Abstract
Recent neuroimaging and neuropsychological findings indicate that the posterior parietal cortex (PPC) plays an important, albeit undefined, role in episodic memory. Here we ask whether this region is specifically involved in associative aspects of episodic memory. Experiment 1 tested whether PPC damage affects the ability to learn and retrieve novel word-pair associations. Experiment 2 tested whether PPC damage affects the retrieval of object-location associations, in a spatial fan task. In both experiments, patients showed normal levels of associative memory. These findings demonstrated that PPC damage did not prevent association memory for verbal items. Finally Experiment 3 tested whether PPC damage affects memory for non-verbal audio-visual pairs. The patients performed with normal accuracy, but with significantly reduced confidence. These findings indicate that the PPC does not have a central role in association formation per se and instead, indicate that the PPC is involved in other aspects of episodic memory.
Keywords: associational memory, relational memory, neuropsychology, Balint’s patient, confidence ratings
Associative memory refers to our ability to build and retrieve mnemonic relationships between disparate pieces of information. These associations permit us to recognize a new colleague’s face, quickly access her name and retrieve relevant information such as the name of her postdoctoral advisor. Thus, associative memory creates the links that form an episodic memory. Research on associative memory has focused primarily on medial temporal lobe structures such as the hippocampus (for a recent review see (Suzuki, 2008). There is considerable evidence indicating that the hippocampus plays a central role in relating two previously unlinked items (reviewed in (Cohen, et al., 1999).
The medial temporal lobe, however, does not work alone. Neuroimaging findings have consistently indicated that the posterior parietal cortex (PPC) may also play some role in episodic memory (reviewed in (Cabeza, 2008; Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; I. R. Olson & Berryhill, 2009; Vilberg & Rugg, 2008; Wagner, Shannon, Kahn, & Buckner, 2005). To demonstrate a causal relationship between parietal activations and episodic memory, several groups have investigated whether patients with parietal lobe damage have memory deficits (Davidson, et al., 2008). These studies have shown that PPC damage can cause episodic memory retrieval deficits, but that the deficits are nuanced (reviewed in(I. R. Olson & Berryhill, 2009). For instance, we found that when patients with bilateral PPC damage freely recall autobiographical events, the memories are impoverished, lacking detail. Not only are spatial details missing, but sensory and emotional descriptions are also reduced. However, when specific probe questions are directly posed to these patients, the patients can supply missing details as readily as control participants (M.E. Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007). These findings pointed towards a specific type of retrieval deficit since the patients were tested on information that they had encoded before their neurological injury, but importantly, because their performance was impaired only under certain retrieval conditions.
Interestingly, these same patients perform normally on some other episodic memory tasks. For instance, in three experiments we investigated the effects of PPC damage on source, or context-dependent, memory. In source memory tasks, participants are asked to retrieve an incidental aspect of the encoding experience such as whether a sentence was spoken by a male or female voice. We found that the patients, along with a group of patients with unilateral parietal lobe damage, had normal levels of source memory, whether tested with visual or auditory information. In contrast, they had diminished levels of memory confidence (Simons, Peers, Mazuz, Berryhill, & Olson, in press). Other groups have reported diminished levels of memory confidence after unilateral PPC damage as well (Ally, Simons, McKeever, Peers, & Budson, 2008; Davidson, et al., 2008). This finding indicates that portions of the parietal cortex may play a role in evoking vivid memories or strong feelings of recollection.
These data support the view that portions of the parietal cortex are functionally involved in some aspect of memory retrieval. The extent of this involvement, and its particular role, remains poorly understood. One plausible explanation is that the PPC may have a role in forming or retrieving associations. The location of the parietal cortex, at the confluence of sensory (visual and auditory), motor, and higher-level cognitive structures indicates that the parietal cortex is well-positioned for such a role. Indeed, apart from primary somatosensory cortex, the parietal cortex is commonly referred to in introductory textbooks as ‘association cortex’. It has been proposed that parietal cortex is the site where perceptual features are bound together to form a unified object (Treisman, 1996, 1998, 1999) based on the finding that patients with simultanagnosia due to bilateral occipito-parietal damage, cannot easily bind together primitive visual features such as color and shape (Friedman-Hill, Robertson, & Treisman, 1995). A striking example comes from a recent case study in which a bilateral parietal patient was unable to attend to more than a single stimulus feature at a time; in other words, the patient could accurately report either the shape or the shading, but not the conjunction of features (Coslett & Lie, 2008). There is also neuroimaging evidence indicating that the PPC’s role in feature binding extends beyond perception into memory. For example, greater PPC activations occur during working memory tasks testing memory for visuospatial conjunctions as compared to object identity alone (Shafritz, Gore, & Marois, 2002). In addition, a study directly contrasting item and associative recognition memory using pairs of images as stimuli reported greater activations in lateral and superior portions of the PPC during encoding and retrieval of associations (Achim & Lepage, 2005).
Here, we tested the hypothesis that bilateral PPC lesions impair associative memory. In three experiments we examined associative memory with stimuli reflecting commonly encountered demands on associative memory: word pairs, object-place word pairs, and audio-visual pairs.
Experiment 1: Associative Memory for Word Pairs
In Experiment 1 we tested whether associative memory for word pairs was impaired following bilateral parietal damage, much like it is following medial temporal lobe damage. A previous observation suggested they might not be as impaired as patients with medial temporal lobe damage. In the verbal paired associates subtest of the WMS, the parietal patients retained 4–6 word pairs following a delay rather than 0–2 retained by medial temporal lobe patients. Here, we used a more elaborate task developed for use in patients with amnesia following medial temporal lobe damage (Giovanello, Keane, & Verfaellie, 2006). During encoding, participants viewed word pairs presented on a monitor and heard the word pair used in a sentence. To promote encoding, participants were required to judge the likelihood of the event described in the sentence. Retrieval was tested using old/new recognition. On trials in which an ‘old’ response was given, participants were required to provide a remember/know response.
Method
Patients
Two patients, EE555 and TQ591, with bilateral parietal lobe damage were tested in this study; see Figure 1 for lesion tracings. These patients have previously been reported in several studies (M. E. Berryhill, Fendrich, & Olson, 2009; M.E. Berryhill & Olson, 2008; M.E. Berryhill, et al., 2007; Drowos, in press; Simons, et al., in press).
Figure 1.
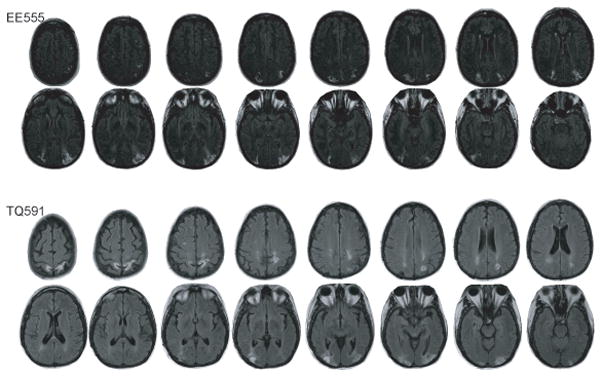
Lesion tracings for the bilateral parietal patients EE555 (top) and TQ591 (bottom).
Patient EE555
EE555 is a 40-year-old former teacher with 16 years of education. In 2004, she suffered three infarcts in the watershed between the posterior and middle cerebral arteries. Temporary symptoms included headaches and blindness. Following her third stroke, she was admitted to the Hospital of the University of Pennsylvania where she was treated for hypertension and anatomical MRI scans were performed. The initial neurological evaluation revealed canonical symptoms of simultanagnosia. EE555’s physical and perceptual symptoms are currently stable. EE555’s MRI revealed symmetrical lesions in lateral aspects of the inferior parietal lobe impinging on the superior occipital lobe and extending through the angular gyrus (Brodmann areas (BA) 39) in and around inferior and middle portions of the intraparietal sulcus (IPS). Superior, more lateral (BA 40) and medial parietal structures (e.g. precuneus) were spared.
Patient EE555’s primary deficit is simultanagnosia. She cannot describe the global meaning in a picture of a scene, instead will describe isolated details (i.e. “There is a necklace”). In line cancellation tasks, she ignores peripheral lines and claims she is completed when the center items have been cancelled. EE555 has moderate optic ataxia, misreaching and mispointing to foveated and peripheral objects. She does not have optic apraxia (inability to change location of fixation). Language comprehension and speech fluency were unimpaired as assessed by her conversational skills, and by ceiling performance on the auditory tests of the Western Aphasia Battery. Reading and writing are impaired due to her simultanagnosia. Her eyesight is normal.
Patient TQ591
TQ591 is a 51-year-old former preschool assistant teacher with 15 years of education. In 2006, she suffered bilateral parieto-occipital damage due to CNS cerebral vasculitis and was treated at the Hospital of the University of Pennsylvania.
TQ591’s MRI revealed signs of previous subacute posterior cerebral artery infarctions. The primary lesions are in bilateral parietal regions. The left parietal lesion affects the IPS (BA 39) and precuneus (BA 7). There are two lesions in the right hemisphere: an inferior lesion in the superior occipital lobe (BA 18 and 19), and a superior lesion in the superior parietal lobe (BA 7). In both hemispheres, the lesions extend slightly into temporo-occipital (BA 19) regions and parietal white matter. Lateral (BA 40) and medial regions (e.g. precuneus) were spared.
TQ591’s primary deficit is simultanagnosia. TQ591 has difficulty describing scenes and complains that parts of scenes ‘disappear’ when she looks away or blinks and she cannot relocate them. In line cancellation tasks, she identifies a small cluster of lines. She suffers from mild optic ataxia and optic apraxia. Language comprehension and speech fluency were unimpaired as assessed by her conversational skills, and ceiling performance on the auditory tests of the Western Aphasia Battery. Reading and writing were somewhat impaired due to her simultanagnosia and spatial disorientation (she loses her place on a page). Her vision is corrected-to-normal.
Control Participants
Ten healthy adults (46.4 years of age, 14.3 years of education, 4 male) participated. There were no significant differences between the patient and control groups in terms of age (t10 = .16, p = .88) or education (t10 = 1.13, p = .29). All protocols were approved by the Internal Review Board of the University of Pennsylvania. Participants in all experiments were reimbursed $15.00/hour for their time.
Materials
We used the stimuli and methodology described in Giovanello et al. (2006) and summarized here. There were two stimulus categories: compound words and unrelated word pairs. For each stimulus category, there were two word lists (A, B) of 12 word triplets each, for a total of 36 elements per list. For the compound word triplets, the first two elements were compound words, such as ‘doorknob’ and ‘dumbbell’. The third word was made by recombining the first two words to make a third compound word. For example, ‘door’ from ‘doorknob’ plus ‘bell’ from ‘dumbbell’ recombined to form ‘doorbell’. The word triplet thus consisted of: doorknob, dumbbell, doorbell. The unrelated word list was similar, except none of the triplet elements had familiar pairings. An example of an unrelated word triplet is the following: drug-oil, bottle-clay, drug-clay.
Procedure
We tested participants in the unrelated and compound conditions in separate blocks during the same testing session, with a thirty-minute interval between blocks. Each condition began with an encoding phase. Encoding trials began with one word pair appearing on a computer monitor. Participants then heard the word pair used in a sentence (“When she entered the room the doorknob fell off”, “The main ingredient in the drug was oil”). The encoding task was to rate the likelihood (1–5) of the event described in the sentence. There were a total of 36 encoding trials; from word list A, participants heard the first two elements of each triplet (24 trials), and from word list B, they only heard the recombined word (12 trials). Following a 5–10 minute delay the test phase began. Test trials began with the appearance of one word pair on the computer monitor. Participants made an old/new recognition judgment. If the response was ‘old’ participants then provided a remember/know response indicating whether they had a clear recollection of the word pair (remember) or whether the word pair seemed familiar (know). There were 24 test trials: 12 ‘old’ word pairs and 12 ‘new’ word pairs. The ‘new’ word pairs were the recombined words from word list A. Lists and condition orders were counterbalanced across participants.
Data Analysis
Patient and control performance was compared on the following measures: hit rates, false alarm rates and corrected recognition. In all studies, the small number of patients lead us to use non-parametric permutation tests (Good, 1994) which have been used in other neuropsychological studies (M.E. Berryhill & Olson, 2008; I.R. Olson, Moore, Stark, & Chatterjee, 2006). For the repeated-measures ANOVA tests, a permutation test was used in which we first computed the F statistic under the standard mixed two-factor ANOVA model. Then the observed values were randomly permuted across the patient and control participants. The F statistics were recomputed for the permuted data set and a one-tailed count over 1000 replicates was used to compute the significance values. We followed the precedent of Giovanello et al. (2006) and used the independent remember-know procedure: (Know/(1-Remember)) (Yonelinas, Kroll, Dobbins, Lazzara, & Knight, 1998). This measure is based on an assumption of independence between remember and know responses. We also calculated the discriminability measure d’ by subtracting normalized false alarm rates from normalized hit rates to explore the effect of the compound and unrelated stimulus categories. These analyses followed the precedent of Giovanello et al. (2006).
Results and Discussion
The analysis of hit rate found no main effect of group or stimulus type and no interaction of group × stimulus type (All F’s < 1, n.s.); see Figure 2. The analysis of false alarm rate found no main effect of group (F1, 10 = 95.09, p = .16) or stimulus type (F1, 10 < 1, n.s.), but the interaction of group × stimulus type did reach significance (F1, 10 = 6.68, p = .03). The interaction captured the finding that the patients demonstrated a greater difference in false alarm rates between conditions (Compound − Unrelated false alarms: M patient = .21-.14 = .06, M control = .24 − .20 = .04). Follow-up permutation tests individually comparing patient and control false alarm rates for compound and unrelated word pairs showed no significant differences (both p’s > .34). The analysis of d’ found no main effect of group (F1, 10 = 5.28, p = .42) or stimulus type (F1, 10 = 1.09, p = .32), and no interaction of group × stimulus type (All F’s < 1, n.s.).
Figure 2.
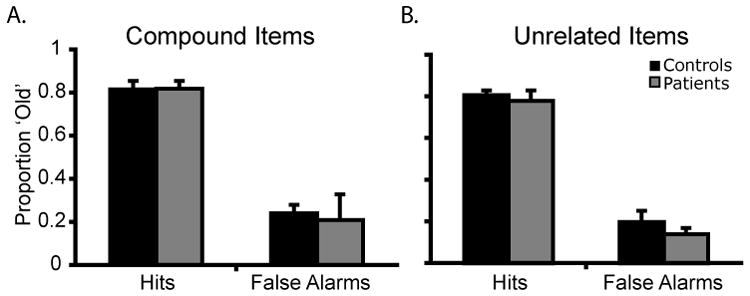
Data from Experiment 1: (A) Proportion of ‘old’ responses in the compound word pairs; (B) Proportion of ‘old’ responses in the unrelated word pairs. Error bars represent the standard error of the mean.
The remember/know data were subjected to permutation tests. First, the proportion of remember responses for the items correctly endorsed as old was compared for each group (patient, control) and each condition (compound, unrelated). There were no statistically significant differences between groups (F1, 10 = 8.52, p = .66) or conditions (F1, 10 = 2.68, p = .14), and the interaction of group × condition did not reach significance (F < 1, p = n.s.). The same analysis examined the familiar responses (IRK corrected) for the items correctly endorsed as old found the same pattern of results: no differences between groups (F1, 10 = 4.68, p = .63) or conditions (F1, 10 < 1, p = n.s.), and no significant interaction of group × condition (F1, 10 < 1, p = n.s.).
There were very few trials in which a new item was incorrectly endorsed as old. Thus, although there were no significant findings (all p’s > .15), the small number of trials must be interpreted with some caution.
In summary, the results of Experiment 1 show that bilateral PPC damage does not affect the ability to remember associations between words. Also, the patients did not differ from controls with regards to their dependence on recollection or familiarity for their recognition judgments. Previously we found that PPC patients performed visual and verbal source memory tasks with normal accuracy but impaired confidence (Simons, et al., in press). One possible explanation for this difference is that the binary memory confidence decision required in the remember/know paradigm is too narrow to reveal lower response confidence. Closer examination of the remember/know responses supports this view as patients had numerically lower proportions of remember responses. Last, unlike the patients with medial temporal lobe damage tested previously in this task (Giovanello, et al., 2006), PPC patients do not appear to rely disproportionately on familiarity to perform this type of associative memory task.
Experiment 2: Associative Memory for Object-Place Word Pairs
Language makes near constant spatial references: the chair next to the door, the house on the corner, go to the door behind the counter. It is important to know where things are, in relationship to other things. Here, we examined associative memory using object-location pairs in a paradigm known as the fan task (J.R. Anderson, 1974; Radvansky, 2005); reviewed in (Radvansky, 1999). In this task, the ability to encode and retrieve object-place word pairs is tested by associating described objects with described places. For example, ‘there is a potted palm in the laundromat’. The task parametrically manipulates the number of items associated with each location. Likewise, items can be associated with several locations. In other words, there may be a second potted palm in the lounge, and there may be a second object, such as a wastebasket, in the laundromat.
Previous neuroimaging work using the fan task has proposed that the PPC, specifically in inferior parietal regions (junction of BA 7, 39, 40) is involved in encoding and updating stimulus representations (Sohn, Goode, Stenger, Carter, & Anderson, 2003). Anderson and colleagues describe these functions as serving as an ‘imaginal buffer’ (J. R. Anderson, Qin, Sohn, Stenger, & Carter, 2003; Sohn, et al., 2003; Sohn, et al., 2005). According to this perspective, parietal damage should impair patients’ performance on the fan task. It is possible that the PPC’s role in spatial perception and memory (I. R. Olson & Berryhill, 2009) might make the use of a spatial fan task more sensitive to associative memory deficits than the word-pair task used in Experiment 1.
Method
Participants
The two patients tested in Experiment 1 and ten new control participants (45.9 age, 13.8 education, 6 male) participated. There were no differences between the patients and controls in terms of age (t10 < 1, p = .98) or education (t10 = 1.05, p = .32).
Materials
We followed the protocol described by Radvansky (Radvansky, 2005). Eighteen sentences were recorded by a female speaker with GarageBand Software (Apple Inc, Cupertino, CA). Each sentence described the location of an object, such as “The fire extinguisher is in the movie theater”. Each of the 12 locations was associated with 1, 2, or 3 different objects. In the following example one location, the barbershop, is associated with two objects, a payphone and a welcome mat: “The payphone is in the barbershop” and “The welcome mat is in the barbershop”. Likewise, each of the 12 objects could be found in 1, 2, or 3 locations. The following sentences, ceiling fan is associated with three difference locations: “The ceiling fan is in the city hall”, “The ceiling fan is in the car dealership”, and “The ceiling fan is in the office building”. Eight objects were associated with one location, two objects were associated with two locations, and three objects were associated with two locations. Similarly, eight locations contained a single object, two locations contained two objects, and two locations contained three objects.
Procedure
The experiment consisted of three phases: auditory training, verbal training, and a recognition memory test. In the auditory training phase, participants passively listened to all 18 object-location sentences as many times as they wished, until they felt they could remember all of the sentences. During the verbal training phase, the experimenter asked two types of questions: (a) where an object was located (“Where is the bulletin board?”); and (b) what objects were found in a particular location (“What is in the high school?”). Participants had to provide all associations to get credit for a correct response. Participants were not tested until they provided all associations accurately three times without any interleaved error trials. Finally, the recognition test investigated retrieval using sentence probes. Sentence probes were previously shown to be most effective at eliciting a differential fan effect for objects and locations (Radvansky, 2001). At test, participants heard a recorded sentence and made an old/new recognition judgment whether the sentence matched what they had learned during training. One half of the object-location sentences were recombined to make false statements (chance = 50%). There were 288 test trials. Patients’ responses were registered by the experimenter, thereby prohibiting reaction times analyses.
Data Analysis
Patients and control performance was compared on the following measures: hit rates, false alarm rates, and corrected recognition. Due to the small number of patients, non-parametric permutation tests were used (Good, 1994). We used the permutation test analogous to a repeated-measures ANOVA as in Experiment 1, and we also used a permutation test analogous to an independent groups t-test. For the t-test version, no t-value is calculated. In the first stage of analysis, the null hypothesis (that there is no difference between the patient and control groups) was tested using a t-test. During the second stage, two groups were randomly defined and subjected to the same comparison. This continues until 1000 random samples are taken. The reported p-values refer to the proportion of scores below the observed experimental value. Statistical analyses were conducted using Matlab (The MathWorks, Natick, MA).
Results and Discussion
The first analysis investigated the number of verbal training repetitions to achieve criterion. This analysis showed that the groups had a similar learning rate (M patients = 5.5; M controls= 4.8, p = .84). The second analysis examined whether the groups differed on recognition memory. This analysis showed that the groups had similar levels of recognition accuracy (M patients = .97; M controls = .91, p = .97).
A third set of analyses examined whether the size of the fan was affected by group membership in a 2 (control, patient) × 3 (number of associations per object or location: 1, 2, 3) permutation-version ANOVA; see Figure 3. There were no main effects of group (F1, 10 = 29.56, p = .37), or number of object associations (F1, 10 = 2.67, p = .09) and no significant interaction between these factors (F1, 10 = 1.87, p = .18). Both groups demonstrated ceiling performance for each number of object associations (M patient = .96, 1, .94; M controls = .96, .95, .91).
Figure 3.
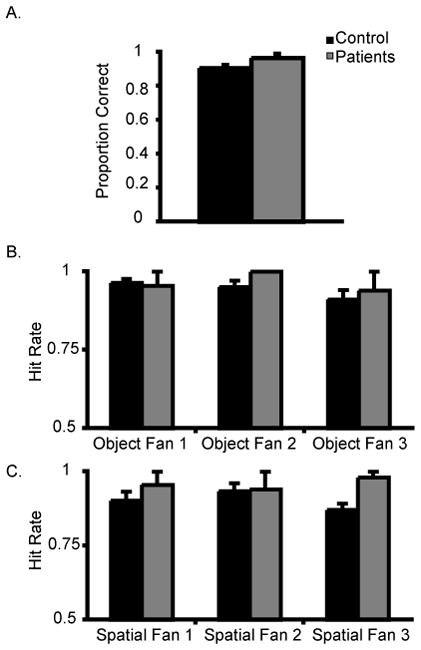
Data from Experiment 2: (A) Proportion of correct responses for the spatial fan task. Hit rates for the (B) object and (C) location fan items. For the object fan task, a single verbally described item was associated with 1, 2, or 3 verbally described locations. For the location fan task, a single verbally described location was associated with 1, 2, or 3 verbally described objects. Error bars represent the standard error of the mean.
The location fan effect (e.g. one location with many objects) was examined. There was no main effect of group (F1, 10 = 3.63, p = .63), but the main effect of the number of associations approached significance (F1, 10 = 3.13, p = .06) due to poorer performance when there were more associations. There was no significant interaction (F1, 10 < 1, p = n.s.). Again, the performance for both groups was very high and patients performed with numerically higher accuracy than control participants (M patient = .96, .94, .98, M control= .90, .93, .87).
These findings indicate that encoding and retrieving verbal object-location word pairs is unimpaired following bilateral parietal lobe damage. A more robust fan effect may have been missed because we examined accuracy data, which produces a less robust fan effect than reaction time data. No difference in performance accuracy between groups was found even when the associations involved spatial information. These null findings do not bear out the predictions of the view of the parietal lobe as the ‘imaginal buffer’ (J. R. Anderson, et al., 2003; Sohn, et al., 2003; Sohn, et al., 2005). It is possible that the location of the proposed imaginal buffer may be more lateral, in supramarginal gyrus, which is intact in our patients. Alternatively, the extensive training paradigm may have rendered the stimuli overlearned, such that memory retrieval was nearly automatic, making it difficult to observe differential effects of PPC damage.
Experiment 3: Associative Memory for Audio-Visual Pairs
In Experiment 3, we tested the impact of bilateral PPC damage on a third commonly encountered type of associative memory: multimodal associations. In this task, participants learned the association between sounds and images and later, performed an old/new recognition memory tests. If bilateral PPC damage does not cause impaired task performance it would suggest that the lesioned regions do not subserve multimodal associative memory.
Method
Participants
Both patients and fourteen control participants (47.8 age, 13.8 education, 12 male) participated. Two control participants who participated in Experiment 1, and two control participants who participated in Experiment 2 were tested. There were no significant differences in age between the patients and the control group (both p’s < .26).
Materials
Audio stimuli were 20 sound effects consisting of animal, transportation, and environmental sounds. Visual stimuli were twenty colorized Snodgrass drawings of everyday objects, (e.g. a swing, a star, a toaster) (Rossion & Pourtois, 2004). There was no semantic congruency between the sounds and the images.
Procedure
During the encoding phase participants passively watched and listened to 20 audio-visual pairs. They were instructed to remember the audiovisual pairings for later testing. The audio and visual components were presented simultaneously and lasted the same duration (1 s). Participants pressed the space bar when they were prepared for the next pair. Immediately following encoding, participants performed an old/new recognition memory test. At test, the audio-visual items remained in their original pairing (50%) or were recombined (50%). For each stimulus pair, participants provided an old/new recognition judgment and then provided a confidence rating of 1–6 (sure new – sure old). Audiovisual pairs were randomized across participants.
Results and Discussion
A permutation test compared corrected recognition scores (hits – correct rejections) for each group (patient, control). The patients were numerically worse than controls but the main effect of group did not reach statistical significance (M patient = .2; M control = .28, p = .36); see Figure 4.
Figure 4.
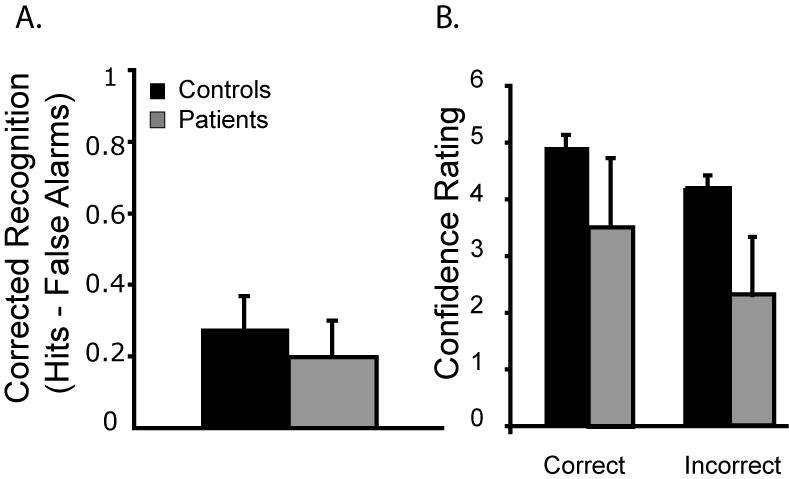
Experiment 3: (A) Corrected recognition scores for the audio-visual pairs. (B) Confidence ratings for correct and incorrect responses. Error bars represent the standard error of the mean.
Additional analyses examined the confidence ratings for correct and incorrect responses. For correct responses, the patients demonstrated lower confidence for correct answers (patients M = 3.5, controls M = 5.0, p = .05). The patients’ confidence ratings for incorrect responses were lower across sessions (M = 2.3) than that of the controls (M = 4.2, p = .03).
These data demonstrate that bilateral parietal damage does not affect the process of encoding new audio-visual associations or retrieving them. However, despite normal accuracy, patients were significantly less confident in their memory judgments. These results also allay concerns from Experiment 2 that group differences might have been obscured by ceiling performances.
General Discussion
In three experiments we tested the hypothesis that the parietal lobe is involved in encoding or retrieving items from associative memory. We compared the performance of two patients with bilateral PPC damage to healthy age- and education-matched control participants. In Experiment 1, a verbal paired associates task was used that previously showed sensitivity to hippocampal pathology (Giovanello, Keane, and Verfaellie, 2006). The parietal patients performed no differently from control participants. In Experiment 2, participants were tested on a spatial language fan task that required them to encode and later retrieve verbally described objects in different locations. There were no differences in encoding rates between groups. Again, the patients showed no impairment when retrieving object-location associations. In Experiment 3, participants were tested in a multimodal associative memory task. Participants were required to encode and retrieve audio-visual associations. The patients performed with normal accuracy, however they had significantly lower confidence ratings.
In sum, these studies show that bilateral PPC lesions do not lead to global deficits in encoding new associations or retrieving items from associative memory. In the following discussion, we focus on mechanisms related to memory retrieval because no encoding related differences were observed in reaching criterion performance in Experiment 2. Note that there is fMRI evidence supporting the view that the PPC plays a role in memory encoding (for a recent review see (Uncapher & Wagner, 2009), however there is currently no converging evidence for this view from neuropsychology. We close by examining associative memory performance differences in patients with medial temporal lobe or parietal damage.
The Episodic Buffer Hypothesis
The influential multi-component model of working memory proposed by Baddeley and colleagues (Baddeley & Hitch, 1974; Baddeley & Logie, 1999) was later expanded to include a module termed the episodic buffer (Baddeley, 2000). The episodic buffer was developed to account for findings in normal and patient populations including resistance to verbal suppression in digit span tasks, and superspan performance when performing prose recall tasks such as sentence repetition (Baddeley, 2000). The episodic buffer was proposed as the site where multisensory information is integrated into a single representation. Recently, these functions were transposed to the episodic memory domain. The output, or episodic, buffer hypothesis proposes that the inferior parietal cortex is the site of an episodic buffer-like structure functioning within the realm of long-term memory retrieval (Vilberg & Rugg, 2008; Wagner, et al., 2005). The episodic buffer hypothesis predicts that damage to the inferior parietal cortex should impair the ability to maintain representations of audio-visual pairs. This pattern was not observed in Experiment 3. Here, the patients performed with normal accuracy. Additional findings, addressing other predictions of the episodic buffer hypothesis, have also been disappointing. For example, it has been specifically stated that the putative episodic buffer is critical for storytelling, or retelling of narratives (Baddeley, 2000). Both bilateral patients are able to recount well-known stories with appropriate narrative structure (Berryhill, Picasso, Arnold, Drowos, Olson, accepted pending revisions). These findings appear to be inconsistent with the predictions of the episodic buffer hypothesis.
The Subjective Memory Hypothesis
The subjective memory hypothesis holds that the PPC provides a signal measuring how vividly a memory is re-experienced (Ally, et al., 2008). Presumably this signal indicates that an episodic event was personally relevant and distinguishable from impersonal semantic knowledge. This hypothesis evolved from anecdotal reports that some patients with inferior parietal lobe damage were not be able to vividly evoke the emotional and perceptual experiences associated with a particular memory, and continually verbalized uncertainty about their memories (Ally, et al., 2008; Davidson, et al., 2008; Hunkin, et al., 1995; Simons, et al., in press). Evidence for this hypothesis is steadily accruing. Previously, we found that in spite of unimpaired performance on a series of source memory tasks, both patients had diminished memory confidence (Simons, et al., in press). They also exhibited diminished subjective memory on two false memory tasks (Drowos, in press) and in Experiment 3, both patients exhibited normal memory of audio-visual associations, but showed significantly reduced memory confidence. However, it is important to note that these bilateral PPC patients were not globally impaired when making confidence judgments. The patients’ confidence ratings were normal when judging whether a trivia statement or visual stimulus had been presented previously (Simons, et al., in press).
There is also neuroimaging evidence in support of the subjective memory view. For example, in one recent fMRI study, participants were scanned as they performed recognition and memory confidence assessments for face/name pairs (Chua, Schacter, Rand-Giovannetti, & Sperling, 2006). The inferior parietal lobe and precuneus were significantly more active when making confidence assessments. The authors suggested that these parietal regions are involved in monitoring retrieved memories. These findings are consistent with the subjective memory hypothesis.
However, this hypothesis does not fully explain why some tasks result in successful retrieval and others fail. We speculate that combining the subjective experience hypothesis with a third viewpoint, the attention to internal memory (AtoM) (M.E. Berryhill, et al., 2007); (Cabeza, et al., 2008; I. R. Olson & Berryhill, 2009) may provide a more comprehensive account. Briefly, the AtoM model distinguishes between bottom-up and top-down memory retrieval and their respective neural correlates. AtoM associates inferior parietal lobe activity with bottom-up, spontaneous retrieval and the superior parietal lobe with top-down, effortful retrieval. We note that in previous work with parietal patients, impaired retrieval was found when there was weak retrieval support, such as in a free recall paradigm. The failure of bottom-up attention to spontaneously retrieve a memory may contribute to the lack of a vivid subjective experience and consequently for patients to register lower confidence ratings. When stronger retrieval support was present, as in the case of cued retrieval, patients performed normally. We propose that the patients performed as well as control participants on the associative memory tasks because the retrieval cues provided sufficient support.
The null findings reported in Experiments 1–3 cannot provide support for or against the AtoM model. However our findings do counter the hypothesis that parietal lobe mnemonic functions are based on association formation or retrieval.
Medial Temporal Lobe and Parietal Involvement in Episodic Memory
Medial temporal lobe damage, particularly to the hippocampus, leads to profound deficits in diverse forms of associative, or relational memory (e.g. (Todorov & Olson, 2008); reviewed in (Cohen, et al., 1999; Ryan, Althoff, Whitlow, & Cohen, 2000). In Experiment 1 we borrowed an associative memory task developed for use in medial temporal lobe amnesics (Giovanello, et al., 2006). When compared to control participants, medial temporal lobe patients were significantly less accurate, made more false alarms, and performed more accurately on compound stimuli than for the novel word pairs; none of these deficits were observed in the parietal patients.
At this juncture, it seems reasonable to suggest that the hippocampus and the parietal lobe structures have complementary functions in associative memory retrieval. The hippocampus forms and retrieves associations. The parietal lobe may shift attention to recently retrieved associations and then signal whether the association has personal relevance. Without this signal, memory confidence may be impaired. Following inferior parietal damage, shifting attention may become more difficult and reliant on retrieval support.
Null Findings and Other Caveats
There are concerns associated with any data reporting null findings. In these three experiments, the PPC patients performed as well as control participants. This raises concerns that the tasks were insensitive to PPC damage. Our patients may have performed well because there were familiar word pairs in Experiment 1. However, they also performed well when the words were unfamiliar. Patients may have benefited from the extensive training to criterion in Experiment 2, making each object-location pair overlearned and less reliant on spatial imagery and attention. The audiovisual pairs used in Experiment 3 may have been relatively easy to learn, but performance for both patients and controls was well below ceiling. As such, we do not think that ceiling effects can account for the null findings between control and patient groups.
Other caveats are intrinsic to neuropsychological research. Only two bilateral parietal patients were tested here because they are very rare. Their lesions are not fully overlapping, and cannot be compared with unilateral parietal patients because the intact hemisphere may compensate for the lesioned area. It is also likely that some degree of reorganization may have occurred following unilateral brain lesions. To more precisely assess parietal involvement in different forms of memory, we concur with Davidson and colleagues (Davidson, et al., 2008) who recommend combining neuropsychology with functional neuroimaging and transcranial magnetic stimulation to better understand the role of the PPC in episodic memory.
Acknowledgments
We would like to thank the patients for their on-going cooperation. We also thank Dr. Marianna Stark and Dr. Anjan Chatterjee for scheduling patients through the Hospital of the University of Pennsylvania patient database. We thank Dr. Kelly Giovanello for providing the stimuli used in Experiment 1. We thank Dr. Roberto Cabeza for suggesting Experiment 2, and Dr. Gabriel Radvansky for providing the stimuli used in Experiment 2. We also thank Jared Danker for additional stimuli. This research was supported by NRSA NS059093 to MEB and ROI MH071615 to IRO.
References
- Achim AM, Lepage M. Neural correlates of memory for items and for associations: an event-related functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2005;17(4):652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: Electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46(7):1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR. Retrieval of propositional information from long-term memory. Cognitive Psychology. 1974;6:451–474. [Google Scholar]
- Anderson JR, Qin Y, Sohn MH, Stenger VA, Carter CS. An information-processing model of the BOLD response in symbol manipulation tasks. Psychonomic Bulletin & Review. 2003;10(2):241–261. doi: 10.3758/bf03196490. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cogntive Science. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Hitch GJ, editors. Working memory. Vol. 8. New York: Academic press; 1974. [Google Scholar]
- Baddeley A, Logie RH. Working memory: The multiple-component model. In: Miyake A, Shah P, editors. Models of Working Memory. New York: Camberidge University Press; 1999. [Google Scholar]
- Berryhill ME, Fendrich R, Olson IR. Impaired distance perception and size constancy following bilateral occipitoparietal damage. Experimental Brain Research. 2009;194:381–393. doi: 10.1007/s00221-009-1707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia. 2008;46(7):1775–1786. doi: 10.1016/j.neuropsychologia.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;(27):14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Picasso L, Arnold RA, Drowos DB, Olson IR. Bilateral parietal lobe damage impairs the ability to imagine the future (accepted pending revisions) [Google Scholar]
- Cabeza R. Role of lateral posterior parietal regions in episodic memory retrieval: The dual attention hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29(4):1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Lie G. Simultanagnosia: when a rose is not red. Journal of Cognitive Neuroscience. 2008;20(1):36–48. doi: 10.1162/jocn.2008.20002. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, et al. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46(7):1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drowos D, Berryhill ME, Andre J, Olson IR. True memory, false memory and the subjective recollection deficits after focal bilateral parietal lobe lesions. Neuropsychology. doi: 10.1037/a0018902. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science. 1995;269(5225):853–855. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Keane MM, Verfaellie M. The contribution of familiarity to associative memory in amnesia. Neuropsychologia. 2006;44(10):1859–1865. doi: 10.1016/j.neuropsychologia.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. Permutation tests: a practical guide to resampling methods for testing hypotheses. New York: Springer-Verlag; 1994. [Google Scholar]
- Hunkin NM, Parkin AJ, Bradley VA, Burrows EH, Aldrich FK, Jansari A, et al. Focal retrograde amnesia following closed head injury: a case study and theoretical account. Neuropsychologia. 1995;33(4):509–523. doi: 10.1016/0028-3932(94)00136-d. [DOI] [PubMed] [Google Scholar]
- Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18(7):1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Radvansky GA. The fan effect: A tale of two theories. Journal of Experimental Psychology: General. 1999;128:198–206. doi: 10.1037//0096-3445.128.2.198. [DOI] [PubMed] [Google Scholar]
- Radvansky GA. Situation models, propositions, and the fan effect. Psychonomic Bulletin & Review. 2005;12:478–483. doi: 10.3758/bf03193791. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow T, Cohen NJ. Amnesia is a deficit in relational memory. Psychological Science. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Gore JC, Marois R. The role of the parietal cortex in visual feature binding. Proceedings of the National Academy of Sciences: U S A. 2002;99(16):10917–10922. doi: 10.1073/pnas.152694799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz Y, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex. doi: 10.1093/cercor/bhp116. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Goode A, Stenger VA, Carter CS, Anderson JR. Competition and representation during memory retrieval: roles of the prefrontal cortex and the posterior parietal cortex. Proceedings of the National Academy of Sciences: U S A. 2003;100(12):7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Goode A, Stenger VA, Jung KJ, Carter CS, Anderson JR. An information-processing model of three cortical regions: evidence in episodic memory retrieval. Neuroimage. 2005;25(1):21–33. doi: 10.1016/j.neuroimage.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Associative learning signals in the brain. Progress in Brain Research. 2008;169:305–320. doi: 10.1016/S0079-6123(07)00019-2. [DOI] [PubMed] [Google Scholar]
- Todorov A, Olson IR. Robust learning of affective trait associations with faces when the hippocampus is damaged, but not when the amygdala and temporal pole are damaged. Social, Cognitive and Affective Neuroscience. 2008;3(3):195–203. doi: 10.1093/scan/nsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. The binding problem. Curr Opin Neurobiol. 1996;6(2):171–178. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Treisman A. Feature binding, attention and object perception. Philosophical Transactions of the Royal Society London B Biological Sciences. 1998;353(1373):1295–1306. doi: 10.1098/rstb.1998.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Solutions to the binding problem: progress through controversy and convergence. Neuron. 1999;24(1):105–110. 111–125. doi: 10.1016/s0896-6273(00)80826-0. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning & Memory. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from event-related fMRI. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12(3):323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]


