Abstract
Objective
The Patient-Reported Outcomes Measurement Information System (PROMIS) allows assessment of the impact of chronic conditions on health-related quality of life (HRQL) across diseases. We report on the HRQL impact of individual and comorbid conditions as well as conditions that are described as limiting activity.
Study Design and Setting
Data were collected through online and clinic recruitment as part of the PROMIS item calibration sample (n=21,133). Participants reported the presence or absence of 24 chronic health conditions and whether or not their activity was limited by each condition.
Results
Across health status domains, the presence of a chronic condition was associated with poorer scores than those without a diagnosis, particularly for those individuals who reported their condition was disabling. The magnitude of detriment in HRQL was more pronounced for individuals with two or more chronic conditions and could not be explained by sociodemographic factors. Patterns of HRQL deficits varied across disease and comorbidity status.
Conclusion
The impact of chronic conditions, particularly when experienced with comorbid disease, is associated with detriments in HRQL. The negative impact on HRQL varies across symptoms and functional areas within a given condition.
Keywords: Chronic disease, Comorbidity, Quality of life, Outcome measures
Background
In the United States, it is estimated that over half (133 million) of the adult population is affected by at least one chronic medical condition.[1] Most chronic conditions have a deleterious effect upon self-reported physical and mental health.[2–4] For example, 11.8% of the general US population reported having limitations in activities of daily living (e.g., personal hygiene, eating), instrumental activities of daily living (e.g., managing medications, doing housework), or working due to a chronic condition.[5] Chronic conditions vary in the degree of limitation associated with them. For working-age and older adults, arthritis and other musculoskeletal conditions were the chronic conditions most frequently reported to cause limitations in activity.[6] These were followed by heart disease and mental illness, depending on the age group. Diabetes and lung disease (e.g., asthma, chronic obstructive lung disease [COPD]) were also frequently reported.
Medical conditions negatively impact not only daily functioning, but also perceived quality of life. This impact may be variable across different components of health-related quality of life (HRQL). For example, being HIV positive but asymptomatic leads to no decrement in physical functioning but has a profound impact on emotional well-being.[7] Cancer survivors who indicated having limitations due to their cancer reported poorer physical and mental health than those without limitations.[8] Multiple conditions represent an added burden on daily functioning and HRQL. For example, cancer patients with more comorbid conditions were found to report poorer physical health than those without comorbid conditions.[9] Given the advancing age of the general population, and the fact that almost half of all people with an index condition actually experience multiple conditions,[10] the impact of multiple comorbidities on HRQL may be a more appropriate “real world” way to appreciate the burden of illness.
It is abundantly clear that chronic illness, including the comorbidity of multiple illnesses, has a detrimental effect upon HRQL.[1–13] However, often the impact of chronic illness and comorbidity has suffered from the use of multiple and different instruments to assess HRQL. [11–13]A population-level appreciation for the HRQL impact of chronic illness comorbidity could be enhanced by a standardized, yet detailed and precise assessment of common HRQL symptoms and functional reports. To that end, we developed the Patient-Reported Outcomes Measurement Information System (PROMIS). PROMIS is an NIH Roadmap Initiative that aims to build and validate common, accessible item banks to measure key symptoms and health concepts applicable to a range of chronic conditions, enabling efficient and interpretable clinical trial research and clinical practice application of patient-reported outcomes. The item banks can be used for both computerized-adaptive testing and fixed length assessment of HRQL domains. PROMIS measures were developed based upon the combined experience of network investigators with prior instruments,[11–20] patient-reported outcome (PRO) measurement and analytic expertise, and published guidelines about developing measures of self-reported health.[21] Using the large PROMIS dataset, we examined the association of individual and cumulative chronic conditions on HRQL and differences in impact on HRQL of having a condition without limitations versus having a condition that limits current activities. Therefore, this paper represents the first test of the ability of PROMIS measures to demonstrate the known impact of comorbidity upon HRQL, including self-reported symptoms and function.
Methods
Measures and Procedure
PROMIS aims to develop and evaluate item banks for clinical research that are appropriate for common chronic disease populations.[22] Early in the development process, a domain framework was constructed (accessible at http://www.nihpromis.org/Web%20Pages/Domain%20Framework.aspx) and five domains were selected for initial bank construction: physical function, fatigue, pain, emotional distress, and social function. Bank development included expert review, cognitive interviews and focus groups with patients.[23–25]
The preliminary PROMIS items were then administered to a large sample representing the U.S. general population and to multiple disease groups.[26] Due to the large number of items, a sampling plan was implemented to limit the number of items any one participant would answer to approximately 150.[21] Some participants received all items from two of the item pools (full bank design), whereas other participants received a set of seven items from each of the preliminary item pools (block design). This strategy allowed for the evaluation of dimensionality, item bank calibration, and evaluation of relationships between domains. The samples of study participants included an internet panel and samples drawn from clinic recruitment at PROMIS network primary research sites that represented known disease groups, including heart disease, cancer, arthritis, psychiatric illness, COPD, and spinal cord injury.
Items were calibrated using a T-score metric with the mean of the U.S. general population equal to 50 and standard deviation fixed at 10 [26]. Higher scores indicate more of the domain being measured. Hence, high scores for the anger, anxiety, depression, fatigue, pain behavior, and pain interference banks indicate poorer health, whereas high scores for the physical function, satisfaction with participation in social roles, and satisfaction with participation in discretionary social activities banks indicate better health.
Participants responded to sociodemographic items, questions assessing comorbid conditions, and finally PROMIS items. For comorbid conditions, participants were asked to indicate for each of 24 conditions, “Have you ever been told by a doctor or a health professional that you had (chronic condition)?” Synonyms or commonly used terms were provided in addition to the clinical name (e.g., “high blood pressure (hypertension)”. If an individual endorsed a condition, a follow-up question was asked, “Are any of your current activities limited by your (chronic condition)?” Common conditions and those expected to have a significant impact on HRQL were selected. The list of diseases included cardiac (hypertension, angina, coronary artery disease, heart failure, heart attack), pulmonary (asthma, COPD), neurological (stroke, spinal cord injury, multiple sclerosis, Parkinson’s disease, epilepsy, amyotrophic lateral sclerosis), psychiatric (depression, anxiety, drug/alcohol problems), and other (cancer, diabetes, arthritis, liver disease, kidney disease, migraines, HIV, sleep disorder) conditions.
Participants
The majority (19,601; 93%) of the sample was recruited through an online polling firm, YouGovPolimetrix. This panel consists of approximately 1,000,000 members who agree to periodically complete online surveys for minimal compensation. Panel members are emailed invitations to participate in various survey studies. If interested, a participant is directed to a study URL where all questionnaire items are presented and answered by clicking on the most appropriate response. YouGovPolimetrix targeted invitations for participation to those panel members meeting key demographic characteristics. Specifically, the panel sample was targeted to include approximately equal numbers of men and women, representation of African-American and Latinos at rates matching the 2000 census figures (10% African-American, 10% Latinos), 10% without a high school education, and an age distribution such that the sample was evenly divided among age groups of 18–29 years, 30–44 years, 45–59 years, 60–74 years, and over 75 years. Most participants were recruited without regard to their health conditions. That is, reporting having a chronic health condition was not a study eligibility criterion.
Additional participants (1,532) were recruited through clinics affiliated with PROMIS network sites in order to increase representation of clinical groups, including arthritis, cancer, coronary artery disease, COPD, spinal cord injury, and psychiatric conditions. These participants completed assessments via computer within clinics or at a PROMIS testing facility. All data collection was computer based and utilized a secure server.
Analysis Plan
For purposes of analyses, participants were grouped into those who reported zero chronic conditions, one condition, or two or more of the 24 listed chronic conditions. Because of the primary, cross-disease purpose of PROMIS, and the ample empirical evidence that chronic disease impact upon HRQL is more generic than disease-specific, we chose to analyze the data according to number of conditions rather than by specific disease. Additionally, participants were divided into those who reported not being limited by any of their chronic conditions, being limited by one condition, or being limited by two or more conditions. In order to be included in analyses, block design participants had to have responses for at least 6 of the 7 block items and full bank participants had to have responses for at least 28 of the 56 items. Participants were excluded if they had repetitive strings of ten or more identical responses and took less than one second per item. Group differences in IRT-based T-scores for anger, anxiety, depression, fatigue, pain behavior, pain interference, physical function, satisfaction with participation in social roles, and satisfaction with participation in discretionary social activities were estimated using the PROC ANOVA and General Linear Model in SAS.
Results
A total of 725 respondents were excluded due to missing data, response pattern and response time. No differences between the analytic sample and excluded sample were found except that the excluded sample was one and a half years younger on average. The final sample consisted of 21,133 participants, approximately equally divided based on gender (52% female; see Table 1). The median age was approximately 55 years, with 28% of the sample being 65 or older. Only 12% were 18–29 and another 12% age 30–39. Most participants were White (80%) and non-Hispanic/Latino (91%). Three percent of the sample had not completed high school, 16% had a high school diploma or GED, 39% had some college, 24% a college degree and 19% held an advanced degree.
Table 1.
Demographics
| N | % | |
|---|---|---|
| Race/Ethnicity | ||
| White | 16795 | 79.70 |
| Black/African-American | 1915 | 9.09 |
| Latino | 1855 | 8.80 |
| Other | 509 | 2.42 |
| Missing | 59 | |
| Education | ||
| Less than High School Degree | 550 | 2.61 |
| High School Degree or GED | 3284 | 15.56 |
| Some College, Technical School, or Associate Degree | 8155 | 38.65 |
| College Degree/Advanced Degree | 9110 | 43.18 |
| Missing | 34 | |
| Gender | ||
| Male | 10130 | 47.96 |
| Female | 10992 | 52.04 |
| Missing | 11 | |
| Living With Partner | ||
| Yes | 13813 | 65.44 |
| No | 7295 | 34.56 |
| Missing | 25 | |
| Age | ||
| Mean (SD) | 53.17 (17.10) | |
| Range | 18–100 | |
| Missing | 29 | |
| # of Chronic Conditions | ||
| 0 | 4015 | 19.00 |
| 1 | 4164 | 19.70 |
| 2 | 4064 | 19.23 |
| 3 | 3156 | 14.93 |
| 4 | 2185 | 10.34 |
| 5 or more | 3549 | 16.79 |
Nineteen percent of the sample reported having none of the 24 conditions while 20% reported one condition, and 61% reported having two or more. The most frequently reported conditions were hypertension (41%), arthritis (32%), depression (26%), anxiety (18%), cancer (17%), migraines (17%) and asthma (16%). Of those with conditions, over half (60%) reported no disabling conditions, 20% one disabling condition, and 19% two or more disabling conditions. The percentage of the sample with each of the 24 conditions is provided in Table 2.
Table 2.
Percent of Sample (N=21,133) with Self-Reported Chronic Conditions
| Condition | Percent with Condition |
|---|---|
| Hypertension | 41.01 |
| Arthritis | 32.21 |
| Depression | 26.32 |
| Anxiety | 18.32 |
| Migraines | 17.13 |
| Cancer | 16.76 |
| Asthma | 16.00 |
| Sleep disorder | 13.62 |
| Diabetes | 13.33 |
| COPD | 11.03 |
| Angina | 9.87 |
| Heart attack | 6.42 |
| Coronary artery disease | 6.17 |
| Heart Failure | 4.53 |
| Spinal cord injury | 4.13 |
| Liver disease | 4.12 |
| Stroke | 4.08 |
| Alcohol/drug problems | 4.03 |
| Kidney disease | 2.90 |
| Epilepsy | 1.22 |
| Multiple sclerosis | 0.92 |
| HIV | 0.73 |
| Parkinson’s disease | 0.24 |
| Amyotrophic lateral sclerosis | 0.07 |
Across most domains, participants without diagnoses had better HRQL than the general population (T-scores .20 to .80 standard deviation [SD] units better than the population mean). T-scores for HRQL domains by number of conditions and number of disabling conditions are reported in Tables 3 to 5. For pain behavior (F=712.02), pain interference (F=1028.25), fatigue (F=818.77), anxiety (F=333.92), depression (F=336.96), physical functioning (F=2018.10), satisfaction with participation in social roles (F=383.07), and satisfaction with participation in discretionary social activities (F=191.57) there were significant differences in scores among participants with zero, one, or two or more chronic conditions (all overall p’s <.0001), with poorer HRQL for participants with more conditions. For anger, there was no difference between groups with zero and one condition. However, patients with two or more chronic conditions reported poorer anger scores than both those with zero and one condition (F=140.36, pairwise p values <.05). The magnitude of difference between zero and the 2+ condition groups ranged from 0.30 to 1.1 SD units.
Table 3.
Pain and Fatigue (T-Scores) by Number of Conditions*
| Pain behavior | Pain interference | Fatigue | ||||
|---|---|---|---|---|---|---|
| Conditions | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Number | ||||||
| None | 39 c | 40 c | 46 c | 47 c | 45 c | 44 c |
| (n) | (2056) | (2071) | (2062) | |||
| One | 41 b | 42 b | 48 b | 49 b | 47 b | 47 b |
| (n) | (2694) | (2682) | (2708) | |||
| Two or more | 46 a | 47 a | 54 a | 55 a | 53 a | 54 a |
| (n) | (10086) | (10095) | (10156) | |||
| F = 786.22 | F = 216.31 | F = 1028.25 | F = 258.68 | F = 818.77 | F = 315.25 | |
| # Disabling | ||||||
| None | 41 c | 42 c | 47 c | 49 c | 47 c | 47 c |
| (n) | (8225) | (8239) | (8264) | |||
| One | 46 b | 47 b | 54 b | 55 b | 53 b | 53 b |
| (n) | (3252) | (3234) | (3260) | |||
| Two or more | 51 a | 52 a | 60 a | 61 a | 59 a | 59 a |
| (n) | (3359) | (3375) | (3402) | |||
| F = 2174.56 | F = 434.17 | F = 3179.09 | F = 622.37 | F =2896.07 | F = 631.58 | |
Means that share the same letter are not significantly different from each other with the overall F significant at p<.0001 and pairwise comparisons p<.05.
Table 5.
Physical Functioning and Social-Role Participation (T-Scores) by Number of Conditions*
| Physical function | Satisfaction with social roles | Satisfaction with discretionary social activities | ||||
|---|---|---|---|---|---|---|
| Conditions | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Number | ||||||
| None | 56 a | 54 a | 53 a | 54 a | 53 a | 54 a |
| (n) | (2326) | (2070) | (2070) | |||
| One | 52 b | 51 b | 52 b | 52 b | 52 b | 52 b |
| (n) | (2856) | (2665) | (2665) | |||
| Two or more | 45 c | 44 c | 49 c | 48 c | 50 c | 49 c |
| (n) | (10486) | (10071) | (10071) | |||
| F = 2029.17 | F = 537.53 | F = 383.07 | F = 134.51 | F = 191.57 | F = 106.45 | |
| # Disabling | ||||||
| None | 52 a | 51 a | 53 a | 53 a | 53 a | 53 a |
| (n) | (8814) | (8192) | (8192) | |||
| One | 45 b | 44 b | 49 b | 49 b | 50 b | 50 b |
| (n) | (3387) | (3236) | (3236) | |||
| Two or more | 40 c | 39 c | 44 c | 44 c | 46 c | 46 c |
| (n) | (3467) | (3378) | (3378) | |||
| F = 3745.79 | F = 1051.54 | F = 1656.83 | F = 331.40 | F = 1024.72 | F = 229.96 | |
Means that share the same letter are not significantly different from each other with the overall F significant at p<.0001 and pairwise comparisons p<.05.
A similar pattern was noted for participants who reported having zero, one, or two or more disabling chronic conditions. For all domains, patients with one disabling chronic condition reported significantly poorer HRQL than those without a disabling chronic condition and patients with two or more disabling conditions reported poorer HRQL than those with zero and one disabling condition (F’s = 585.34 to 3695.96, p’s <.0001). The range of difference between the zero and 2+ disabling chronic conditions groups was 0.50 to 1.3 SD units.
When adjusting for multiple sociodemographic factors (female gender, living with a partner, age [continuous], race [Latino, African-American, White, other], and education [less than high school, high school diploma/GED, some college, college/post-graduate degree]), results followed a similar pattern and remained significant. Across domains, more chronic conditions were associated with poorer HRQL (F’s=106.45 to 534.24, p’s <.0001). The difference in scores between the zero and 2+ condition groups ranged from 0.50 to 1.0 SD units. Similarly, participants with more disabling chronic conditions reported poorer HRQL than those with fewer or no disabling chronic conditions (F’s=196.86 to 1038.05, p’s <.0001). The difference for those with 2+ disabling conditions compared to no disabling conditions ranged from 0.6 to 1.2 SD units.
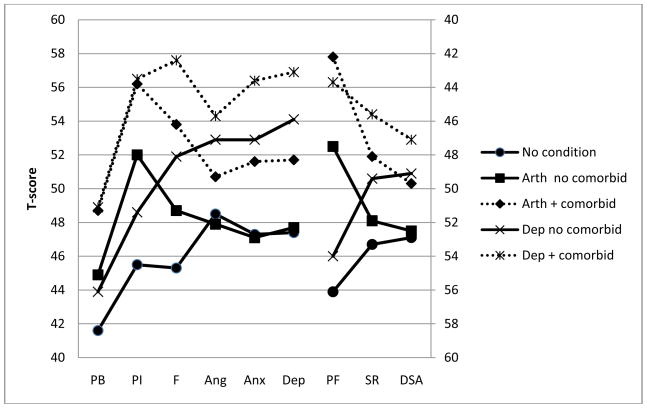
Domain scores by condition for participants with and without comorbidity are presented in Table 6. Across conditions, different symptom patterns were noted. For example, compared with individuals without chronic conditions, patients with arthritis alone reported poorer scores for pain, fatigue, physical function, and satisfaction with participation in social roles. However, the groups were not different for measures of emotional distress or satisfaction with participation in discretionary social activities (see Figure 1). Individuals who reported being diagnosed with a depressive condition reported poorer functioning and greater symptoms than individuals without a condition across all areas of HRQL that were measured. For all listed conditions (hypertension, arthritis, depression, anxiety, migraine, cancer, asthma, sleep disorder, diabetes, and COPD), having multiple conditions was almost always associated with poorer HRQL than having no condition.
Table 6.
Domain scores and standard deviations for disease groups with and without comorbid conditions
| No Condition | HTN | Arthritis | Depression | Anxiety | Migraine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comorbidity | Comorbidity | Comorbidity | Comorbidity | Comorbidity | |||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||
| Pain Behavior | 41.6 (6.1) | 41.7 (6.1) | 47.0 (7.4) | 44.9 (6.8) | 48.7 (7.1) | 43.9 (6.9) | 48.9 (7.5) | 43.4 (7.0) | 49.1 (7.6) | 44.4 (7.2) | 49.4 (7.3) |
| n= | 1934 | 545 | 5803 | 348 | 4993 | 191 | 3973 | 121 | 2768 | 239 | 2471 |
| Compare w/no condition | n.s. | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.01 | p<.0001 | p<.0001 | p<.0001 | |
| Pain Interfere. | 45.5 (6.5) | 45.9 (6.7) | 53.5 (9.3) | 52.0 (8.2) | 56.2 (8.8) | 48.6 (7.4) | 56.5 (9.6) | 45.6 (7.9) | 56.6 (9.7) | 50.2 (8.4) | 56.8 (9.3) |
| n= | 1947 | 538 | 5806 | 350 | 5000 | 193 | 3979 | 113 | 2799 | 234 | 2485 |
| Compare w/no condition | n.s. | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.05 | p<.0001 | p<.0001 | p<.0001 | |
| Fatigue | 45.3 (8.3) | 44.7 (7.5) | 52.2 (9.7) | 48.7 (8.1) | 53.8 (9.6) | 51.9 (8.2) | 57.6 (8.9) | 49.6 (7.6) | 57.8 (9.1) | 49.4 (8.3) | 56.4 (9.6) |
| n= | 1936 | 536 | 5829 | 353 | 5027 | 194 | 3992 | 119 | 2818 | 242 | 2481 |
| Compare w/no condition | p<.05 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | |
| Anger | 48.5 (8.3) | 47.5 (7.8) | 50.3 (8.6) | 47.9 (7.6) | 50.7 (8.6) | 52.9 (8.8) | 54.3 (9.1) | 51.3 (8.0) | 54.9 (9.0) | 49.3 (8.1) | 48.5 (8.3) |
| n= | 1944 | 539 | 5833 | 348 | 5006 | 191 | 3994 | 122 | 2816 | 245 | 2456 |
| Compare w/no condition | p<.01 | p<.0001 | n.s. | p<.0001 | p<.0001 | p<.0001 | p<.001 | p<.0001 | n.s. | p<.0001 | |
| Anxiety | 47.3 (7.7) | 46.3 (7.3) | 50.7 (8.9) | 47.1 (7.3) | 51.6 (9.0) | 52.9 (8.4) | 56.4 (9.1) | 52.2 (8.2) | 57.8 (9.1) | 49.6 (8.6) | 54.4 (9.5) |
| n= | 1904 | 535 | 5824 | 346 | 5006 | 190 | 3997 | 120 | 2806 | 236 | 2481 |
| Compare w/no condition | p<.01 | p<.0001 | n.s. | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | |
| Depression | 47.4 (7.8) | 46.1 (7.3) | 50.8 (9.0) | 47.7 (7.3) | 51.7 (9.1) | 54.1 (9.1) | 56.9 (9.2) | 50.5 (7.9) | 57.1 (9.3) | 49.3 (8.4) | 54.7 (9.6) |
| n= | 1901 | 535 | 5828 | 349 | 5006 | 189 | 4004 | 120 | 2814 | 237 | 2487 |
| Compare w/no condition | p<.001 | p<.0001 | n.s. | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.001 | p<.0001 | |
| Physical Function | 56.1 (6.7) | 53.6 (6.8) | 44.2 (8.8) | 47.5 (7.8) | 42.2 (8.4) | 54.0 (7.3) | 43.7 (9.3) | 54.3 (6.8) | 44.1 (9.5) | 54.1 (7.1) | 44.4 (9.4) |
| n= | 2161 | 594 | 6045 | 366 | 5162 | 212 | 4121 | 125 | 2890 | 253 | 2561 |
| Compare w/no condition | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.01 | p<.0001 | p<.0001 | p<.0001 | |
| Social Role Satisfaction | 53.3 (7.9) | 53.9 (7.5) | 49.1 (8.6) | 51.9 (7.5) | 48.1 (8.6) | 49.4 (7.2) | 45.6 (8.4) | 52.2 (7.6) | 45.7 (8.5) | 51.7 (7.9) | 46.7 (8.7) |
| n= | 1936 | 531 | 5793 | 346 | 4984 | 195 | 3975 | 115 | 2801 | 240 | 2481 |
| Compare w/no condition | n.s. | p<.0001 | p<.01 | p<.0001 | p<.0001 | p<.0001 | n.s. | p<.0001 | p<.01 | p<.0001 | |
| Social DSA Satisfaction | 52.9 (7.9) | 53.4 (7.4) | 50.4 (8.2) | 52.5 (7.7) | 49.7 (8.2) | 49.1 (6.4) | 47.1 (7.8) | 51.0 (6.4) | 47.0 (7.8) | 52.2 (7.5) | 48.3 (8.2) |
| n= | 1936 | 531 | 5793 | 346 | 4984 | 195 | 3975 | 115 | 2801 | 240 | 2481 |
| Compare w/no condition | n.s. | p<.0001 | n.s. | p<.0001 | p<.0001 | p<.0001 | p<.01 | p<.0001 | n.s. | p<.0001 | |
| No Condition | Cancer | Asthma | Sleep | DM | COPD | ||||||
| Comorbidity | Comorbidity | Comorbidity | Comorbidity | Comorbidity | |||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||
| Pain Behavior | 41.6 (6.1) | 40.9 (5.6) | 45.3 (7.3) | 43.3 (6.7) | 48.7 (7.5) | 42.7 (5.8) | 49.9 (7.4) | 41.7 (5.8) | 48.2 (7.5) | 45.2 (7.5) | 49.8 (7.5) |
| n= | 1934 | 411 | 2536 | 204 | 2307 | 26 | 2204 | 73 | 2074 | 110 | 1733 |
| Compare w/no condition | p<.05 | p<.0001 | p<.001 | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | p<.0001 | p<.0001 | |
| Pain Interfere. | 45.5 (6.5) | 45.5 (6.4) | 51.5 (8.8) | 47.6 (7.5) | 55.4 (9.6) | 46.8 (5.7) | 57.7 (9.3) | 45.8 (6.7) | 55.0 (9.5) | 48.5 (8.4) | 55.7 (9.7) |
| n= | 1947 | 419 | 2535 | 194 | 2326 | 26 | 2198 | 75 | 2069 | 112 | 1741 |
| Compare w/no condition | n.s. | p<.0001 | p<.0001 | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | p<.001 | p<.0001 | |
| Fatigue | 45.3 (8.3) | 44.0 (7.7) | 50.2 (9.4) | 47.9 (8.3) | 54.9 (9.9) | 47.2 (7.5) | 57.9 (9.3) | 46.7 (7.7) | 53.8 (9.6) | 49.3 (9.6) | 55.3 (9.8) |
| n= | 1936 | 410 | 2538 | 208 | 2330 | 25 | 2216 | 82 | 2081 | 113 | 1757 |
| Compare w/no condition | p<.0001 | p<.0001 | p<.0001 | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | p<.0001 | p<.0001 | |
| Anger | 48.5 (8.3) | 46.9 (7.7) | 48.7 (8.1) | 49.2 (8.3) | 52.3 (9.1) | 50.7 (6.1) | 53.7 (9.2) | 49.6 (7.5) | 51.1 (8.9) | 50.0 (8.0) | 52.3 (8.7) |
| n= | 1944 | 412 | 2538 | 203 | 2308 | 26 | 2224 | 74 | 2090 | 113 | 1762 |
| Compare w/no condition | p<.001 | n.s. | n.s. | p<.0001 | p<.10 | p<.0001 | n.s. | p<.0001 | p<.10 | p<.0001 | |
| Anxiety | 47.3 (7.7) | 45.2 (7.0) | 49.1 (8.2) | 48.8 (7.6) | 53.1 (9.7) | 48.7 (7.4) | 55.5 (9.5) | 47.1 (7.2) | 51.4 (9.1) | 49.1 (8.1) | 52.9 (9.5) |
| n= | 1904 | 413 | 2550 | 195 | 2306 | 27 | 2207 | 72 | 2077 | 117 | 1765 |
| Compare w/no condition | p<.001 | p<.0001 | p<.05 | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | p<.05 | p<.0001 | |
| Depression | 47.4 (7.8) | 46.1 (7.3) | 48.9 (8.4) | 48.5 (8.4) | 53.2 (9.5) | 47.3 (7.4) | 55.5 (9.5) | 47.1 (8.1) | 51.8 (9.2) | 48.9 (7.9) | 52.9 (8.8) |
| n= | 1901 | 412 | 2549 | 195 | 2307 | 27 | 2210 | 72 | 2078 | 117 | 1762 |
| Compare w/no condition | p<.01 | p<.0001 | p<.05 | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | p<.05 | p<.0001 | |
| Physical Function | 56.1 (6.7) | 54.0 (6.8) | 46.3 (8.5) | 54.5 (7.1) | 43.5 (9.3) | 53.7 (6.2) | 41.9 (9.1) | 53.7 (7.4) | 42.3 (8.9) | 47.1 (9.4) | 41.0 (8.3) |
| n= | 2161 | 418 | 2604 | 224 | 2397 | 31 | 2284 | 77 | 2141 | 115 | 1798 |
| Compare w/no condition | p<.0001 | p<.0001 | p<.001 | p<.0001 | p<.05 | p<.0001 | p<.01 | p<.0001 | p<.0001 | p<.0001 | |
| Social Role Satisfaction | 53.3 (7.9) | 52.4 (6.4) | 50.4 (7.8) | 52.4 (7.3) | 47.5 (8.7) | 50.3 (6.1) | 45.8 (8.7) | 54.0 (7.8) | 47.9 (8.8) | 51.2 (6.4) | 47.5 (8.2) |
| n= | 1936 | 408 | 2515 | 195 | 2307 | 25 | 2207 | 74 | 2073 | 111 | 1742 |
| Compare w/no condition | p<.05 | p<.0001 | n.s. | p<.0001 | p<.10 | p<.0001 | n.s. | p<.0001 | p<.01 | p<.0001 | |
| Social DSA Satisfaction | 52.9 (7.9) | 53.8 (6.9) | 51.5 (7.7) | 51.7 (7.3) | 49.0 (8.1) | 51.4 (7.3) | 47.2 (8.0) | 54.0 (7.8) | 49.3 (8.2) | 52.5 (6.9) | 49.1 (7.4) |
| n= | 1936 | 408 | 2515 | 195 | 2307 | 25 | 2207 | 74 | 2073 | 111 | 1742 |
| Compare w/no condition | p<.05 | p<.0001 | p<.05 | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | n.s. | p<.0001 | |
Note: HTN=hypertension, Social Role Satisfaction=Satisfaction with participation in social roles, Social DSA Satisfaction=Satisfaction with participation in discretionary social activities, n.s.=not statistically significant
Note: Sleep=sleep disorder, DM=diabetes, COPD=chronic obstructive pulmonary disorder, Social Role Satisfaction=Satisfaction with participation in social roles, Social DSA Satisfaction=Satisfaction with participation in discretionary social activities, n.s.=not statistically significant
Figure 1.
T-score by Comorbidity Status for Arthritis and Depression
Note: PB=pain behavior, PI=pain interference, F=fatigue, Ang=anger, Anx=anxiety, Dep=depression, PF=physical function, SR=satisfaction with participation in social roles, DSA=satisfaction with participation in discretionary social activities, Arth=arthritis, Dep=depression
Discussion
Across all of the reported PROMIS domains (pain, fatigue, anger, anxiety, depression, physical function, satisfaction with participation in social roles and satisfaction with participation in discretionary social activities), people without chronic conditions reported better scores than those with one or more chronic conditions. Furthermore, within those with at least one chronic condition, those who stated their activities were limited consistently scored worse in all domains than those who stated they were not limited. The magnitude of detriment associated with multiple conditions appears to be slightly worse for conditions reported as disabling (effect size range = 0.50 to 1.3 SD units) compared to a simple count of conditions (effect size range = 0.30 to 1.1 SD units). Although multiple strategies for calculating minimally important differences have been identified, [27] it has been suggested that approximately 0.5 SD on an HRQL instrument is a reasonable threshold, [28] thereby supporting the clinical meaningfulness of these differences. Additionally, the magnitude of difference between those with multiple conditions compared to a single condition was greater (0.2 to 0.7 SD units) than the difference between those with a single condition and no conditions (0.1 to 0.4 SD units). Whereas having a single condition appears to exert an overall impact on one’s HRQL, the presence of comorbidity is associated with more detrimental HRQL.
Older individuals are more likely to experience comorbid conditions and may have non-disease-based physical limitations. This might lead one to hypothesize that sociodemographic factors such as age could be confounded with the HRQL effects of disease and comorbidity. However, after controlling for age, gender, relationship status, race, and education, the differences between groups remained statistically significant at virtually the same level of magnitude as when not controlling for demographic factors.
In this sample, 81% of participants reported having at least one chronic condition. This sample prevalence is higher than other published prevalence estimates in the US general population.[1] Because our purpose was to derive item calibrations targeted to clinical research samples that either had or would likely develop conditions with related HRQL problems, we oversampled from chronic illness groups across the PROMIS network (See Cella et al in this issue).[26]
Results support the importance of assessment and analysis of comorbidity in HRQL research. Presence of comorbidity appears to be a significant source of disparities in HRQL, independent of any effect due to sociodemographic factors like age or ethnicity. Individuals with chronic conditions reported poorer HRQL than those without chronic conditions. Those with more than one chronic condition reported worse HRQL than those with one, and the presence of self-reported impairment due to one’s condition was clearly associated with decrements in HRQL. While these results support the often-observed relationship between illness and HRQL impact across a variety of conditions, at times the differences were small (e.g., less than 0.5 SD units). This could be due to a variety of reasons. It is possible that the true difference between some groups is indeed smaller than one would guess based on clinical observation or experience. Adaptation or adjustment to illness may play a role. As Lacey and colleagues noted,[29] “[quality of life] does suffer as a result of chronic disease, but not as much as nonpatients imagine” (p.673). In addition, it is possible that the illness experience with symptoms and their functional impact may cause some people to “recalibrate” the meaning or severity level they ascribe to a question, leading them to give a less-impaired response than they would have given prior to their illness experience (e.g., Andrykowski et al., 2009).[30] Further research into these and other possible explanations would be of value in appreciating the full impact of chronic disease and comorbidity upon HRQL. These findings also suggest that the common practice of excluding people with various comorbid conditions from clinical trials may disproportionately exclude patients with the poorer HRQL, who may be most in need of clinical services. This raises questions about the generalizability of clinical trial results in expanded community practice. Today’s emphasis on comparative effectiveness research can help better appreciate the broader impact of new and emerging treatments. HRQL instruments like PROMIS offer the opportunity to measure these wider population-level effects with common, generic measures of symptoms and functional status. The results reported here offer initial reassurance that these PROMIS tools will be able to detect differences associated with comorbidity associated with common adult chronic conditions.
Our results suggest that within a given condition, the negative impact on HRQL is not equal across symptoms and functional areas. For example, patients with some conditions (e.g., depressive disorders, migraines, COPD) report poorer HRQL compared to individuals without any condition in almost all areas. However, individuals with sleep disorders alone only report a few differences with condition-free individuals. Results support assessing multiple areas of HRQL. In this sample, physical function was always better for healthy individuals compared to all disease groups. This was usually the case for fatigue, as well. Emotional distress varied between groups even when the chronic condition was not psychiatric in nature. Pain as well varied between groups in non-pain conditions like asthma. HRQL detriments were even more pronounced for individuals with multiple conditions. With rare exception, individuals with multiple conditions experience global HRQL detriments compared to healthy individuals.
Future Directions
Disease-specific analyses that address issues of disease severity and course are warranted. Because PROMIS measures were constructed to be applicable across a range of chronic illness, comparisons across diseases can be facilitated in the future by utilization of these tools.
Limitations
All disease groups were based upon participant self-report of diagnosis. While there is reasonably good evidence to suggest that people are reliable reporters of medical diagnosis,[31–33] it would have been ideal to obtain confirmation of diagnosis from a clinician or confirmatory diagnostic tests. Furthermore, only the most frequently diagnosed conditions, not all possible comorbid conditions, were assessed. It is possible that the results obscure the impact of less common conditions on HRQL. Although the sample is large and reasonably diverse, it is not nationally representative. By utilizing a panel for data collection for a large portion of the sample, we selected for individuals that are comfortable completing online questionnaires. Validation studies of the PROMIS instruments are ongoing in clinically confirmed disease populations. Data from these studies will help supplement our understanding of how these instruments perform in specific populations.
Table 4.
Emotional Distress (T-Scores) by Number of Conditions*
| Anger | Anxiety | Depression | ||||
|---|---|---|---|---|---|---|
| Conditions | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Number | ||||||
| None | 48 b | 47 c | 47 c | 46 c | 47 c | 46 c |
| (n) | (2071) | (2031) | (2027) | |||
| One | 49 b | 49 b | 48 b | 48 b | 48 b | 48 b |
| (n) | (2700) | (2673) | (2675) | |||
| Two or more | 51 a | 52 a | 52 a | 53 a | 52 a | 53 a |
| (n) | (10130) | (10132) | (10137) | |||
| F = 140.36 | F = 152.04 | F = 333.92 | F = 229.50 | F = 336.96 | F = 235.43 | |
| # Disabling | ||||||
| None | 49 c | 49 c | 48 c | 48 c | 48 c | 48 c |
| (n) | (8237) | (8199) | (8190) | |||
| One | 50 b | 51 b | 51 b | 51 b | 51 b | 51 b |
| (n) | (3258) | (3246) | (3250) | |||
| Two or more | 54 a | 55 a | 57 a | 57 a | 57 a | 57 a |
| (n) | (3406) | (3391) | (3390) | |||
| F = 585.34 | F = 196.86 | F = 1348.00 | F = 360.50 | F = 1392.05 | F = 375.06 | |
Means that share the same letter are not significantly different from each other with the overall F significant at p<.0001 and pairwise comparisons p<.05.
Acknowledgments
The Patient-Reported Outcomes Measurement Information System (PROMIS) is a National Institutes of Health (NIH) Roadmap initiative to develop a computerized system measuring patient-reported outcomes in respondents with a wide range of chronic diseases and demographic characteristics. PROMIS was funded by cooperative agreements to a Statistical Coordinating Center (Northwestern University PI: David Cella, PhD, U01AR52177) and six Primary Research Sites (Duke University, PI: Kevin Weinfurt, PhD, U01AR52186; University of North Carolina, PI: Darren DeWalt, MD, MPH, U01AR52181; University of Pittsburgh, PI: Paul A. Pilkonis, PhD, U01AR52155; Stanford University, PI: James Fries, MD, U01AR52158; Stony Brook University, PI: Arthur Stone, PhD, U01AR52170; and University of Washington, PI: Dagmar Amtmann, PhD, U01AR52171). NIH Science Officers on this project are Deborah Ader, Ph.D., Susan Czajkowski, PhD, Lawrence Fine, MD, DrPH, Louis Quatrano, PhD, Bryce Reeve, PhD, William Riley, PhD, and Susana Serrate-Sztein, PhD. This manuscript was reviewed by the PROMIS Publications Subcommittee prior to external peer review See the web site at www.nihpromis.org for additional information on the PROMIS cooperative group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Wu SY, Green A. Projection of chronic illness prevalence and cost inflation: RAND Corporation. 2000. [Google Scholar]
- 2.Maddigan SL, Feeny DH, Johnson JA. Health-related quality of life deficits associated with diabetes and comorbidities in a Canadian National Population Health Survey. Qual Life Res. 2005;14(5):1311–20. doi: 10.1007/s11136-004-6640-4. [DOI] [PubMed] [Google Scholar]
- 3.Siroux V, Boudier A, Anto JM, Cazzoletti L, Accordini S, Alonso J, et al. Quality-of-life and asthma-severity in general population asthmatics: results of the ECRHS II study. Allergy. 2008;63(5):547–54. doi: 10.1111/j.1398-9995.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Measuring healthy days: Population assessment of health-related quality of life. Atlanta, Georgia: CDC; 2000. [Google Scholar]
- 5.Adams PF, Lucas JW, Barnes PM. Summary health statistics for the U.S. population: National Health Interview Survey, 2006. Vital Health Stat. 2008;10(236):1–104. [PubMed] [Google Scholar]
- 6.National Center for Health Statistics (U.S.) Health, United States, 2007: With chartbook on trends in the health of Americans. Hyattsville, MD: Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2007. [Google Scholar]
- 7.Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med. 2000;108(9):714–22. doi: 10.1016/s0002-9343(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 8.Richardson LC, Wingo PA, Zack MM, Zahran HS, King JB. Health-related quality of life in cancer survivors between ages 20 and 64 years: population-based estimates from the Behavioral Risk Factor Surveillance System. Cancer. 2008;112(6):1380–9. doi: 10.1002/cncr.23291. [DOI] [PubMed] [Google Scholar]
- 9.Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 10.Partnership for Solutions, Robert Wood Johnson Foundation & Johns Hopkins University. Chronic conditions: making the case for ongoing care. Baltimore, Md: Johns Hopkins University; 2002. [Google Scholar]
- 11.Krishnan E, Tugwell P, Fries JF. Percentile benchmarks in patients with rheumatoid arthritis: Health Assessment Questionnaire as a quality indicator (QI) Arthritis Res Ther. 2004;6(6):505–13. doi: 10.1186/ar1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan E, Fries JF. Reduction in long-term functional disability in rheumatoid arthritis from 1977 to 1998:a longitudinal study of 3035 patients. Am J Med. 2003;115(5):371–6. doi: 10.1016/s0002-9343(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 13.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 14.Cella D. Assessment methods for quality of life in cancer patients: The FACIT measurement system. International Journal of Pharmaceutical Medicine. 2000;14(2):78–81. [Google Scholar]
- 15.Cella D, Nowinski C. Measuring quality of life in chronic illness: The Functional Assessment of Chronic Illness Therapy Measurement System. Arch Phys Med Rehabil. 2002;83(Suppl 2):S10–S7. doi: 10.1053/apmr.2002.36959. [DOI] [PubMed] [Google Scholar]
- 16.Cella D, Lai JS, Davis K, Dineen K, Hudgens S, Gershon R, et al. Assessing Quality of Life. Oxford University Press; 2004. Developing a questionnaire using Item Response Theory: A case study of fatigue. [Google Scholar]
- 17.Hays RD, Shaul JA, Williams VS, Lubalin JS, Harris-Kojetin LD, Sweeny SF, et al. Psychometric properties of the CAHPS 1.0 survey measures. Consumer Assessment of Health Plans Study. Med Care. 1999;37(3 Suppl):MS22–MS31. doi: 10.1097/00005650-199903001-00003. [DOI] [PubMed] [Google Scholar]
- 18.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 19.Fries JF, Bruce B, Cella D. The promise of PROMIS: Using item response theory to improve assessment of patient-reported outcomes. Clin Exp Rheumatol. 2005;23(S38):S33–S7. [PubMed] [Google Scholar]
- 20.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30(1):167–78. [PubMed] [Google Scholar]
- 21.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric Evaluation and Calibration of Health-Related Quality of Life Item Banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(5 Suppl 1):S22–S31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap Cooperative Group During its First Two Years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of Item Candidates: The PROMIS Qualitative Item Review. Med Care. 2007;45(5 Suppl 1):S12–S21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castel LD, Williams KA, Bosworth HB, Eisen SV, Hahn EA, Irwin DE, et al. Content validity in the PROMIS social-health domain: A qualitative analysis of focus-group data. Qual Life Res. 2008;17(5):737–49. doi: 10.1007/s11136-008-9352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christodoulou C, Junghaenel DU, DeWalt DA, Rothrock N, Stone AA. Cognitive interviewing in the evaluation of fatigue items: Results from the patient-reported outcomes measurement information system (PROMIS) Qual Life Res. 2008;17(10):1239–46. doi: 10.1007/s11136-008-9402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Riley W, Stone AA, Rothrock N, Reeve BB, Yount S, et al. Initial item banks and first wave testing of the Patient Reported Outcomes Measurement Information System (PROMIS) network: 2005–2008. J Clin Epidemiol. 2010 doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revicki D, Hays R, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–9. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Norman G, Sloan J, Wyrwich K. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41 (5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 29.Lacey HP, Fagerlin A, Loewenstein G, Smith DM, Riis J, Ubel PA. Are they really that happy? Exploring scale recalibration in estimates of well-being. Health Psychol. 2008;27(6):669. doi: 10.1037/0278-6133.27.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrykowski MA, Donovan KA, Jacobsen PB. Magnitude and Correlates of Response Shift in Fatigue Ratings in Women Undergoing Adjuvant Therapy for Breast Cancer. J Pain Symptom Manage Journal of Pain and Symptom Management. 2009;37(3):341–51. doi: 10.1016/j.jpainsymman.2008.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Fakiri F, Bruijnzeels MA, Hoes AW. No evidence for marked ethnic differences in accuracy of self-reported diabetes, hypertension, and hypercholesterolemia. J Clin Epidemiol. 2007;60(12):1271–9. doi: 10.1016/j.jclinepi.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Straus SE, McAlister FA, Sackett DL, Deeks JJ. Accuracy of History, Wheezing, and Forced Expiratory Time in the Diagnosis of Chronic Obstructive Pulmonary Disease. J Gen Intern Med. 2002;17:684–8. doi: 10.1046/j.1525-1497.2002.20102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow JH, Turner AP, Wright CC. Comparison of clinical and self-reported diagnoses for participants on a community-based arthritis self-management programme. Br J Rheumatol. 1998;37(9):985–7. doi: 10.1093/rheumatology/37.9.985. [DOI] [PubMed] [Google Scholar]