Abstract
Amphibian metamorphosis is an excellent example of hormone-dependent control of development. Thyroid hormones (THs) regulate almost all aspects of metamorphosis, including brain development and larval neuroendocrine function. Sex steroids are also important for early brain function, although little is known about interactions between the two hormonal systems. In the present study, we established brain developmental profiles for thyroid hormone receptors (tralpha and trbeta), deiodinases (dio1, dio2 and dio3), aromatase (cyp19) mRNA and activity, oestrogen receptors (eralpha and erbeta), androgen receptor (ar) and 5α-reductases (srd5alpha1 and srd5alpha2) mRNA during Silurana (Xenopus) tropicalis metamorphosis. Real-time reverse transcriptase-polymerase chain reaction analyses revealed that all of the genes were expressed in the brain and for most of the genes expression increased during development, with the exception of dio2, srd5alpha1 and srd5alpha2. The ability of premetamorphic tadpoles to respond to exogenous THs was used to investigate the regulation of TH- and sex steroid-related genes in the brain during development. Exposure of premetamorphic tadpoles to triiodothyronine (T3; 0, 0.5, 5 and 50 nm) for 48 h resulted in concentration-dependent increases in trbeta, dio2, dio3, eralpha and erbeta. Expression of srd5alpha2 showed large increases (six- to 7.5-fold) for all three concentrations of T3. No changes were detected in dio1, ar and cyp19 transcript levels; however, cyp19 activity increased significantly at 50 nm T3. The results obtained suggest that expression of TH-related genes and er during development could be regulated by rising levels of THs, as previously documented in Lithobates (Rana) pipiens. The positive regulation of srd5alpha by T3 in the brain suggests that endogenous TH levels help maintain or control the rate at which srd5alpha mRNA levels decrease as metamorphosis progresses. Finally, we have identified sex steroid-related genes that are responsive to T3, providing additional evidence of crosstalk between THs and sex steroids in the tadpole brain.
Keywords: Xenopus tropicalis, metamorphosis, receptor, aromatase, 5α-reductase, deiodinases
Amphibian metamorphosis is a complex developmental process, tightly controlled by thyroid hormones (THs) (1). The process is separated into three specific periods: premetamorphosis, prometamophosis and metamorphic climax. Premetamorphosis [Nieuwkoop and Faber (NF) stages 46–54] is the period of early tadpole growth and development characterised by low levels of circulating THs. The growth of the hind limbs and toe differentiation is accelerated during prometamorphosis (NF 55–57). This period is characterised by a rapid increase in the concentrations of endogenous THs. During metamorphic climax (NF 58–65), TH levels peak and rapid morphological changes occur, such as the emergence of the forelimbs and tail resorption (1, 2). The central nervous system (CNS) in tadpoles is an important target of THs. During metamorphosis, the CNS is extensively remodelled; for example, with re-structuring (disappearance of certain larval neuronal structures and the development of adult structures), axon guidance and growth, cell proliferation and death (3, 4).
The two THs, 3,5,3′-triiodothyronine (T3) and thyroxine (T4), mediate their physiological effects by binding to nuclear thyroid hormone receptors (tr) encoded by two genes tralpha and trbeta (5). The metabolism of THs is regulated by three types of deiodinases (type I, II and III). Deiodinase type I (dio1) catalyses outer-ring deiodination to produce T3 from T4 and also inner ring deiodination to produce reverse T3 (rT3, inactive) from T4. Type II deiodinase (dio2) exclusively activates THs by catalyzing the conversion from T4 to T3. By contrast, type III deiodinase (dio3) inactivates THs by inner-ring deiodination of T4 and T3 to produce rT3 and T2 (diiodothyronine), respectively (6). Localised activity of the different types of dio in target tissues controls local THs levels that allow the various tissues to undergo independent and differentially-timed development during metamorphosis (7). The tadpole brain has been shown to have one of the highest concentration of tr mRNA relative to other tissues (8) and dio transcripts have also been detected in the brain (9–12). Apart from being a target of THs, the CNS is also a target for sex steroids (i.e. oestrogens and androgens) that regulate brain sexual development and reproduction. For example, oestrogen is known to regulate and organise the neuroendocrine circuits and controlling reproductive functions in fish (13). In rodents, testosterone masculinises the brain via conversion to oestrogen by the enzyme aromatase (CYP19) (14) and/or by binding directly to the androgen receptor (AR) (15). Previous research in our laboratory has shown that T3 regulates the expression of the oestrogen-responsive genes: oestrogen receptor alpha (eralpha) and cyp19 in the brain of Lithobates (Rana) pipiens (11). In addition, in Silurana (Xenopus) tropicalis whole body larvae, T3 increases the expression of androgen-related genes, 5α-reductase type 1 (srd5alpha1) and ar (16). Therefore, the present study aimed to investigate whether TH- and sex steroid-related genes are also regulated by T3 in the brain of S. tropicalis tadpoles.
During premetamorphosis, tadpoles have very low levels of THs but they are responsive to exogenous THs; therefore, this is an excellent developmental period to examine the functions and mechanism of action of THs (4). We used this model system to examine the effects of THs on the tadpole brain. We first established developmental profiles of TH- and sex steroid-related genes in the brain during S. tropicalis natural metamorphosis and adulthood. Then, premetamorphic tadpoles were exposed to T3 and transcript levels of TH-, oestrogen- and androgen-related genes and activity of cyp19 were measured in the brain of S. tropicalis.
Materials and methods
Animals
Silurana tropicalis frogs were reared in dechlorinated and aerated water from the University of Ottawa Animal Care facility (Ontario, Canada). Fertilised eggs were obtained from five pairs of frogs by injecting human chorionic gonadotrophin hormone (hCG; Sigma, St Louis, MO, USA) into the dorsal lymph sac of adult S. tropicalis. Both males and females received a priming injection of 12.5 IU of hCG followed by a boosting injection of 100 IU of hCG after 20 h. Eggs and larvae were raised in Petri dishes at 24–25 °C containing modified Ringer’s solution (0.1 m NaCl, 1.8 mm KCl, 2.0 mm CaCl2, 1.0 mm MgCl2, 300 mg/l NaHCO3; 1 : 9 v/v) and 0.04 mg/l of the antibiotic gentamycin (Sandoz Canada Inc., Boucherville, QC, Canada). When the tadpoles began feeding, they were transferred to 12-litre tanks containing aerated water (pH = 7.5–8.0, dissolved oxygen = 80–85%, temperature = 23–24 °C, conductivity = 850–900 μS) and fed Sera Micron (Pondside Herp Supplies, Indian Harbor Beach, FL, USA) twice a day. Staging was determined by following the NF (17) developmental table. A 12 : 12 h light/dark cycle was maintained (lights on 07.00 h). The care and treatment of animals used in this study were in accordance with the guidelines of the Animal Care Committee of the University of Ottawa and the Canadian Council on Animal Care.
Brain collection for developmental profiles
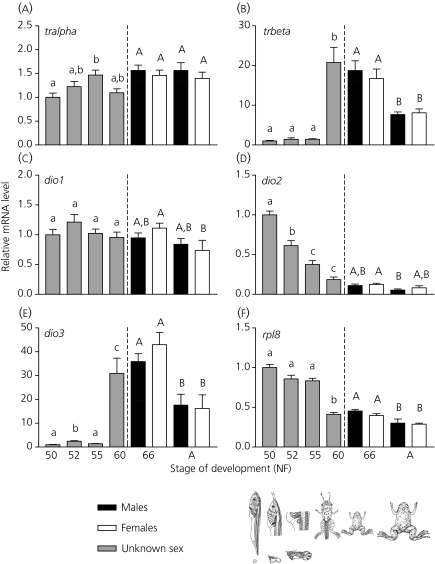
Samples of whole brain for gene expression were taken at different NF stages of development (Fig. 1): 50 and 52 (premetamorphosis), 55 (prometamorphosis), 60 (beginning of metamorphic climax) and 66 (juvenile). Tadpoles were anaesthetised by immersion into 1% of 3-aminobenzoic acid ethyl ester (MS-222; Sigma), euthanised by decapitation, and the brain was dissected, frozen on dry ice and kept at −80 °C. For NF 50–55, brains were pooled (two to five per pool; n = 8 pools) to ensure sufficient material for RNA isolation. For NF 60 and 66, brains were analysed individually (n = 8). In addition, to compare the gene expression during development with adult gene expression, five mature males and five mature females from our colony were sacrificed using 4% MS-222 and the brain was dissected and frozen. For the cyp19 activity assay, whole brains were also collected for NF stages 52, 55, 60, 66 following the same procedure as for the gene expression samples.
Fig. 1.
Brain developmental profiles of thyroid hormone-related genes during Silurana tropicalis metamorphosis and adulthood. Transcript levels of tralpha (a), trbeta (b), dio1 (c), dio2 (d) and dio3 (e) were measured in whole brain from Nieuwkoop and Faber (NF) stage 50 until adulthood. Levels of mRNA are expressed relative to NF 50 and are normalised to RNA content. Results for the reference gene ribosomal protein L8 (rpl8; f) are also presented. Bars represent the mean ± SEM. Different letters indicate statistically significant differences between stages (n = 5–8 pools; P<0.05). Brain samples for NF 66 and adulthood were statistically analysed separately from NF 52–60 samples (for details, see Materials and methods). Main morphological characteristics (i.e. whole body and hind limb diagrams) are included for each NF stage of development. Note that the scales of the y-axis vary between genes. A, adult.
T3 exposure
Premetamorphic tadpoles (NF stage 52–54) were exposed to three nominal concentrations of T3 (0.5, 5 and 50 nm; 3,3′,5-triiodo-L-thyronine; Sigma) or a dimethyl sulfoxide (DMSO; Sigma) solvent control for 48 h. The final DMSO concentration in the tanks was 0.005% in all treatments. The density in all the tanks was one tadpole per litre. Chemical additions were not renewed during the 48-h period. At the end of the exposure, tadpoles were anaesthetised by immersion into 1% of MS-222 and euthanised by decapitation. Brain was dissected, frozen on dry ice and kept at −80 °C.
RNA isolation and cDNA synthesis
Total RNA for the developmental profile and T3 exposure samples was obtained from whole brain using the Qiagen RNeasy Micro kit (including the DNase treatment set) in accordance with the manufacturer’s instructions (Qiagen, Valencia, CA, USA). Individual and pooled brains were homogenised and disrupted using a MM301 Mixer Mill (Retsch, Newton, PA, USA) at 20 Hz for 3 min. Isolated RNA was resuspended in RNase free water and stored at −80 °C. Total cDNA was prepared from 1 μg (for the developmental profile) and 2 μg (for the T3 exposure) of total RNA and 200 ng random hexamer primers (Invitrogen, Carlsbad, CA, USA) using Superscript II reverse transcriptase (Invitrogen). For the developmental samples, the reverse transcriptase reaction was modified to be carried out at 42 °C for 90 min (instead of 50 min) to increase the cDNA yield. The cDNA products were diluted 20-fold prior to PCR amplification.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Gene specific primers for real-time RT-PCR were designed and optimised as previously described (18). Real-time PCR primers for additional reference genes were designed based on GenBank sequences: gapdh (accession no. CR760856; forward 5′-TCACTGCCACCCAGAAGAC-3′; reverse 5′-GGATGACTTTCCCAACAGC-3′; product size: 123 bp) and 18S (accession number X04025; forward: 5′-TCAACACGGGAAACCTCAC-3′; reverse: 5′-AGACAAATCGCTCCACCAAC-3′; product size: 117 bp). Primers were designed using Primer 3 (http://fokker.wi.mit.edu/primer3/input.htm) and primer concentrations were optimised to obtain a minimum threshold cycle and a maximum change in fluorescence. The optimised primer concentration for gapdh was 200 nm and for 18S it was 150 nm. The specificity of the primer sets was confirmed by cloning and sequencing the single amplicon obtained. Expression of target genes in the brain was analysed using dual-labeled fluorescent probes [for eralpha, erbeta, cyp19 and rpl8 (ribosomal protein L8)] and SYBR Green I [for tralpha, trbeta, dio1, dio2, dio3, ar, srd5alpha1, srd5alpha2, actb (β-actin), ef1alpha, gapdh and 18S] real-time RT-PCR assays using a MX3000P real-time PCR system (Stratagene, La Jolla, CA, USA) as previously described (18).
Data analysis
The relative standard curve method was used to interpolate relative mRNA abundance of target and reference genes within each sample. The standard curves were generated using a cDNA mix of NF 60–66 brain samples (for the developmental profiles), and using equal parts of cDNA from each treatment (for the T3 exposure). Reaction efficiencies were 90–110% with an R2 ≥ 0.990. Samples were run in duplicate along with a negative template control (RNase-free water instead of cDNA template) and a negative reverse transcriptase control (cDNA template for which water was added instead of Superscript II). Data for each target were averaged and normalised to RNA content (19). Data for the developmental profiles are presented as fold change relative to NF 50 and data for the T3 exposure are expressed relative to the control group.
Aromatase activity assay
Aromatase activity in the brain during metamorphosis and after T3 exposure was measured by a radiometric method modified from Du et al. (20) and previously optimised for frog brain in our laboratory (18). Briefly, cyp19 activity was measured in pools of four to six brains (n = 5–6 pools) for the developmental profile and six or seven brains (n = 5 pools) for the T3 exposure. Cofactor (NADPH system) and 3H-androstenedione (3H-A) were first incubated for 30 min at 37 °C. After this pre-incubation, brain homogenates were added to a mix of cofactor and 3H-A, and incubated for 80 min at 25 °C. Aromatase activity was determined by the release of tritiated water from the C-1β carbon atom of 1β-3H-androstenedione during its conversion to oestrogen. Tritiated water was extracted twice with a charcoal solution and radioactivity was counted. Aromatase activity is expressed as fmol 3H2O/h per mg protein.
Statistical analysis
Statistical analysis was performed using S-Plus 8.0 (Insightful Corporation, Seattle, WA, USA). Data for all the genes were first tested for normality and homogeneity of variance using the Kolmogorov–Smirnov test and Levene’s test, respectively. When the assumptions were not met, the data were transformed as required (e.g. log10, square root) and retested for normality and homogeneity of variance. Data were analysed by one-way anova, except for NF 66 and adult brain samples, which were analysed using a two-way anova to examine sex differences. Analyses were followed by the Bonferroni multiple comparisons test. When data failed to meet assumptions even after being transformed, the nonparametric Kruskal–Wallis test on ranks was used. P<0.05 was considered statistically significant.
Results
Brain developmental profiles during metamorphosis and adulthood
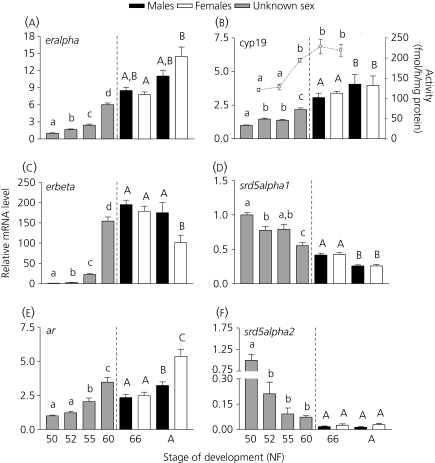
We established developmental profiles of TH- and sex steroid-related genes by sampling at five NF stages of development (for main morphological characteristics, see Fig. 1): 50 and 52 (premetamorphosis; foot paddle stages), 55 (prometamorphosis; hind limb development), 60 (beginning of metamorphic climax; forelimb emergence) and 66 (juvenile frog; tail completely resorbed). For all of the genes assessed, transcripts were detected throughout metamorphosis and in the adult brain (Figs 1 and 2). All the reference genes tested (rpl8, ef1alpha, actb and gapdh) changed during development; therefore, we decided to present the gene expression data normalised to RNA content only (19). Fig. 1(f) presents the brain developmental profile of rpl8 (i.e. the reference gene that varied the least during metamorphosis). The expression profiles of the receptors and enzymes show very distinct patterns and magnitude of change. Two genes that remain relatively constant throughout larval development are tralpha and dio1 (Fig. 1a, c). For tralpha, the only difference detected was at NF 55, which showed a higher expression relative to NF 52. For dio1, expression in the female brain decreases significantly after metamorphosis is complete (NF 66 versus adult brain); however, these changes are relatively minor (1.5-fold). The genes that show increases during development are trbeta, dio3, eralpha, erbeta, ar and cyp19 (Figs 1 and 2). Transcript levels of trbeta and dio3 are low and steady during premetamorphosis (NF 50-52) and early prometamorphosis (NF 55) and levels only increase significantly (by 30- and 20-fold respectively) at the beginning of metamorphic climax (NF 60; Fig. 1b,e). In the case of eralpha, erbeta, ar and cyp19, mRNA levels gradually increase during development (Fig. 2). We also found that cyp19 activity increases during metamorphosis, very closely following the mRNA profile (Fig. 2b). Finally, the expression of three genes (dio2, sdr5alpha1 and srd5alpha2) decreases during development and remains low during adulthood (Figs 1d and 2d,f). Sex differences in the brain of the expression of target genes are only detected at the adult stages for erbeta (males had a 1.7-fold higher expression than females) and ar (females had a 1.7-fold higher expression than males).
Fig. 2.
Brain developmental profiles of sex steroid-related genes during Silurana tropicalis metamorphosis and adulthood. Transcript levels of eralpha (a), cyp19 mRNA and activity (b) erbeta (c), srd5alpha1 (d), ar (e) and srd5alpha2 (f) were measured in whole brains from Nieuwkoop and Faber (NF) stage 50 until adulthood (y-axis on the left). Levels of mRNA are expressed relative to NF 50 and are normalised to RNA content. Enzyme activity for cyp19 (b) was measured from NF 52–66 (y-axis on the right) and is expressed in fmol/h normalised to protein content. Bars represent the mean ± SEM. Different letters indicate statistically significant differences between stages (n = 5–8 pools; P<0.05). Brain samples for NF 66 and adulthood were statistically analysed separately from NF 52 to NF 60 samples (for details, see Materials and methods). Significant differences in enzyme activity levels are indicated by small letters (n = 5–6; P<0.05). Note that the scales of the y-axis vary between genes. A, adult.
Effects of T3 on brain transcript levels of thyroid hormone- and sex steroid-related genes
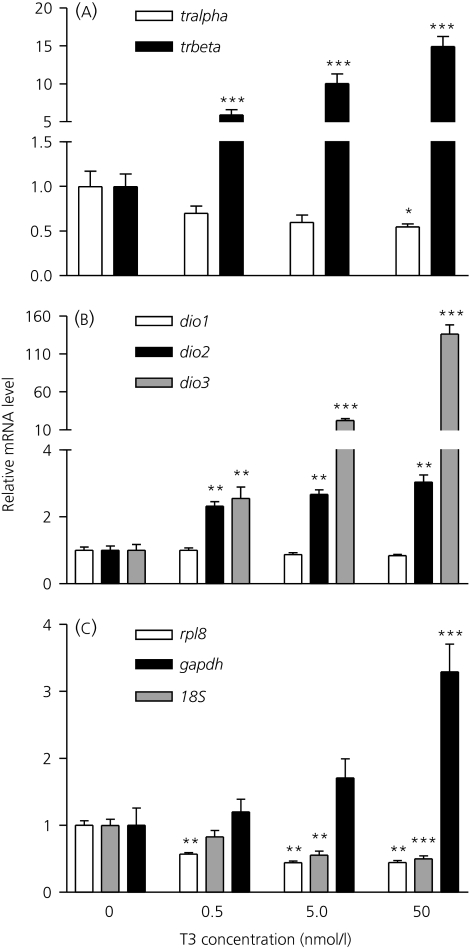
To investigate whether T3 regulates the expression of TH- and sex steroid-related genes in the brain of S. tropicalis, premetamorphic tadpoles (NF 52–54) competent to respond to THs were exposed to exogenous T3 for 48 h. Treatment with T3 had no effect on mortality and 100% survivorship post-exposure was observed in all treatment groups. Exposure to T3 resulted in concentration-dependent increases in trbeta (5.0–15-fold; anova; P<0.001), dio2 (2.0–3.0-fold; anova; P<0.01) and dio3 (2.5–120-fold; anova; P<0.01) mRNA relative to control (Fig. 3). Levels of tralpha remained unchanged for the 0.5 and 5 nm groups but decreased significantly (2.0-fold; anova; P<0.05) at the 50 nm T3 concentration (Fig. 3a). Transcript levels for dio1 did not change with T3 treatment (Fig. 3b). The reference genes rpl8, gapdh and 18S changed with T3 (Fig. 3c; anova; P<0.01); therefore, the gene expression data is normalised to RNA content, as suggested by Huggett et al. (19), and as we have previously reported (16, 18).
Fig. 3.
Effects of triiodothyronine (T3) exposure on the expression of thyroid hormone-related genes in Silurana tropicalis premetamorphic tadpoles. Silurana tropicalis (Nieuwkoop and Faber stage 52–54) were exposed to T3 (0, 0.5, 5, 50 nm) for 48 h. Effects of T3 on tralpha and trbeta (a), dio1, dio2 and dio3 (b) and the reference genes rpl8, gapdh and 18S (c) are presented. Data are presented as the fold changes relative to control and are normalised to RNA content (note the broken y-axis for a and b). Bars represent the mean ± SEM. Asterisks represent significant differences from the control group (n = 8 pools; *P<0.05; **P<0.01; ***P<0.001). Note that the scales of the y-axis vary between genes.
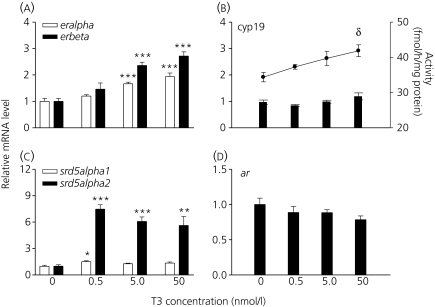
The effects of T3 on sex steroid-related gene expression and cyp19 activity are shown in Fig. 4. Exposure to T3 resulted in concentration-dependent increases in eralpha (1.8–2.0-fold; anova; P<0.001) and erbeta (2.4–2.8-fold; anova; P<0.001) mRNA (Fig. 4a). Expression of cyp19 was not affected by T3; however, we observed a significant increase (21% relative to control; anova; P<0.05) in cyp19 activity at the 50 nm concentration (Fig. 4b). Gene expression levels of srd5alpha2 increased significantly (between six- and 7.5-fold; anova; P<0.01) at all concentrations of T3, whereas levels of srd5alpha1 increased significantly at 0.5 nm T3 relative to the control group (1.5-fold; anova; P<0.05; Fig. 4c). Transcript levels of ar did not change after exposure to T3 (Fig. 4d).
Fig. 4.
Effects of triiodothyronine (T3) exposure on the expression of sex steroid-related genes in Silurana tropicalis premetamorphic tadpoles. Silurana tropicalis (NF 52–54) were exposed to T3 (0, 0.5, 5, 50 nm) for 48 h. Effects of T3 on eralpha and erbeta (a), cyp19 mRNA and activity (b), srd5alpha1 and srd5alpha2 (c) and ar (d) are presented. Gene expression data (y-axis on the left) are presented as the fold changes relative to control and are normalised to RNA content. Enzyme activity (y-axis on the right; b) is expressed in fmol/h normalised to protein content. Bars represent the mean ± SEM. Asterisks represent significant differences in gene expression levels from the control group (n = 8 pools; *P<0.05; **P<0.01; ***P<0.001). Significant differences in enzyme activity levels are indicated by delta (δ; n = 5; P<0.01) relative to the control group. Note that the scales of the y-axis vary between genes.
Discussion
In the present study, we used S. tropicalis tadpoles to investigate the effects of THs on brain gene expression. There is evidence that the thyroid and reproductive axes interact during tadpole development (11, 16, 18, 21). Therefore, the target genes that we chose to analyse are those related to TH, oestrogen and androgen receptors and synthesis enzymes. In the second part of the study, tadpoles were exposed to three concentrations of T3 (0.5, 5.0 and 50 nm) for 48 h. We followed the same protocol (e.g. T3 concentrations, stages of tadpoles, duration of the exposure) as in our previous study using L. pipiens (11) to allow valid comparisons between the two species. In the present study, we report that developmental expression and T3-regulation of TH-related genes are similar in S. tropicalis as in other frogs. By contrast, this is not the case for oestrogen responsive genes. Moreover, T3 can regulate expression of androgen synthesis enzymes in the brain.
Expression of thyroid hormone receptors and deiodinases during metamorphosis
Developmental profiles of tr and dio in the brain during natural metamorphosis have been previously established in S. tropicalis and other frog species. In the case of trbeta, the developmental profile and response to T3 obtained in the present study are very similar to data from Xenopus laevis (22), L. pipiens (11), and to those previously published for S. tropicalis (23). In X. laevis, trbeta mRNA increases in parallel with TH levels during metamorphosis and after T3 treatment (24).
This is the first time that expression profiles have been established for all three dio in the brain of S. tropicalis. To the best of our knowledge, we also present for the first time the developmental profile of dio1 in a frog brain. In the case of dio2 and dio3, the present results are similar to the profiles in L. pipiens (11). Interestingly, dio1 remained fairly constant during metamorphosis and after T3 exposure, suggesting that this enzyme does not play a major role in brain remodelling during metamorphosis. The results for dio1 are in marked contrast to dio2 and dio3, which show a very dynamic pattern of expression and T3 regulation. The positive regulation of dio2 by T3 is surprising because we would expect dio2 to decrease with T3 exposure (as observed in the developmental profile, dio2 decreases at metamorphic climax when T3 levels are highest). However, this has been observed previously in whole larvae of S. tropicalis (16) and X. laevis (10) and in the brain of L. pipiens (11) and S. tropicalis tadpoles (25) exposed to T3. The molecular mechanism underlying this response needs to be investigated further. Increases in dio3 mRNA during development and after T3 exposure are also consistent with previous studies (10, 11, 16) and can be explained by the presence of a TH-responsive element in the promoter region of the X. laevis and Rana catesbeiana dio3 genes (26, 27). Low levels of dio2 and high levels of dio3 during metamorphic climax indicate that the brain has reduced T3 synthesis but has started inactivating THs to protect itself from high levels of circulating THs because developmental remodelling of the brain has been completed (9).
Expression of oestrogen- and androgen-related genes during metamorphosis
Our treatments were effective and the T3 responses in TH-related genes in S. tropicalis brain were highly comparable to several other species. The second question that we addressed in the present study was whether T3 could affect the expression of sex steroid-related genes in the brain during metamorphosis. We measured for the first time oestrogen- and androgen-related genes in the brain of S. tropicalis during metamorphosis. Although both eralpha and erbeta increase during development in the brain, the magnitude of change of their mRNA levels is very different. Of all the genes analysed in the present study, erbeta showed the highest increase (150-fold) at NF 60, whereas eralpha showed a more moderate increase (six-fold). Comparing the developmental profiles to the T3 exposure results helps elucidate whether endogenous THs are important regulators of gene expression. Interestingly, we found that both er mRNAs increased at the beginning of metamorphic climax and they were also positively regulated by T3. However, the relative increases were not as dramatic in the short T3 exposure compared to the metamorphic peak). The expression of er mRNAs has been shown to be regulated by oestrogen and oestrogenic compounds in frog and fish brain (28–30). We suggest that part of the increase in er mRNAs during development could be a result of rising levels of THs during metamorphosis and the other part could be a result of rising levels of oestrogen in the brain via increasing cyp19 activity during development (present study). This study with S. tropicalis confirms the previous findings in the brain of L. pipiens where eralpha also increased during metamorphosis and after T3 exposure (11). In the adult brain, we found that erbeta mRNA was higher in males than females, implying that there may be sex differences in the T3 regulation of er mRNA in the tadpole brain. However, this idea and the molecular mechanism by which T3 induces er expression need to be investigated further.
We found that both cyp19 mRNA and activity increase in the brain during S. tropicalis metamorphosis and this is similar to gene expression profiles in X. laevis (31) and L. pipiens (11). T3 did not affect cyp19 mRNA, which is in marked contrast with results in L. pipiens where cyp19 mRNA decreased with T3 concentration (11). This difference between the two studies suggests there might be species differences in T3 regulation of cyp19; however, additional studies using other frog species will help further elucidate cyp19 regulation in the frog brain. Furthermore, we found that T3 increases cyp19 activity in the brain of S. tropicalis. To our knowledge, this is the first time that an increase in cyp19 activity has been reported after T3 treatment in the frog brain. In mammals, most of the research has focused on the effects of TH on gonadal development and function. Exposure to TH decreases gonadal CYP19 activity in vitro in pigs (32) rats (33, 34) and mice (35). Therefore, the molecular mechanism and physiological consequences of T3 regulation of cyp19 activity in the brain are unknown at this point. One way that T3 could affect cyp19 activity without affecting mRNA level is by regulating the enzyme at the post-translational level (e.g. phosphorylation of the enzyme) (36) or by another indirect mechanism.
The profiles of srd5alpha1 and srd5alpha2 differ significantly with respect to the profiles of the other sex steroid-related genes. The expression of the two enzymes involved in the processing of testosterone for 5α-dihydrotestosterone (5α-DHT) synthesis decreases during development and remains low during adulthood in the brain. Urbatzka et al. (31) measured srd5alpha1 and srd5alpha2 mRNAs by semi-quantitative RT-PCR in the brain of X. laevis and, in general, their profiles are very similar to the ones reported in the present study for S. tropicalis. Immunohistochemical analyses have also shown that srd5alpha1 is present in the brain of Rana esculenta tadpoles during metamorphosis (37). Exposure to all three concentrations of T3 resulted in a large increase (six- to 7.5-fold) in srd5alpha2 but only a small increase (1.5-fold) in srd5alpha1. Previous studies have found that T3 induces srd5alpha1 mRNA in whole larvae of S. tropicalis (16) and, in rats, hypothyroidism causes decreases in hepatic Srd5alpha expression and activity, an effect that was restored with T4 treatment (38). Srd5alpha2 is also involved in the conversion of progesterone into 5α-reduced metabolites such as allopregnanolone. Allopregnanolone has been shown to be involved in neurogenesis, regulating cell death and proliferation in vitro in rat and human neuronal stem cells (39) as well as in the developing sheep brain (40). TH promotes neurogenesis in the spinal cord of X. laevis (41), and is involved in neuronal proliferation and migration during vertebrate development (42). Taken together, the induction of srd5alpha1 and srd5alpha2 by T3 could be related to the roles of TH and neurosteroids in brain development. The comparison of developmental expression profiles and the results obtained after T3 exposure of srd5alpha1 and srd5alpha2 reveal marked differences. During metamorphosis, srd5alpha1 and srd5alpha2 decrease in the brain, whereas they increase following T3 exposure. These results indicate that rising endogenous THs are not directly responsible for developmental decreases in srd5alpha expression in the tadpole brain. Whole body measurement of testosterone and 5α-DHT indicates that androgens decrease during X. laevis development (43). In S. tropicalis larvae exposed to finasteride (a 5α- and 5β-DHT synthesis inhibitor), we have observed a decrease in srd5alpha2 (18, 21). In rats, 5α-DHT can induce Srd5alpha in the prostate (44) and Srd5alpha2 in the adult brain (45). These studies suggest that expression of srd5alpha can be regulated by its own enzymatic reaction product (i.e. feedforward control of 5α-DHT on srd5alpha). Therefore, the decrease in srd5alpha mRNA during development observed in the present study could be linked to the decrease in 5α-DHT levels. On the other hand, rising TH levels likely counteract this to maintain or control the rate at which srd5alpha mRNA levels decrease as metamorphosis progresses. Another explanation involves the action of oestrogens. Oestradiol has been shown to inhibit Srd5alpha activity in rat skin (46) and adrenal tissue (47). In addition, in S. tropicalis, exposure to the aromatase inhibitor fadrozole increases hepatic srd5alpha1 and srd5alpha2 mRNA levels (21) and, in fish, fadrozole increases circulating androgen levels (48). These studies suggest that oestrogen can inhibit androgen synthesis in the tadpole as in adult frogs (49). In the present study, we found that brain cyp19 activity increases during S. tropicalis metamorphosis; therefore, this putative increase in oestrogen could inhibit srd5alpha activity in the brain, leading to a decrease in 5α-DHT and srd5alpha mRNA as a result of feedforward control. Future experiments are needed to test these hypotheses of the complex interplay between THs, androgens and oestrogens, as well as the physiological consequences of the regulation of sr5alpha by T3.
Taken together, the present results indicate that TH-related genes display very similar developmental profiles and T3 responses in the brain of different frog species. We found that the brain is an important site of TH regulation of not only TH-responsive genes, but also sex steroid receptor and enzyme genes. In the case of sex steroid-related genes, T3 regulation of eralpha appears to be common to both S. tropicalis and L. pipiens; however, this is not the case for cyp19 and additional research is needed for the androgen-related genes. Finally, we present supporting evidence of a crosstalk between TH and sex steroids in the developing brain of S. tropicalis. The present study provides an important baseline to determine the physiological consequences of this interaction during the remodelling of the frog brain.
Acknowledgments
The authors would like to thank Bill Fletcher for animal care and Valérie Langlois for providing helpful comments on the manuscript and for help with the aromatase activity assay. This research was supported by the NSERC-DG program (to V.L.T.), the University of Ottawa International Scholarship and NSERC-PGSD program (to P.D.-G.).
References
- 1.Shi Y-B. Amphibian Metamorphosis: From Morphology to Molecular Biology. New York, NY: John Wiley; 2000. [Google Scholar]
- 2.Denver RJ. Proximate mechanisms of phenotypic plasticity in amphibian metamorphosis. Am Zool. 1997;37:172–184. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- 3.Denver RJ. The molecular basis of thyroid hormone-dependent central nervous system remodeling during amphibian metamorphosis. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:219–228. doi: 10.1016/s0742-8413(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 4.Tata JR. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol. 2006;246:10–20. doi: 10.1016/j.mce.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 6.Galton VA. The roles of the iodothyronine deiodinases in mammalian development. Thyroid. 2005;15:823–834. doi: 10.1089/thy.2005.15.823. [DOI] [PubMed] [Google Scholar]
- 7.Brown DD. The role of deiodinases in amphibian metamorphosis. Thyroid. 2005;15:815–821. doi: 10.1089/thy.2005.15.815. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara A, Baker BS, Tata JR. Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development. 1991;112:933–943. doi: 10.1242/dev.112.4.933. [DOI] [PubMed] [Google Scholar]
- 9.Cai L, Brown DD. Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol. 2004;266:87–95. doi: 10.1016/j.ydbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Morvan-Dubois G, Sebillot A, Kuiper GG, Verhoelst CH, Darras VM, Visser TJ, Demeneix BA. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology. 2006;147:4941–4949. doi: 10.1210/en.2006-0609. [DOI] [PubMed] [Google Scholar]
- 11.Hogan NS, Crump KL, Duarte P, Lean DR, Trudeau VL. Hormone cross-regulation in the tadpole brain: developmental expression profiles and effect of T3 exposure on thyroid hormone- and estrogen-responsive genes in Rana pipiens. Gen Comp Endocrinol. 2007;154:5–15. doi: 10.1016/j.ygcen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Tindall AJ, Morris ID, Pownall ME, Isaacs HV. Expression of enzymes involved in thyroid hormone metabolism during the early development of Xenopus tropicalis. Biol Cell. 2007;99:151–163. doi: 10.1042/BC20060074. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini E, Menuet A, Lethimonier C, Adrio F, Gueguen MM, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen Comp Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda K, Sakamoto H, Kawata M. Androgen action in the brain and spinal cord for the regulation of male sexual behaviors. Curr Opin Pharmacol. 2008;8:747–751. doi: 10.1016/j.coph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Duarte-Guterman P, Langlois VS, Pauli BD, Trudeau VL. Expression and T3 regulation of thyroid hormone- and sex steroid-related genes during Silurana (Xenopus) tropicalis early development. Gen Comp Endocrinol. 2010;166:428–435. doi: 10.1016/j.ygcen.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. New York, NY: Garland Publishing, Inc.; 1994. [Google Scholar]
- 18.Langlois VS, Duarte-Guterman P, Ing S, Pauli BD, Cooke GM, Trudeau VL. Fadrozole and finasteride exposures modulate sex steroid- and thyroid hormone-related gene expression in Silurana (Xenopus) tropicalis early larval development. Gen Comp Endocrinol. 2010;166:417–427. doi: 10.1016/j.ygcen.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 20.Du JL, Lee CY, Tacon P, Lee YH, Yen FP, Tanaka H, Dufour S, Chang CF. Estradiol-17beta stimulates gonadotropin II expression and release in the protandrous male black porgy Acanthopagrus schlegeli Bleeker: a possible role in sex change. Gen Comp Endocrinol. 2001;121:135–145. doi: 10.1006/gcen.2000.7583. [DOI] [PubMed] [Google Scholar]
- 21.Duarte-Guterman P, Langlois VS, Hodgkinson K, Pauli BD, Cooke GM, Wade MG, Trudeau VL. The aromatase inhibitor fadrozole and the 5-reductase inhibitor finasteride affect gonadal differentiation and gene expression in the frog Silurana tropicalis. Sex Dev. 2009;3:333–341. doi: 10.1159/000280586. [DOI] [PubMed] [Google Scholar]
- 22.Krain LP, Denver RJ. Developmental expression and hormonal regulation of glucocorticoid and thyroid hormone receptors during metamorphosis in Xenopus laevis. J Endocrinol. 2004;181:91–104. doi: 10.1677/joe.0.1810091. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Matsuda H, Shi YB. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology. 2008;149:5610–5618. doi: 10.1210/en.2008-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- 25.Bonett RM, Hoopfer ED, Denver RJ. Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen Comp Endocrinol. 2010 doi: 10.1016/j.ygcen.2010.03.014. doi: 10.1016/j.ygcen.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker KB, Schneider MJ, Davey JC, Galton VA. The type III 5-deiodinase in Rana catesbeiana tadpoles is encoded by a thyroid hormone-responsive gene. Endocrinology. 1995;136:4424–4431. doi: 10.1210/endo.136.10.7664662. [DOI] [PubMed] [Google Scholar]
- 27.St Germain DL, Schwartzman RA, Croteau W, Kanamori A, Wang Z, Brown DD, Galton VA. A thyroid hormone-regulated gene in Xenopus laevis encodes a type III iodothyronine 5-deiodinase. Proc Natl Acad Sci USA. 1994;91:11282. doi: 10.1073/pnas.91.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duarte P, Hogan NS, Lean DRS, Trudeau VL. Regulation and endocrine disruption of aromatase in the brain of developing Rana pipiens. #559. 27th Annual Meeting of the Society of Environmental Toxicology and Chemistry of North America, Montreal, PQ, Canada, 2006.
- 29.Marlatt VL, Martyniuk CJ, Zhang D, Xiong H, Watt J, Xia X, Moon T, Trudeau VL. Auto-regulation of estrogen receptor subtypes and gene expression profiling of 17beta-estradiol action in the neuroendocrine axis of male goldfish. Mol Cell Endocrinol. 2008;283:38–48. doi: 10.1016/j.mce.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Chandrasekar G, Archer A, Gustafsson JA, Andersson Lendahl M. Levels of 17beta-estradiol receptors expressed in embryonic and adult zebrafish following in vivo treatment of natural or synthetic ligands. PLoS ONE. 2010;5:e9678. doi: 10.1371/journal.pone.0009678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbatzka R, Lutz I, Kloas W. Aromatase, steroid-5-alpha-reductase type 1 and type 2 mRNA expression in gonads and in brain of Xenopus laevis during ontogeny. Gen Comp Endocrinol. 2007;153:280–288. doi: 10.1016/j.ygcen.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Gregoraszczuk EL, Slomczynska M, Wilk R. Thyroid hormone inhibits aromatase activity in porcine thecal cells cultured alone and in coculture with granulosa cells. Thyroid. 1998;8:1157–1163. doi: 10.1089/thy.1998.8.1157. [DOI] [PubMed] [Google Scholar]
- 33.Ulisse S, Jannini EA, Carosa E, Piersanti D, Graziano FM, D’Armiento M. Inhibition of aromatase activity in rat Sertoli cells by thyroid hormone. J Endocrinol. 1994;140:431–436. doi: 10.1677/joe.0.1400431. [DOI] [PubMed] [Google Scholar]
- 34.Hatsuta M, Tamura K, Shimizu Y, Toda K, Kogo H. Effect of thyroid hormone on CYP19 expression in ovarian granulosa cells from gonadotropin-treated immature rats. J Pharmacol Sci. 2004;94:420–425. doi: 10.1254/jphs.94.420. [DOI] [PubMed] [Google Scholar]
- 35.Cecconi S, Rucci N, Scaldaferri ML, Masciulli MP, Rossi G, Moretti C, D’Armiento M, Ulisse S. Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology. 1999;140:1783–1788. doi: 10.1210/endo.140.4.6635. [DOI] [PubMed] [Google Scholar]
- 36.Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 37.Bruzzone F, Do Rego JL, Luu-The V, Pelletier G, Vallarino M, Vaudry H. Immunohistochemical localization and biological activity of 3beta-hydroxysteroid dehydrogenase and 5alpha-reductase in the brain of the frog, Rana esculenta, during development. J Chem Neuroanat. 2010;39:35–50. doi: 10.1016/j.jchemneu.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ram PA, Waxman DJ. Pretranslational control by thyroid hormone of rat liver steroid 5 alpha-reductase and comparison to the thyroid dependence of two growth hormone-regulated CYP2C mRNAs. J Biol Chem. 1990;265:19223–19229. [PubMed] [Google Scholar]
- 39.Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3:185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- 40.Yawno T, Hirst JJ, Castillo-Melendez M, Walker DW. Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience. 2009;163:838–847. doi: 10.1016/j.neuroscience.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Schlosser G, Koyano-Nakagawa N, Kintner C. Thyroid hormone promotes neurogenesis in the Xenopus spinal cord. Dev Dyn. 2002;225:485–498. doi: 10.1002/dvdy.10179. [DOI] [PubMed] [Google Scholar]
- 42.Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol. 2009;313:36–49. doi: 10.1016/j.mce.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 43.Bogi C, Levy G, Lutz I, Kloas W. Functional genomics and sexual differentiation in amphibians. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:559–570. doi: 10.1016/s1096-4959(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 44.George FW, Russell DW, Wilson JD. Feed-forward control of prostate growth: dihydrotestosterone induces expression of its own biosynthetic enzyme, steroid 5 alpha-reductase. Proc Natl Acad Sci USA. 1991;88:8044–8047. doi: 10.1073/pnas.88.18.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres JM, Ortega E. Differential regulation of steroid 5alpha-reductase isozymes expression by androgens in the adult rat brain. FASEB J. 2003;17:1428–1433. doi: 10.1096/fj.02-1119com. [DOI] [PubMed] [Google Scholar]
- 46.Dube JY, Ngo-Thi NH, Tremblay RR. In vivo effects of steroid hormones on the testosterone 5alpha-reductase in skin. Endocrinology. 1975;97:211–214. doi: 10.1210/endo-97-1-211. [DOI] [PubMed] [Google Scholar]
- 47.Maynard PV, Cameron EH. Adrenal microsomal C19-steroid 5alpha-reductase activity in the Snell transplantable rat adrenocortical tumour 494 and the effect of oestradiol, testosterone propionate and adrenocorticotrophin in intact and gonadectomized rats. Biochem J. 1973;132:293–300. doi: 10.1042/bj1320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ankley GT, Kahl MD, Jensen KM, Hornung MW, Korte JJ, Makynen EA, Leino RL. Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fathead minnow (Pimephales promelas) Toxicol Sci. 2002;67:121–130. doi: 10.1093/toxsci/67.1.121. [DOI] [PubMed] [Google Scholar]
- 49.Pierantoni R, Varriale B, Minucci S, Di MatteoL, Fasano S, D’Antonio M, Chieffi G. Regulation of androgen production by frog (Rana esculenta) testis: an in vitro study on the effects exerted by estradiol, 5 alpha-dihydrotestosterone, testosterone, melatonin, and serotonin. Gen Comp Endocrinol. 1986;64:405–410. doi: 10.1016/0016-6480(86)90076-6. [DOI] [PubMed] [Google Scholar]