Abstract
Streptococcus pneumoniae (SPN), the leading cause of meningitis in children and adults worldwide, is associated with an overwhelming host inflammatory response and subsequent brain injury. Here we examine the global response of the blood-brain barrier to SPN infection and the role of neuraminidase A (NanA), a SPN surface anchored protein recently described to promote central nervous system tropism. Microarray analysis of human brain microvascular endothelial cells (hBMEC) during infection with SPN or an isogenic NanA-deficient (ΔnanA) mutant revealed differentially activated genes, including neutrophil chemoattractants IL-8, CXCL-1, CXCL-2. Studies using bacterial mutants, purified recombinant NanA proteins and in vivo neutrophil chemotaxis assays indicated that pneumococcal NanA is necessary and sufficient to activate host chemokine expression and neutrophil recruitment during infection. Chemokine induction was mapped to the NanA N-terminal lectin-binding domain with a limited contribution of the sialidase catalytic activity, and was not dependent on the invasive capability of the organism. Further, pretreatment of hBMEC with recombinant NanA protein significantly increased bacterial invasion suggesting that NanA-mediated activation of hBMEC is a prerequisite for efficient SPN invasion. These findings were corroborated in an acute murine infection model where we observed less inflammatory infiltrate and decreased chemokine expression following infection with the ΔnanA mutant.
Introduction
S. pneumoniae (SPN, pneumococcus), a Gram-positive, alpha-hemolytic diplococcus, is a significant human pathogen ranked as the 4th most common cause of global mortality by an infectious pathogen (Carapetis et al., 2005). It is responsible for frequently occurring diseases including pneumonia, otitis media, sinusitis and meningitis. All SPN strains tested have been shown to possess neuraminidase (sialidase) activity (Kelly et al., 1967). Although three distinct neuraminidases, nanA, nanB and nanC are present in the SPN genome (Xu et al., 2008), only NanA is surfaced-anchored and present in all strains (Camara et al., 1994, Pettigrew et al., 2006, King et al., 2005). NanA has been shown to contribute to pneumococcal colonization of the nasopharynx and development of otitis media (Tong et al., 2000, Tong et al., 2001), spread from the nasopharynx to lungs in a mouse model of infection (Manco et al., 2006, Orihuela et al., 2004) and more recently to BBB penetration (Uchiyama et al., 2009). Neuraminidases in general represent attractive new targets for drug discovery (Hsiao et al., 2009), thus increased understanding of the functional role of NanA could lead to important discoveries for therapeutic intervention.
Pneumococcus is currently the leading cause of bacterial meningitis in young children and adults worldwide with a mortality rate of 20–35% (Gans et al., 2002). Despite antibiotic therapy high morbidity is prevalent due to intracranial complications such as brain edema, hydrocephalus, and cerebrovascular hemorrhage (Weisfelt et al., 2006). Brain damage following pneumococcal meningitis results generally from the loss of BBB integrity due to the toxicity of the bacterial products and/or activation of host inflammatory mediators that compromise BBB function (Meli et al., 2002). The human BBB, which is primarily composed of a single layer of specialized human brain microvascular endothelial cells (hBMEC), serves as a critical barrier to protect the central nervous system (CNS) against microbial invasion. In addition to providing a barrier function, the BBB is thought to play an active role in initiating a specific innate defense response that promotes neutrophil recruitment and activation (Doran et al., 2003). We have recently shown that NanA promotes BBB penetration, but the exact mechanism for bacterial entry and the contribution of SPN NanA to inflammatory activation during BBB interaction is unknown.
In this study we examine for the first time the global gene expression profile of brain endothelium to infection with SPN and an isogenic ΔnanA mutant using microarray, real-time RT-PCR, and protein analysis. Our studies suggest that BBB endothelium responds to the SPN NanA with functional gene expression to promote the characteristic neutrophilic inflammatory response of acute pneumococcal meningitis. NanA-mediated BBB activation was attributed primarily to the Laminin G like lectin-binding domain that also promotes SPN-hBMEC interaction. Our results demonstrate that SPN exploits the NanA-induced BBB defensive response by promoting bacterial uptake, emphasizing a novel role for this neuraminidase in the pathogenesis of pneumococcal meningitis.
Results
Expression profile of brain endothelium induced by SPN and the NanA-deficient mutant
Understanding the acute response of the BBB endothelium to SPN infection and major virulence factor NanA should provide insight into the pathogenesis of pneumococcal meningitis. In this study we used microarray analysis to examine the global transcriptional response of hBMEC to infection with WT and a ΔnanA mutant strain. Growth kinetics were similar between the two strains under the conditions used in our experiments (Supplementary Fig. 1A). Additionally, both the WT and ΔnanA mutant exhibited similar levels of hemolytic activity (data not shown). By 6 hours post infection with SPN WT, 43 genes exhibited more than 1.5 fold increase in transcript abundance (Supplementary Table 1). The most highly induced hBMEC genes included IL-8, CXCL-1, and CXCL-2, the CXC chemokine family members that act mainly on cells of the neutrophil lineage. Other highly induced proinflammatory genes were CCL-20, a chemokine which is chemotactic for lymphocytes and neutrophils (Hieshima et al., 1997), IL-6, a pro-inflammatory cytokine which has been shown in mice to be required for resistance against SPN (Poll et al., 1997) and ICAM-1 which plays an essential role as an endothelial receptor to bind and activate circulating neutrophils at the site of infection.
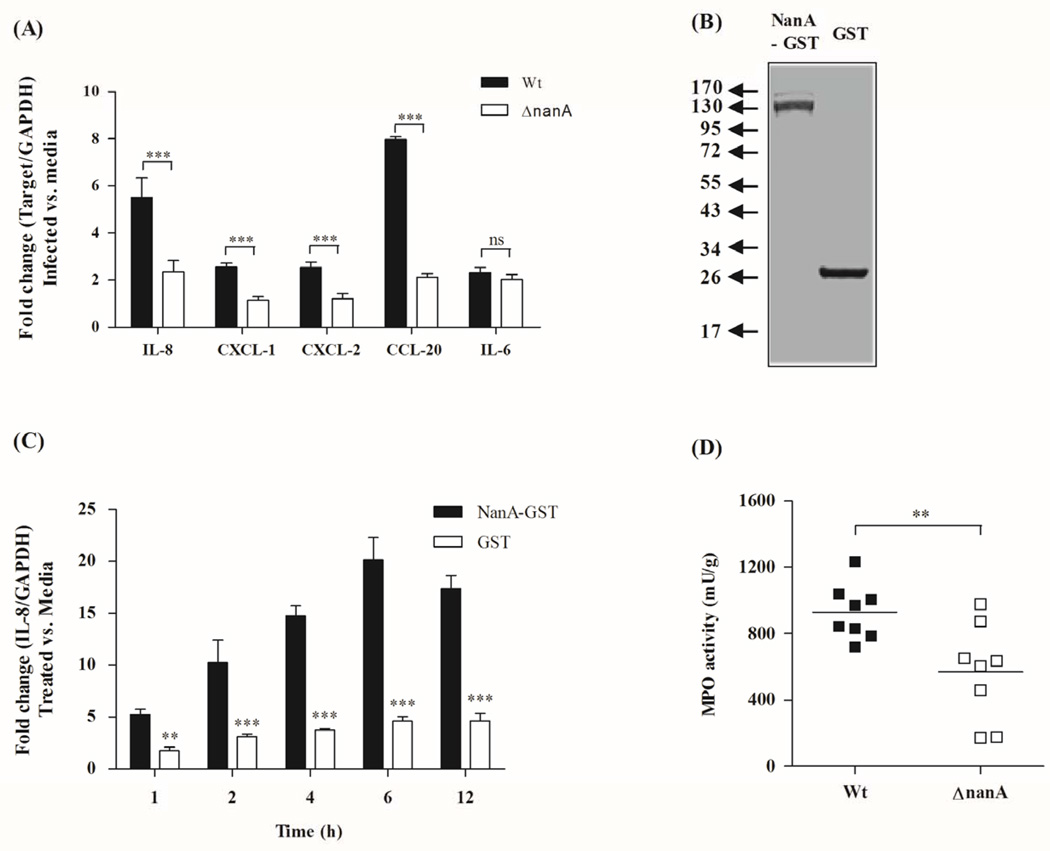
Interestingly, infection with the NanA-deficient mutant resulted in decreased expression of a small subset of genes (n=14), compared to that observed after WT infection, which included chemokines IL-8, CCL-20, CXCL-1, and CXCL-2 (Supplementary Table 1). These results were confirmed in independent experiments using real time RT-PCR (Fig. 1A). In general, the relative abundance of the different transcripts correlated with the fold increases observed by the microarray analysis (Fig. 1A, Supplementary Table 1). We also observed a marked increase in chemokine secretion by hBMEC upon infection with WT SPN, but secretion of chemokines IL-8, CXCL-1, CXCL-2 and CCL-20 was significantly reduced when cells were infected with the ΔnanA mutant (Supplementary Fig. 1B). Consistent with the microarray experiments, no difference in IL-6 secretion was observed, indicating that NanA only affects transcription of specific cytokines. Overall, these independent experiments confirmed our microarray results and suggest that the pneumococcal NanA contributes to the induction and rapid activation of the host innate defense system for neutrophil recruitment and activation.
Figure 1.
(A) Real time RT-PCR analysis of chemokines IL-8, CXCL1, CXCL2, CCL20 in hBMEC 6 h post infection with WT SPN or isogenic ΔnanA mutant. Transcript levels were normalized to GAPDH and fold change was determined as described in Material and Methods. Bars represent mean and standard deviation of one representative experiment. (B) NanA was expressed as GST tagged fusion protein and purified using Glutathione Sepharose affinity chromatography. The recombinant protein was >95% pure as demonstrated by SDS-PAGE. (C) Treatment of hBMEC with NanA-GST induced significant IL-8 expression over time compared to GST control. (D) Neutrophil recruitment was assessed by measuring myeloperoxidase (MPO) activity in skin homogenates 4 h post subcutaneous injection with either SPN WT or ΔnanA mutant. Bars indicate mean levels of neutrophil recruitment. ** p<0.005, *** p<0.001.
NanA is sufficient to induce IL-8 expression and neutrophil chemotaxis
We next sought to determine whether SPN NanA is sufficient to induce IL-8, the most potent neutrophil chemo-attractant, using purified recombinant NanA protein. NanA was expressed as an N-terminal GST-tagged fusion protein and purified to >95% homogeneity (Fig. 1B) as described in the Experimental procedures. Using a fluorescent assay to measure sialidase activity (Uchiyama et al., 2009), we found that the NanA-GST exhibited sialidase activity (Supplementary Fig. 2). Treatment of hBMEC with NanA-GST resulted in increased IL-8 transcription over time while incubation with the similarly purified GST alone had no effect (Fig. 1C). Both purified proteins, NanA-GST and GST alone, contained a similar low level (2EU/ml or ∼0.2 ng/ml) of endotoxin.
As we have demonstrated that NanA is both necessary and sufficient for chemokine induction in hBMEC, we next hypothesized that infection with WT SPN would result in increased neutrophil recruitment compared to infection with the isogenic ΔnanA mutant strain. We therefore analyzed neutrophil recruitment to the site of infection using an in vivo neutrophil recruitment assay as described previously (Sorge et al., 2008). Neutrophil migration was assessed upon subcutaneous injection of SPN WT or the ΔnanA mutant strain into the right or left flank of mice, respectively. After 4 hours, homogenized skin sections were analyzed for the neutrophil enzyme myeloperoxidase (MPO), which serves as an effective indicator of neutrophil infiltration (Bradley et al., 1982, Sorge et al., 2008) and compares well with other in vivo assays of neutrophil chemotaxis (Sorge et al., 2008). MPO levels and therefore the number of accumulating neutrophils were significantly lower after infection with the ΔnanA mutant compared to the WT strain (Fig. 1D). Parallel experiments demonstrated that similar bacterial CFU were recovered from the skin for the both the WT and ΔnanA mutant under these conditions (Supplementary Fig. 3). Taken together these results indicate that pneumococcal NanA is sufficient to activate IL-8 transcription and functional neutrophil signaling pathways in vivo resulting in neutrophil recruitment during active pneumococcal infection.
The NanA Laminin G like lectin binding domain promotes IL-8 induction
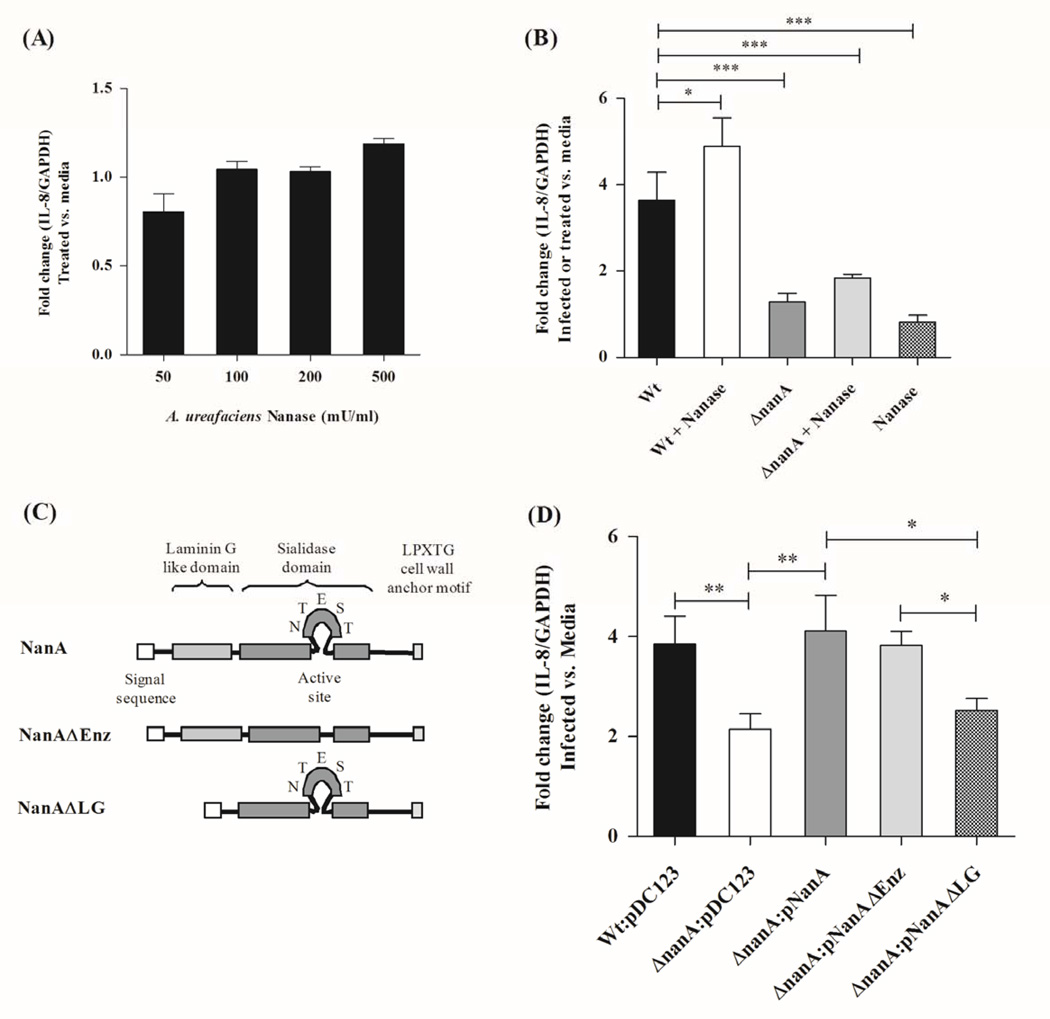
As neuraminidase activity is a key function of NanA, we hypothesized that exogenous neuraminidase treatment may similarly result in increased IL-8 expression in brain endothelium. A recent study demonstrated that treatment with exogenous neuraminidase increased IL-8 production in lung epithelial cells (Kuroiwa et al., 2009). We treated hBMEC with increasing concentrations of commercially-available neuraminidase from Arthrobacter ureafaciens, which lacks a lectin binding domain, but targets similar sialic acid linkages as SPN (Scanlon et al., 1989, Rogerieux et al., 1993); however, no significant induction in IL-8 gene transcription was observed even at the highest concentration (500 U/ml) of sialidase (Fig. 2A). Prior treatment of hBMEC with exogenous sialidase followed by infection with SPN stimulated a slight, but statistically significant, increase in IL-8 transcription (Fig. 2B). However this treatment failed to rescue the activation defect of the ΔnanA mutant. While these results suggest that sialidase activity may contribute to some extent to IL-8 induction, it is not sufficient for complete hBMEC activation.
Figure 2.
Measurement of IL-8 transcript after 6 h treatment of hBMEC with exogenous sialidase from Arthrobacter urefaciencs alone (A) or with concurrent infection with the WT and ΔnanA mutant (B). Schematic diagram of NanA showing different protein domains including the signal sequence, laminin G like domain, catalytic domain and LPXTG motif (C). IL-8 transcript abundance following infection with SPN WT and the ΔnanA mutant complemented with WT nanA or deletion mutants lacking the active catalytic site (pNanAΔEnz) or the laminin G domain (pNanAΔLG) (D). Data represent mean and standard deviation of triplicate wells of a representative experiment performed three times. * p<0.05, ** p<0.005, *** p<0.001.
In addition to the catalytic domain responsible for enzymatic activity, sequence analysis has revealed that SPN NanA also possesses an YSIRK type signal sequence, a laminin G-like lectin binding domain, and the C-terminal LPXTG motif that anchors the enzyme to the bacterial cell surface (Camara et al., 1994, Yesilkaya et al., 2006) (Fig. 2C). Thus we sought to characterize the contribution of the NanA sialidase catalytic domain and the laminin G-like lectin binding domain to IL-8 gene activation using targeted deletion constructs lacking the active catalytic site (ΔEnz) or the laminin G domain (ΔLG) constructed previously (Uchiyama et al., 2009). The specific constructs, pNanA, pNanAΔEnz and pNanAΔLG, were used to complement the ΔnanA mutant strain. Cell surface location of NanA targeted mutant proteins in these strains as well as sialidase activity in the case of pNanAΔLG is preserved in these strains. As shown in Fig. 2D, only complementation with pNanA or pNanAΔEnz restored IL-8 activation to levels induced by WT SPN. Additionally treatment of hBMEC with purified NanAΔEnz-GST resulted in similar IL-8 induction levels compared to that observed with NanA-GST treatment (Supplementary Fig. 4). These studies demonstrate that the laminin G-like lectin binding domain of SPN NanA is required for IL-8 activation.
Induction of IL-8 transcription depends on NanA-mediated adherence
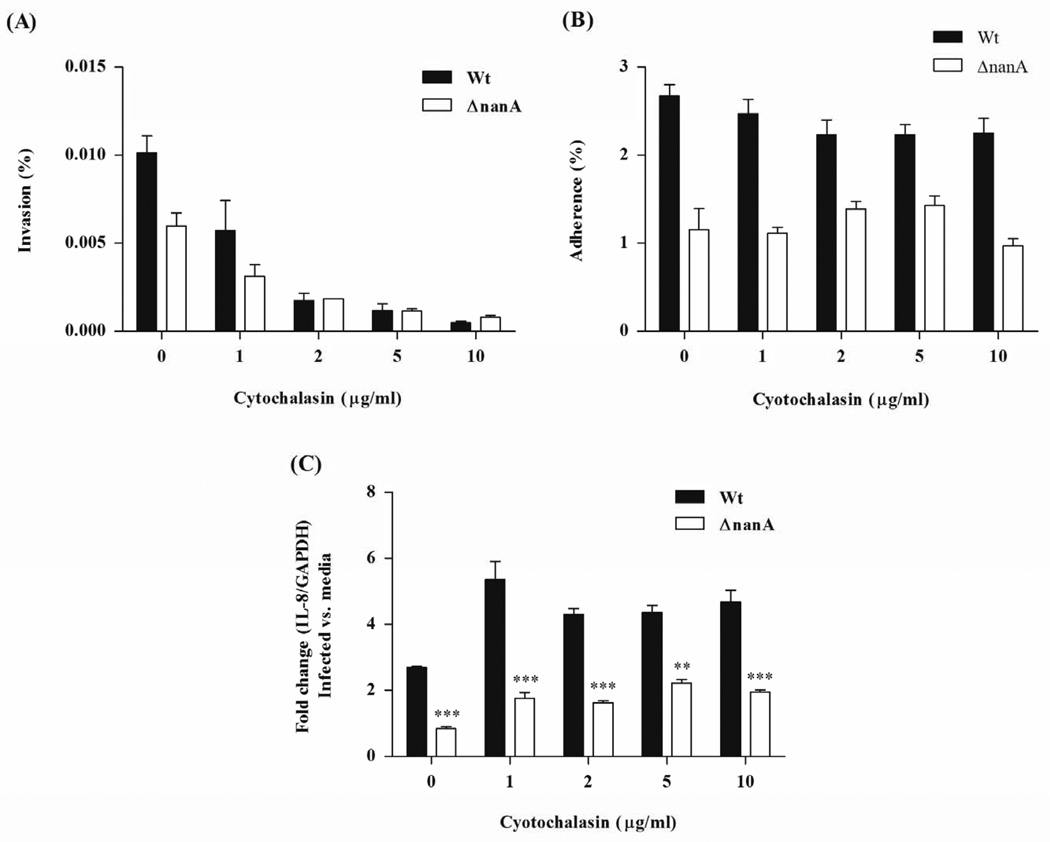
Recognition of SPN by brain endothelial cells and subsequent expression of chemokines may contribute significantly to the prominent leukocyte recruitment observed in pneumococcal meningitis. As we have shown recently that SPN NanA also promotes pneumococcal BBB invasion (Uchiyama et al., 2009), we asked whether NanA-mediated bacterial invasion is required for the observed IL-8 activation. We used cytochalasin D (Sorge et al., 2008, Nizet et al., 1997), a potent actin polymerization inhibitor, to block pneumococcal invasion of hBMEC in a dose dependent manner (Fig. 3A). Blocking concentrations of cytochalasin D did not inhibit pneumococcal adherence or IL-8 induction in parallel experiments (Fig. 3B, C). These results confirm that pneumococcal invasion of hBMEC is not required for IL-8 induction and imply that SPN NanA-mediated adherence initiates signaling events that lead to increased chemokine production.
Figure 3.
Determination of bacterial invasion (A), adherence (B), and IL-8 transcript abundance (C) following cytochalasin D treatment. HBMEC monolayers were treated with indicated concentrations of cytochalasin D for 30 min prior to infection with WT SPN or the ΔnanA mutant. IL-8 transcript levels were measured by real time RT-PCR following 6 h infection. Experiments were performed three times in triplicate. ** p<0.005, *** p<0.001.
NanA mediated activation promotes bacterial entry to brain endothelium
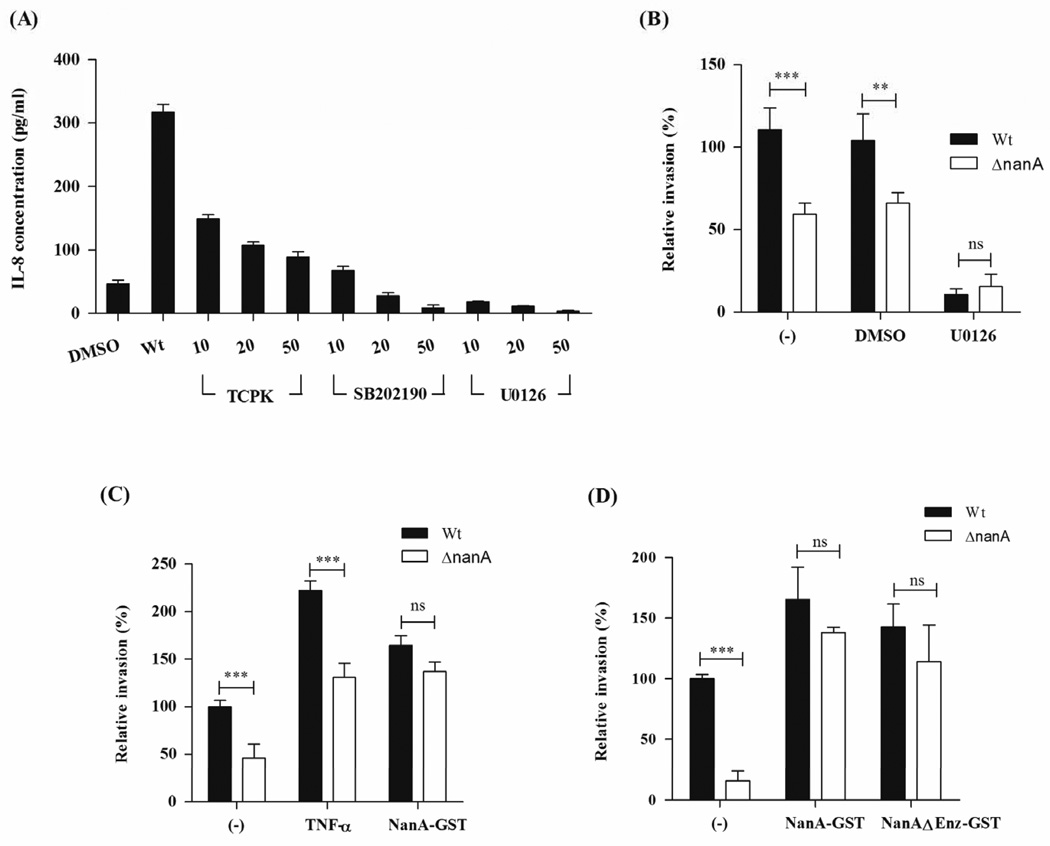
To elucidate the signaling pathways that trigger IL-8 induction, we performed experiments in the presence of pharmacological inhibitors of key signaling molecules involved in cytokine production, including transcription factor NF-κB and members of the Mitogen-activated protein kinase (MAPK) family, MAPK/ERK (extracellular signal-regulated kinase) kinase1/2 (MEK1/2) and p38 MAPK. As shown in Fig. 4A SPN-induced IL-8 secretion in hBMEC is reduced in a dose-dependent manner by all tested inhibitors. Treatment of hBMEC with U0126, a compound that inhibits the enzymatic activity of MEK1/2 and subsequent activation of ERK signaling pathways (Favata et al., 1998), had the strongest inhibitory effect on IL-8 secretion compared to the DMSO treated control (Fig. 4A). Experiments by others have demonstrated that host cell activation by proinflammatory cytokines promotes SPN uptake (Cundell et al., 1995, Ring et al., 1998, Radin et al., 2005). Consistent with these observations, pretreatment of hBMEC monolayers with U0126 inhibitor (50 µM) prior to infection reduced bacterial invasion for both the WT and ΔnanA mutant strain (Fig. 4B), further indicating that cellular activation is a prerequisite for efficient hBMEC invasion by SPN.
Figure 4.
(A) HBMEC monolayers were treated with signaling pathway inhibitors TCPK, SB202190 and U0126 for 30 min prior to infection with SPN. Protein expression of IL-8 in hBMEC supernatants, 6 h post infection was determined using ELISA. Quantification of bacterial invasion following hBMEC treatment with U0126 (30 min) compared to DMSO control (B), TNFα and NanA-GST (C) or NanA-GST and NanAΔEnz-GST (D). Experiments were performed three times in triplicate. Bars represent mean and standard deviation of one representative experiment. ** p<0.005, *** p<0.001.
To assess whether NanA could provide the necessary activation to stimulate bacterial uptake, hBMEC were treated with TNFα, shown previously to activate host cells and promote SPN uptake (Cundell et al., 1995), or with purified NanA protein (NanA-GST), shown above to induce chemokine transcription and immune activation. Following a 3 hour incubation period, proteins were removed and monolayers infected with WT SPN or the ΔnanA mutant. As expected, TNFα treatment resulted in increased bacterial uptake, however there was still a significant difference in the invasive capability observed between the WT and the NanA-deficient mutant (Fig. 4C). Interestingly, pretreatment of hBMEC with NanA-GST or NanAΔEnz-GST significantly increased bacterial invasion of both the WT and NanA-deficient strains (Fig. 4C, D). These results strongly suggest that NanA, and specifically the lectin binding domain, mediates immune activation of hBMEC, and promotes SPN uptake.
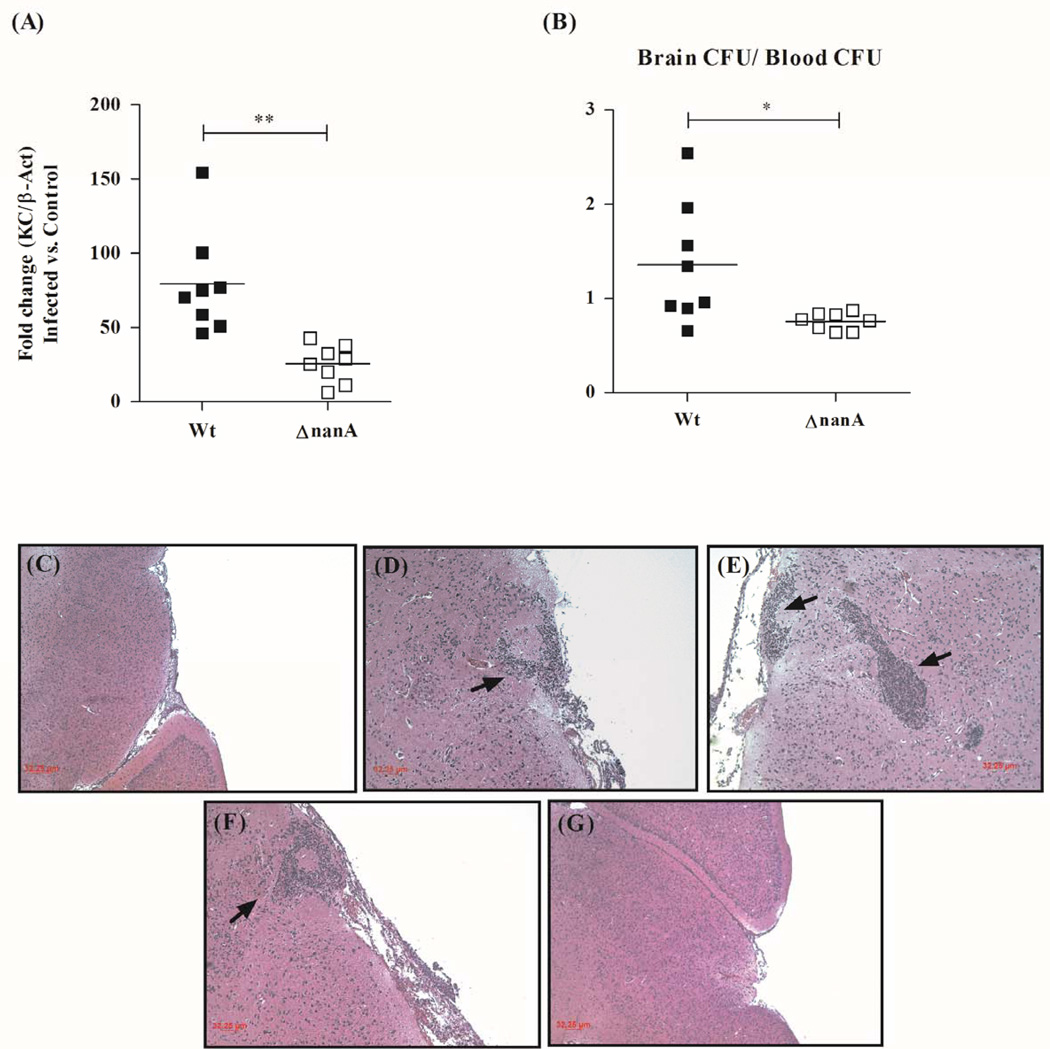
Our in vitro results using the hBMEC tissue culture model indicate that SPN NanA contributes to BBB endothelium activation and subsequent bacterial uptake. We sought to corroborate our in vitro findings in vivo using a murine model of pneumococcal infection as described previously (Uchiyama et al., 2009). BALB/c male mice (8 weeks old) were injected intravenously with SPN WT or isogenic ΔnanA mutant (8 mice per group). Following 6 or 24hrs post injection, mice were euthanized, blood and brain were collected, and total RNA was isolated from brain tissue at the 6hr time point. At 6 hr real time RT-PCR analysis revealed that mice infected with the ΔnanA mutant exhibited decreased transcript levels of the murine functional CXC homologue, KC, compared to mice infected with WT SPN (Fig. 5A). At this early time point levels of SPN detected in the blood of each group were essentially identical, while bacterial counts isolated from the brain of ΔnanA infected mice were significantly lower than those isolated from mice infected with the WT strain (Supplementary Fig. 5A). We found that at 24 h the levels of WT and ΔnanA mutant SPN in blood and brain were similar (Supplementary Fig. 5B), but the mean ratio of brain/blood cfu in the WT strain was significantly greater than the ΔnanA mutant (Fig. 5B). Histopathologic analysis revealed brain leukocytic infiltrates in sections from WT-infected mice (Fig. 5C,D,E,F); inflammation was scant or absent in those animals infected with the isogenic ΔnanA mutant (Fig. 5G).
Figure 5.
(A) Transcript abundance of murine chemokine KC in total RNA isolated from mice brain 6h post intravenous (i.v.) injection with WT SPN or ΔnanA mutant. Transcript levels were normalized to β-Actin and expressed as fold change compared to mice injected with PBS only. (B) Ratio of brain bacterial counts (CFU) to blood CFU 24h post infection with WT SPN or ΔnanA mutant. Mice were injected with 5×104 CFU of WT SPN or ΔnanA mutant intravenously and 24h post injection animals were sacrificed. Histopathology of H&E stained representative brain tissue samples following infection with WT SPN (C–F), depicting areas of areas of leukocyte infiltration and microabscess formation (arrows), and ΔnanA mutant (G) ** p<0.005, *** p<0.001.
Discussion
Our studies using DNA microarray analysis, real-time RT-PCR and immunoassays reveal a unique requirement for the SPN NanA protein in activation of specific BBB defense pathways by SPN, the leading agent of bacterial meningitis in children and adults. SPN infection resulted in NanA-dependent secretion of IL-8, CXCL1, CXCL2 and CCL20 in hBMEC that correlated with functional neutrophil chemotaxis in vivo. Complementary, purified recombinant NanA resulted in increased expression of chemokine IL-8. NanA-mediated IL-8 induction was mapped primarily to the N-terminal Laminin G-like lectin binding domain with only a modest contribution from sialidase activity. Using pharmacological inhibitors, we observed a dominant role for the MAPK/ERK signaling pathway in IL-8 secretion. Finally, our data suggest that the gene induction mediated by the NanA-hBMEC interaction results in efficient SPN invasion. These results suggest a novel role for the bacterial NanA protein in exploiting BBB activation to promote bacterial CNS entry, allowing escape from the blood stream to an immune-privileged site.
The BBB, composed primarily of a specialized layer of brain microvascular endothelial cells separates the brain and its surrounding tissues from the circulating blood, tightly regulating the flow of nutrients and molecules and thereby maintaining the proper biochemical conditions for normal brain function (Betz, 1992, Betz et al., 1986). Bacterial meningitis, a serious CNS infection, can develop rapidly into a life-threatening situation even in previously healthy children or adults. We have adapted a tissue culture model of the human BBB using immortalized hBMEC (Stins et al., 1997) to investigate for the first time the acute global response of BBB endothelium to SPN infection in vitro. The hBMEC line has proven valuable in the analysis of a wide variety of human CNS pathogens including Neisseria meningitidis (Unkmeir et al., 2002), Escherichia coli K1 (Kim, 2001), group B streptococcus(Nizet et al., 1997, Doran et al., 2003), Bacillus anthracis (Sorge et al., 2008) and SPN (Ring et al., 1998, Uchiyama et al., 2009). HBMEC infection by SPN WT resulted in upregulation of proinflammatory cytokines and chemokines functioning to orchestrate neutrophil recruitment and activation. A similar innate response was previously observed upon hBMEC infection with GBS, suggesting a generalized response to bacterial infection (Doran et al., 2003). Compared to WT SPN infection, we found that infection of hBMEC with a NanA-deficient mutant resulted in significantly less gene induction and secretion of key immune CXC chemokine proteins and functional neutrophil chemotaxis to the site of infection. Other factors such as the SPN toxin, pneumolysin, choline-binding protein A, CbpA, and pnemococcal surface protein A, PspA, have also been shown to induce chemokine expression in human cells (Graham et al., 2006, Bernatoniene et al., 2008). Interestingly, chemokine modulation by SPN infection may be cell context dependent as CbpA was shown to down regulate chemokine expression in respiratory epithelium and nasopharyngeal cells (Graham et al., 2006). Our studies presented here were all performed with inocula of SPN expressing the opaque phenotype. It is likely that multiple phase-variant SPN factors may function synergistically to elicit BBB immune defense.
Bacterial neuraminidases play important roles in host-pathogen interactions and offer attractive targets for therapeutic intervention (Taylor, 1996, Hsiao et al., 2009). The major pneumococcal neuraminidase, NanA, has been shown to desialylate both host proteins and surface components from neighboring/competing bacteria (Shakhnovich et al., 2002, King et al., 2004) and contribute to virulence in animal models of disease (Mitchell, 2000, Orihuela et al., 2004, Manco et al., 2006, Uchiyama et al., 2009). However, previous studies have not examined the contribution of NanA to inflammatory activation. Our results clearly show that NanA is necessary and sufficient to promote chemokine signaling in brain endothelium, resulting in functional neutrophil recruitment during infection. Interestingly, activation was mediated largely by the N-terminal Laminin G like portion of NanA, with only a modest contribution by the sialidase catalytic domain. These results parallel recent studies on the trans-sialidase from protozoan Trypanosoma cruzi, which demonstrated that endothelial cell activation and parasite uptake were initiated by an inactive form of the sialidase (Dias et al., 2008).
Earlier literature has suggested that bacterial neuraminidase may be a factor in the pathogenesis of pneumococcal meningitis as elevated levels of free sialic acid in cerebrospinal fluid were associated with adverse outcome including coma and bacteremia (O'Toole et al., 1971). Our results from this study suggest that the Laminin G-like domain of NanA must interact directly with hBMEC to initiate chemokine signaling and inflammatory activation. This may involve the synergistic effect of the sialidase activity and binding function imparted by the laminin G domain. We have recently shown that the Laminin G-like lectin binding domain of NanA plays an important role in SPN invasion of brain endothelium and bacterial entry into the CNS (Uchiyama et al., 2009). This evokes the following question: does NanA-mediated invasion initiate hBMEC activation, or does NanA-mediated cellular activation initiate bacterial uptake? To address these possibilities we inhibited bacterial invasion with cytochalasin D and analyzed IL-8 transcript after infection with WT and NanA-deficient strains. Cytochalasin D treatment did not inhibit IL-8 induction. These results confirm that pneumococcal invasion of hBMEC is not required for IL-8 induction, and that SPN NanA-mediated adherence initiates signaling events that increase chemokine production.
To address whether NanA-mediated activation is required for bacterial invasion, we first investigated the signaling pathways involved in hBMEC IL-8 production upon SPN infection. We employed specific pharmacological inhibitors to block major signaling pathways involved in immune responses including NFκB and MAPK/ERK kinase1/2 (MEK1/2) and p38 MAPK. Pretreatment of hBMEC with these individual inhibitors resulted in a dose-dependent decrease in IL-8 secretion by WT SPN; however inhibition of MEK1/2 and subsequent inhibition of ERK signaling pathways with inhibitor U0126 had the most potent effect. Interestingly, inhibition of ERK signaling in hBMEC also markedly reduced invasion of both the WT and NanA-deficient strains (Fig. 4B). Pretreatment of hBMEC with TNFα or NanA recombinant proteins (NanA-GST and NanAΔEnz-GST) also increased bacterial invasion in hBMEC (Fig. 4C, D). These results suggest that hBMEC activation through NanA is necessary for efficient SPN uptake and are consistent with previous studies showing that activation of MAP kinases was required for pneumococcal uptake (Radin et al., 2005). It has been well established that inflammatory activation of host cells results in the upregulation of PAF receptor (PAFr), promoting efficient SPN host cell entry, invasion and trafficking across the BBB via receptor-mediated endocytosis (Ring et al., 1998). Whether NanA-mediated activation results in upregulation of PAFr remains to be determined although our microarray analysis does not show a change in PAFr transcription following infection with SPN WT or the ΔnanA mutant (data not shown).
Our results suggest a novel role for SPN NanA in immune evasion, allowing bacterial escape from the blood stream to an immune privileged site by exploiting host cell defense to enhance bacterial BBB penetration. These results were corroborated in vivo as infection with the NanA-deficient mutant resulted in significantly less expression of KC, the murine functional homologue of CXC family chemokines and reduced BBB invasion, as we had observed previously (Uchiyama et al., 2009). We cannot exclude the possibility that the reduced level of KC observed during infection with the NanA-deficient mutant in our in vivo studies was due to an overall reduced bacterial load in the brain. However, our in vitro and in vivo studies presented here show decreased chemokine expression, bacterial invasion and PMN migration following infection with the ΔnanA mutant, despite similar bacterial levels as the WT strain. These data strongly support the idea that NanA-mediated SPN adherence contributes to chemokine induction that promotes subsequent cellular activation and bacterial internalization. Future studies on the contribution of NanA to SPN induced BBB permeability during disease progression will be informative.
We speculate that the Laminin G-like domain of SPN NanA engages a cellular receptor to initiate a signal transduction cascade leading to immune activation of brain endothelium. Subsequent SPN-hBMEC interaction likely involves additional pneumococcal factors such as PavA (Pracht et al., 2005) and/or CbpA via interaction with PAFr (Ring et al., 1998) or the Laminin receptor (Orihuela et al., 2009). Candidate receptors known to interact with Laminin G like domains include sulfatides, heparin, β1 integrins and α-dystroglycan (Talts et al., 1999), some of which are known to activate the ERK-MAP kinase signaling cascade (Ferletta et al., 2003, Spence et al., 2004). In summary we have established a previously unidentified role for NanA in the pathogenesis of pneumococcal infection. Our discovery of the novel requirement of a neuraminidase for immune activation and subsequent CNS entry suggests that therapies directed at neutralizing this molecule may be beneficial in preventing the progression of bacterial meningitis.
Experimental Procedures
Bacterial strains and growth conditions
Streptococcus pneumoniae (SPN) serotype 2 strain D39 (NCTC 7466)(Berry et al., 1989) and its isogenic ΔnanA mutant were used for these experiments. The ΔnanA mutant, deficient in neuraminidase A, was constructed by nonpolar insertion-duplication mutagenesis of the nanA gene as previously described (Winter et al., 1997). SPN cultures were grown in Todd-Hewitt broth (THB) supplemented with 1.5% yeast extract (THY media). All studies were all performed with inocula of SPN expressing the opaque phenotype. NanA deletion derivatives have been described previously (Uchiyama et al., 2009). Briefly, elimination of the critical active site residues for sialidase activity (E609 and R625) (Yesilkaya et al., 2006) or of the Laminin G-like domain (amino acid residues 37 to 222) of nanA was accomplished by inverse PCR and confirmed by sequence analysis. Surface expression of NanA in mutant derivative was determined previously by FACS analysis (Uchiyama et al., 2009). SPN transformed with either pNanA or NanA deletion derivatives, pNanAΔLG and pNanAΔEnz, or empty vector control (pDC123) were cultured in THY containing chloramphenicol (2µg/ml).
Endothelial cell culture and infection assays
The human brain microvascular endothelial cell line (hBMEC), kindly provided by Kwang Sik Kim (Johns Hopkins University), were originally isolated as previously described (Stins et al., 1997, Stins et al., 1994). hBMEC monlayers were cultured using RPMI 1640 (Gibco), supplemented with 10% fetal calf serum (FBS; Gibco), 10% Nuserum (BD Biosciences, San Jose, California, USA), and 1% modified Eagle's medium nonessential amino acids (Gibco). Infection assays for microarray analysis, cytokine secretion, bacterial adherence and invasion were performed as previously described (Doran et al., 2003, Doran et al., 2005, Uchiyama et al., 2009). Bacterial adherence and invasion was calculated as (recovered CFU / initial inoculum CFU) x 100%. For inhibitor studies cells were pretreated (30 min) with indicated amounts of cytochalasin D, TCPK, SB202190 and U0126 (Sigma), prior to incubation with bacteria. For activation experiments, hBMEC were treated with TNFα (Peprotech Inc. Rocky Hill, NJ; 200ng/ml), or recombinant NanA (0.5 µM) for 3 hours, after which time proteins were removed and monolayers washed 1X with PBS prior to incubation with bacteria.
RNA isolation, cDNA preparation, RT-PCR and chemokine expression
RNA was isolated from hBMEC monolayers or mouse brain tissue infected with SPN or isogenic ΔnanA mutant, using the RNEasy kit (Qiagen, Valencia, CA) according to the manufacturer's instruction. 1 µg of RNA was reverse transcribed to cDNA (Superscript First-strand synthesis kit, Invitrogen) and quantitative PCR (qPCR) was performed using the following primer sets: KC-F, 5'- CCGCGCCTATCGCCAATG-3' and KC-R: 5'-CTTGGGGACACCTTTTAGCATCTTTTGG -3'; Beta-Actin F: 5'-ACCCACACTGTGCCCATCTAC-3' and Beta Actin R: 5'-AGCCAAGTCCAGACGCAGG-3'; primer sets for IL-6, IL-8, CXCL1, CXCL2, CCL20 and PCR amplification conditions including primer efficiencies have been described previously (Sorge et al., 2008). PCR primer efficiencies for KC and Beta actin were 2.10 and 2.08 respectively. Calculation of relative gene expression included adjustments for PCR efficiencies and using the following equation: Relative gene expression = target gene efficiency x (CT control - CT sample) / efficiency for Beta actin or GAPDH x (CT control - CT sample).
Concentrations of cytokines and chemokines in hBMEC supernatants collected 6h post infection with SPN WT or the ΔnanA mutant were measured using enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions for IL-8, IL-6, CCL20, CXCL1 (R&D systems, Minneapolis, MN, USA) and CXCL2 (BioSupplyUK).
Microarray analysis
Microarray experiments were performed using Sentrix Human-8 Expression BeadChips, which analyzed 25,440 transcripts (Illumina, San Diego, CA) according to manufacturer's instructions and as described previously (Sorge et al., 2008). Data were analyzed using a statistical algorithm developed for high-density oligonucleotide arrays (Sasik et al., 2002).
Expression and purification of NanA-GST fusion proteins
The nanA gene was amplified from the genomic DNA of SPN D39 using the following primers, BamHI-NanA-F (5′-GAATTCGGATCCCAAGAAGGGGCAAGT-3′), XhoI-NanA-R (5′-CAGATCCTCGAGTGCCTGCTGAGCAAG-3′) and cloned in BamHI/XhoI site of pGEX4T-2 (GE HealthSciences). NanAΔEnz was amplified using the same set of primers from pNanAΔEnz and was also cloned into BamHI/XhoI site of pGEX4T-2. The recombinant plasmids, pGEX-NanA and pGEX-NanAΔEnz, were verified by DNA sequencing. Expression and purification of NanA-GST and NanAΔEnz-GST fusion proteins were performed according to manufacturer's instructions. Briefly, E. coli BL21(DE3) cells carrying pGEX-NanA or pGEX-NanAΔEnz were grown at 37°C and fusion protein expression was induced with 1 mM IPTG. After 4 h of induction, cells were harvested by centrifugation and lysed by sonication. Proteins were purified from the crude extract using Glutathione-Sepharose (GE HealthSciences) column chromatography. Fractions collected were concentrated using Amicon Ultra centrifugal devices (Millipore) and buffer exchanged in PBS using PD10 columns (GE HealthSciences). The purified proteins were >95% pure as evident from SDS-PAGE (Fig. 3A). GST alone was similarly purified and served as a control protein. The level of endotoxin in purified protein preparations was determined using ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (Genscript) according to manufacture’s directions.
Sialidase activity assay
A quantitative assay utilizing 4-Methylumbelliferyl-N-acetyl-α-D-neuraminic acid sodium salt hydrate (4-MU; Fluka USA) was used to assay sialidase activity as described previously (Uchiyama et al., 2009). Briefly, protein dilutions were mixed with 50 µl of 4-MU diluted to 0.35% in phosphate citrate buffer. The plate was incubated at 37°C and fluorescence (excitation 360 nm, emission 460 nm) recorded every 15 minutes from time 0 to 1 h; with reported values corrected for the reaction blank containing phosphate citrate buffer alone.
In vivo neutrophil chemotaxis and myeloperoxidase assay
Neutrophil recruitment was determined using an in vivo chemotaxis assay as described previously (Sorge et al., 2008). SPN WT and ΔnanA mutant were grown to early log phase, washed and resuspended in PBS to OD600 = 0.4. Eight week old CD-1 male mice were injected subcutaneously with 1×106 CFU (0.1 ml) of either WT SPN or ΔnanA mutant on the right or left shaved flank, respectively. After 4 hours, mice were euthanized and the site of subcutaneous injection was excised for further analysis of bacterial counts or myeloperoxidase (MPO) activity as described previously (Sorge et al., 2008).
Mouse Infection Studies
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, all animal work was approved by the appropriate committee (SDSU protocol APF #07-07-014D). A murine model of early CNS infection using SPN WT and the isogenic ΔnanA mutant has been described previously (Uchiyama et al., 2009). Briefly, 8 week old male BALB/c (Charles River) mice were injected intravenously with 5–6 ×107 CFU of SPN WT or isogenic ΔnanA mutant (n=8 per group). Six hours after injection, mice were euthanized and blood and brain were collected, ∼30 mg brain tissue was homogenized in RLT buffer (Qiagen, Valencia, CA) containing β-mercaptoethanol (10µl/ml) using a bead beater, and total RNA extracted using the RNEasy kit (Qiagen) according to the manufacturer's instruction. Remaining brain tissue was homogenized in PBS and lysate plated on THY plates for enumeration of bacterial colonies. For a longer endpoint experiment mice were injected intravenously with 5 ×104 CFU of SPN WT or isogenic ΔnanA mutant (n=8 per group). 24 hours after injection, mice were euthanized and blood and brain were collected. One half of brain was fixed in PBS + 4% PFA for histopathological analysis and the remaining half was homogenized in PBS and lysate plated on THY plates for enumeration of bacterial colonies.
Statistical analysis
Graphpad Prism version 4.03 was used for statistical analysis. Differences in adherence/invasion, mRNA expression, chemokine secretion in hBMEC supernatants were evaluated using Students t-test. Differences in neutrophil recruitment were determined using a paired t-test for the MPO assay. Statistical significance was accepted at p < 0.05.
Supplementary Material
Acknowledgments
The authors are grateful to Monique Stins and Kwang Sik Kim for providing hBMEC and Roman Sasik for assistance with microarray data analysis. The microarray analysis was performed at the Biogem Core Facility of the University of California San Diego, director Gary Hardiman, and histopathologic analysis performed at the UCSD Core Facility, director Nissi Varki. This work was supported by grant R01NS051247 from the NINDS/NIH to K.S.D.
References
- Bernatoniene J, Zhang Q, Dogan S, Mitchell TJ, Paton JC, Finn A. Induction of CC and CXC chemokines in human antigen-presenting dendritic cells by the pneumococcal proteins pneumolysin and CbpA, and the role played by toll-like receptor 4, NF-kappaB, and mitogen-activated protein kinases. J Infect Dis. 2008;198:1823–1833. doi: 10.1086/593177. [DOI] [PubMed] [Google Scholar]
- Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL. An overview of the multiple functions of the blood-brain barrier. NIDA Res Monogr. 1992;120:54–72. [PubMed] [Google Scholar]
- Betz AL, Goldstein GW. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. doi: 10.1146/annurev.ph.48.030186.001325. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Camara M, Boulnois GJ, Andrew PW, Mitchell TJ. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 1994;62:3688–3695. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–168. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- Dias WB, Fajardo FD, Graca-Souza AV, Freire-de-Lima L, Vieira F, Girard MF, et al. Endothelial cell signalling induced by trans-sialidase from Trypanosoma cruzi. Cell Microbiol. 2008;10:88–99. doi: 10.1111/j.1462-5822.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Invest. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran KS, Liu GY, Nizet V. Group B streptococcal beta hemolysin/cytolysin activates neutrophil signalling pathways in brain endothelium and contributes to developments of meningitis. J. Clin. Invest. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Ferletta M, Kikkawa Y, Yu H, Talts JF, Durbeej M, Sonnenberg A, et al. Opposing roles of integrin alpha6beta1 and dystroglycan in laminin mediated extracellular signal regulated kinase activation. Mol. Biol. Cell. 2003;14:2088–2103. doi: 10.1091/mbc.E03-01-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans Jd, Beek Dvd. Dexamethasone in Adults with Bacterial Meningitis. New Eng. J. Med. 2002;347:1549–1556. doi: 10.1056/NEJMoa021334. [DOI] [PubMed] [Google Scholar]
- Graham RM, Paton JC. Differential role of CbpA and PspA in modulation of in vitro CXC chemokine responses of respiratory epithelial cells to infection with Streptococcus pneumoniae. Infect Immun. 2006;74:6739–6749. doi: 10.1128/IAI.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieshima K, Imai T, Opdenakker G, Damme JV, Kusuda J, Tei H, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J. Biol. Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- Hsiao Y-S, Parker D, Ratner AJ, Prince A, Tong L. Crystal structures of respiratory pathogen neuraminidases. Biochem. Biophys. Res. Comm. 2009;380 doi: 10.1016/j.bbrc.2009.01.108. 467–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RT, Farmer S, Greiff D. Neuraminidase activities of clinical isolates of Diplococcus pneumoniae. J. Bacteriol. 1967;94:272–273. doi: 10.1128/jb.94.1.272-273.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS. Escherichia coli translocation at the blood-brain barrier. Infect. Immun. 2001;69:5217–5222. doi: 10.1128/IAI.69.9.5217-5222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Hippe KR, Gould JM, Bae D, Peterson S, Cline RT, et al. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol Microbiol. 2004;54:159–171. doi: 10.1111/j.1365-2958.2004.04252.x. [DOI] [PubMed] [Google Scholar]
- King SJ, Whatmore AM, Dowson CG. NanA, a neuraminidase from Streptococcus pneumoniae, shows high levels of sequence diversity, at least in part through recombination with Streptococcus oralis. J Bacteriol. 2005;187:5376–5386. doi: 10.1128/JB.187.15.5376-5386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem M, Beerthuyzen MM, Vaughan EE, Vos WMd, Kuipers OP. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 1997;63:4581–4584. doi: 10.1128/aem.63.11.4581-4584.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa A, Hisatsune A, Isohama Y, Katsuki H. Bacterial neuraminidase increases IL-8 production in lung epithelial cells via NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2009;379:754–759. doi: 10.1016/j.bbrc.2008.12.120. [DOI] [PubMed] [Google Scholar]
- Manco S, Hernon F, Yesilkaya H, Paton JC, Andrew PW, Kadioglu A. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect. Immun. 2006;74:4014–4020. doi: 10.1128/IAI.01237-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli DN, Christen S, Leib SL, Tauber MG. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr Opin Infect Dis. 2002;15:253–257. doi: 10.1097/00001432-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Mitchell TJ. Virulence factors and the pathogenesis of disease caused by Streptococcus pneumoniae. Res Microbiol. 2000;151:413–419. doi: 10.1016/s0923-2508(00)00175-3. [DOI] [PubMed] [Google Scholar]
- Nizet V, Kim K, Stins M, Jonas M, Chi EY, Nguyen D, Rubens CE. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole RD, Goode L, Howe C. Neuraminidase activity in bacterial meningitis. J Clin Invest. 1971;50:979–985. doi: 10.1172/JCI106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. Tissue specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 2004;190:1661–1669. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Variation in the Presence of Neuraminidase Genes among Streptococcus pneumoniae Isolates with Identical Sequence Types. Infect. Immun. 2006;74:3360–3365. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll Tvd, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- Pracht D, Elm C, Gerber J, Bergmann S, Rohde M, Seiler M, et al. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect Immun. 2005;73:2680–2689. doi: 10.1128/IAI.73.5.2680-2689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen EI. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerieux F, Belaise M, Terzidis-Trabelsi H, Greffard A, Pilatte Y, Lambre CR. Determination of the sialic acid linkage specificity of sialidases using lectins in a solid phase assay. Anal Biochem. 1993;211:200–204. doi: 10.1006/abio.1993.1257. [DOI] [PubMed] [Google Scholar]
- Sasik R, Calvo E, Corbeil J. Statistical analysis of high-density oligonucleotide arrays: a multiplicative noise model. Bioinformatics. 2002;18:1633–1640. doi: 10.1093/bioinformatics/18.12.1633. [DOI] [PubMed] [Google Scholar]
- Scanlon KL, Diven WF, Glew RH. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme. 1989;41:143–150. doi: 10.1159/000469069. [DOI] [PubMed] [Google Scholar]
- Shakhnovich E, King S, Wiser JN. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect. Immun. 2002;70:7161–7164. doi: 10.1128/IAI.70.12.7161-7164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge NMv, Ebrahimi CM, McGillivray SM, Quach D, Sabet M, Guiney DG, Doran KS. Antrax toxins inhibit neutrophil signalling pathways in brain endothelium and contribute to the pathogenesis of meningitis. Plos One. 2008;3:e2964. doi: 10.1371/journal.pone.0002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence HJ, Dhillon AS, James M, Windler SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5:484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stins MF, Prasadarao NV, Ibric L, Wass CA, Luckett P, Kim KS. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am. J. Pathol. 1994;145:1228–1336. [PMC free article] [PubMed] [Google Scholar]
- Stins MF, Prasadarao NV, Zhou J, Arditi M, Kim KS. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev. Biol. Anim. 1997;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin 1 and 2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix protein. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. Sialidases: structures, biological significance and therapeutic potential. Curr. Opin. Struct. Biol. 1996;6:830–837. doi: 10.1016/s0959-440x(96)80014-5. [DOI] [PubMed] [Google Scholar]
- Tong HH, Blue LE, James MA, DeMaria TF. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase deficient mutant in nasophraryngeal colonisation and development of otitis media in the chinchila model. Infect. Immun. 2000;68:921–924. doi: 10.1128/iai.68.2.921-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong HH, James MA, Grants I, Liu X, Shi G, DeMaria TF. Comparison of structural changes of cell surface carbohydrates in the eustachian tube epithelium of chinchilas infected with a Streptococcus pneumoniae neuraminidase deficient mutant or its isogenic parent strain. Microb. Pathog. 2001;31:309–317. doi: 10.1006/mpat.2001.0473. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Carlin AF, Khosravi A, Weiman S, Banerjee A, Quach D, et al. The surface anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J. Exp. Med. 2009;206:1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkmeir A, Latsch K, Dietrich G, Wintermeyer E, Schinke B, Schwender S, et al. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol Microbiol. 2002;46:933–946. doi: 10.1046/j.1365-2958.2002.03222.x. [DOI] [PubMed] [Google Scholar]
- Weisfelt M, Beek Dvd, Spanjaard L, Reitsma JB, Gans Jd. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 2006;5:123–129. doi: 10.1016/S1474-4422(05)70288-X. [DOI] [PubMed] [Google Scholar]
- Winter AJ, Comis SD, Osborne MP, Tarlow MJ, Stephen J, Andrew PW, et al. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 1997;65:4411–4418. doi: 10.1128/iai.65.11.4411-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Potter JA, Russell RJM, Oggioni MR, Andrew PW, Taylor GL. Crystal structure of the NanB sialidase from Streptococcus pneumoniae. J. Mol. Biol. 2008;384:436–449. doi: 10.1016/j.jmb.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Yesilkaya H, Soma-Haddrick S, Crennell SJ, Andrew PW. Identification of amino acids essential for catalytic activity of pneumococcal neuraminidase A. Res. Microbiol. 2006;157:569–575. doi: 10.1016/j.resmic.2005.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.