/s0165-5728(97)00133-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Deng GM, Levi M, Wahren B, Diab A, Vandermeide PH, et al. Prevention of Experimental Autoimmune Neuritis By Nasal Administration of P2 Protein Peptide 57–81. J Neuro Exp Neuro. 1998;57(3):291–301. doi: 10.1097/00005072-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hasselberg A, Ekman L, Yrlid LF, Schon K, Lycke NY. ADP-ribosylation controls the outcome of tolerance or enhanced priming following mucosal immunization. J Immunol. 2010 Mar 15;184(6):2776–84. doi: 10.4049/jimmunol.0901445. [DOI] [PubMed] [Google Scholar]
- 26.Milling SW, Yrlid U, Jenkins C, Richards CM, Williams NA, MacPherson G. Regulation of intestinal immunity: effects of the oral adjuvant Escherichia coli heat-labile enterotoxin on migrating dendritic cells. Eur J Immunol. 2007 Jan;37(1):87–99. doi: 10.1002/eji.200636199. [DOI] [PubMed] [Google Scholar]
- 27.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006 Apr 1;176(7):4275–83. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 28.Lycke N. From toxin to adjuvant: basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol Lett. 2005 Mar 15;97(2):193–8. doi: 10.1016/j.imlet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Lycke N. ADP-ribosylating bacterial enzymes for the targeted control of mucosal tolerance and immunity. Ann N Y Acad Sci. 2004 Dec;1029:193–208. doi: 10.1196/annals.1309.036. [DOI] [PubMed] [Google Scholar]
- 30.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153(2):647–57. [PubMed] [Google Scholar]
- 31.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting Edge: The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165:4778–82. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 32.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995 Nov 15;155(10):4621–9. [PubMed] [Google Scholar]
- 33.Simecka JW, Jackson RJ, Kiyono H, McGhee JR. Mucosally Induced Immunoglobulin E-Associated Inflammation in the Respiratory Tract. Infect Immun. 2000 February 1;68(2):672–9. doi: 10.1128/iai.68.2.672-679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. [comment] New England Journal of Medicine. 2004;350(9):860–1. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 35.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. [see comment] New England Journal of Medicine. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4(9):e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staats HF, Ennis FA., Jr IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J Immunol. 1999 May 15;162(10):6141–7. [PubMed] [Google Scholar]
- 38.Bradney CP, Sempowski GD, Liao HX, Haynes BF, Staats HF. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol. 2002 Jan;76(2):517–24. doi: 10.1128/JVI.76.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scan J Immunol. 1997;46(5):506–13. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu HY, Russell MW. Nasal Lymphoid Tissue, Intranasal Immunization, and Compartmentalization of the Common Mucosal Immune System. Immunol Res. 1997;16(2):187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 41.Zuercher AW, Cebra JJ. Structural and functional differences between putative mucosal inductive sites of the rat. Eur J Immunol. 2002;32(11):3191–6. doi: 10.1002/1521-4141(200211)32:11<3191::AID-IMMU3191>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev. 2005;206(1):22–31. doi: 10.1111/j.0105-2896.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 43.Kiyono H, Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004 Sep;4(9):699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandtzaeg P. Immunology of tonsils and adenoids: everything the ENT surgeon needs to know. Int J Pediatr Otorhinolaryngol. 2003 Dec;67(Supplement 1):S69–S76. doi: 10.1016/j.ijporl.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Boyaka PN, Wright PF, Marinaro M, Kiyono H, Johnson JE, Gonzales RA, et al. Human nasopharyngeal-associated lymphoreticular tissues. Functional analysis of subepithelial and intraepithelial B and T cells from adenoids and tonsils. Am J Pathol. 2000 Dec;157(6):2023–35. doi: 10.1016/S0002-9440(10)64841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staats HF, Nichols WG, Palker TJ. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A) J Immunol. 1996 Jul 1;157(1):462–72. [PubMed] [Google Scholar]
- 47.Egan MA, Chong SY, Hagen M, Megati S, Schadeck EB, Piacente P, et al. A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine. 2004;22(27–28):3774–88. doi: 10.1016/j.vaccine.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Janakova L, Bakke H, Haugen IL, Berstad AK, Hoiby EA, Aaberge IS, et al. Influence of intravenous anesthesia on mucosal and systemic antibody responses to nasal vaccines. Infection & Immunity. 2002 Oct;70(10):5479–84. doi: 10.1128/IAI.70.10.5479-5484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sloat BR, Cui Z. Evaluation of the immune response induced by a nasal anthrax vaccine based on the protective antigen protein in anaesthetized and non-anaesthetized mice. Journal of Pharmacy & Pharmacology. 2006 Apr;58(4):439–47. doi: 10.1211/jpp.58.4.0003. [DOI] [PubMed] [Google Scholar]
- 50.Southam DS, Dolovich M, O'Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002 April 1;282(4):L833–9. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 51.Matsuura S, Shirakami G, Iida H, Tanimoto K, Fukuda K. The effect of sevoflurane on ciliary motility in rat cultured tracheal epithelial cells: a comparison with isoflurane and halothane. Anesthesia & Analgesia. 2006 Jun;102(6):1703–8. doi: 10.1213/01.ane.0000216001.36932.a3. [DOI] [PubMed] [Google Scholar]
- 52.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002 Jun;1(1):111–8. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 53.Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. Vaccine. 2006 Apr 12;24(Suppl 2):S2–44–5. doi: 10.1016/j.vaccine.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 54.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Review of Vaccines. 2002;1:111–8. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 55.von Uexkull C, Nourshargh S, Williams TJ. Comparative responses of human and rabbit interleukin-1 in vivo: effect of a recombinant interleukin-1 receptor antagonist. Immunol. 1992 Dec;77(4):483–7. [PMC free article] [PubMed] [Google Scholar]
- 56.Vandenabeele P, Guisez Y, Declercq W, Bauw G, Vandekerckhove J, Fiers W. Response of murine cell lines to an IL-1/IL-2-induced factor in a rat/mouse T hybridoma (PC60): differential induction of cytokines by human IL-1 alpha and IL-1 beta and partial amino acid sequence of rat GM-CSF. Lymphokine Res. 1990 Fall;9(3):381–9. [PubMed] [Google Scholar]
- 57.Libert C, Brouckaert P, Shaw A, Fiers W. Induction of interleukin 6 by human and murine recombinant interleukin 1 in mice. Eur J Immunol. 1990 Mar;20(3):691–4. doi: 10.1002/eji.1830200333. [DOI] [PubMed] [Google Scholar]
- 58.Lederer JA, Czuprynski CJ. Species-specific binding of IL-1, but not the IL-1 receptor antagonist, by fibroblasts. Cytokine. 1994 Mar;6(2):154–61. doi: 10.1016/1043-4666(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 59.Kokuryo S, Inoue H, Fukuizumi T, Tsujisawa T, Tominaga K, Fukuda J. Evaluation of interleukin 1 as a mucosal adjuvant in immunization with Streptococcus sobrinus cells by tonsillar application in rabbits. Oral Microbiology & Immunology. 2002;17(3):163–71. doi: 10.1034/j.1399-302x.2002.170305.x. [DOI] [PubMed] [Google Scholar]
- 60.Wagner EM, Foster WM. Importance of airway blood flow on particle clearance from the lung. J Appl Physiol. 1996 Nov;81(5):1878–83. doi: 10.1152/jappl.1996.81.5.1878. [DOI] [PubMed] [Google Scholar]
- 61.Lausch RN, Staats H, Metcalf JF, Oakes JE. Effective antibody therapy in herpes simplex virus ocular infection. Characterization of recipient immune response. Intervirol. 1990;31:159–65. doi: 10.1159/000150150. [DOI] [PubMed] [Google Scholar]
- 62.Jackson RJ, Fujihashi K, Xu-Amano J, Kiyono H, Elson CO, McGhee JR. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infection & Immunity. 1993 Oct;61(10):4272–9. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto M, Briles DE, Yamamoto S, Ohmura M, Kiyono H, McGhee JR. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998 Oct 15;161(8):4115–21. [PubMed] [Google Scholar]
- 64.Yamamoto M, McDaniel LS, Kawabata K, Briles DE, Jackson RJ, McGhee JR, et al. Oral Immunization With Pspa Elicits Protective Humoral Immunity Against Streptococcus Pneumoniae Infection. Infection & Immunity. 1997;65(2):640–4. doi: 10.1128/iai.65.2.640-644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swiatlo E, King J, Nabors GS, Mathews B, Briles DE. Pneumococcal Surface Protein A Is Expressed In Vivo, and Antibodies to PspA Are Effective for Therapy in a Murine Model of Pneumococcal Sepsis. Infect Immun. 2003 December 1;71(12):7149–53. doi: 10.1128/IAI.71.12.7149-7153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Little SF, Ivins BE, Fellows PF, Pitt MLM, Norris SLW, Andrews GP. Defining a serlogical correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22:422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Atmar RL, Keitel WA, Cate TR, Munoz FM, Ruben F, Couch RB. A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults. Vaccine. 2007;25(29):5367. doi: 10.1016/j.vaccine.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mikszta JA, Sullivan VJ, Dean C, Waterston AM, Alarcon JB, Dekker JP, 3rd, et al. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005 Jan 15;191(2):278–88. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 69.Staats HF, Montgomery SP, Palker TJ. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997 Jul 20;13(11):945–52. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 70.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008 May;14(5):536–41. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 71.Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, et al. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect Immun. 2007 Nov;75(11):5443–52. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kouiavskaia DV, Berard CA, Datena E, Hussain A, Dawson N, Klyushnenkova EN, et al. Vaccination with agonist peptide PSA: 154–163 (155L) derived from prostate specific antigen induced CD8 T-cell response to the native peptide PSA: 154–163 but failed to induce the reactivity against tumor targets expressing PSA: a phase 2 study in patients with recurrent prostate cancer. J Immunother. 2009 Jul–Aug;32(6):655–66. doi: 10.1097/CJI.0b013e3181a80e0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicologic Pathology. 2006;34(3):252–69. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 75.Casteleyn C, Broos AM, Simoens P, Van den Broeck W. NALT (nasal cavity-associated lymphoid tissue) in the rabbit. Vet Immunol Immunopathol. 2010 Feb 15;133(2–4):212–8. doi: 10.1016/j.vetimm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Park CY, Jung SH, Bak JP, Lee SS, Rhee DK. Comparison of the rabbit pyrogen test and Limulus amoebocyte lysate (LAL) assay for endotoxin in hepatitis B vaccines and the effect of aluminum hydroxide. Biologicals. 2005 Sep;33(3):145–51. doi: 10.1016/j.biologicals.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa Y, Maeda H, Murai T. Evaluation of the In Vitro Pyrogen Test System Based on Proinflammatory Cytokine Release from Human Monocytes: Comparison with a Human Whole Blood Culture Test System and with the Rabbit Pyrogen Test. Clin Diagn Lab Immunol. 2002 May 1;9(3):588–97. doi: 10.1128/CDLI.9.3.588-597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spreitzer I, Fischer M, Hartzsch K, Luderitz-Puchel U, Montag T. Comparative study of rabbit pyrogen test and human whole blood assay on human serum albumin. Altex. 2002;19 (Suppl 1):73–5. [PubMed] [Google Scholar]
- 79.Lofthouse SA, Andrews AE, Barcham GJ, Nash AD. Parameters related to the application of recombinant ovine interleukin-1 beta as an adjuvant. Vaccine. 1995;13(14):1277–87. doi: 10.1016/0264-410x(95)00074-b. [DOI] [PubMed] [Google Scholar]
- 80.Staruch MJ, Wood DD. The adjuvanticity of Interleukin 1 in vivo. J Immunol. 1983;130(5):2191–4. [PubMed] [Google Scholar]
- 81.Dinarello CA. Cytokines as Endogenous Pyrogens. The Journal of Infectious Diseases. 1999;179(s2):S294–S304. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- 82.Wimer-Mackin S, Hinchcliffe M, Petrie CR, Warwood SJ, Tino WT, Williams MS, et al. An intranasal vaccine targeting both the Bacillus anthracis toxin and bacterium provides protection against aerosol spore challenge in rabbits. Vaccine. 2006;24(18):3953–63. doi: 10.1016/j.vaccine.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 83.Garmise RJ, Staats HF, Hickey AJ. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8(4):E81. doi: 10.1208/pt0804081. [DOI] [PubMed] [Google Scholar]
- 84.Bryant ML, Brown P, Gurevich N, McDougall IR. Comparison of the clearance of radiolabelled nose drops and nasal spray as mucosally delivered vaccine. Nuclear Medicine Communications. 1999;20(2):171–4. doi: 10.1097/00006231-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007 Jul 26;25(30):5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Huang J, Mikszta JA, Ferriter MS, Jiang G, Harvey NG, Dyas B, et al. Intranasal administration of dry powder anthrax vaccine provides protection against lethal aerosol spore challenge. Hum Vaccines. 2007 May–Jun;3(3):90–3. doi: 10.4161/hv.3.3.4011. [DOI] [PubMed] [Google Scholar]
- 87.Coucke D, Schotsaert M, Libert C, Pringels E, Vervaet C, Foreman P, et al. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine. 2009 Feb 18;27(8):1279–86. doi: 10.1016/j.vaccine.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 88.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective Mucosal Immunity to Anthrax: Neutralizing Antibodies and Th Cell Responses Following Nasal Immunization with Protective Antigen. J Immunol. 2003 June 1;170(11):5636–43. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- 89.Veltri S, Smith JW., 2nd Interleukin 1 trials in cancer patients: a review of the toxicity, antitumor and hematopoietic effects. Stem Cells. 1996;14(2):164–76. doi: 10.1002/stem.140164. [DOI] [PubMed] [Google Scholar]
- 90.Hashimoto M, Ishikawa Y, Yokota S, Goto F, Bando T, Sakakibara Y, et al. Action site of circulating interleukin-1 on the rabbit brain. Brain Research. 1991;540(1–2):217–23. doi: 10.1016/0006-8993(91)90510-3. [DOI] [PubMed] [Google Scholar]
- 91.Murakami N, Sakata Y, Watanabe T. Central action sites of interleukin-1 beta for inducing fever in rabbits. Journal of Physiology. 1990;428:299–312. doi: 10.1113/jphysiol.1990.sp018213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaganathan KS, Vyas SP. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine. 2006 May 8;24(19):4201–11. doi: 10.1016/j.vaccine.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Soane RJ, Hinchcliffe M, Davis SS, Illum L. Clearance characteristics of chitosan based formulations in the sheep nasal cavity. International Journal of Pharmaceutics. 2001;217(1–2):183–91. doi: 10.1016/s0378-5173(01)00602-0. [DOI] [PubMed] [Google Scholar]
- 94.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scandinavian Journal of Immunology. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 95.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. [see comment] Vaccine. 2002;20(9–10):1340–53. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]