Abstract
FTY720 is an immunomodulator that is phosphorylated in vivo and inhibits lymphocyte mobilization by targeting sphingosine 1-phospate receptors. At doses higher than required for immunomodulation, FTY720 inhibits tumor progression through an unknown mechanism. Here we show that FTY720-phosphate is a competitive inhibitor (Ki ~0.2 µM) of autotaxin (ATX or NPP2), a nucleotide phosphodiesterase/pyrophosphatase (NPP) that enhances metastasis and angiogenesis and acts as a lysophospholipase D to produce the lipid mediator lysophosphatidic acid (LPA). FTY720-phosphate did no affect the activity of NPP1, the closest relative of ATX. After oral administration in mice, FTY720 (3 mg/kg) significantly reduced plasma LPA levels. These results suggest that FTY720 may exert its anticancer effects, at least in part, by targeting the ATX-LPA axis.
Keywords: FTY720, autotaxin, lysophosphatidic acid, tumor progression
1. Introduction
FTY720 (also known as fingolimod) is a novel immunomodulator that interferes with lymphocyte trafficking. This investigational drug is currently in Phase III clinical trials for patients with relapsing multiple sclerosis [1]. FTY720 is structurally similar to the natural lipid sphingosine and is phosphorylated in vivo to the active principle FTY720-phospate (FTY-P). FTY-P binds with high affinity to G protein-coupled receptors for the lipid mediator sphingosine 1-phosphate (S1P), particularly receptor subtype S1P1 [2;3]. Upon FTY-P binding, S1P1 receptors are targeted for internalization and degradation [4]. The “functional antagonist” hypothesis holds that lymphocytes exposed to FTY-P fail to respond to S1P and thereby remain sequestered in secondary lymphoid organs thus being unable to migrate to sites of inflammation [5].
In addition to its immunomodulating action, FTY720 exerts striking anticancer effects in animal models. When administered at doses at least 10-fold higher than required for immunomodulation, FTY720 (3–10 mg/kg/d) inhibits tumor growth, angiogenesis and metastasis in various carcinoma models [6–9] and the syngeneic B16 melanoma model [10]. Furthermore, FTY720 remarkably suppresses Bcr/Abl-driven leukemogenesis in mice without overt toxicity [11] and it prolongs survival in a mouse model of disseminated B-cell malignancy [12]. However, the mechanism whereby FTY720 restrains tumor progression is unclear although various scenarios have been proposed, ranging from S1P receptor modulation to selective induction of apoptosis [6–12].
We previously reported a novel action of S1P, namely as an inhibitor of autotaxin (Ki ~0.1 µM) [13]. Autotaxin (ATX, also known as NPP2) is a secreted nucleotide phosphodiesterase/pyrophosphatase (NPP) [14] that primarily functions as a lysophospholipase D (lysoPLD), producing the lipid mediator lysophosphatidic acid (LPA) from more complex lysophospholipids [15;16]. LPA acts on specific G protein-coupled receptors to stimulate the proliferation, migration and survival of many cell types, both normal and malignant [17–19], and is an effector of tumor progression in mice [20;21]. Like its product LPA, ATX is strongly implicated in tumor progression: it is overexpressed in various cancers (reviewed in [16;18]), it promotes tumor aggressiveness, metastasis and angiogenesis in preclinical models [22;23], while a pharmacological inhibitor of ATX suppresses melanoma metastasis [24]. Moreover, gene targeting studies in mice have uncovered a vital role for ATX in vascular development [25;26]. Thus, the ATX-LPA axis is an attractive target for anticancer and anti-angiogenesis therapy.
Given the structural similarity of FTY-P to S1P, we sought to determine whether FTY-P can mimic S1P in inhibiting ATX. Here we report that FTY-P is a competitive inhibitor of ATX in vitro and that the parent drug reduces circulating LPA levels in vivo. We suggest that the anticancer effects of FTY720 may be attributed, at least in part, to inhibition of the ATX-LPA axis by FTY-P.
2. Materials and Methods
2.1. Recombinant ATX
Human ATX cDNA was cloned with a C-terminal His tag in pcDNA3 (InVitrogen). HEK293T cells were transfected with pcDNA3-ATX-His and maintained in serum-free DMEM for 48 hrs. Recombinant ATX was purified from conditioned medium using TALON Resin beads (Clontech).
2.2. ATX activity assays
The phosphodiesterase activity of ATX toward para-nitrophenyl tymidine-5’-monophosphate (pNP-TMP) was measured by light absorbance in 96-well plates, as described previously [13]. ATX-His (75 nM) was added to 100 µl of Tris-buffered saline (pH 8.0) containing 1 mg/ml fatty-acid free BSA and 1 mM pNP-TMP. After incubation for 30 min. at 37 °C, the amount of liberated para-nitrophenol was quantified by measuring the absorbance at 405 nm. To measure lysoPLD activity, the fluorigenic lysophospholipid substrate FS3 (2.5 µM) was used in Tris-buffered saline (pH 8.0) containing 1 mg/ml fatty-acid free BSA. After addition of ATX-His (75 nM), the increase in fluorescence as monitored at 37 °C (excitation, 485 nm; emission, 520 nm) as described previously [27].
2.3. Studies in mice
Female mice (C57Bl/6 × sv129) were dosed daily for five days by oral gavage, 0.2 ml either with vehicle of 3.0 mg/kg FTY720. At day 6, mice were sacrificed 24 hrs after the final dose. Citrate-treated plasma was extracted by acidic butanol and LPA quantified using a highly sensitive radio-enzymatic assay based on the transfer of a [14C] fatty acyl chain onto LPA resulting in the production of [14C] phosphatidic acid [28]. Lymphocytes were counted as described [3].
3. Results and discussion
3.1. Inhibition of ATX activity
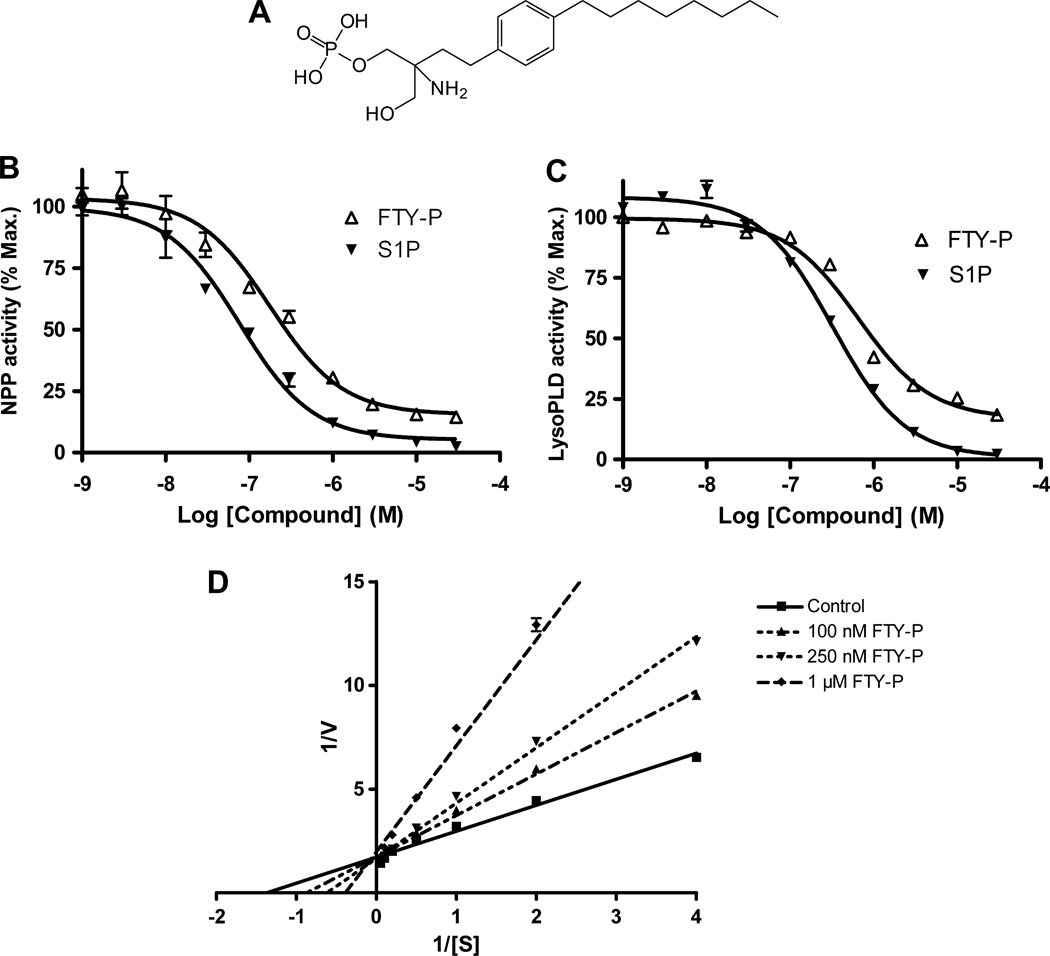
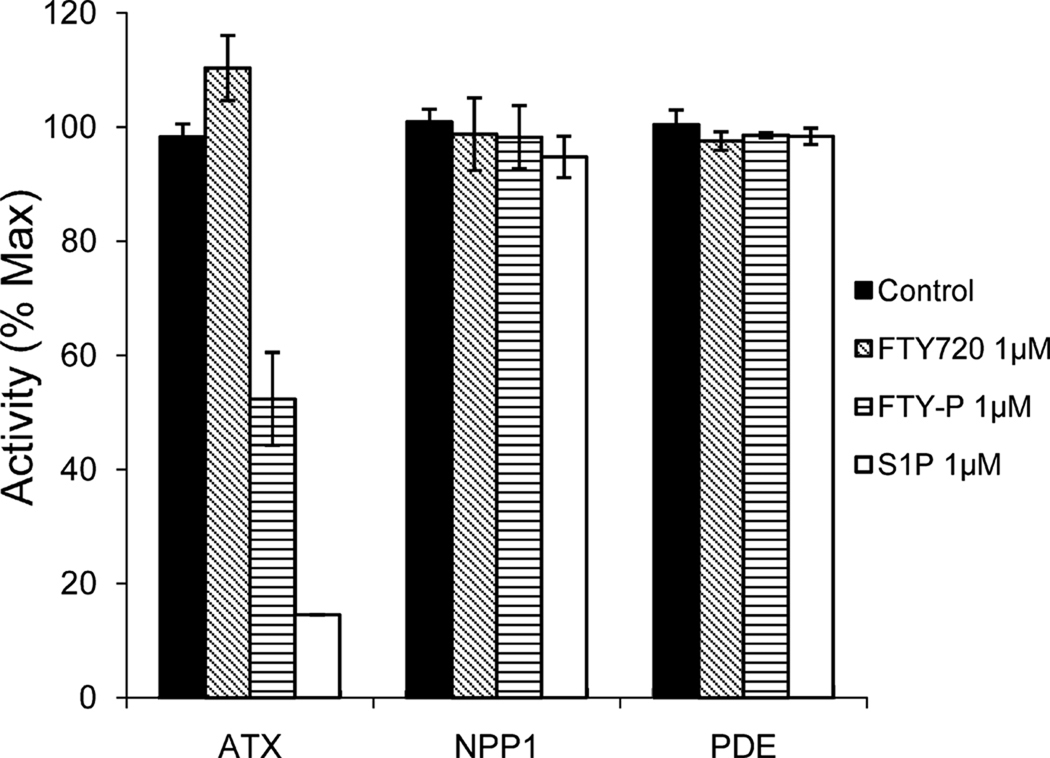
ATX/NPP2 uses a single catalytic site for the hydrolysis of both lipid and non-lipid substrates [14]. We measured the nucleotide phosphodiesterase and lysoPLD activity of recombinant ATX using pNP-TMP and FS3 as substrates, respectively. We previously found that ATX is inhibited by LPA and S1P (Ki ~ 0.1 uM) [13]. We examined the effect of FTY-P (the bioactive S-enantiomer; Fig. 1A) and compared its effect to that of S1P. FTY-P inhibited the catalytic activity of ATX in a dose-dependent manner using either a nucleotide or a lysophosphospholipid as substrate (Fig. 1B,C). Using substrate concentrations close to the Km values [13;27], FTY-P inhibited ATX activity with IC50 values of 0.3–0.4 µM. FTY-P was found to be somewhat less potent than S1P (IC50 ~ 0.1 µM) (Fig.1B,C; see also ref. [13]). Substrate titration curves and double-reciprocal plot analysis revealed that FTY-P is a competitive inhibitor, producing an increase in Km without affecting Vmax (Fig. 1D). The inhibition constant (Ki) was approx. 0.2 µM, a value about two-fold higher than that for S1P [13]. The parent compound (FTY720, 1 µM) had no discernible effect on ATX activity (Fig. 2).
Fig. 1. Inhibitory effects of FTY-P on ATX activity.
(A) Structure of FTY-P. (B) Nucleotide phosphodiesterase (NPP) activity of recombinant ATX (70 nM) using pNP-TMP (1 mM) as substrate. (C) LysoPLD acitivity of ATX using FS3 (2.5 µM) as substrate.
(D) Double-reciprocal plot analysis of ATX inhibition by FTY-P. FTY-P was added at the indicated concentrations and ATX catalytic rate (V) was determined using pNP-TMP (1 mM) as substrate (S). FTY-P-inhibited ATX shows the same Y-intercept as untreated ATX (Control), indicating that FTY-P is a competitive inhibitor.
Fig. 2. Specificity of FTY-P and S1P toward ATX as measured by pNP-TMP hydrolysis.
FTY720, FTY-P and S1P were used at the indicated concentrations. Purified NPP1 was used at 200 nM, as in a previous study [13]. PDE denotes a broad-specificity phosphodiesterase from snake venom (1 U/ml).
3.2. Selectivity
ATX/NPP2 is the only NPP family member with lysoPLD activity [14]. The inhibitory effect of FTY-P was selective since the compound did not affect the activity of NPP1, the closest relative of ATX/NPP2, nor did it inhibit a broad-specificity phosphodiesterase (Fig. 2). This implies that ATX has a unique binding site for FTY-P, not present in NPP1. In this respect we note that, apart from its catalytic site (residue Thr210), ATX contains an additional substrate-binding sequence (aa 318–334) not found in other NPP family members [29]. It will be interesting to determine whether this region may serve as a binding site for FTY-P.
3.3. Administration in mice
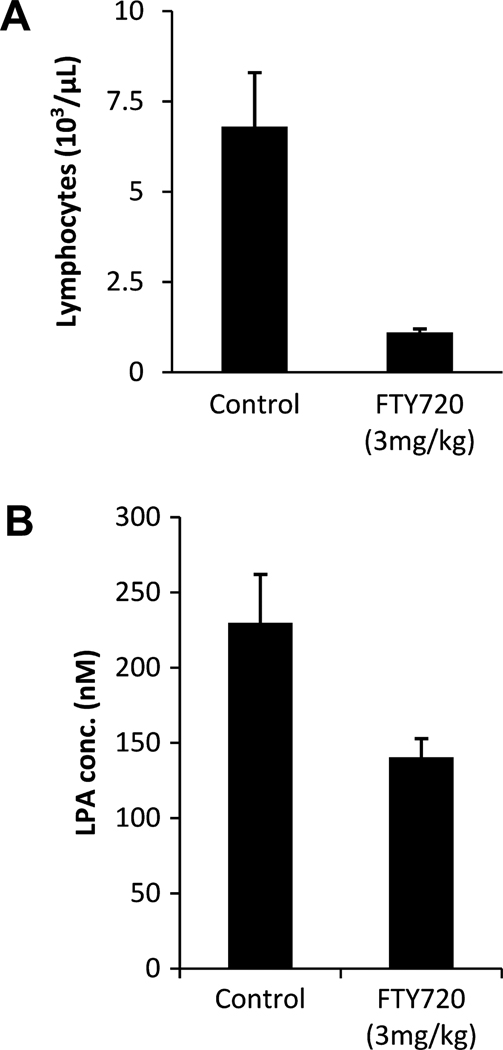
ATX is the major LPA-producing enzyme in vivo, as shown by the finding that half-normal ATX expression results in 50% reduced plasma LPA levels [25;26]. Therefore, pharmacological inhibition of ATX in vivo is expected to lower plasma LPA levels. In a mouse cancer model, drug-induced depletion of plasma LPA is accompanied by strongly reduced bone metastasis, supporting the notion that circulating LPA is a determinant of tumor progression [20]. To examine whether FTY720 may affect plasma LPA levels, we treated mice orally once daily with 3 mg/kg FTY720. After 5 days, the number of circulating lymphocytes and plasma LPA levels were determined. FTY720 administration caused significant lymphopenia (Fig. 3A) and, more importantly, an approx. 50% reduction in plasma LPA levels (from 240 to 110 nM), consistent with plasma ATX activity being inhibited by FTY-P (Fig. 3B).
Fig. 3. Plasma LPA levels and blood lymphocyte counts in FTY720-treated mice.
Three sets of 5 mice were dosed with vehicle and FTY720 (3.0 mg/kg) daily for 5–6 days. (A) Blood lymphocyte counts in FTY720- and vehicle-treated mice. (B) Plasma LPA levels (nM; p<0.01) in treated and non-treated animals, as determined by a radioenzymatic assay based on the transfer of a [14C] fatty acyl chain onto LPA resulting in the production of [14C] phosphatidic acid [28].
3.4. Concluding remarks
Inhibition of the ATX-LPA axis by FTY-P may provide a mechanistic explanation for the reported anticancer effects of FTY720. Although the doses at which FTY-P inhibits ATX activity are substantially higher than those required to target S1P receptors and arrest lymphocyte migration, they are within the predicted pharmacological range obtained after high FTY720 dosage. The blood levels of FTY-P can exceed those of the parent compound several-fold [3]. Based on published data [3], we estimate that high FTY720 doses may result in FTY-P blood levels of about 1 µM, a concentration that inhibits ATX activity by approx. 70% (Fig. 1). Given the high doses needed, the anticancer activity of FTY720 is unlikely to be credited only to S1P receptor modulation but must involve alternative or additional targets such as the ATX-LPA axis identified here. Not only is the ATX-LPA signaling system strongly implicated in tumor progression [16–18;20–24], evidence for S1P receptors playing an active role in cancer is limited. Another possibility is that the antitumor activity of FTY720 may involve a (direct or indirect) action of the parent compound on intracellular targets such as, for example, the tumor suppressor protein phosphatase 2A (PP2A). FTY720 stimulates PP2A activity specifically in certain leukemic cells, albeit at high concentrations (2.5–10 µM), and thereby may enhance apoptosis and suppress leukemogenesis [11;12;30]. The present results lead us to suggest that the anticancer effects of FTY720 may relate to FTY-P inhibiting the ATX-LPA axis, possibly in conjunction with other tumor-suppressing actions, a scenario that clearly warrants further investigation.
Acknowledgements
We thank Paula Ruurs and Sandra Grés for excellent technical assistance and Dr. James Goding for providing purified NPP1. This work was supported by the Dutch Cancer Society, the Center for Biomedical Genetics and the US National Institutes of Health (R01 GM067958 to K.R.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 4.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 5.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 6.Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T, Nonomura N, Suzuki S, Okuyama A, Katsuoka Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410–1419. [PubMed] [Google Scholar]
- 7.Chua CW, Lee DT, Ling MT, Zhou C, Man K, Ho J, Chan FL, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits in vivo growth of androgen-independent prostate cancer. Int J Cancer. 2005;117:1039–1048. doi: 10.1002/ijc.21243. [DOI] [PubMed] [Google Scholar]
- 8.Lee TK, Man K, Ho JW, Wang XH, Poon RT, Xu Y, Ng KT, Chu AC, Sun CK, Ng IO, Sun HC, Tang ZY, Xu R, Fan ST. FTY720: a promising agent for treatment of metastatic hepatocellular carcinoma. Clin Cancer Res. 2005;11:8458–8466. doi: 10.1158/1078-0432.CCR-05-0447. [DOI] [PubMed] [Google Scholar]
- 9.Schmid G, Guba M, Ischenko I, Papyan A, Joka M, Schrepfer S, Bruns CJ, Jauch KW, Heeschen C, Graeb C. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J Cell Biochem. 2007;101:259–270. doi: 10.1002/jcb.21181. [DOI] [PubMed] [Google Scholar]
- 10.LaMontagne K, Littlewood-Evans A, Schnell C, O'Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau G, Billy E, Theuer A, Hla T, Wood J. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 11.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, Lehman A, Perrotti D, Chen CS, Dalton JT, Muthusamy N, Byrd JC. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T, Moolenaar WH. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 14.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 18.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 19.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 20.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam SW, Clair T, Campo CK, Lee HY, Liotta LA, Stracke ML. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene. 2000;19:241–247. doi: 10.1038/sj.onc.1203263. [DOI] [PubMed] [Google Scholar]
- 23.Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61:6938–6944. [PubMed] [Google Scholar]
- 24.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, Uchiyama A, Murakami-Murofushi K, Koh E, Bandle RW, Byun HS, Bittman R, Fan D, Murph M, Mills GB, Tigyi G. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson CG, Bigman CS, Richardson RD, van Meeteren LA, Moolenaar WH, Prestwich GD. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org Lett. 2006;8:2023–2026. doi: 10.1021/ol060414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saulnier-Blache JS, Girard A, Simon MF, Lafontan M, Valet P. A simple and highly sensitive radioenzymatic assay for lysophosphatidic acid quantification. J Lipid Res. 2000;41:1947–1951. [PMC free article] [PubMed] [Google Scholar]
- 29.Clair T, Krutzsch HC, Liotta LA, Stracke ML. Nucleotide binding to autotaxin: crosslinking of bound substrate followed by lysC digestion identifies two labeled peptides. Biochem Biophys Res Commun. 1997;236:449–454. doi: 10.1006/bbrc.1997.6982. [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]