Abstract
Adipose tissue is a major reservoir of cholesterol and, as such, it may play a significant role in cholesterol homeostasis. The aims of this study were to obtain a quantitative characterization of apolipoprotein A-I (apoA-I)-dependent lipid efflux from adipocytes and examine the role of ATP-binding cassette transporter A1 (ABCA1) in this process. The rates of apoA-I-induced cholesterol and phospholipid efflux were determined and normalized by cellular protein or ABCA1 levels. In order to allow a comparative analysis, parallel experiments were also performed in macrophages. These studies showed that apoA-I induces cholesterol efflux from adipocytes at similar rates as from macrophages. Enhancement of the expression of ABCA1 increased the rates of cholesterol efflux from both adipocytes and macrophages. The results also suggested that a non-ABCA1-dependent mechanism could make significant contributions to the rate of apoA-I-dependent cholesterol efflux when the expression levels of ABCA1 are low. Furthermore, the study of the effect of inhibitors of lipid efflux showed that glyburide and brefeldin A, which affect ABCA1 function, exerted strong and similar inhibitory effects on lipid efflux from both adipocytes and macrophages, whereas BLT1, an SRB-I inhibitor, only exerted a moderate inhibition. Overall these studies suggest that ABCA1 plays a major role in apoA-I-dependent lipid efflux from adipocytes and showed high similarities between the abilities of adipocytes and macrophages to release cholesterol in an apoA-I-dependent fashion.
Keywords: apoA-I, Adipocytes, Macrophages, Lipid efflux, ABCA1, Cholesterol
Introduction
Cholesterol (CL) plays important roles in maintaining cellular functions. The cellular levels of CL are defined by the relative rates of synthesis, uptake, catabolism, and efflux to external acceptors. Most cells have only a limited ability to catabolize CL [1], and the removal of excess CL is mainly accomplished through a pathway termed reverse cholesterol transport (RCT). This pathway involves lipid efflux from cells to extracellular acceptors that transport CL to the liver for processing and excretion as bile salts or for recycling to maintain CL homeostasis [2].
Although the entire RCT pathway is needed for efficient removal of excess cellular CL, the initial step in which lipoproteins (mainly apoA-I) are thought to bind to the membrane transporter ABCA1 and stimulate efflux to form HDL, is believed to be a crucial step [3–7]. This is supported by the fact that mutations in ABCA1 lead to Tangier’s disease (TD) [8–10], which is characterized by cellular cholesterol ester accumulation and very low levels of HDL [11]. The importance of ABCA1’s role in HDL biogenesis is also evident by the inability of TD fibroblasts to efflux lipids to apoA-I [12, 13].
It has been recently reported that liver and intestine are the main tissues contributing to the production of circulating HDL in mice [14, 15]. These studies, however, did not take into account the potential contribution of adipose tissue, which is the largest reservoir of stored free cholesterol in the body [16, 17]. The mass of the stored CL varies with the body mass, but it is proportional to the amount of triglycerides [18, 19] and, therefore, to the mass of adipose tissue.
Extensive studies have provided a good characterization of the process of apoA-I-induced CL efflux in macrophage and liver cells [20–24]. Previous studies have shown that incubation of 3T3 L-1 adipocytes with apoA-I promotes lipid efflux into the media [25, 26]. These previous studies, however, have not sought to provide a detailed quantitative account of the process of cholesterol efflux in adipocytes. This study intends to provide a significant characterization of the process of apolipoprotein A-I (apoA-I) dependent lipid efflux from adipocytes and also to examine the role of ATP-binding cassette transporter A1 (ABCA1) in this process. Because both the rate of apoA-I-induced cholesterol efflux and the role of ABCA1 have been amply studied in macrophages [7, 20, 23], we have carried out parallel experiments with adipocytes and J774 macrophages to allow a simple comparison of the kinetics of cholesterol efflux and the role of ABCA1 across different cell types.
Materials and methods
Materials
J774 and 3T3 L-1 cells were purchased from American Type Cell Culture (Manassas, VA). Dimethyl sulfoxide (DMSO), brefeldin A (BFA), glyburide (GLYB), fatty acid free bovine serum albumin (BSA), isobutyl methyl xanthine (IBMX), dexamethasone, trypsin, insulin, GW3965, streptomycin, and penicillin were purchased from Sigma Chemicals Co. (St. Louis, MO). Fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Cellgro Mediatech, Inc (Herndon, VA). Block lipid transport-4 (BLT4) was from Chembridge Corp. (San Diego, CA). BLT-1 was a gift from Dr. George H. Rothblat. Anti-ABCA1 NB400-105 was purchased from Novus Biologicals (Littleton, CO). Infinity Cholesterol Reagent was purchased from Hitachi (Indianapolis, IN). Phospholipid C kit was purchased from WAKO diagnostics (Richmond, VA). [3H]-choline and [3H]-cholesterol were purchased from PerkinElmer (Waltham, MA).
Cell culture
J774 macrophages or 3T3 L-1 pre-adipocytes were cultured in six-well plates at 37°C in 8% CO2 atmosphere in DMEM supplemented with 10% FBS and 0.01% antibiotics. J774 were allowed to reach 90% confluence prior to experiments. For differentiation of 3T3 L-1 into adipocytes, cells were allowed to grow to confluence and then the differentiation into adipocytes was induced by addition of IBMX (111 µg/ml), dexamethasone (0.46 µg/ml), and insulin (1.5 µg/ml) [27]. After 48 h, the cells were incubated in DMEM/10% FBS containing insulin for an additional 48 h. Afterward, the cells were maintained in DMEM/10% FBS. All experiments using adipocytes were conducted 12–14 days after the differentiation period.
Analysis of cellular lipids
Cell monolayers from individual wells were scrapped and homogenized. Aliquots of homogenate were used for lipid extraction with chloroform:methanol (2:1 by vol) (28). Lipids were separated by TLC along with known amounts of standards and visualized with iodine vapor. The spots of samples and standards were then scrapped off the plates and lipids extracted from the silica with either 1:1 chloroform:methanol (CL) or 80:20 methanol:chloroform (PL) and dried. These lipid extracts were then reconstituted as per manufacturer’s instructions for either a choline-containing phospholipid colorimetric assay (Phospholipid C assay kit, WAKO) or a cholesterol colorimetric assay (Infinity Reagent, Hitachi).
Cholesterol efflux assays
Prior to the efflux experiment, fully differentiated 3T3 L-1 adipocytes or J774 macrophages were incubated for 22–24 h in 2.5% FBS–DMEM containing [3H]-cholesterol (2 µCi/ml) and 2 µM GW3965 (or buffer) for adipocytes or 180 µM 8-Br-cAMP (or buffer) for macrophages. Recombinant apoA-I was cloned and purified as previously described was used to initiate cellular lipid efflux [29]. At the start of the experiment, fresh 0.05% BSA–DMEM (to determine background levels of efflux) or 0.05%BSA–DMEM containing 75 µg/ml apoA-I was added to the cells. Aliquots of media were taken at indicated time points, centrifuged, and used to determine [3H]-cholesterol efflux by scintillation counting. After allowing efflux to proceed for 5 h, the wells were washed and cellular cholesterol extracted with isopropanol. Whole well [3H]-cholesterol (cpm) was determined by adding the cpm found in the media to the cpm present in the isopropanol extract. The percent of cellular CL efflux was calculated at each time point as:
ApoA-I-induced lipid efflux was determined as the difference between the mean percent of CL efflux in medium containing apoA-I and the average percent of lipid efflux found in wells without apoA-I (background efflux). The standard deviation of apoA-I-induced efflux at each time point was estimated from the standard deviations of the background and apoA-I total efflux using the propagation of error approach. This approach was used for each individual time point. The time course of apoA-I-induced CL release (as % total) was fitted by linear regression to estimate the corresponding rate of efflux.
Phospholipid efflux assays
In order to measure phospholipid (PL) efflux, macrophages or adipocytes were labeled in 2.5% FBS-M199 for 24 h with [3H]-choline [5 µCi/ml]. At time zero, DMEM–0.05%BSA containing 75 µg/ml of recombinant apoA-I was added to each well. Background efflux was determined in wells containing medium without apoA-I. Aliquots of media were collected at indicated time points, and at the final time point the whole well homogenate and remaining media were collected. PL contained in the media and homogenates were extracted using the Folch procedure [28]. The organic phases containing the lipids were dried completely and used to determine cpm associated to [3H]-choline labeled PL by liquid scintillation counting. The percent of cellular PL efflux was calculated from the amounts of [3H]-choline labeled PL determined in media and cell homogenates as:
The mean values and standard deviations of apoA-I-induced PL efflux were determined as indicated for CL efflux.
Lipid efflux inhibition assays
Lipid efflux was studied as mentioned above, but after labeling the lipid pools, the cells were pre-incubated with the drugs for 2 h prior to experiment. These pre-incubations were carried out in DMEM–0.05% BSA media containing BFA (10 µg/ml), GLYB (500 µM), BLT1(10 µM), BLT4 (150 µM), or DMSO as a control. After 2 h, zero time for efflux, the media was replaced with fresh media containing only the inhibitor, or the inhibitor and 75 µg/ml of recombinant apoA-I. Media and homogenates were collected and protein and lipids analyzed as described above. ApoA-I-induced lipid efflux was calculated by subtracting the mean values of lipid efflux observed in the presence of inhibitor alone (background efflux %) from the mean efflux percent determined in wells containing both inhibitor and apoA-I. The standard deviation of apoA-I-induced efflux was estimated from the standard deviations of the background and total efflux using the propagation of error approach. This approach was used for each individual time point. The resulting time course was fitted by linear regression to obtain the rate of apoA-I-induced efflux. The efficiency of the inhibition was estimated as the ratio percent of the mean rate in the presence of inhibitor to the mean rate in the absence of inhibitor. The corresponding standard deviations were calculated using the propagation of errors.
Estimation of the relative levels of cellular ABCA1 protein expression
A semi-quantitative analysis of cellular ABCA1 protein was done by Western blotting of aliquots of homogenates. The samples analyzed were obtained from some of the wells not used for lipid efflux studies but contained in the same plate. The volumes of homogenate separated by electrophoresis were adjusted such that equal amounts of protein (70 µg) were loaded for all samples. After SDS-PAGE, the proteins were transferred to a nitrocellulose membrane and incubated with rabbit polyclonal anti-ABCA1. All samples to be compared, adipocytes (control and GW3965 treated) and macrophages (control and 8-Br-cAMP treated), were loaded in the same gels and simultaneously blotted and analyzed. After detection by chemiluminescence, the films were scanned, and the band intensities were determined by densitometry using AlphaEase software (Santa Clara, CA). The band intensities in arbitrary density units (a.u.) were directly compared within the same blots. In order to compare the results of different blots the band intensities were first normalized using the intensity of adipocytes control as internal reference. These levels of ABCA1 protein were used to calculate the rates of lipid efflux normalized by ABCA1 protein: nmol lipid/h-ABCA1.
Statistical analysis
The statistical significance of differences was determined between the means of control and treated samples using the Student’s t-test.
Results
Lipid efflux from adipocytes
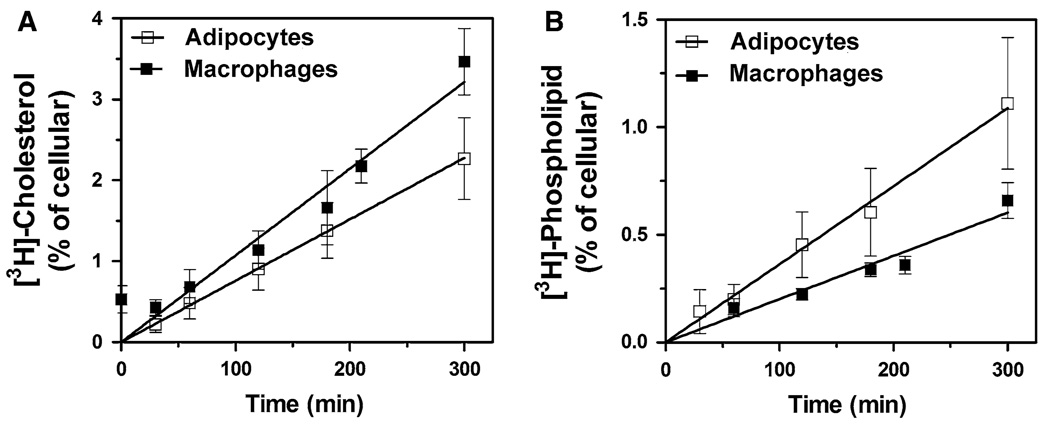
In order to characterize apoA-I-dependent lipid efflux from adipocytes, the apparent rates (%cellular lipid released/h) of apoA-I-induced cholesterol release from [3H]-cholesterol-labeled adipocytes was determined. ApoA-I-induced CL efflux was estimated as the difference between the fractions of cellular CL released into the cell culture medium observed in the presence and in the absence of apoA-I at a given time. Similar studies were also performed in the murine macrophage cell line J774 to allow the comparison of adipocytes with a well-studied cell type. As shown in Fig. 1a, after 5-h period, apoA-I promotes the release of ~2.4% of the cellular cholesterol content of adipocytes and a significantly higher fraction from macrophages (~ 3.3%). Conversely, the study of apoA-I-induced PL efflux showed that the apolipoprotein induces the release of 1.04 and 0.60% of the cellular PL contents of adipocytes and macrophages, respectively (Fig. 1b). These differences in percent, however, are related to both the lipid content of the cells and the actual rates of lipid efflux induced by apoA-I. In order to determine the rates of lipid efflux, in nmoles of lipid/h mg protein, the PL and CL contents of adipocytes and macrophages were determined. As expected, due to the presence of free cholesterol in the lipid droplets, the CL content of adipocytes is significantly higher than that of macrophages (Table 1). This feature of adipocytes can also be observed through the comparison of the molar ratios of cellular CL to PL. Adipocytes have a molar ratio of CL to PC + SM ~ 1.0, which is greater than that of macrophages (~ 0.75). From the lipid contents of the cells and the fractions of lipid efflux the rates of CL and PL efflux in nmoles lipid/h-mg protein were calculated. These estimates (Table 1) show that apoA-I induces similar rates of CL efflux from adipocytes and macrophages. Table 1 also shows that apoA-I induces the release two moles of CL per mol of PL (PC + SM) in adipocytes, whereas this ratio is four in macrophages. This difference is due to the higher rate of PL efflux in adipocytes. The results obtained in macrophages are consistent with previously reported data [22–24, 30]. Moreover, it has been shown that in macrophages, overexpression of ABCA1, by cAMP-treatment, leads to a decrease in the molar ratio of CL to PL from 4 to 2 [23, 30].
Fig. 1.
Lipid efflux from adipocytes and macrophages. a Cholesterol efflux: [3H]-cholesterol-labeled 3T3 L-1 adipocytes or J774 macrophages were incubated with apoA-I (75 µg/ml) or with buffer as described in “Methods” section “Cholesterol efflux assays”. The data points representing apoA-I-induced CL efflux from adipocytes (□) and macrophages (■) were calculated by subtracting background CL efflux (no apoA-I) from the CL efflux of wells containing apoA-I. The mean efflux and SD, for each time point, include data from 25 wells for adipocytes and from 10 wells for macrophages. b Phospholipid efflux: Cells were labeled with [3H]-choline and used to determine apoA-I-induced PL (PC + SM) efflux as indicated in Section “Phospholipid efflux assays”. apoA-I-induced PL efflux was calculated by subtracting the background PL efflux, determined in the absence of apoA-I, from the PL efflux determined in wells containing apoA-I. At each time point, the mean efflux and SD were calculated with data from nine wells for adipocytes (□) and seven wells for macrophages (■)
Table 1.
Cellular lipid compositions and rates of apoA-I-dependent efflux of adipocytes and macrophages
| Adipocytes | Macrophages | |
|---|---|---|
| Cellular Phospholipid (nmol PC/mg protein)a | 45.7 ± 8.3 (7) | 33.9 ± 8.4 (12) |
| Cellular Cholesterol (nmol CL/mg protein)a | 46.2 ± 11.2 (27) | 25.7 ± 7.6 (15) |
| Phospholipid Efflux (nmol PC/h-mg protein)b | 0.10 ± 0.018 (33) | 0.041 ± 0.010 (44) |
| Cholesterol Efflux (nmol CL/h-mg protein)b | 0.21 ± 0.051 (138) | 0.17 ± 0.050 (70) |
| CL/PL Efflux ratio | 2.1 ± 0.64 | 4.0 ± 1.6 |
Cellular contents of choline-containing phospholipids (PC and SM) and cholesterol (CL) were determined as indicated in “Materials and methods” Section. Data are expressed as mean values ± SD. The number of independent data points, different wells, is included in parenthesis
The rates of lipid efflux (mean ± SD) were obtained by linear regression. Data from five experiments (J774 cells) and eight experiments using 3T3-L1 cells are included. The number of data points used in the calculation of the rates is included in parenthesis
The efflux stoichiometry in moles of CL per mol of PL was calculated from the average rates of efflux. The SD of the CL/PL ratio was estimated from the SDs of the rates of CL and PL efflux and the propagation of error equation
Effect of ABCA1 protein levels in the rate of cholesterol efflux from adipocytes
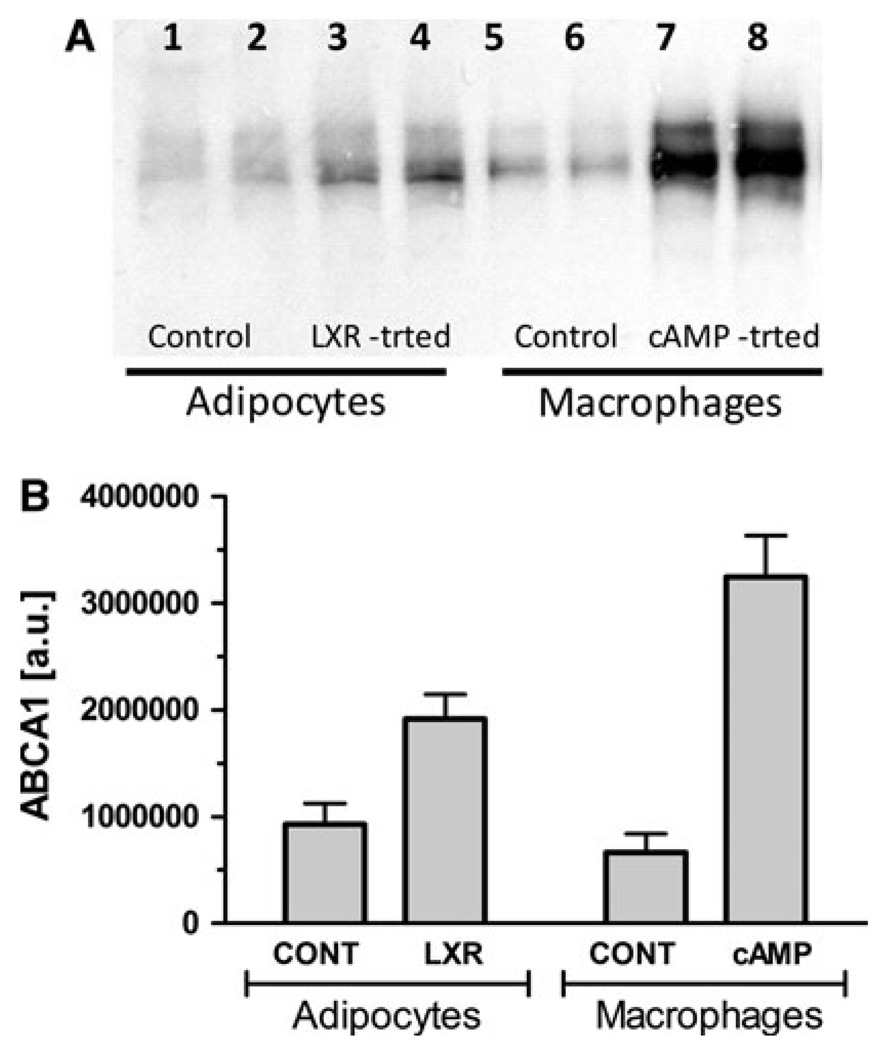
In order to investigate the role of ABCA1 in lipid efflux from adipocytes, the levels of ABCA1 protein in both adipocytes and macrophages were increased by treatments with inducers of ABCA1 expression, and the rates of lipid efflux determined. Previous studies have shown that incubation of J774 macrophages with cAMP analogs increases both ABCA1 mRNA and protein levels and stimulates apoA-I-induced lipid efflux through the ABCA1-mediated pathway [7, 31–34]. On the other hand, other studies [35–37] have shown that LXR agonists, such as GW3965, increase ABCA1 mRNA levels in adipocytes. Therefore, in order to investigate the role of ABCA1 in lipid efflux the rates of lipid efflux of macrophages and adipocytes treated with the cAMP analog 8-Br-cAMP and the LXR agonist, GW3965, respectively, were compared to the rates of control cells. The lipid efflux assays were performed as indicated before, and the levels of ABCA1 protein were estimated in the cell homogenates by Western blot analysis. Aliquots of the homogenates from control and treated adipocytes and macrophages were loaded in the same gels and simultaneously blotted to allow semi-quantitatively estimation of the relative levels of ABCA1 for different treatments and cells. Figure 2a shows a representative blot of samples from one experiment, whereas Fig. 2b shows the average values of combined experiments. As expected, ABCA1 is present in both adipocytes and macrophages. A clear increase in the expression of ABCA1 was observed in both LXR agonist-treated adipocytes and 8-Br-cAMP-treated macrophages. However, a much stronger induction of ABCA1 protein expression was observed in macrophages (4.9-fold) than in adipocytes (2.1-fold).
Fig. 2.
ABCA1 protein expression levels in adipocytes and macrophages. a Western blot: Aliquots of the cell homogenates from control and treated adipocytes and macrophages were loaded in the same gels and simultaneously blotted to allow semi-quantitatively estimation of the relative levels of ABCA1 for different treatments and cells. Each lane was loaded with 70 µg of homogenate protein. The Western blot shows the results of one experiment. Three separate experiments provided similar results. b Relative levels of ABCA1 protein: The level of ABCA1 protein was estimated by densitometric scanning of the western blots as explained in methods. The figure shows the relative average levels of ABCA1 normalized by cellular protein (mean ± SD). A total of seven samples from three separate experiments were included in the calculations of the means and corresponding SD. The differences between the means of control and LXR-treated samples of adipocytes, and also between control and cAMP-treated macrophages were estimated to be significant (P < 0.001)
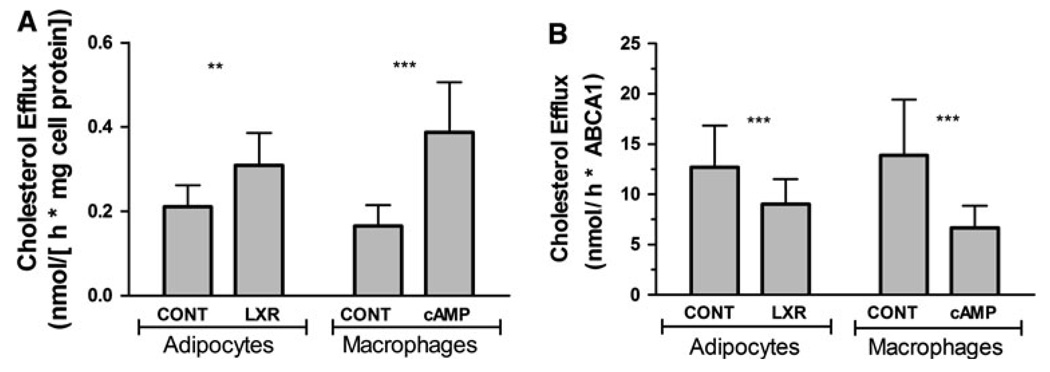
Accompanying the increase in expression of ABCA1, the rates of CL efflux also increased in adipocytes (1.38-fold) and macrophages (2.0-fold), see Fig. 3a. In order to further investigate the contribution of ABCA1 expression to the process of CL efflux, the average rates of apoA-I-induced CL efflux were normalized using the average relative levels of ABCA1 expression shown in the Fig. 2b. The resulting ABCA1-normalized rates are shown in Fig. 3b. These results show that ABCA1-normalized rates of apoA-I-induced CL efflux are higher for cells expressing basal levels of ABCA1. This difference is observed in both adipocytes and macrophages. The lower ABCA1-normalized rate of CL efflux in cells expressing higher levels of ABCA1 could be due to a significant contribution of an ABCA1-independent mechanism of CL efflux in control cells. Alternatively, it may indicate that induction of ABCA1 protein expression is not the only factor determining the rate of CL efflux to apoA-I.
Fig. 3.
Effect of ABCA1 expression levels in apoA-I-dependent CL Efflux from adipocytes or macrophages. a Rates of CL efflux normalized by total cellular protein content. The experiments were performed as indicated in “Materials and methods” section. The cells were mock-treated (CONT) or treated with either the LXR-agonist, 2 µM GW3965 (LXR), or 180 µM 8-Br-cAMP (cAMP) for 24 h prior to assays. The rates of apoA-I-induced CL efflux normalized by protein content were determined from the slopes of the time courses (% of cellular CL released into medium vs time). The mean values ± SD for adipocytes were obtained from three independent experiments and include 19 data points. The data for macrophages were obtained from four experiments (n = 15). The differences between the means of control and treated cells were significant (P < 0.001) for both adipocytes and macrophages. b Rates of CL efflux normalized by ABCA1 protein levels. The average rates of apoA-I-induced CL efflux shown in Fig. 3a were converted into nmoles of CL/h-ABCA1 protein using the average relative levels of ABCA1 expression shown in the Fig. 2b and the cholesterol contents of the cells. The lipid compositions of control and treated homogenates showed no significant differences. Each bar represents the mean ± SD. The SDs were calculated using the propagation of errors equation and the SD of ABCA1 levels and rates of efflux. The differences between the means of control and treated cells were significant (P < 0.001) for both adipocytes and macrophages
Effect of inhibitors of SR-BI and ABCA1 mediated cholesterol efflux
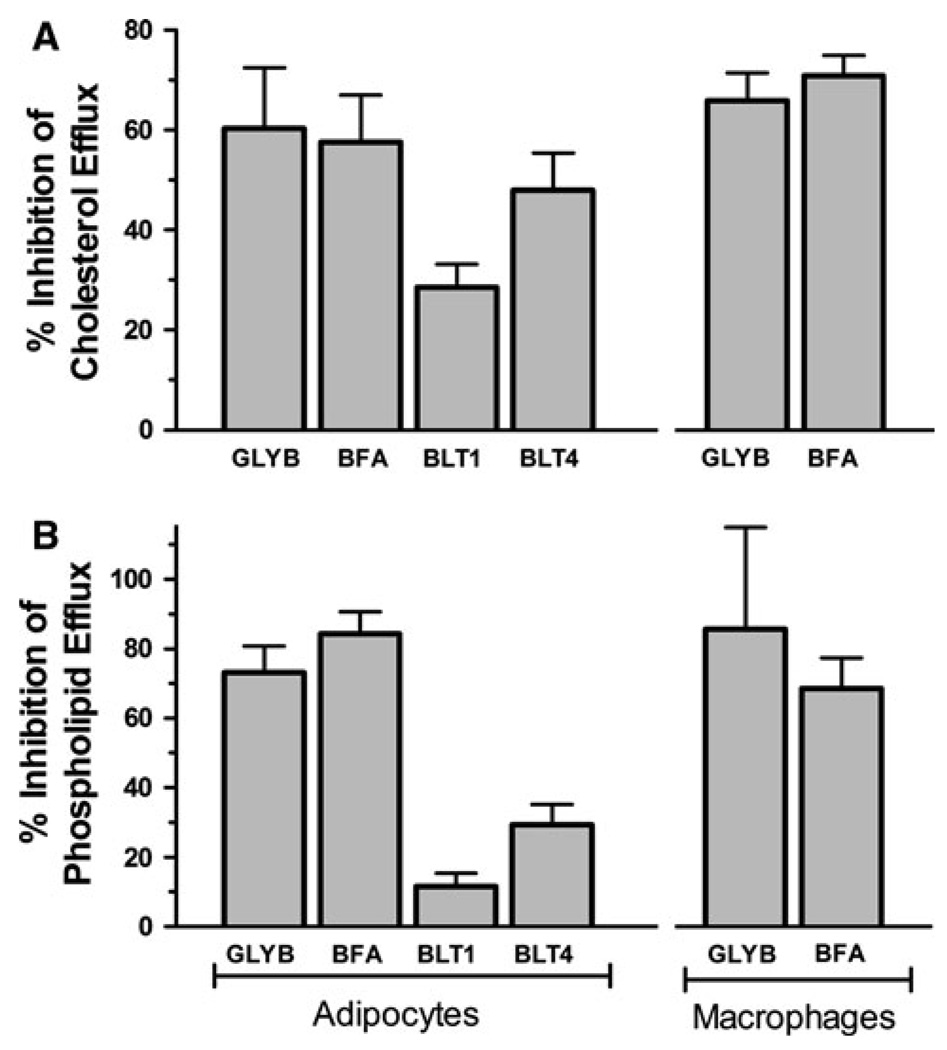
The role of ABCA1 in apoA-I-dependent lipid efflux from adipocytes was also investigated by performing efflux assays in the presence of several drugs that are thought to reduce apoA-I-dependent lipid efflux via inhibition of the ABCA1 transporter. The effects of the inhibitors on the rates of apoA-I-dependent CL and PL efflux were studied as described above, using [3H]-choline- or [3H]-cholesterol-labeled cells, but the cells were pre-incubated with the drugs prior to the addition of apoA-I. The time courses of inhibitor-treated and mock-treated cells were used to obtain the rates of efflux. The inhibition of apoA-I-dependent lipid efflux was expressed as percentage of the corresponding rates determined in mock-treated cells. Figure 4 shows that all inhibitors tested inhibited to some extent the release of cellular lipids. The sulfonylurea glyburide (GLYB) is known to block several ABC transporters including ABCA1 [3, 38–44]. GLYB strongly inhibited lipid efflux in both adipocytes and macrophages, suggesting a major role of ABCA1 in lipid efflux from adipocytes and confirming previous reports on the effects of GLYB and the function of ABCA1 in macrophages [45, 46].
Fig. 4.
Effect of ABCA1-inhibiting drugs on apoA-I-dependent efflux from adipocytes compared to macrophages. The effect of GLYB (500 µM), BFA (36 µM), BLT1 (10 µM), and BLT4 (150 µM) on apoA-I-dependent lipid efflux from macrophages and adipocytes was determined as described in “Methods” section Lipid efflux inhibition assays. a Inhibition of apoA-I-induced CL efflux from adipocytes and macrophages. The bars represent the mean ± SD of 2–4 independent experiments for adipocytes(n = 6–14) and one experiment for macrophages (n = 3). b Effects of the same drugs on inhibition of apoA-I-dependent phospholipid efflux from adipocytes and macrophages. The bars represent the mean ± SD of one experiment with n = 3–4
Brefeldin A also promoted a strong inhibition of CL and PL efflux in both macrophages and adipocytes. The inhibition exerted by BFA falls within the expected inhibition range (~ 58–85%) when compared to previously reported data obtained in fibroblasts, but is somewhat higher than data previously reported in macrophages [47, 48]. BFA has been shown to inhibit recycling of ABCA1 between the plasma membrane and intracellular compartments. This effect was suggested as the cause of its inhibitory effect on apoA-I-induced CL efflux [47, 49]. Overall, the observed inhibition of CL and PL efflux promoted by BFA in adipocytes, and its similarity to the effects observed in macrophages, suggests that ABCA1 transport is also important for the process of CL efflux in adipocytes.
In addition to GLYB and BFA, adipocytes were also incubated with BLT1 and BLT4, two members of the block lipid transport (BLT) family of inhibitors [50, 51]. BLT1 inhibits the scavenger receptor, class B, type I (SR-BI) [51], which mediates cellular uptake of cholesterol esters and, in the opposite direction, CL efflux to HDL [52, 53]. BLT1 was significantly less efficient than GLYB and BFA to inhibit either CL or PL efflux (Fig. 4). This result suggests a minor role of SR-BI in the process of apoA-I-induced cholesterol efflux in adipocytes.
Whereas GLYB and BFA affect ABCA1 function, and BLT1 is specific for SR-BI, it has been suggested that BLT4 cross inhibits both ABCA1-mediated and SR-BI-dependent CL efflux [50]. In our studies in adipocytes, BLT4 was more efficient than BLT1 at inhibiting lipid efflux, but less efficient than BFA and GLYB (Fig. 4). The higher inhibition observed with BLT4, as compared to BLT1, is consistent with the expected effects of BLT4 and also with the fact that ABCA1 plays a major role in apoA-I-induced lipid efflux from adipocytes. The low inhibition promoted by BLT4, as compared to BFA and GLYB, also suggests that the efficiency of BLT4 to bock ABCA1 is much lower than that of GLYB and BFA. BLT4 had been previously reported to strongly inhibit cholesterol release to apoA-I [50]. However, the effects of BLT4 on phospholipid efflux had not been examined until now. Another difference between the inhibitory effects of BLT4, as compared to the effects of BFA and GLYB, resides in the fact that BLTs inhibited CL efflux more effectively than PL efflux, whereas GLYB and BFA inhibited PL and CL efflux to nearly the same extent.
Discussion
As the largest storage site of free cholesterol in the body, adipocytes have the potential to influence circulating HDL [16, 17]. Previous studies have shown correlations between obesity and changes in the levels or composition of circulating HDL [54–56]. This suggests that lipid efflux from adipocytes may significantly alter overall HDL levels and compositions.
In this study, we intended to estimate the rate of apoA-I-induced cholesterol efflux from adipocytes and place this rate in the context of a reference cell type, macrophages, to allow a better evaluation of the significance of the data. This study also intended to examine the role of ABCA1 in CL efflux from adipocytes. The results of CL efflux are commonly expressed as percent of the cellular CL content of the wells. This expression of the data is useful when comparing data from the same cell type. However, it is less useful in comparisons across cell types, and in particular for comparisons with adipocytes, whose CL contents are significantly larger than those of most cell types. Here, we have shown that, if the rates are expressed in nmoles of lipid per h mg of protein, then macrophages and adipocytes have comparable rates of efflux. It was also important for us to compare the rates of lipid efflux normalized by ABCA1 protein content (nmoles CL/ABCA1 protein h). This comparison also showed similar basal rates of CL efflux in both cell types, suggesting that these cell types share similar pathways of CL efflux under basal conditions.
The effects of ABCA1 mutations and expression on apoA-I-dependent lipid efflux have demonstrated that ABCA1 plays a major role in the rate of lipid efflux in macrophages and few other cell types [33, 57–60]. However, the role of ABCA1 in lipid efflux from adipocytes has not been fully characterized. Here, we show that comparable levels of ABCA1 protein are found under basal conditions in adipocytes and macrophages. This result and the nearly identical rates of CL efflux observed, argue in favor of similar roles of ABCA1 in both cell types. Moreover, and also supporting a significant role of ABCA1 in CL efflux in adipocytes, when the expression of ABCA1 was induced, the rate of cholesterol efflux, in nmoles/h mg protein, increased in both cell types (Fig. 3a). The induction of ABCA1 expression was greater in macrophages (Fig. 2) and, correspondingly, the rates of efflux were also higher in these cells. However, when the rates of efflux were normalized by ABCA1 content, nearly identical rates were estimated for both cell types (Fig. 3b). A somewhat surprising result was the observation that the rate of CL efflux normalized by ABCA1 levels was higher for cells expressing basal levels of ABCA1. Although, a definite answer is not possible at this time, this result suggests the presence of an ABCA1-independent pathway that makes a significant contribution to the rate of CL efflux under basal conditions. Alternatively, this result, observed in both cell types, would suggest that expression of ABCA1 alone is not sufficient to promote a proportional increase in the rate of lipid efflux. The cellular treatments used may not have induced the expression of a second factor also needed for ABCA1-mediated lipid efflux.
In order to further investigate the role of ABCA1 in apoA-I-dependent lipid efflux from adipocytes, the effects of four inhibitors of lipid efflux were evaluated in both adipocytes and macrophages. The results showed that GLYB, an ABCA1-targeting drug [3] and BFA which affects ABCA1 transport [49], strongly inhibit apoA-I-dependent lipid efflux from adipocytes and macrophages. The strong inhibitory effects of these drugs apply to both apoA-I-induced PL and CL efflux. On the other hand, we also studied the effects of BLT1 and BLT4, the two inhibitors of lipid efflux that have not been as well tested as BFA and GLYB, but are interesting because they have been suggested to inhibit only SR-BI-mediated CL efflux, BLT1, or both SR-BI- and ABCA1-dependent lipid efflux, BLT4 [50]. BLT1 (10 µM) only had a minor effect on PL efflux to apoA-I and slightly inhibited CL efflux, suggesting a minor role of SR-BI- in apoA-I-induced lipid efflux. BLT4 has been shown to strongly inhibit CL efflux in HEK293 cells over-expressing ABCA1 [50]. Our assays in adipocytes showed, however, that BLT4 is a weak inhibitor of CL efflux. This conclusion is reached not only by comparing the inhibitory effects of BLT4 with the inhibitory effects observed with GLYB or BFA (Fig. 4), but also from the fact that BLT4 is used at a considerably high concentration (150 µM). We used BLT1 and BLT4 at the concentrations suggested from the original studies describing the use of these inhibitors [50, 51]. However, we think that the studies with BLT4 must be carefully evaluated because, given its hydrophobic nature and the concentration required in the assays, it is likely to affect many membrane and metabolic functions.
Overall, this study provides two significant conclusions. The first novel conclusion is that apoA-I induces efflux of cellular cholesterol at similar rates from adipocytes and macrophages. The second conclusion is that ABCA1 is a major player in determining the rate of apoA-I-dependent lipid efflux from adipocytes. This latter conclusion is supported by the studies on the levels of ABCA1 expression, their correlation with the rates of CL efflux, and the effects of inhibitors of lipid efflux.
Acknowledgments
The authors thank Noah Barnes for his assistance in cell culture and data collection. This study was funded by Oklahoma State Experiment Station, and NIH Grant GM55622.
Abbreviations
- ApoA-I
Apolipoprotein A-I
- ABCA1
ATP-binding cassette transporter A1
- GLYB
Glyburide
- BFA
Brefeldin A
- LXR
Liver X receptor
- BLT
Block lipid transport
- BSA
Bovine serum albumin
- FBS
Fetal bovine serum
- CL
Cholesterol
- PL
Phospholipid
- SR-BI
Scavenger receptor-BI
- BLT4
Block lipid transport-4
- BLT1
Block lipid transport-1
References
- 1.Babiker A, Andersson O, Lund E, Xiu RJ, Deeb S, Reshef A, Leitersdorf E, Diczfalusy U, Bjorkhem I. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem. 1997;272:26253–26261. doi: 10.1074/jbc.272.42.26253. [DOI] [PubMed] [Google Scholar]
- 2.Chiang JY. Regulation of bile acid synthesis. Front biosci. 1998;3:d176–d193. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- 4.Chambenoit O, Hamon Y, Marguet D, Rigneault H, Rosseneu M, Chimini G. Specific docking of apolipoprotein A-I at the cell surface requires a functional ABCA1 transporter. J Biol Chem. 2001;276:9955–9960. doi: 10.1074/jbc.M010265200. [DOI] [PubMed] [Google Scholar]
- 5.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 7.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 8.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 9.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 10.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 11.Fredrickson DS, Attrocchi PH, Avioli LV, Goodman DS, Goodman HC. Tangier Disease. Ann Intern Med. 1961;55:1016. [Google Scholar]
- 12.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J Clin Investig. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remaley AT, Schumacher UK, Stonik JA, Farsi BD, Nazih H, Brewer HB., Jr Decreased reverse cholesterol transport from Tangier disease fibroblasts Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler Thromb Vasc Biol. 1997;17:1813–1821. doi: 10.1161/01.atv.17.9.1813. [DOI] [PubMed] [Google Scholar]
- 14.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Investig. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Investig. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas J, Angel A, Avigan M. I. Studies on the compartmentation of lipid in adipose cells. II. Cholesterol accumulation and distribution in adipose tissue components. J Lipid Res. 1973;14:344–356. [PubMed] [Google Scholar]
- 17.Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- 18.Kovanen PT, Nikkila EA, Miettinen TA. Regulation of cholesterol synthesis and storage in fat cells. J Lipid Res. 1975;16:211–223. [PubMed] [Google Scholar]
- 19.Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984;25:97–110. [PubMed] [Google Scholar]
- 20.Yamauchi Y, Abe-Dohmae S, Yokoyama S. Differential regulation of apolipoprotein A-I/ATP binding cassette transporter A1-mediated cholesterol and phospholipid release. Biochim Biophys Acta. 2002;1585:1–10. doi: 10.1016/s1388-1981(02)00304-9. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama S. ABCA1 and biogenesis of HDL. J Atheroscler Thromb. 2006;13:1–15. doi: 10.5551/jat.13.1. [DOI] [PubMed] [Google Scholar]
- 22.Hara H, Yokoyama S. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J Biol Chem. 1991;266:3080–3086. [PubMed] [Google Scholar]
- 23.Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, Rothblat GH, Phillips MC. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Komaba A, Yokoyama S. Cholesterol is poorly available for free apolipoprotein-mediated cellular lipid efflux from smooth muscle cells. Biochemistry. 1993;32:4597–4603. doi: 10.1021/bi00068a016. [DOI] [PubMed] [Google Scholar]
- 25.Prattes S, Horl G, Hammer A, Blaschitz A, Graier WF, Sattler W, Zechner R, Steyrer E. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. J Cell Sci. 2000;113(Pt 17):2977–2989. doi: 10.1242/jcs.113.17.2977. [DOI] [PubMed] [Google Scholar]
- 26.Le Lay S, Robichon C, Le Liepvre X, Dagher G, Ferre P, Dugail I. Regulation of ABCA1 expression and cholesterol efflux during adipose differentiation of 3T3-L1 cells. J Lipid Res. 2003;44:1499–1507. doi: 10.1194/jlr.M200466-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Rubin CS, Hirsch A, Fung C, Rosen OM. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3–L1 cells. J Biol Chem. 1978;253:7570–7578. [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 29.Verghese PB, Arrese EL, Howard AD, Soulages JL. Brefeldin A inhibits cholesterol efflux without affecting the rate of cellular uptake and re-secretion of apolipoprotein A-I in adipocytes. Arch Biochem Biophys. 2008;478:161–166. doi: 10.1016/j.abb.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vedhachalam C, Liu L, Nickel M, Dhanasekaran P, Anantharamaiah GM, Lund-Katz S, Rothblat GH, Phillips MC. Influence of ApoA-I structure on the ABCA1-mediated efflux of cellular lipids. J Biol Chem. 2004;279:49931–49939. doi: 10.1074/jbc.M406924200. [DOI] [PubMed] [Google Scholar]
- 31.Sakr SW, Williams DL, Stoudt GW, Phillips MC, Rothblat GH. Induction of cellular cholesterol efflux to lipid-free apolipoprotein A-I by cAMP. Biochim Biophys Acta. 1999;1438:85–98. doi: 10.1016/s1388-1981(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith JD, Miyata M, Ginsberg M, Grigaux C, Shmookler E, Plump AS. Cyclic AMP induces apolipoprotein E binding activity and promotes cholesterol efflux from a macrophage cell line to apolipoprotein acceptors. J Biol Chem. 1996;271:30647–30655. doi: 10.1074/jbc.271.48.30647. [DOI] [PubMed] [Google Scholar]
- 33.Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- 34.Abe-Dohmae S, Suzuki S, Wada Y, Aburatani H, Vance DE, Yokoyama S. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 2000;39:11092–11099. doi: 10.1021/bi0008175. [DOI] [PubMed] [Google Scholar]
- 35.Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hummasti S, Laffitte BA, Watson MA, Galardi C, Chao LC, Ramamurthy L, Moore JT, Tontonoz P. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J Lipid Res. 2004;45:616–625. doi: 10.1194/jlr.M300312-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Sheppard DN, Welsh MJ. Effect of ATP-sensitive K + channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturgess NC, Ashford ML, Cook DL, Hales CN. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985;2:474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- 40.Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- 41.Payen L, Delugin L, Courtois A, Trinquart Y, Guillouzo A, Fardel O. The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells. Br J Pharmacol. 2001;132:778–784. doi: 10.1038/sj.bjp.0703863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golstein PE, Boom A, van Geffel J, Jacobs P, Masereel B, Beauwens R. P-glycoprotein inhibition by glibenclamide and related compounds. Pflugers Archiv. 1999;437:652–660. doi: 10.1007/s004240050829. [DOI] [PubMed] [Google Scholar]
- 43.Becq F, Hamon Y, Bajetto A, Gola M, Verrier B, Chimini G. ABC1, an ATP binding cassette transporter required for phagocytosis of apoptotic cells, generates a regulated anion flux after expression in Xenopus laevis oocytes. J Biol Chem. 1997;272:2695–2699. doi: 10.1074/jbc.272.5.2695. [DOI] [PubMed] [Google Scholar]
- 44.Fielding PE, Nagao K, Hakamata H, Chimini G, Fielding CJ. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry. 2000;39:14113–14120. doi: 10.1021/bi0004192. [DOI] [PubMed] [Google Scholar]
- 45.Smith JD, Le Goff W, Settle M, Brubaker G, Waelde C, Horwitz A, Oda MN. ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid Res. 2004;45:635–644. doi: 10.1194/jlr.M300336-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Kiss RS, Maric J, Marcel YL. Lipid efflux in human and mouse macrophagic cells: evidence for differential regulation of phospholipid and cholesterol efflux. J Lipid Res. 2005;46:1877–1887. doi: 10.1194/jlr.M400482-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Zha X, Gauthier A, Genest J, McPherson R. Secretory vesicular transport from the Golgi is altered during ATP-binding cassette protein A1 (ABCA1)-mediated cholesterol efflux. J Biol Chem. 2003;278:10002–10005. doi: 10.1074/jbc.C300024200. [DOI] [PubMed] [Google Scholar]
- 48.Mendez AJ, Uint L. Apolipoprotein-mediated cellular cholesterol and phospholipid efflux depend on a functional Golgi apparatus. J Lipid Res. 1996;37:2510–2524. [PubMed] [Google Scholar]
- 49.Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, Brewer HB., Jr Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem. 2001;276:27584–27590. doi: 10.1074/jbc.M103264200. [DOI] [PubMed] [Google Scholar]
- 50.Nieland TJ, Chroni A, Fitzgerald ML, Maliga Z, Zannis VI, Kirchhausen T, Krieger M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J Lipid Res. 2004;45:1256–1265. doi: 10.1194/jlr.M300358-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc Natl Acad Sci USA. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 53.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 54.Pascot A, Lemieux I, Prud’homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Despres JP. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res. 2001;42:2007–2014. [PubMed] [Google Scholar]
- 55.Despres JP. Dyslipidaemia and obesity. Bailliere Clin Endocrinol Metabol. 1994;8:629–660. doi: 10.1016/s0950-351x(05)80289-7. [DOI] [PubMed] [Google Scholar]
- 56.Williams PT, Vranizan KM, Austin MA, Krauss RM. Associations of age, adiposity, alcohol intake, menstrual status, and estrogen therapy with high-density lipoprotein subclasses. Arterioscler thromb. 1993;13:1654–1661. doi: 10.1161/01.atv.13.11.1654. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald ML, Morris AL, Rhee JS, Andersson LP, Mendez AJ, Freeman MW. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J Biol Chem. 2002;277:33178–33187. doi: 10.1074/jbc.M204996200. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka AR, Abe-Dohmae S, Ohnishi T, Aoki R, Morinaga G, Okuhira K, Ikeda Y, Kano F, Matsuo M, Kioka N, Amachi T, Murata M, Yokoyama S, Ueda K. Effects of mutations of ABCA1 in the first extracellular domain on subcellular trafficking and ATP binding/hydrolysis. J Biol Chem. 2003;278:8815–8819. doi: 10.1074/jbc.M206885200. [DOI] [PubMed] [Google Scholar]
- 59.Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz G, Fischer H, Beuck M, Hoecker KP, Robenek H. Dysregulation of lipid metabolism in Tangier monocyte-derived macrophages. Arterioscler (Dallas, Tex) 1990;10:1010–1019. doi: 10.1161/01.atv.10.6.1010. [DOI] [PubMed] [Google Scholar]