Abstract
Objective
To compare MR signal characteristics of contrast agent-labeled apoptotic and viable human mesenchymal stem cells (hMSCs) in matrix associated stem cell implants (MASI).
Methods
hMSCs were labeled with FDA approved ferumoxides nanoparticles. One group (A) remained untreated while a second group (B) underwent Mitomycin C-induced apoptosis induction. Viability of group A and apoptosis of group B was confirmed by Caspase-assays and TUNEL stains. Labeled viable hMSCs, unlabeled viable hMSCs, labeled apoptotic hMSCs and unlabeled apoptotic hMSCs (n=7 samples each) in an agarose scaffold were implanted into cartilage defects of porcine patellae specimens and underwent MR imaging at 7T, using T1 weighted SE sequences, T2-weighted SE sequences and T2*-weighted GE sequences. Signal to noise ratios (SNR) of the implants were calculated and compared between different experimental groups using linear mixed regression models (LMM).
Results
Ferumoxides-labeled hMSCs provided a strong negative T2 and T2*-enhancement. Corresponding SNR data of labeled hMSCs were significantly lower compared to unlabeled controls (p<0.05). Apoptosis induction resulted in a significant signal decline of ferumoxides-labeled hMSC transplants on short TE T2-weighted sequences. SNR data of labeled apoptotic hMSCs were significantly lower compared to labeled viable hMSCs (p<0.05).
Conclusion
Apoptosis of transplanted ferumoxides-labeled stem cells in cartilage defects can be visualized non-invasively by a significant signal decline on T2-weighted MR images. The described MR signal characteristics may serve as a non-invasive outcome measure for the assessment of MASI therapies in clinical practice. Additional studies are needed to further enhance the observed differences between viable and apoptotic cells, e.g. by further optimizing the applied MR pulse sequence parameters or by determining more robust T2-relaxation times.
Keywords: MR Imaging, molecular imaging, apoptosis, stem cells, cartilage
Introduction
Mesenchymal stem cells (hMSCs) are a promising approach in the treatment of osteochondral defects. Matrix associated stem cell implants (MASI) are clinically applicable and lead to the synthesis of cartilage-like regeneration tissue [1]. Unlike chondrocytes, which have to be harvested from cartilage, hMSCs can be harvested from bone marrow, muscle or adipose tissue, thus avoiding further joint damage. In addition, hMSCs can be easily expanded and can be differentiated into chondrocytes, osteocytes, adipocytes, or myoblasts based on exposure to specific growth factors and culture conditions [2].
However, one major obstacle to successful MASI outcomes is a substantial post-implantation loss of stem cells due to cell efflux, necrosis and apoptosis [3, 4]. hMSC efflux occurs shortly after implantation mainly because of possible leakage of the fluid embedded cells from the transplantation site into the joint space [5]. Necrosis of transplanted hMSCs occurs as a result of physical cell damage and subsequent cell membrane disruption. Apoptosis of transplanted hMSC represents endogenous, programmed cell death as a result of either local cytotoxins or as part of the physiological aging process and is characterised by cell condensation and fragmentation [3].
At present, the outcome of matrix associated chondrocyte implants (MACI) and MASI is investigated clinically by invasive arthroscopy and biopsy several weeks after cell transplantation [1, 6, 7]. An earlier, non-invasive diagnosis of the three major causes for poor MASI outcomes, namely cell efflux, necrosis and apoptosis, would allow for earlier interventions, improve longitudinal surveillance, decrease secondary costs and reduce patient disability from invasive procedures. MR imaging provides non-invasive and direct depiction of cartilage with high anatomical resolution and high soft tissue contrast, and may serve as an alternative to direct tissue biopsy for the diagnosis of successful cartilage regeneration after MACI or MASI [8].
Direct depiction of transplanted stem cells on MR images would allow for early detection of complications several days after implantation into cartilage defects rather than late detection of successful outcomes weeks or months after implantation. Iron oxide nanoparticles provide labeling and tracking of transplanted stem cells in arthritic joints, which could be used for non-invasive diagnosis of stem cell efflux and necrosis. Previous studies demonstrated a decreased T2-signal of necrotic compared to viable iron oxide labeled stem cells, which was apparently due to a limited proton-interaction of cell-bound iron oxide nanoparticles in viable cells versus an unlimited proton-interaction of iron oxides released from mechanically disrupted necrotic cells [9].
The goal of our study was to investigate whether the same physicochemical principles apply to apoptotic iron oxide labeled stem cells. Stem cell apoptosis leads to cell fragmentation rather than cell disruption with subsequent dispersion, but not release of iron oxide labels. The hypothesis was that dispersed iron oxides in fragments of apoptotic cells would provide a stronger T2-effect compared to clustered iron oxides in viable cells. If successful, this non-invasive approach for the diagnosis of apoptosis in MASI constructs could significantly improve our ability to identify favorable MASI constructs, improve implant surveillance, and ultimately help to optimize our efforts to restore joint functions of patients with arthritis.
Materials and Methods
Contrast Agent
The FDA-approved superparamagnetic particles of iron oxide (SPIO) compound ferumoxides was used (Feridex™, Bayer Healthcare, Seattle, WA and Endorem®, Laboratoire Guerbet, Aulnay-sous-Bois, France). Ferumoxides are composed of a non-stoichiometric crystalline iron oxide core containing both ferrous iron (Fe2+) and ferric iron (Fe3+) and are coated with a dextran T-10 coat. The particles have a hydrodynamic diameter of 120–180 nm and an R2/R1 relaxivity ratio (L × mmol−1 × sec−1) of 160/40.
Cell Culture and Cell Labeling
Human mesenchymal stem cells (Lonza, Basel, Switzerland) were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and 1% Penicillin-Streptomycin in a humidified 5% CO2 atmosphere at 37°C with cultures up to passage 10. Before labeling, hMSCs were plated at 80% confluency and incubated for 12h in normal growth media. After this incubation period, the cells were washed once with PBS and incubated for 4h with labeling media, which consisted of DMEM, 1% Penicillin-Streptomycin, and ferumoxides at a concentration of 100μg Fe/mL. Following this incubation period, labeling media was removed and the cells were washed three times with PBS. All subsequently described experiments, assays, and measurements were performed seven times.
Spectrometry
Iron uptake into hMSCs was quantified by inductively coupled plasma-optical emission spectroscopy (ICP-OES). For ICP-OES, the cell samples were dissolved in 80% HNO3, nebulized into argon plasma, and analyzed by a spectrometer (Perkin-Elmer, Waltham, MA with Winlab software, North Brunswick, NJ). ICP-OES analysis of the cell samples was done at three different time points in the process of MASI preparation: (1) Before suspending the cells in the agarose scaffold; (2) after cell suspension in the agarose scaffold and before implantation into cartilage defects; (3) after implantation into cartilage defects. In the latter case, MASIs were explanted from the patella and subsequently analyzed with ICP-OES.
Induction of Apoptosis
Apoptosis of labeled and unlabeled hMSCs was induced according to a method previously described by Chang et al. [10], using Mitomycin C. Mitomycin is a classic inducer of p53-mediated apoptosis[11]. Labeled and unlabeled hMSCs were co-incubated with 0.06 mg Mitomycin C per mL media for 24 hours at standard cell culture conditions. After this incubation period, the cells were washed 3 times with PBS. Higher concentrations (0.2 to 0.5 mg/ml) were not used because they lead to a higher number of cell death in the form of necrosis [12].
Apoptosis induction in hMSCs was measured with the Caspase 3/7 assay kit (SensoLyte AMC Caspase 3/7 assay kit, Anaspec, San Diego, CA). Caspase 3 plays a key role in the apoptosis pathway. Apoptosis leads to caspase 3 cleavage and generation of the fluorescent substrate Ac-DEVD-AMC, which can be detected by spectrometry. Samples of 5 × 103 iron oxides labeled or unlabeled hMSCs in 150μl medium were plated in microplates and incubated with 50μl caspase 3 substrate solution for 30 min according to the manufacturer’s instructions. 1h and 18h later, production of Ac-DEVD-AMC as an indicator for caspase 3 cleavage and apoptosis induction was measured with a microplate spectrofluorometer (Molecular devices, Sunnyvale, CA) at excitation/emission wavelengths 354/442 nm. Samples with 50μl caspase 3 substrate solution in culture medium served as a fluorescence standard. Results from experimental groups were subtracted from control cell samples.
Implantation of Viable and Apoptotic hMSCs into Cartilage Defects
Pig knee specimens were obtained from a local butcher. The patellae were dislocated and four ~30 mm3 (3×3×3 mm3) full thickness cartilage defects were created in each patella with a ceramic scalpel (to avoid susceptibility artifacts from metal scalpel blades). To produce MASI constructs, hMSCs in DMEM (30 million cells/mL) were combined with a sterile agarose solution (42°C, 4% w/v in PBS, type VII, Sigma Aldrich, St. Louis, MO) at a 1:1 volume ratio, as described by Mauck et al. [13]. 4.5 ×105 ferumoxides labeled viable hMSCs (n=7 cartilage defects), ferumoxides labeled apoptotic hMSCs (n=7), unlabeled viable hMSCs (n=7), and unlabeled apoptotic hMSCs (n=7) were implanted into each of the created patella defects. After solidifying for 5 min at 4°C the patella specimens with MASI were placed in 50mL centrifuge tubes (VWR, West Chester, PA). The test tubes were filled with ultrasound gel (Cardinal Health, McGaw Park, IL) to avoid susceptibility artifacts from surrounding air and to mimic MR signal characteristics of joint effusion [14].
MR Imaging and Data Analysis
Patella specimens with ferumoxides-labeled or unlabeled viable or apoptotic hMSCs in cartilage defects were placed in a dedicated 1H birdcage coil (inner diameter of 32 mm) and imaged with a 7T MR scanner (Varian, Palo Alto, CA) using the following pulse sequences: T1-weighted spin echo (SE) 500/15 (TR/TE) sequences, T2-weighted SE sequences with TR of 4000 ms and multiple TE values of 18.27, 36.54, 54.81, 73.08 ms and T2*-weighted gradient echo sequences with a flip angle of 30 degrees, a TR of 300 ms, and increasing TEs of 3.82, 7.64, 11.46 and 15.28 ms. All sequences were obtained with a slice thickness of 1mm, a field of view of 40×40 mm, a matrix of 256×256 pixels, and one acquisition.
MR images in FDF format were processed with Matlab software (MathWorks Inc., Natick, MA). The signal intensity of the MASI constructs and background noise were measured by operator defined regions of interest (ROI) on all acquired images. Signal to noise ratios (SNR) were calculated by dividing the signal intensity of the implant (SIMASI) by the signal intensity of the background noise (SINoise) in phase encoding direction in the field of view according to the equation: SNR= SIMASI/SINoise.
Histology
MASI constructs were fixed in 10% paraformaldehyde (VWR, West Chester, PA), dehydrated in a graded series of ethanol and embedded in paraffin (Surgipath, Richmond, IL). Samples were sectioned at 8 μm thickness. Apoptosis in MASI constructs was assessed with the TUNEL assay (terminal dUTP nick-end labeling) according to the manufacturer’s protocol (Roche, Basel, Switzerland). Slides were counterstained with nuclear fast red (American Mastertech Scientific, Inc., Lodi, CA). The TUNEL assay labels DNA fragmentation that occurs in apoptotic cells. In random fields, TUNEL-positive nuclei as a percentage of total nuclei were determined and used as the apoptotic index.
Iron uptake into hMSCs was confirmed qualitatively by Prussian blue staining.
Confocal microscopy
MASI constructs were frozen with O.C.T. Compound (Finetek, Torrance, CA) and sectioned at 8 μm thickness. Samples were then stained with wheat germ agglutinin (WGA), Alexa Fluor® 594 (Molecular Probes, Inc., Eugene, OR) for 10 min, washed with Hank’s balanced salt solution (HBSS) and stained with anti-dextran FITC antibody (Stem Cell Technologies, Tukwila, WA) for 60 min. WGA is a carbohydrate-binding protein of approx. 36 kDa that selectively recognizes sialic acid and N-acetylglucosaminyl sugar residues, which are predominantly found on the plasma membrane. WGA conjugated with fluorophores, such as FITC, delineate cell membranes on fluorescence and cofocal microscopy. Anti-dextran-FITC stains delineate dextran coated iron oxide nanoparticles. Slides were washed three times with PBS, counterstained with DAPI (Vectashield with DAPI, Vector Laboratories, Burlingame, CA) and evaluated by confocal microscopy at 20 × magnification, using a Zeiss LSM 510 microscope (Thornwood, NY).
Statistical analysis
Linear mixed regression models (LMM) were employed to simultaneously investigate the course of SNR data over time as well as differences in SNR levels between experimental groups. The sampling time point was considered a random effect. A Bonferroni correction was applied to account for multiple group comparisons. Statistical significance of Bonferroni-adjusted p-values was considered for a two-sided level of 0.05. Required assumptions for an appropriate model fit were verified with descriptive residual analysis (Q-Q plots and histograms). Coefficients from the regression model were reported as mean +/− standard error. Error bars depicting conditional means with 95% confidence intervals were used for illustrative purposes. All statistical analyses were performed using SPSS software for Windows (V. 17.0, SPSS Inc. Chicago, IL).
Results
Cell Labeling
ICP-OES revealed a significantly higher intracellular iron content of labeled viable hMSCs (1.36 ± 0.10 pg/cell) compared to unlabeled controls (below threshold of detection for ICP-OES, p<0.05). The iron content of labeled viable and apoptotic hMSCs (1.11 ± 0.08 pg/cell) was not significantly different (p>0.05) and did not change significantly at different time points of observation: before suspending the cells in an agarose scaffold, after cell suspension in agarose, and after implantation into cartilage defects (p>0.05).
Apoptosis Induction
The trypan blue exclusion test, a measure of cell membrane integrity, showed no significant difference between viable hMSCs (no dye uptake in 96 ± 3%) and apoptotic hMSCs (91 ± 6%; p>0.05). This indicates that the cell membrane was intact in the majority of cells in both groups. The caspase 3 test, a measure of apoptosis induction, revealed a significant threefold increase of caspase activity of the Mitomycin C-treated hMSCs compared to untreated hMSCs (p<0.05; Fig. 1). There was no significant difference in the level of caspase activity between ferumoxides-labeled and unlabeled hMSCs (p>0.05; Fig. 1).
Fig 1.
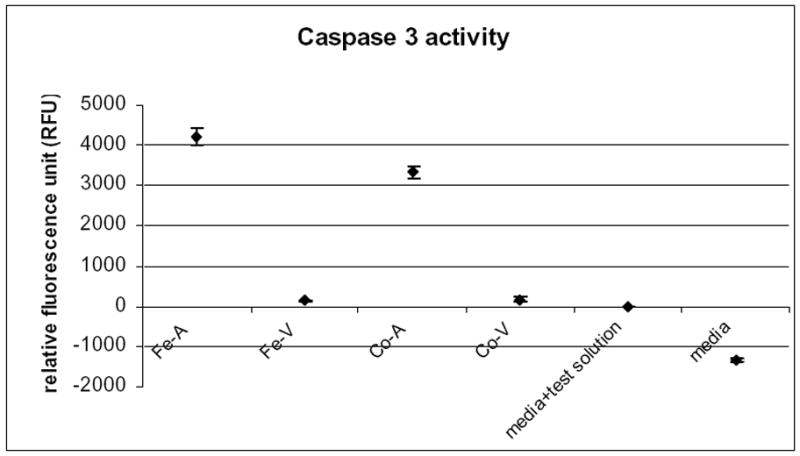
Apoptosis induction of iron oxide labeled or unlabeled, Mitomycin C treated or untreated hMSCs, as measured by the cells caspase activity and generation of the fluorescent substrate Ac-DEVD-AMC. Data points represent mean values ± standard deviations of triplicate experiments for each experimental group. Fe-A: iron labeled apoptotic hMSCs, Fe-V: iron labeled viable hMSCs, Co-A: control unlabeled apoptotic hMSCs, Co-V: control unlabeled viable hMSCs. There is a significant increase of caspase activity after apoptosis induction with Mitomycin C
MR Imaging of MASI, which contained Viable or Apoptotic hMSCs
T2-weighted SE images revealed a markedly lower signal of ferumoxides-labeled hMSCs compared to adjacent cartilage, while unlabeled hMSCs showed a similar T2-signal compared to adjacent cartilage (Fig. 2). MASI with ferumoxides-labeled apoptotic hMSCs showed a stronger signal loss on T2-weighted SE images compared to ferumoxides-labeled viable hMSCs (Fig. 2). This difference in T2-signal effects of labeled viable and apoptotic cells decreased with increasing echo times (Fig. 3). T1-SE images showed similar “shine through” T2-effects, while T2*-GE images showed no significant differences between ferumoxides labeled viable and apoptotic hMSCs (p>0.05).
Fig 2.
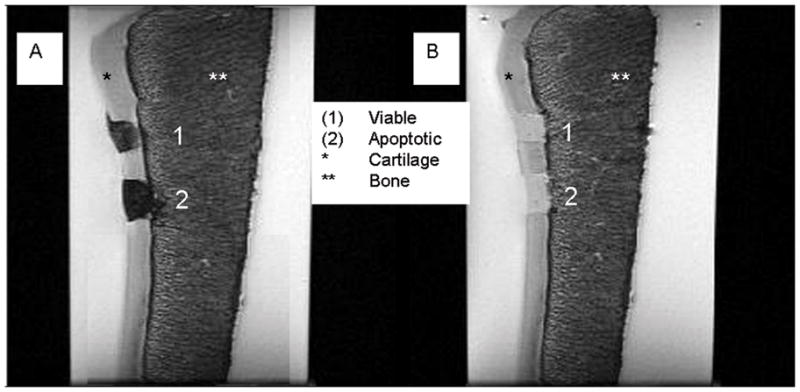
Coronal T2 weighted SE images (TR 4000 ms/TE 18.27 ms) of a patella specimen with implanted hMSCs in cartilage defects. A: MASI constructs with ferumoxides labeled hMSCs show a marked negative signal effect. Labeled viable cells (A1) show relatively less T2-signal compared to labeled apoptotic cells (A2) B: Non-labeled control hMSCs show no T2-effect and no difference in signal between viable (B1) and apoptotic (B2) cells.
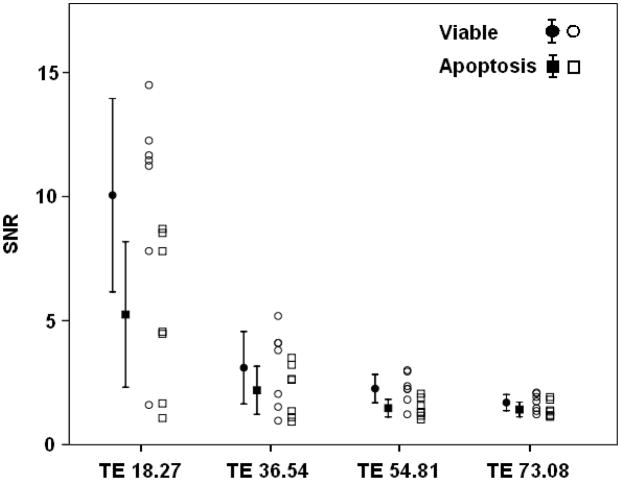
Fig 3.
Individual SNR data with mean values and 95% confidence intervals of viable (round symbols) or apoptotic (square symbols) iron oxide labeled stem cells. The SNR difference between the two groups decreased with increasing echo time (TE).
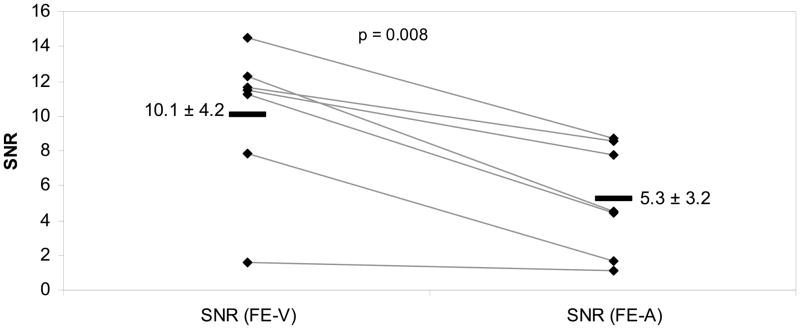
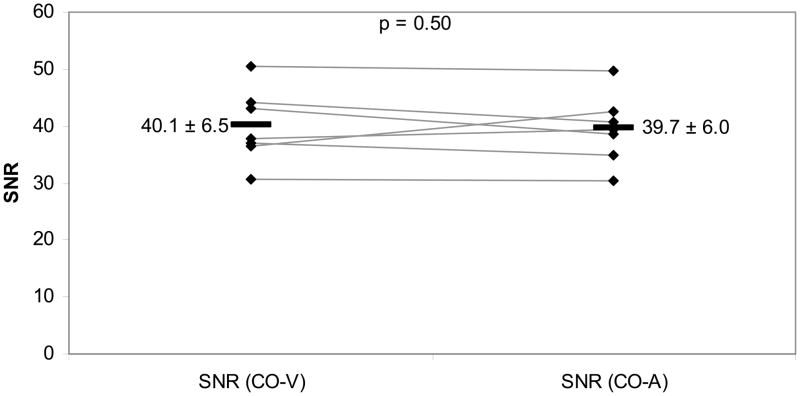
Corresponding SNR-data were significantly different between iron oxide labeled viable and apoptotic cells for T2-weighted SE images with relatively short TE values of 18.27 ms (mean difference: p=0.008; Fig 4a) and 36.54 ms (p=0.024) as well as T1-SE sequences (p=0.037). As shown in Fig. 4a, the SNR values of labeled viable cells were consistently higher compared to corresponding labeled apoptotic cells for SE-images with a short TE of 18.27 ms. This difference decreased with increasing T2-weighting. SNR values of iron oxide labeled viable and apoptotic cells on T2-SE images with higher TE values (TE 54.81: p=0.064 and TE 73.08: p=0.103) and T2*-GE images were not significantly different (p>0.05). Control MASI showed no significant difference in SNR values of unlabeled apoptotic and viable cells (p>0.05; Fig 4b).
Fig 4.
Fig 4a. SNR values of iron oxide labeled viable hMSCs (Fe-V) and iron oxide labeled apoptotic hMSCs (Fe-A) for short-TE T2-weighted SE images (TR 4000, TE 18.27). Individual data points (rhombi) are displayed as means (−) and standard deviation (±). The mean Fe-V and Fe-A data were differently different, as determined by the mixed linear regression model (p<0.05).
Fig 4b. SNR values of unlabeled viable hMSCs (CO-V) and unlabeled apoptotic hMSCs (CO-A) for short-TE T2-weighted SE images (TR 4000, TE 18.27). Individual data points (rhombi) are displayed with mean (−) and standard deviation (±). Comparison of mean CO-V versus CO-A data showed no significant difference (p>0.05).
Histology
TUNEL assays revealed apoptosis of the majority of Mitomycin C treated MASI constructs (apoptotic index: 53.4 ± 3.6%), while untreated MASI constructs showed no signs of apoptosis (Fig 5). Prussian blue stains confirmed the cellular uptake of ferumoxides particles into the cytoplasm of labeled hMSCs and lack of ferumoxides uptake in unlabeled control cells (Fig 6). Viable cells showed iron oxide particles clustered in their cytoplasm while apoptotic cells showed ferumoxides dispersed in a larger quantity of smaller cell fragments (Fig. 6).
Fig 5.
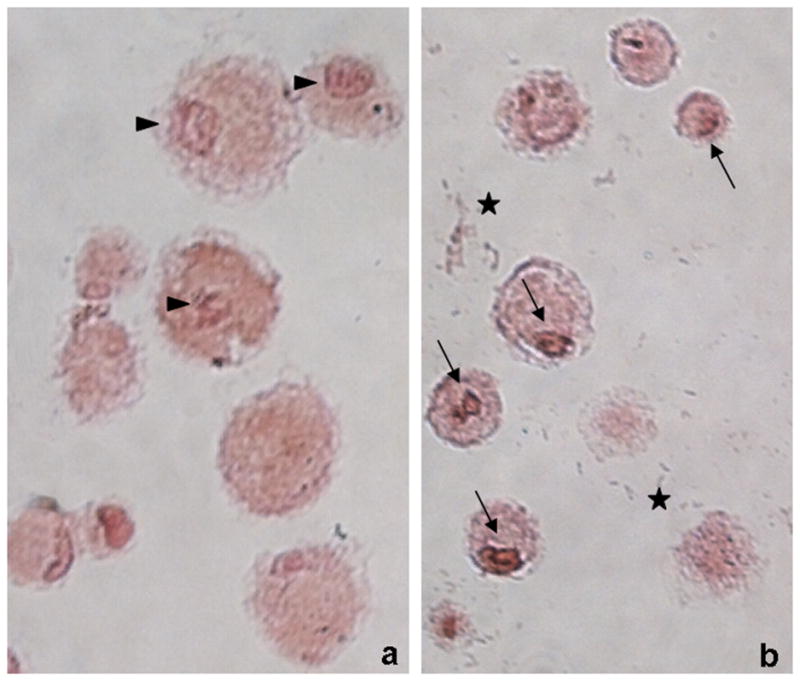
Viable (a) and apoptotic (b) cells in agarose scaffold, stained with TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) and counterstained with nuclear fast red. Apoptotic brown nuclei are highlighted with black arrows while non-apoptotic nuclei in the control group are highlighted with arrow heads. Note also the high number of cells fragments seen in the apoptotic cell group (marked with stars)
Fig 6.
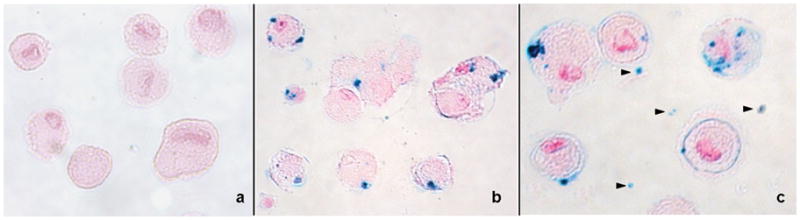
Prussian blue staining of hMSCs counterstained with nuclear fast red. a: non iron labeled hMSCs do not show any Prussian blue staining. b: iron labeled viable hMSCs show intracellular localization of ferumoxides c: iron labeled apoptotic hMSCs demonstrate intracellular localization of some ferumoxides, but also additional Prussian blue positive iron particles in cell fragments and around the cells (arrow heads).
Confocal microscopy
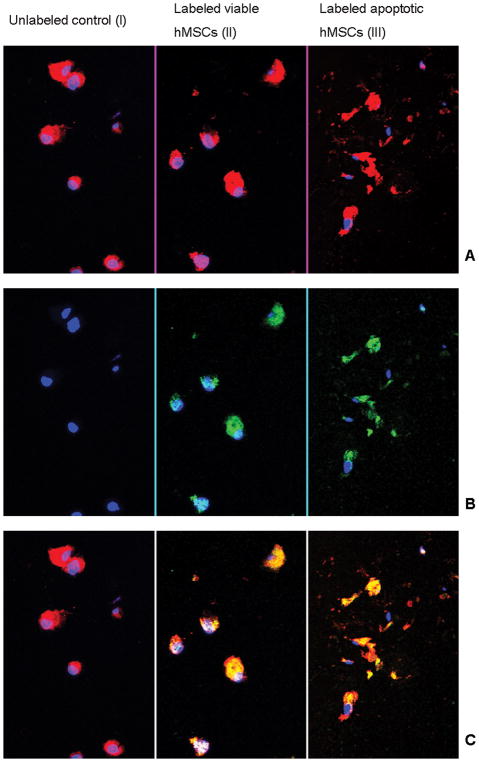
Confocal microscopy depicted the cellular integrity and iron oxide compartimentalization in viable and apoptotic cells (Fig 7). The WGA cell membrane stain showed intact cells in the “viable group” and numerous cell fragments in the “apoptotic cell group”. The anti-dextran FITC antibody stain confirmed intracellularly compartimentalized dextran-coated iron oxides in viable cells. Conversely, the apoptotic cell group showed dispersed ferumoxides inside cell fragments or released iron oxides.
Fig 7.
Confocal microscopy of hMSCs in agarose: Row A: wheat germ agglutinin cell membrane staining (red); Row B: anti-dextran staining (green); Row C: Co-localization of anti-dextran stain and wheat germ agglutinin (yellow). B II demonstrates intracellular localization of ferumoxides within the labeled viable cells. Apoptotic cells show multiple cell fragments (A III) with internalized ferumoxides (C III). The unlabeled control does not show any FITC staining (B I).
Discussion
Our data shows that MR imaging can differentiate between iron oxide nanoparticle-labeled viable and apoptotic cells. Apoptosis of ferumoxides-labeled hMSCs resulted in a significant decrease in T2-signal compared to labeled viable cells. The iron content in both experimental groups was not significantly different, excluding differences in iron concentration as the underlying cause for the observed MR signal differences. To the best of our knowledge, this is the first study that has visualized apoptosis of transplanted stem cells based on signal effects of an FDA-approved MR contrast agent.
A variety of other approaches for the detection of apoptosis by imaging methods have been described before. Most of these studies focus on the detection of apoptosis in endogenous cells (e.g. tumor cells) as opposed to transplanted stem cells. One approach utilizes Annexin V-linked tracers. Annexin V preferentially binds to phosphatidylserine which is translocated from the inner (cytoplasmic) leaflet of the plasma membrane to the outer (cell surface) leaflet in the event of apoptosis [15]. Annexin V linked to radiotracers, such as 99m Tc, 123 I and 18 F, was used to detect apoptotic cells via SPECT or PET [16–18]. A corresponding MR imaging based technique was based on SPIOs linked to Annexin V and the C2 domain of synaptotagmin I [19]. A different approach utilized fluorescently labeled activity-based probes (ABPs) that covalently labeled active caspases in vivo. The fluorescent substrate could be used to detect cytotoxic therapy induced apoptosis with optical imaging [20].
All of these previously applied techniques are based on intravenously-injected probes, which distribute not only to the target cells but also to other organs. These protein-based tracers have a limited delivery to target tissues with low vascularity and could trigger immune reactions, thereby limiting repetitive administrations and observations. Our approach is specifically tailored for detecting apoptosis in transplanted stem cells and is based on exogenous labeling of target cells with FDA approved iron oxide nanoparticles. Of note, our data and data of others showed that the FDA approved ferumoxides themselves have no adverse effect on stem cell viability, if applied in limited quantities [14, 21, 22]. Our technique allows longitudinal studies without the need of repetitive administrations of external tracers as well as non-invasive observations of implanted cells in cartilage, while avoiding systemic delivery of large quantities of contrast agent. Since we used FDA-approved iron oxides and a simple incubation labeling technique, our approach would be immediately clinically applicable.
Magnetic resonance spectroscopy (MRS) is another available method for non-invasive in vivo detection and quantification of apotosis. Apoptosis leads to decreased membrane microviscosity and increased amounts of membrane lipid, both of which result in large methylene and methyl resonances (at 1.3 ppm and 0.9 ppm, respectively), which can be detected with 1H NMR spectroscopy [23]. However, the minimum voxel area for spectroscopy applications of about 1 cm3 would be difficult to apply to the particular anatomical site of stem cells at the cartilage – bone and cartilage – joint space interfaces.
Apoptosis consists of three stages: (1) release, marked by rounding of the cell, (2) blebbing, or formation of small outpocketings on the cellular membrane and (3) condensation, a breakdown of the cell into small apoptotic bodies that contain fragmented intracellular contents [24–27]. These apoptotic bodies with a diameter of 1 to 4 μm are considerably smaller compared to adherent mesenchymal stem cells with diameters in the order of 20–100 μm (majority being 41–50 μm) [28]. Because cell volume is proportional to r3, even small hMSCs will produce hundreds of apoptotic bodies. In the case of ferumoxides-labeled hMSCs, the intracellular nanoparticles disperse in apoptotic bodies and their distribution in a MASI construct becomes more uniform. This in turn reduces the distance of nearby water molecules to the iron oxide nanoparticles, thereby shortening the T2 relaxation time and decreasing the observed T2 signal [29–31].
The effect of iron oxides on T2 relaxation times has been extensively studied and modeled [32–35]. T2 relaxation effects of iron oxides have been shown to be more sensitive to changes in iron compartmentalization than to changes in iron concentration [33, 36].
Another contribution to the decreased T2 effects seen in the labeled apoptotic cells could be a result of the changes in water dynamics in early apoptosis. Hortelano et al. described decreased T2 times in apoptotic macrophages upon caspase activation using 1H NMR [37]. It was found that the decrease in T2 signal was due to changes in water structure caused by cell shrinkage and caspase-mediated proteolysis. The authors found regions of increased hydrophobicity indicating restricted rotational movement of the intracellular water. Although we did not observe any differences in T2-effects of unlabeled viable and apoptotic hMSCs in our study, the described effects could have contributed to enhanced T2 effect of iron oxide labeled apoptotic cells compared to viable cells.
We recognize several limitations of our study:
The observed apoptosis-induced MR signal changes were relatively small because labeled viable cells showed already strong T2-relaxation effects with little room for further enhancement. Additional studies are under way to optimize the cell labeling and MR imaging technique, e.g. by loading the cells with less iron or using further decreased TE times in order to enhance our ability to detect increased T2-effects of iron labeled cells.
Our investigations have been obtained in vitro and ex vivo, in pig knee specimen. Further studies have to prove our concept in vivo. In vivo, iron oxide labeled dead cells may be phagocytosed by local macrophages which could alter the described T2-effects [24–27].
Our studies were done with FDA-approved ferumoxides. Further studies have to evaluate whether the observed signal effects also apply to new, second generation ultrasmall superparamagentic iron oxide nanoparticles, which have been recently FDA-approved.
In conclusion, our data show that ferumoxides labeling can be used to differentiate viable and apoptotic stem cells on MR images based on differences in their T2 effects. This non-invasive imaging technique may be used as a new and non-inasive measure for stem cell engraftment outcomes. Potential applications include comparative investigations of the engraftment process of embryonic and adult stem cells, comparisons of transplanted autologous and allogeneic cells, investigations of various stem cell subtypes or genetically engineered stem cells, and assessments of therapy effects on stem cell engraftment outcomes. Since we used clinical MR equipment and FDA-approved MR contrast agents, our concept would be in principle readily accessible for patients with arthritis. The presence and grade of potential clinical improvement after MASI could be correlated with the presence of viable stem cells at the site of disease.
Acknowledgments
This work was supported primarily by NIH grant R01AR054458 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; and in part by grants from the National Heart, Lung and Blood Institute (HL24136, HL59157) and the National Cancer Institute (CA82923)
References
- 1.Wakitani S, Imoto K, Yamamoto T, et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 2004;15:406–410. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Steinert AF, Ghivizzani SC, Rethwilm A, et al. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koga H, Engebretsen L, Brinchmann JE, et al. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009:1289–1297. doi: 10.1007/s00167-009-0782-4. [DOI] [PubMed] [Google Scholar]
- 5.Peretti GM, Xu JW, Bonassar LJ, et al. Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Eng. 2006;12:1151–1168. doi: 10.1089/ten.2006.12.1151. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Niemeyer P, Pestka JM, Kreuz PC, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36:2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 8.Pinker K, Szomolanyi P, Welsch GC, et al. Longitudinal evaluation of cartilage composition of matrix-associated autologous chondrocyte transplants with 3-T delayed gadolinium-enhanced MRI of cartilage. AJR Am J Roentgenol. 2008;191:1391–1396. doi: 10.2214/AJR.07.3930. [DOI] [PubMed] [Google Scholar]
- 9.Henning TD, Wendland MF, Golovko D, et al. Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5T and 3T: Discrimination of viable from lysed cells. Magn Reson Med. 2009:325–332. doi: 10.1002/mrm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SW, Chou SF, Chuang JL. Mitomycin C potentiates ultraviolet-related cytotoxicity in corneal fibroblasts. Cornea. 2008;27:686–692. doi: 10.1097/01.ico.0000611400.04418.79. [DOI] [PubMed] [Google Scholar]
- 11.Cheng MH, Cheng HT, Lin SS, et al. Apoptotic death mode of mitomycin C-treated HeLa cells and cellular localization of mitomycin C-induced P-glycoprotein. Drug Chem Toxicol. 2009;32:158–168. doi: 10.1080/01480540802594491. [DOI] [PubMed] [Google Scholar]
- 12.Occleston NL, Daniels JT, Tarnuzzer RW, et al. Single exposures to antiproliferatives: long-term effects on ocular fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci. 1997;38:1998–2007. [PubMed] [Google Scholar]
- 13.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Nedopil AJ, Mandrussow LG, Daldrup-Link HE. Implantation of ferumoxides labeled human mesenchymal stem cells in cartilage defects. J Vis Exp. 2010;5:pii, 1793. doi: 10.3791/1793. doi:1710.3791/1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 16.Blankenberg FG, Katsikis PD, Tait JF, et al. In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci U S A. 1998;95:6349–6354. doi: 10.1073/pnas.95.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tait JF, Smith C, Blankenberg FG. Structural requirements for in vivo detection of cell death with 99mTc-annexin V. J Nucl Med. 2005;46:807–815. [PMC free article] [PubMed] [Google Scholar]
- 18.Lahorte CM, Vanderheyden JL, Steinmetz N, et al. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur J Nucl Med Mol Imaging. 2004;31:887–919. doi: 10.1007/s00259-004-1555-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, Beauregard DA, Loizou L, et al. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nature Medicine. 2001;7:1241–1244. doi: 10.1038/nm1101-1241. [DOI] [PubMed] [Google Scholar]
- 20.Edgington LE, Berger AB, Blum G, et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat Med. 2009;15:967–973. doi: 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henning TD, Sutton EJ, Kim A, et al. The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast Media Mol Imaging. 2009;4:165–173. doi: 10.1002/cmmi.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutry S, Brunin S, Mahieu I, et al. Magnetic labeling of non-phagocytic adherent cells with iron oxide nanoparticles: a comprehensive study. Contrast Media & Molecular Imaging. 2008;3:223–232. doi: 10.1002/cmmi.256. [DOI] [PubMed] [Google Scholar]
- 23.Blankenberg FG, Storrs RW, Naumovski L, et al. Detection of apoptotic cell death by proton nuclear magnetic resonance spectroscopy. Blood. 1996;87:1951–1956. [PubMed] [Google Scholar]
- 24.Häcker G. The morphology of apoptosis. Cell and Tissue Research. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 25.Hristov M, Erl W, Linder S, et al. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 26.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anatomia, Histologia, Embryologia. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 28.Furlani D, Ugurlucan M, Ong L, et al. Is the intravascular administration of mesenchymal stem cells safe?: Mesenchymal stem cells and intravital microscopy. Microvascular Research. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Kirsch JE. Basic principles of magnetic resonance contrast agents. Topics in Magnetic Resonance Imaging: TMRI. 1991;3:1–18. [PubMed] [Google Scholar]
- 30.Renshaw PF, Charles SO, Alan CM, et al. Ferromagnetic contrast agents: A new approach. Magnetic Resonance in Medicine. 1986;3:217–225. doi: 10.1002/mrm.1910030205. [DOI] [PubMed] [Google Scholar]
- 31.Tanimoto A, Daniel P, Burkhard PK, et al. Effects of spatial distribution on proton relaxation enhancement by particulate iron oxide. Journal of Magnetic Resonance Imaging. 1994;4:653–657. doi: 10.1002/jmri.1880040506. [DOI] [PubMed] [Google Scholar]
- 32.Bloembergen N, Purcell EM, Pound RV. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Physical Review. 1948;73:679–679. [Google Scholar]
- 33.Bowen CV, Zhang X, Saab G, et al. Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cells. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2002;48:52–61. doi: 10.1002/mrm.10192. [DOI] [PubMed] [Google Scholar]
- 34.Brooks RA, Francis M, Pierre G. On T2-shortening by weakly magnetized particles: The chemical exchange model. Magnetic Resonance in Medicine. 2001;45:1014–1020. doi: 10.1002/mrm.1135. [DOI] [PubMed] [Google Scholar]
- 35.Gillis P, Francis M, Rodney AB. On T2-shortening by strongly magnetized spheres: A partial refocusing model. Magnetic Resonance in Medicine. 2002;47:257–263. doi: 10.1002/mrm.10059. [DOI] [PubMed] [Google Scholar]
- 36.Kuhlpeter R, Dahnke H, Matuszewski L, et al. R2 and R2* Mapping for Sensing Cell-bound Superparamagnetic Nanoparticles: In Vitro and Murine in Vivo Testing. Radiology. 2007;245:449–457. doi: 10.1148/radiol.2451061345. [DOI] [PubMed] [Google Scholar]
- 37.Hortelano S, García-Martín ML, Cerdán S, et al. Intracellular water motion decreases in apoptotic macrophages after caspase activation. Cell Death and Differentiation. 2001;8:1022–1028. doi: 10.1038/sj.cdd.4400913. [DOI] [PubMed] [Google Scholar]