Abstract
We previously reported that in the sheep fetus, long term hypoxia (LTH) resulted in elevated basal plasma ACTH1–39 while cortisol levels were not different from normoxic controls. We also showed that LTH enhances endothelial nitric oxide synthase (eNOS) expression in the fetal adrenal. This study was designed to determine the effect of nitric oxide on cortisol production in adrenocortical cells from LTH fetal sheep. Ewes were maintained at high altitude (3,820 m) from ~40 days’ gestation (dG) to near term. Between 138–141 dG, fetal adrenal glands were collected from LTH and age-matched normoxic control fetuses. Adrenal cortical cells were pre-treated with sodium nitroprusside (SNP), L-NAME, L-arginine, or Diethyleneamine nitric oxide (DETA-NO) then challenged with 10nM ACTH. Cortisol responses were compared after 1 h. ACTH induced cortisol secretion was significantly higher in LTH vs. control (p<0.01). Enhancement of nitric oxide with L-arginine resulted in a significant reduction of ACTH-mediated cortisol production in the LTH group. DETA-NO also caused a significant decrease in ACTH-mediated cortisol production (p<0.05). Inhibition of NOS with L-NAME significantly increased cortisol production in the LTH group (p<0.05 compared to ACTH alone) while the effect on the control group was not significant. NOS activity was significantly higher in the LTH group compared to control but this difference was eliminated following ACTH treatment. These data indicate that LTH enhances adrenal cortical sensitivity to the inhibitory effects of NO on cortisol production. NO may therefore play an important role in regulating ACTH-induced cortisol production in the LTH fetal adrenal.
Keywords: nitric oxide synthase, L-NAME, sodium nitroprusside
Introduction
In the ovine fetus, there is an exponential increase in fetal plasma cortisol concentration during the final ~20 days of gestation in conjunction with the maturation of the fetal hypothalamo-pituitary-adrenal axis. 1 This increased cortisol production assures maturation of fetal organs and stimulates the parturition process. It is imperative that plasma cortisol is maintained at normal levels throughout gestation to allow for normal growth and development as evidenced by premature elevations in fetal plasma cortisol resulting in preterm delivery of a growth-restricted fetus.2–5 Our laboratory has demonstrated that fetal sheep adapt to development under conditions of long-term hypoxia (LTH) by maintaining normal basal plasma cortisol concentrations despite elevated basal plasma adrenocorticotropic hormone (ACTH).6–8 The mechanism(s) that govern this adaptation is not fully understood and a number of potential mechanisms may be involved in the regulation of fetal cortisol production under conditions of LTH. One potential regulatory effector is enhanced production of nitric oxide (NO).
Nitric oxide (NO) is a diatomic free-radical gas that has a wide range of physiological functions.9, 10 NO is synthesized from L-arginine by a family of nitric oxide synthases (NOS);11 the constitutively expressed neuronal NOS (nNOS/ NOS-I) and endothelial NOS (eNOS/NOS-III) and a third inducible isoform (iNOS/NOS-II). eNOS and nNOS are regulated by Ca2+ and calmodulin, while iNOS is Ca2+/calmodulin independent.12 NO produced by eNOS and nNOS regulates a range of physiologic functions while iNOS tends to be invoked in more pathological situations.
NO has a profound effect on steroidogenesis in endocrine tissues. NO inhibited steroidogenesis in ovarian tissue of women,13 pigs,14, 15 rabbits, 16, 17 and rats, 18 while inhibition of NOS increased testosterone production in Leydig cells.19 Immobilization stress increased NO in adult rat testis and reduced production of testosterone.20 Although less is known about the effects of NO on adrenal steroidogenesis, NO inhibits basal, ACTH and angiotensin II-induced aldosterone production in zona glomerulosa cells of adult rat adrenal cortex transfected with eNOS.21 NO mediated inhibition of aldosterone was also shown to be cGMP-independent,22 and reversed by NOS inhibitor thiocitrulline.23 NOS inhibition also increased aldosterone production in humans.24 NO donors decrease both un-stimulated and ACTH-stimulated corticosterone production in rat zona fasciculata cells while NOS inhibition enhances glucocorticoid output,25 implicating NO-mediated inhibition of key rate-limiting steps in the steroidogenic pathway.26 Adams and coworkers also found NO to act as a negative inhibitor of corticosterone synthesis.27
These data, together with evidence that NOS or the effects of NO may be regulated by hypoxia 28–30 and other stressors,31 suggest NO may be involved in the adrenocortical adaptation to LTH in the ovine fetus by preventing premature maturation of the fetal adrenal cortex resulting in excess cortisol production in the face of the significantly elevated ACTH. We recently observed that LTH enhances expression of eNOS in sheep fetal adrenal cortex and that eNOS was located in the cortisol producing cells of the zona fasciculata.32 This was the first study to demonstrate the presence of eNOS in the ovine fetal adrenal cortex and the pronounced upregulation of eNOS in response to LTH. However, it is not known whether differential NOS expression represents a functional mechanism of cortisol regulation in response to LTH. The present study was designed to determine the effect of nitric oxide on basal and ACTH-induced cortisol production in adrenocortical cells from LTH fetal sheep and to assess the effect of LTH on NOS activity.
Materials and Methods
Animals
Pregnant ewes were maintained at high altitude (3,820 m) from approximately day 40 of gestation to near term (term ~ 146 days). Following transportation to the laboratory, hypoxia was maintained by nitrogen infusion through a non-occlusive maternal tracheal catheter as previously described.6, 33 Age matched, normoxic ewes served as controls. Between days 138–141 of gestation, the ewes were sedated with pentobarbital, intubated, and maintained under general anesthesia with 1.5–2% halothane in oxygen while the fetuses were delivered through a midline laparotomy. Procedures were preformed as previously described in detail. 8, 34, 35 Fetal adrenal glands were collected from the LTH and age-matched normoxic control animals for cell dispersion and subsequent study.
Cell Dispersion
Fetal adrenal glands were placed in phosphate buffered saline, divided in half along the longitudinal axis and the cortex was micro-dissected from the capsule and medulla.
The cortical tissue was minced then gently digested in a suspension of collagenase type IV and DNAse and filtered through a 100 µm cell strainer followed by treatment with red blood cell lysis buffer and centrifugation for cell collection. Cells were incubated in Ham’s F10 medium (Mediatech, Manassas, VA) for a 2 hr recovery period, then aliquotted into individual tubes (2.5 × 105 cells per tube; in duplicate). Cell viability was confirmed by Trypan blue exclusion.
Effects of NO on Cortisol Production
Fetal adrenal cortical cells (FACs) were subjected to a 30 min pre-treatment with either media alone (control) the NO donor sodium nitroprusside (SNP, 1mM), NOS substrate (L-arginine (L-Arg), 2mM), or the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 1mM) at 37C. Concentrations were based on preliminary dose response experiments and other published reports. 22, 25, 36 After the pre-incubation, cells were stimulated with ACTH (10 nM) for one hour at 37° C. Following the 1 h incubation, the tubes were centrifuged, cells and media separated, and both stored at −80° C until analysis. Cortisol analysis was performed using a commercially available ELISA kit (Oxford Biomedical Research; #EA 65) as previously described and validated in our laboratory.37 Additional studies were performed to confirm the effects of the NO-donor SNP on cortisol production by using another NO donor, Diethyleneamine nitric oxide (DETA-NO, 0.5mM). This does was found to be effective in inhibition of steroidogenesis in adult rat adrenal fasciculata.25 We also treated FACs with a combination of L-NAME (1.0 mM) and L-Arg (2mM) to confirm that the effect of L-NAME was due to inhibition of NOS.
NOS Activity in Fetal Adrenocortical Cells
Additional FACs, incubated for 1 h at 37° C in the presence or absence of ACTH, were immediately snap frozen in liquid nitrogen for determination of NOS activity. At the time of assay, the cortical cells were homogenized in 200µl lysis buffer (20mM HEPES-KOH, 10mM KCl, 1.5mM MgCl2, 1mM EDTA, 167mM dithiothreitol, 100mM PMSF, 5µg/ml Leupeptin, 0.8µg/ml aprotinin) for 30 seconds. The mixture was then centrifuged at 14000 × g for 15 minutes and the supernatant retained. Total protein was determined by Bradford method (Bio-Rad). NOS activity was determined using an enzyme linked immunoassay kit (Oxford Biomedical Research, NB 78) following the manufacture’s instructions. NOS activity was measured as µM nitrite/µg protein.
Statistical analysis
Differences in cortisol responses and NOS activity were determined by two-way ANOVA and with Bonferonni post tests where appropriate (Prism 4.0 software) and p<0.05 was considered significant.
Results
Effects of NO Cortisol Production
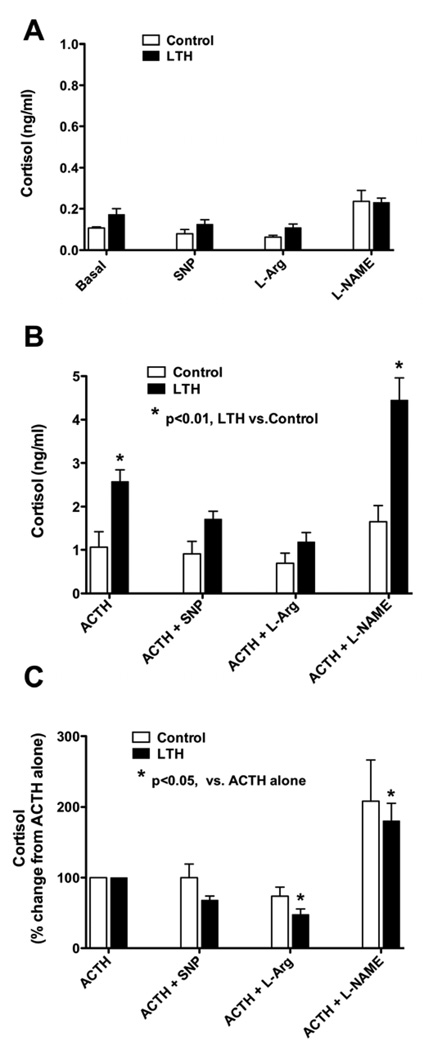
Basal (un-stimulated) cortisol production and the effects of alterations in NO on adrenal cortical cells cortisol production from control and LTH adrenal cortical cells are illustrated in Figure 1A. Although there was a trend towards higher cortisol production from FACs obtained from LTH fetuses under basal, SNP and L-Arg treatment, there were no differences in cortisol production between control and LTH FACs in any of the treatment groups examined in the absence of ACTH. Neither SNP, L-Arg or L-NAME had a significant effect on cortisol production from either control or LTH FACs.
Figure 1.
Cortisol production in fetal adrenal cortical cells (FACs) from control and LTH ovine fetuses as described in the Methods section. A) untreated basal, or treated with sodium nitroprusside (SNP, 1mM), NOS substrate (L-arginine (L-Arg), 2mM) or the NOS inhibitor L-NAME (1mM) B) treated with ACTH (10nM) or pre-treated with sodium nitroprusside (SNP, 1mM), NOS substrate (L-arginine, 2mM) or the NOS inhibitor L-NAME (1mM) followed by ACTH treatment as described in the Methods section. C) Cortisol data normalized to % change from ACTH treatment alone. (n=6 for control and n=7 for LTH). LTH indicates long-term hypoxia; L-NAME, nitro-L-arginine methyl ester; NOS, nitric oxide synthase; ACTH, adrenocorticotropic hormone.
In response to ACTH (Figure 1B), cortisol production was significantly greater in the LTH FACs compared with controls (p<0.01). Pre-treatment with SNP or L-Arg, abolished the enhanced cortisol production in response to ACTH observed in the LTH FACs. In contrast, the cortisol production was significantly greater in the LTH group compared to control in response to ACTH following L-NAME pre-treatment (p<0.01).
Since the LTH FACs produced significantly more cortisol in response to ACTH than the control group, we normalized the data to percent cortisol response to ACTH alone, and then compared the effects of alterations in NO synthesis for each group (Figure 1C). In the control fetal FACs, enhancement of NO synthesis with either SNP or L-Arg pre-treatment had no effect on cortisol production in response to ACTH. In marked contrast, in the LTH group compared to ACTH treatment alone, enhancement of NO synthesis with L-Arg pre-treatment significantly reduced cortisol production (p<0.05). A similar trend was observed with SNP but was not significant. In the LTH FACs, NOS inhibition with L-NAME resulted in a significant increase (p<0.01) in cortisol production compared to ACTH alone. A similar trend was noted in the control group but did not reach statistical significance.
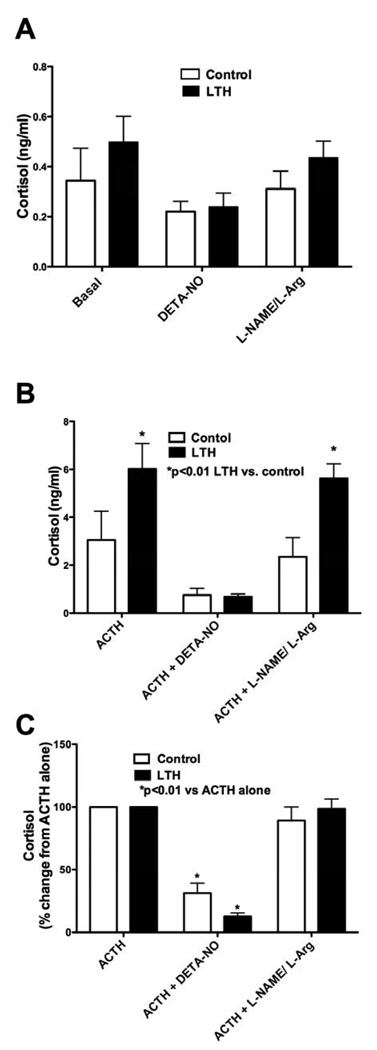
In order to confirm the effect of NO on inhibition of cortisol synthesis, we also studied another NO donor, DETA-NO. Under basal conditions, there was no effect on cortisol production on either control or LTH FACs (Figure 2A). As observed above, LTH FACs had a significantly higher cortisol production in response to ACTH compared to controls (Figure 2B, p<0.01) while pre-treatment with DETA-NO eliminated this difference. Again, since LTH FACs produced significantly more cortisol in response to ACTH than the control group, we normalized the data to percent cortisol response to ACTH alone, and then compared the effects of alterations in NO synthesis within each group (Figure 2C). Pre-treatment with DETA-NO significantly reduced the cortisol response to ACTH in both control and LTH FACs (p<0.01). We also pretreated cells with both L-NAME and L-Arg to ensure that the previously observed effects (Figure 1) were indeed the result of specific effects on NOS. Although this treatment had no effect on basal cortisol production (Figure 3A), the combination of the NOS substrate and NOS inhibitor restored cortisol production to levels observed with ACTH alone (Figure 3B).
Figure 2.
Cortisol production in FACS from control and LTH ovine fetuses as described in the Methods section. A) untreated basal, treated with Diethyleneamine (DETA-NO 0.5 nM), or a combination of L-Arg (2mM) and L-NAME (1mM). B) treated with ACTH (10nM) alone or pre-treated with Diethyleneamine (DETA-NO 0.5 nM), or a combination of L-arginine (2mM) and L-NAME (1mM) followed by ACTH treatment. C) Cortisol data normalized to % change from ACTH treatment alone. (n=4 for control and 6 for LTH). LTH indicates long-term hypoxia; FACs, fetal adrenal cortical cells; L-Arg, L-arginine; L-NAME, nitro-L-arginine methyl ester; ACTH, adrenocorticotropic hormone.
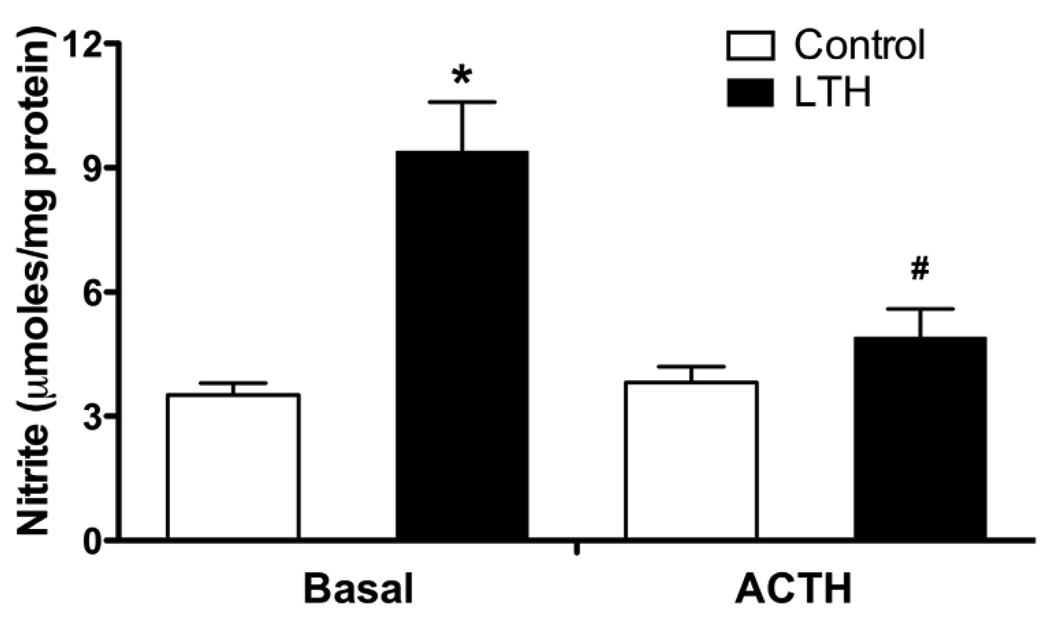
Figure 3.
NOS activity in control and LTH fetal adrenal cortical cells under basal and ACTH (10nM) stimulated conditions. Under basal conditions, LTH NOS activity was significantly greater than all other treatments (*p<0.05). Following ACTH treatment there was a significant reduction in NOS activity in the LTH group compared to basal (#p<0.05). (n=7 for each group). LTH indicates long-term hypoxia; ACTH, adrenocorticotropic hormone.
NOS Activity
Under basal conditions, NOS activity was significantly greater in the LTH FACs compared with control (p<0.05, Figure 3). Following ACTH treatment, NOS activity was unchanged in the control cells. In marked contrast, there was a significant reduction in NOS activity in LTH FACs (p<0.05), restoring levels to that observed in the control FACs.
Discussion
Previous studies from our laboratory demonstrated that in response to development under conditions of LTH, basal plasma cortisol concentrations in fetal sheep are in the normal range despite elevated basal ACTH. 6–8 Interestingly, in response to a secondary stressor, the cortisol response is enhanced in LTH fetuses compared to normoxic controls.6, 38 Although the factors involved in these important adaptive responses remain undefined, recent studies from our laboratory together with other observations suggest that regulation of nitric oxide in response to LTH in the fetal adrenal cortex may play a critical role. In a recent study, we demonstrated that LTH significantly elevated eNOS expression in sheep fetal adrenal cortex32. However, the mere presence of NOS does not necessarily indicate a functional role. In the present study, we demonstrate that stimulation of NO synthesis suppressed ACTH-stimulated cortisol synthesis in the LTH fetal adrenal while inhibition of NOS activity enhanced cortisol production. We further show for the first time that basal NOS activity was elevated in LTH fetal adrenocortical cells compared to normoxic control FACs and that ACTH treatment reduced NOS activity in adrenals cells from this group.
The effects of NO on vascular tissue are well established and largely depend on cGMP pathways (See 11 for review). In endocrine tissue however, the effects of NO on steroidogenesis appear to be cGMP-independent 21. NO interacts competitively with the oxygen binding site of key steroidogenic enzymes such as P450scc (CYP11A1) and P450c17 (CYP17)39. Peterson et al., 40 have suggested that since these enzymes use several rounds of attack of the heme-oxygen complex on the steroid substrate, such multi-step targets would be more sensitive to NO inhibition than other steroidogenic enzymes. These authors also suggested that since the lyase activity of CYP17 is a key branch point in differentiating glucocorticoid and androgen biosynthesis in the ovine fetal adrenal, inhibition by NO could play a role in maintaining the very low levels of DHEA that are associated with ovine adrenal activity while still allowing cortisol biosynthesis through 17-OH progesterone.40 Overall, this competitive interaction makes NO an effective inhibitor of steroidogenesis. Clearly, NO has been shown to inhibit key rate-limiting steps in the steroidogenic pathway and can inhibit steroidogenesis in a range of endocrine tissue including ovary 17, testis 41, 42 and adrenal glands 22, 25, 36. All of these studies however used adult tissue or cells. To our knowledge, there have been no previous studies utilizing fetal tissue or cells and examining the response to LTH.
The present studied examined the effect LTH and NO on cortisol synthesis in FACs. Under basal conditions (absence of ACTH), cortisol output is relatively low and no differences were noted between control and LTH fetal adrenal cells. However, with ACTH stimulation, a number of important changes occurred. In response to ACTH alone, there was a significant increase in cortisol output compared to un-stimulated conditions in both control and LTH adrenal cortical cells with a greater increase observed in LTH adrenal cortical cells. This finding confirms previous in vitro data 43 as well as in vivo studies 6, 38 showing enhanced cortisol responsiveness to stress levels of ACTH stimulation in LTH fetuses. Following pre-treatment with either an NO donor or NOS substrate, the difference in cortisol production from the fetal adrenal cells was eliminated between control and LTH groups (Figure 1B,). When data were normalized to cortisol output in response to ACTH alone, there was a significant inhibition of cortisol synthesis in fetal adrenal cells in the LTH group that was not present in the control. The LTH-enhanced increase in eNOS message and protein expression 32 coupled with the increased NOS activity under basal conditions (Figure 3) may be responsible for the differential effects of NO on cortisol production.
Data from the LTH fetal adrenal cells are similar to the observations of Cymeryng et al 25, 36 in adult rat adrenal cells and Y1 adrenal cell line in that that NO suppresses glucocorticoid production. NOS inhibition with L-NAME resulted in increased cortisol production in fetal adrenal cells in the LTH group (p<0.05) compared to ACTH alone. A similar trend was noted in adrenal cells from the control fetuses but was not significant. The effects of L-NAME on steroidogenesis in the present study are in agreement with the effects of NOS inhibition on aldosterone production in the adrenal 24, 44 and androgen in the testis 20. It has become clear that the extent of the effect of NO is probably dependent on species, tissue type, and physiological condition.
Higher basal levels of NOS activity in the FACs from the LTH group were expected based on our previous study showing enhanced expression of adrenal cortical eNOS 32. What was unexpected however, was the effect of ACTH on NOS activity. After ACTH treatment, NOS activity in the LTH FACs was reduced to levels comparable to control cells under both basal and ACTH stimulation (Figure 3). A number of mechanisms are involved in the regulation of NOS activity that include post-translational modification via phosphorylation, availability of substrates/cofactors and protein–protein interactions including binding to calcium-dependent calmodulin, caveolin-1 and heat shock protein 90 (Hsp90)45. Relevant to the potential effects of ACTH, one of the key factors that may link ACTH stimulation and a decrease in NOS activity is ERK1/2. Although the role of ERK 1/2 on NOS expression/activity is controversial, it is apparent that it plays a role in regulating NOS activity. ERK1/2 inhibition upregulated ATP stimulated eNOS activity in COS-7 cells 46. MEK/ERK 1/2 inhibition also enhanced eNOS activity in porcine pulmonary arteries 47. These studies suggest therefore, that stimulation of ERK 1/2 could therefore inhibit NOS activation. Further, ERK 1/2 stimulation plays an important role in adrenal steroidogenesis.48 We have recently shown using a MEK inhibitor that the ERK1/2 signaling pathway plays a key role in ACTH and cAMP induced cortisol synthesis in the ovine fetal adrenal cortisol cells.49 inhibiting ERK activity also had a more pronounced effect in the LTH cells suggesting that this pathway is upregulated in response to LTH. 43
Hypoxia has also been shown to have significant effects on NOS activity. Chen and Meyrick28 found that acute hypoxia stimulated Hsp90 binding to eNOS and activation of the PI3–Akt pathway resulting in increased eNOS phosphorylation. They suggested that this may be a mechanism whereby eNOS activity and subsequent NO production is upregulated in hypoxic coronary arteries. Other studies using hypoxic conditions for a longer duration confirmed that hypoxia increases eNOS generation of NO by enhancing Hsp-90 binding in myocardial tissue 50. The same group also found the hypoxia decreased caveolin-3 51, another mechanism that can contribute to enhanced NOS activity. Clearly future studies are warranted to address these key mechanisms regulating NOS function in the LTH fetal adrenal cells.
In the present study, the locus of the ACTH effect on NOS activity in the LTH cannot be established. However, together with the data showing significant inhibition of cortisol output with NO stimulation and enhanced cortisol output in response to NOS inhibition, it appears that this may be a mechanism of regulating cortisol responses under conditions of LTH. In vivo under basal conditions, despite higher basal levels of ACTH, LTH fetuses maintain plasma cortisol concentrations at a level similar to normoxic controls. It is possible however, that although the elevated basal ACTH concentrations may not significantly affect basal cortisol biosynthesis, they may play a role in regulating adrenal growth under hypoxic conditions. The previously reported upregulation of adrenal eNOS in the LTH group 32 may be responsible for enhanced basal NOS activity observed in the present study, which would exert an inhibitory effect on basal cortisol production overcoming the low level of stimulation by elevated basal ACTH. Stimulation with stress levels of ACTH in vitro as in the present study or those observed in vivo in response to a secondary stressor6, 38 decrease NO activity, thus enhancing cortisol output.
In conclusion, the present study demonstrated for the first time in fetal FACs, that NO plays a role in the regulation of cortisol biosynthesis following LTH exposure. Further, we have demonstrated that LTH enhances adrenocortical sensitivity to NO and that ACTH treatment reduces NOS activity. Taken together these data strengthen the concept that NO may be a modulator of cortisol production and ACTH stimulation increases cellular sensitivity to NO following LTH. ACTH stimulation also reduces NOS activity in LTH adrenocortical cells, making NO a potential driving force in LTH fetal responsiveness to a secondary stressor. Future studies will focus on the precise mechanisms involved in LTH induced ACTH reduction of NOS enzyme activity.
Acknowledgments
Supported by NIH grant HD-31226
REFERENCES
- 1.Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, Lowe KC, Buster JE, Nathanielsz PW. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980;107:155–159. doi: 10.1210/endo-107-1-155. [DOI] [PubMed] [Google Scholar]
- 2.Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 3.Jenkin G, Young IR. Mechanisms responsible for parturition; the use of experimental models. Anim Reprod Sci. 2004;82–83:567–581. doi: 10.1016/j.anireprosci.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz J, McMillen IC. Fetal hypothalamus-pituitary-adrenal axis on the road to parturition. Clin Exp Pharmacol Physiol. 2001;28:108–112. doi: 10.1046/j.1440-1681.2001.03412.x. [DOI] [PubMed] [Google Scholar]
- 5.Whittle WL, Patel FA, Alfaidy N, Holloway AC, Fraser M, Gyomorey S, Lye SJ, Gibb W, Challis JR. Glucocorticoid regulation of human and ovine parturition: The relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglandin production. Biol Reprod. 2001;64:1019–1032. doi: 10.1095/biolreprod64.4.1019. [DOI] [PubMed] [Google Scholar]
- 6.Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. AmJPhysiol RegulIntegrComp Physiol. 2004;287:R209–R217. doi: 10.1152/ajpregu.00701.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ducsay CA. Fetal and maternal adaptations to chronic hypoxia: Prevention of premature labor in response to chronic stress. Comp BiochemPhysiol A MolIntegrPhysiol. 1998;119:675–681. doi: 10.1016/s1095-6433(98)01004-6. [DOI] [PubMed] [Google Scholar]
- 8.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. AmJPhysiol RegulIntegrComp Physiol. 2005;288:R1178–R1184. doi: 10.1152/ajpregu.00697.2004. [DOI] [PubMed] [Google Scholar]
- 9.Moncada S, Palmer RM. Biosynthesis and actions of nitric oxide. Semin Perinatol. 1991;15:16–19. [PubMed] [Google Scholar]
- 10.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 11.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 12.Michel T, Feron O. Nitric oxide synthases: Which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Voorhis BJ, Dunn MS, Snyder GD, Weiner CP. Nitric oxide: An autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology. 1994;135:1799–1806. doi: 10.1210/endo.135.5.7525252. [DOI] [PubMed] [Google Scholar]
- 14.Masuda M, Kubota T, Aso T. Effects of nitric oxide on steroidogenesis in porcine granulosa cells during different stages of follicular development. EurJEndocrinol. 2001;144:303–308. doi: 10.1530/eje.0.1440303. [DOI] [PubMed] [Google Scholar]
- 15.Masuda M, Kubota T, Karnada S, Aso T. Nitric oxide inhibits steroidogenesis in cultured porcine granulosa cells. MolHumReprod. 1997;3:285–292. doi: 10.1093/molehr/3.4.285. [DOI] [PubMed] [Google Scholar]
- 16.Gobbetti A, Boiti C, Canali C, Zerani M. Nitric oxide synthase acutely regulates progesterone production by in vitro cultured rabbit corpora lutea. JEndocrinol. 1999;160:275–283. doi: 10.1677/joe.0.1600275. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi J, Miyazaki T, Iwasaki S, Kishi I, Kuroshima M, Tei C, Yoshimura Y. Effects of nitric oxide on ovulation and ovarian steroidogenesis and prostaglandin production in the rabbit. Endocrinology. 1997;138:3630–3637. doi: 10.1210/endo.138.9.5392. [DOI] [PubMed] [Google Scholar]
- 18.Mitsube K, Mikuni M, Matousek M, Brannstrom M. Effects of a nitric oxide donor and nitric oxide synthase inhibitors on luteinizing hormone-induced ovulation in the ex-vivo perfused rat ovary. HumReprod. 1999;14:2537–2543. doi: 10.1093/humrep/14.10.2537. [DOI] [PubMed] [Google Scholar]
- 19.Dobashi M, Fujisawa M, Yamazaki T, Okuda Y, Kanzaki M, Tatsumi N, Tsuji T, Okada H, Kamidono S. Inhibition of steroidogenesis in leydig cells by exogenous nitric oxide occurs independently of steroidogenic acute regulatory protein (star) mrna. ArchAndrol. 2001;47:203–209. doi: 10.1080/014850101753145915. [DOI] [PubMed] [Google Scholar]
- 20.Kostic T, Andric S, Kovacevic R, Maric D. The involvement of nitric oxide in stress-impaired testicular steroidogenesis. EurJPharmacol. 1998;346:267–273. doi: 10.1016/s0014-2999(98)00057-0. [DOI] [PubMed] [Google Scholar]
- 21.Hanke CJ, Drewett JG, Myers CR, Campbell WB. Nitric oxide inhibits aldosterone synthesis by a guanylyl cyclase-independent effect. Endocrinology. 1998;139:4053–4060. doi: 10.1210/endo.139.10.6252. [DOI] [PubMed] [Google Scholar]
- 22.Hanke CJ, O'Brien T, Pritchard KA, Jr, Campbell WB. Inhibition of adrenal cell aldosterone synthesis by endogenous nitric oxide release. Hypertension. 2000;35:324–328. doi: 10.1161/01.hyp.35.1.324. [DOI] [PubMed] [Google Scholar]
- 23.Hanke CJ, Campbell WB. Endothelial cell nitric oxide inhibits aldosterone synthesis in zona glomerulosa cells: Modulation by oxygen. AmJPhysiol EndocrinolMetab. 2000;279:E846–E854. doi: 10.1152/ajpendo.2000.279.4.E846. [DOI] [PubMed] [Google Scholar]
- 24.Muldowney JA, III, Davis SN, Vaughan DE, Brown NJ. No synthase inhibition increases aldosterone in humans. Hypertension. 2004;44:739–745. doi: 10.1161/01.HYP.0000143852.48258.f1. [DOI] [PubMed] [Google Scholar]
- 25.Cymeryng CB, Dada LA, Podesta EJ. Effect of nitric oxide on rat adrenal zona fasciculata steroidogenesis. JEndocrinol. 1998;158:197–203. doi: 10.1677/joe.0.1580197. [DOI] [PubMed] [Google Scholar]
- 26.Drewett JG, ms-Hays RL, Ho BY, Hegge DJ. Nitric oxide potently inhibits the rate-limiting enzymatic step in steroidogenesis. MolCell Endocrinol. 2002;194:39–50. doi: 10.1016/s0303-7207(02)00214-9. [DOI] [PubMed] [Google Scholar]
- 27.Adams ML, Nock B, Truong R, Cicero TJ. Nitric oxide control of steroidogenesis: Endocrine effects of ng-nitro-l-arginine and comparisons to alcohol. Life Sci. 1992;50:PL35–PL40. doi: 10.1016/0024-3205(92)90384-2. [DOI] [PubMed] [Google Scholar]
- 28.Chen JX, Meyrick B. Hypoxia increases hsp90 binding to enos via pi3k-akt in porcine coronary artery endothelium. Lab Invest. 2004;84:182–190. doi: 10.1038/labinvest.3700027. [DOI] [PubMed] [Google Scholar]
- 29.Xiao D, Bird IM, Magness RR, Longo LD, Zhang L. Upregulation of enos in pregnant ovine uterine arteries by chronic hypoxia. AmJPhysiol Heart CircPhysiol. 2001;280:H812–H820. doi: 10.1152/ajpheart.2001.280.2.H812. [DOI] [PubMed] [Google Scholar]
- 30.Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilatation by chronic hypoxia in ovine cranial arteries. JApplPhysiol. 2006;100:225–232. doi: 10.1152/japplphysiol.00221.2005. [DOI] [PubMed] [Google Scholar]
- 31.Kostic TS, Andric SA, Maric D, Kovacevic RZ. Inhibitory effects of stressactivated nitric oxide on antioxidant enzymes and testicular steroidogenesis. JSteroid BiochemMolBiol. 2000;75:299–306. doi: 10.1016/s0960-0760(00)00185-0. [DOI] [PubMed] [Google Scholar]
- 32.Monau TR, Vargas VE, King N, Yellon SM, Myers DA, Ducsay CA. Long-term hypoxia increases endothelial nitric oxide synthase expression in the ovine fetal adrenal. Reprod Sci. 2009;16:865–874. doi: 10.1177/1933719109336678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1997–R2005. doi: 10.1152/ajpregu.00313.2007. [DOI] [PubMed] [Google Scholar]
- 34.Mlynarczyk M, Imamura T, Umezaki H, Kaushal KM, Zhang L, Ducsay CA. Long-term hypoxia changes myometrial responsiveness and oxytocin receptors in the pregnant ewe: Differential effects on longitudinal versus circular smooth muscle. Biol Reprod. 2003;69:1500–1505. doi: 10.1095/biolreprod.103.018556. [DOI] [PubMed] [Google Scholar]
- 35.Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. AmJPhysiol RegulIntegrComp Physiol. 2005;289:R1707–R1714. doi: 10.1152/ajpregu.00343.2005. [DOI] [PubMed] [Google Scholar]
- 36.Cymeryng CB, Dada LA, Colonna C, Mendez CF, Podesta EJ. Effects of l-arginine in rat adrenal cells: Involvement of nitric oxide synthase. Endocrinology. 1999;140:2962–2967. doi: 10.1210/endo.140.7.6848. [DOI] [PubMed] [Google Scholar]
- 37.Ducsay CA, Mlynarczyk M, Kaushal KM, Hyatt K, Hanson K, Myers DA. Long-term hypoxia enhances acth response to arginine vasopressin but not corticotropin-releasing hormone in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2009;297:R892–R899. doi: 10.1152/ajpregu.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. JSocGynecolInvestig. 2004;11:131–140. doi: 10.1016/j.jsgi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Tsubaki M, Hiwatashi A, Ichikawa Y, Hori H. Electron paramagnetic resonance study of ferrous cytochrome p-450scc-nitric oxide complexes: Effects of cholesterol and its analogues. Biochemistry. 1987;26:4527–4534. doi: 10.1021/bi00388a054. [DOI] [PubMed] [Google Scholar]
- 40.Peterson JK, Moran F, Conley AJ, Bird IM. Zonal expression of endothelial nitric oxide synthase in sheep and rhesus adrenal cortex. Endocrinology. 2001;142:5351–5363. doi: 10.1210/endo.142.12.8537. [DOI] [PubMed] [Google Scholar]
- 41.Del Punta K, Charreau EH, Pignataro OP. Nitric oxide inhibits leydig cell steroidogenesis. Endocrinology. 1996;137:5337–5343. doi: 10.1210/endo.137.12.8940355. [DOI] [PubMed] [Google Scholar]
- 42.Kostic TS, Andric SA, Maric D, Stojilkovic SS, Kovacevic R. Involvement of inducible nitric oxide synthase in stress-impaired testicular steroidogenesis. JEndocrinol. 1999;163:409–416. doi: 10.1677/joe.0.1630409. [DOI] [PubMed] [Google Scholar]
- 43.Vargas VE, Monau T, Kaushal KM, Myers DA, Ducsay CA. Long term hypoxia enhances acth-induced cortisol secretion in the near term ovine fetal adrenal in vitro. J Repro Sci. 2008 doi: 10.1177/1933719110386242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sainz JM, Reche C, Rabano MA, Mondillo C, Patrignani ZJ, Macarulla JM, Pignataro OP, Trueba M. Effects of nitric oxide on aldosterone synthesis and nitric oxide synthase activity in glomerulosa cells from bovine adrenal gland. Endocrine. 2004;24:61–71. doi: 10.1385/ENDO:24:1:061. [DOI] [PubMed] [Google Scholar]
- 45.Chen JX, Lawrence ML, Cunningham G, Christman BW, Meyrick B. Hsp90 and akt modulate ang-1-induced angiogenesis via no in coronary artery endothelium. J Appl Physiol. 2004;96:612–620. doi: 10.1152/japplphysiol.00728.2003. [DOI] [PubMed] [Google Scholar]
- 46.Cale JM, Bird IM. Inhibition of mek/erk1/2 signalling alters endothelial nitric oxide synthase activity in an agonist-dependent manner. Biochem J. 2006;398:279–288. doi: 10.1042/BJ20060371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem. 2000;275:30707–30715. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- 48.Gyles SL, Burns CJ, Whitehouse BJ, Sugden D, Marsh PJ, Persaud SJ, Jones PM. Erks regulate cyclic amp-induced steroid synthesis through transcription of the steroidogenic acute regulatory (star) gene. J Biol Chem. 2001;276:34888–34895. doi: 10.1074/jbc.M102063200. [DOI] [PubMed] [Google Scholar]
- 49.Vargas VE, Myers DA, Kaushal KM, Ducsay CA. Acth induced cortisol synthesis in ovine fetal adrenocortical cells is mediated in part by extracellular signal regulated kinase (erk) 1 and 2: Effect of long term hypoxia (lth) Reprod Sci. 2009;16 250A Number 3. [Google Scholar]
- 50.Shi Y, Baker JE, Zhang C, Tweddell JS, Su J, Pritchard KA., Jr Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ Res. 2002;91:300–306. doi: 10.1161/01.res.0000031799.12850.1e. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Pritchard KA, Jr, Holman P, Rafiee P, Griffith OW, Kalyanaraman B, Baker JE. Chronic myocardial hypoxia increases nitric oxide synthase and decreases caveolin-3. Free Radic Biol Med. 2000;29:695–703. doi: 10.1016/s0891-5849(00)00364-6. [DOI] [PubMed] [Google Scholar]