Abstract
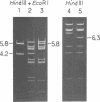
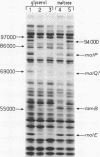
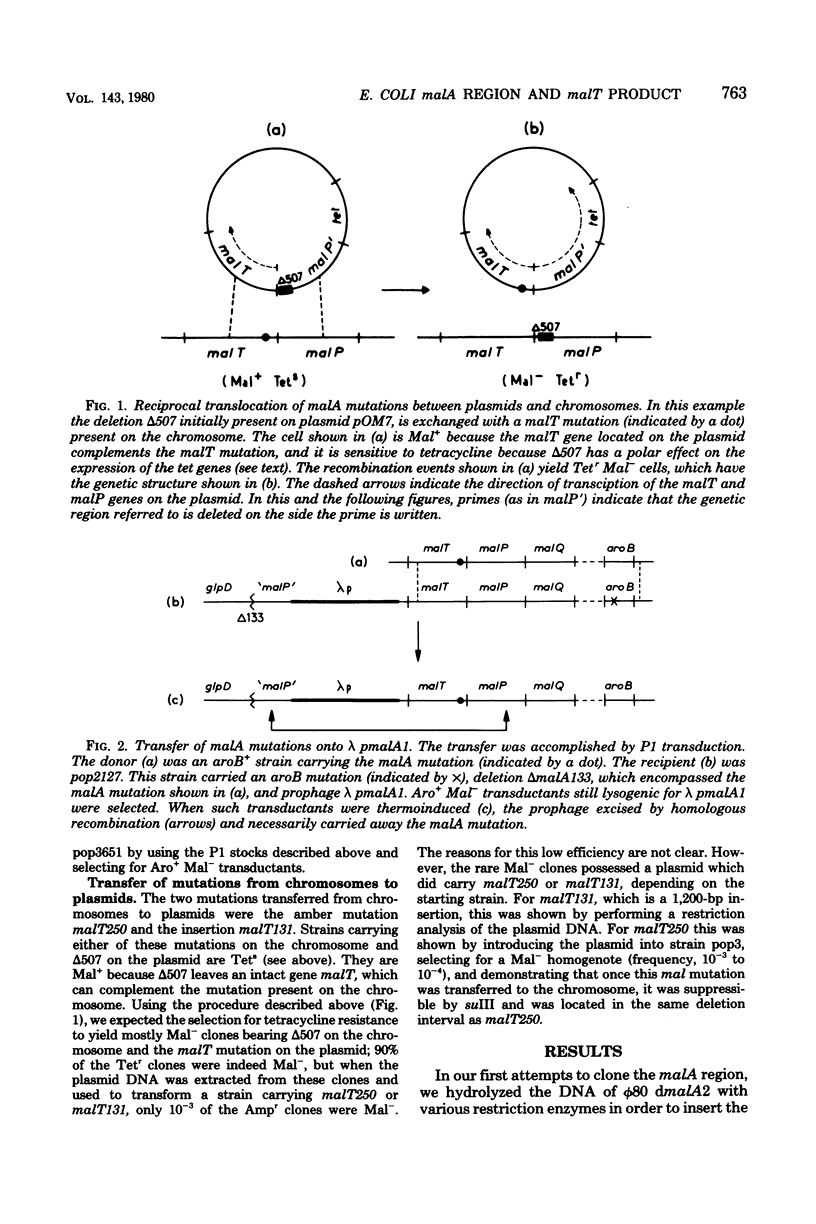
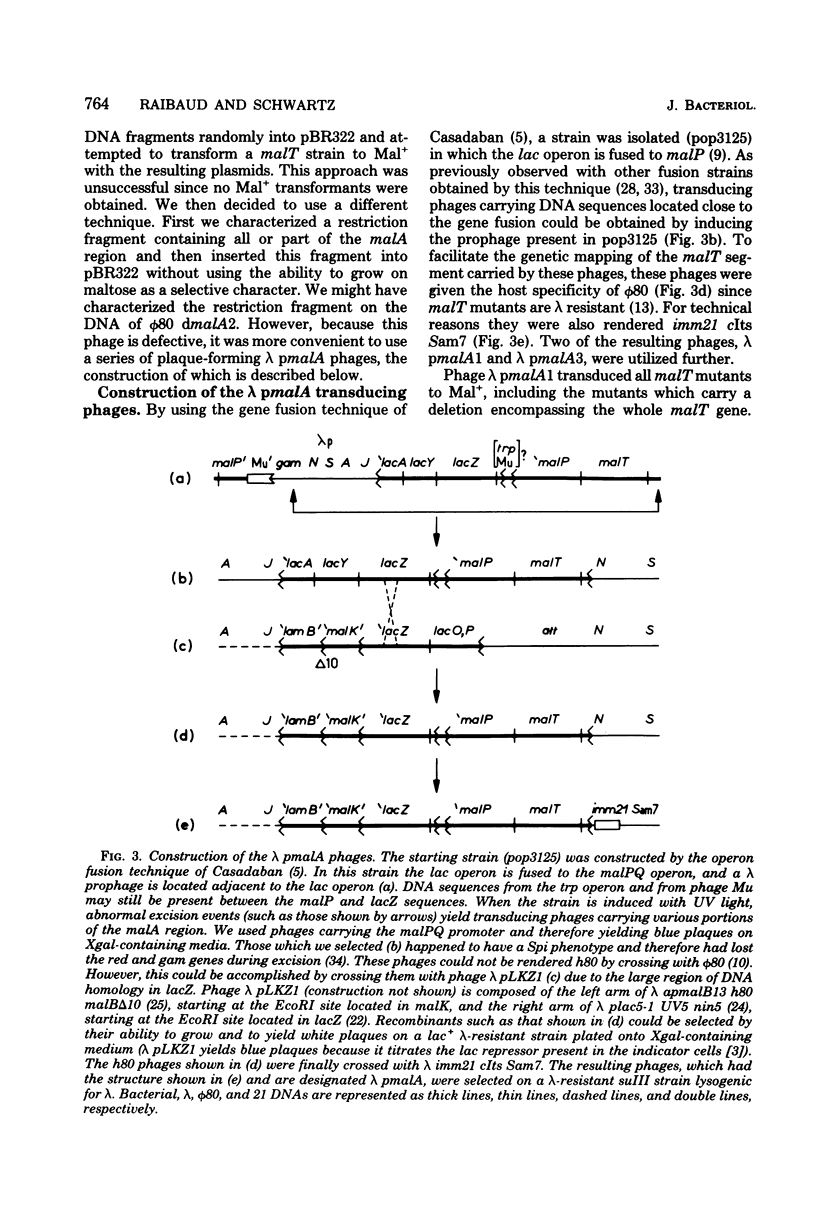
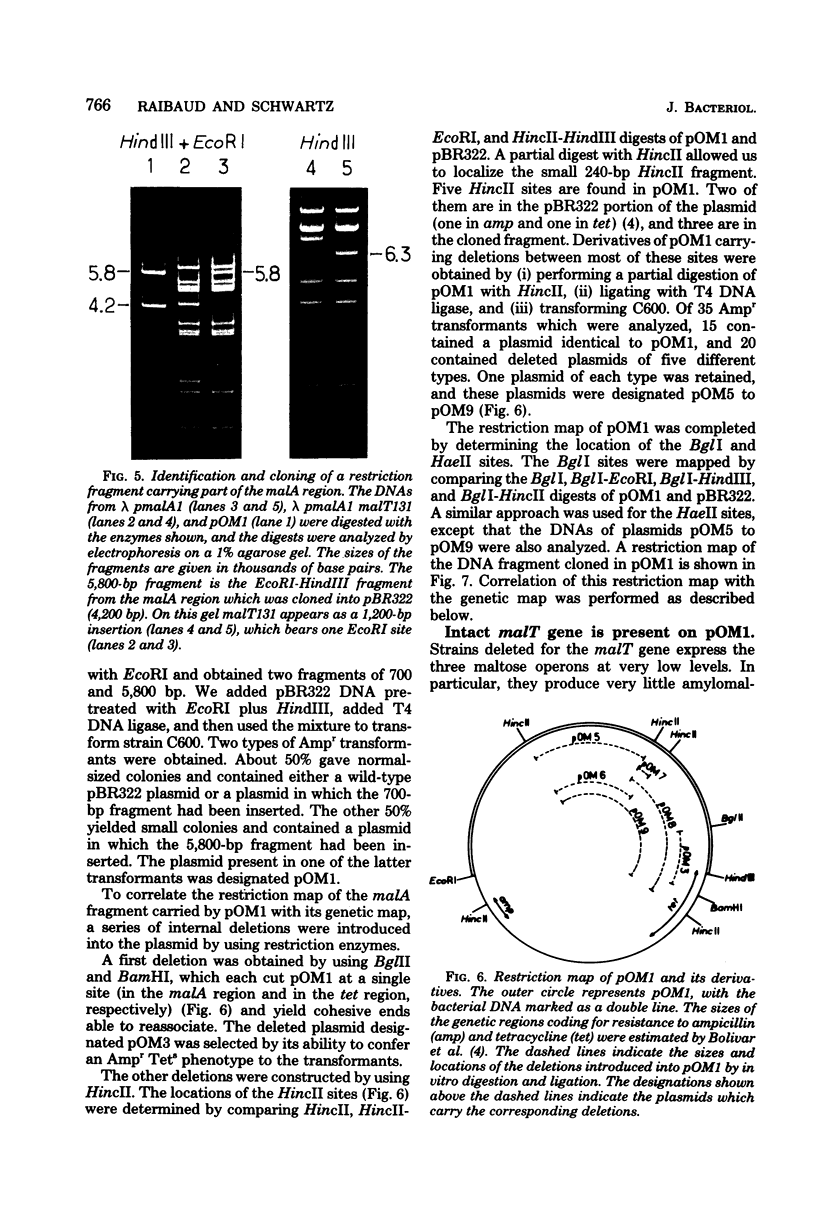
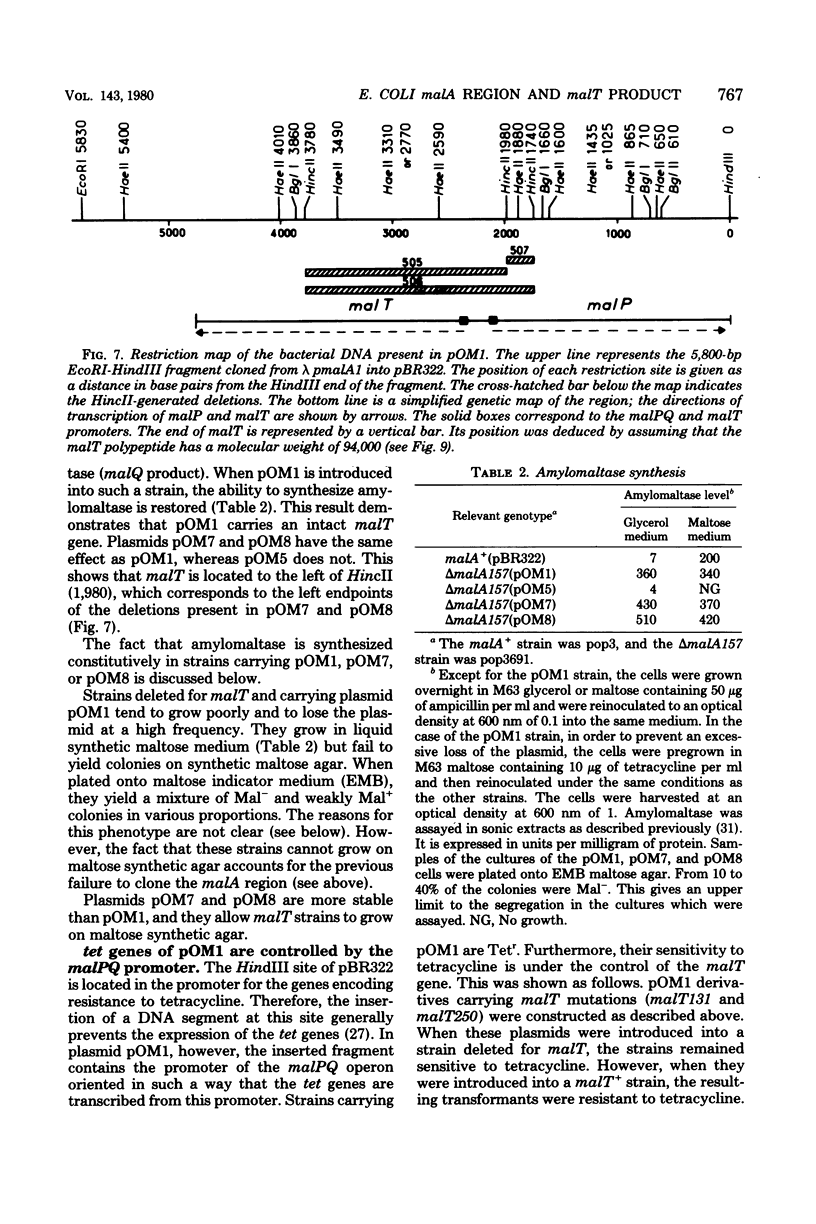
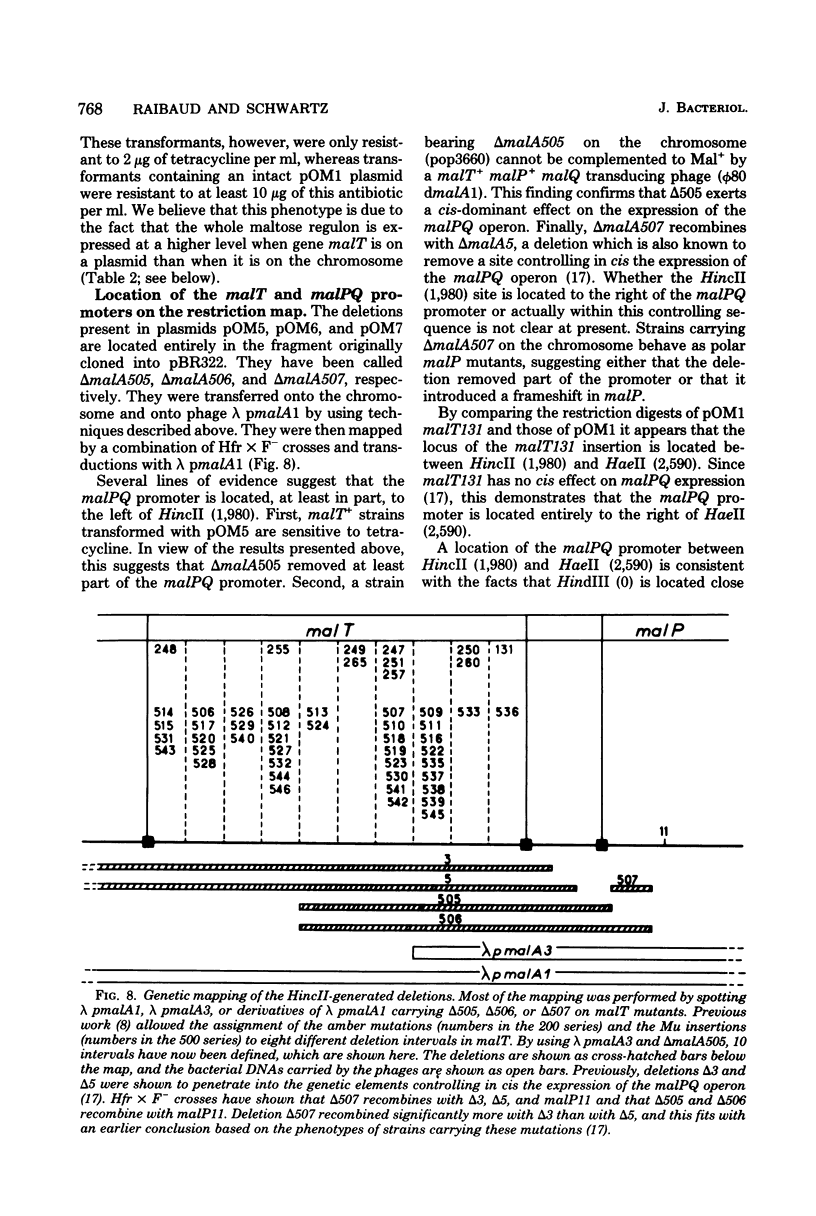
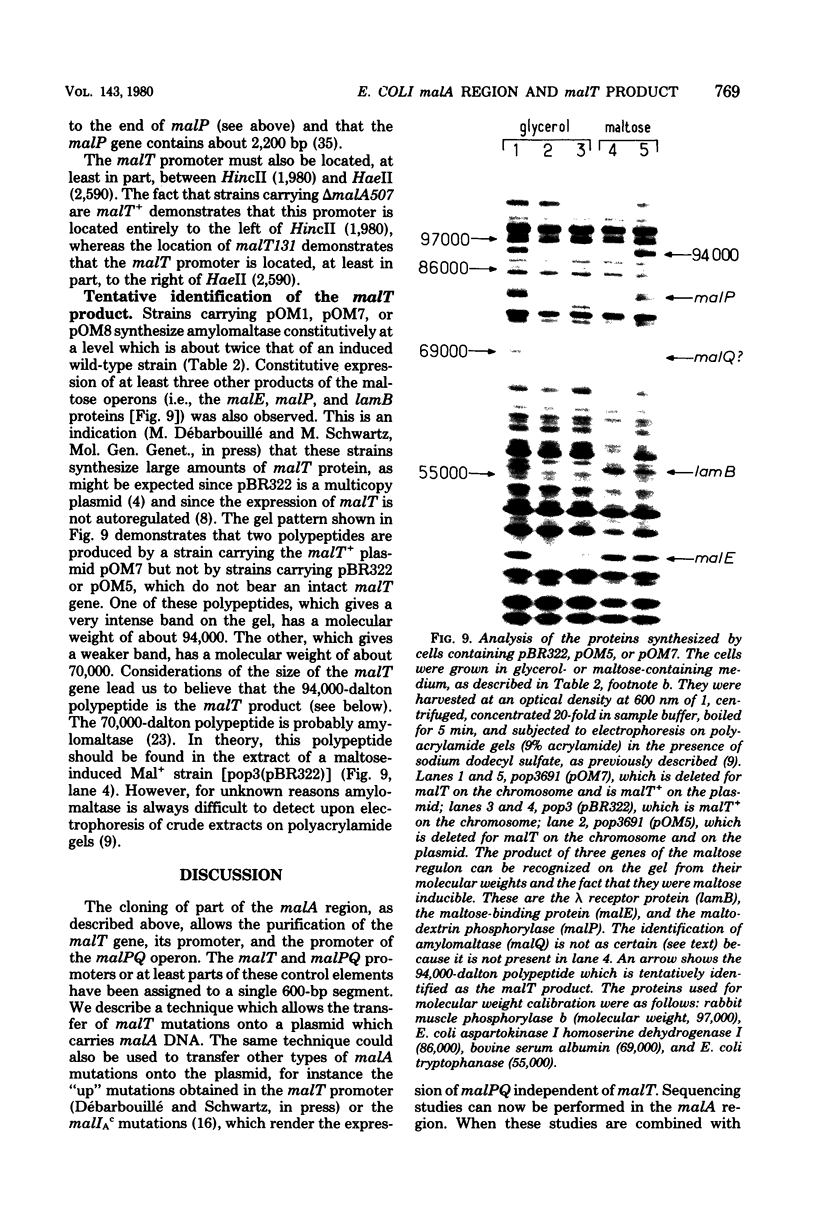
A series of plaque-forming lambda h80 transducing phages carrying various portions of the malA region were isolated. A 5,800-base pair HindIII-EcoRI DNA fragment from one of these phages was cloned into pBR322 and shown to contain malT, which is the positive regulator gene of the maltose regulon, and most of malP, the structural gene for maltodextrin phosphorylase. A restriction map of the HindIII-EcoRI fragment was established, and it was correlated with the genetic map of the malA region (i) by mapping deletions which had been generated in vitro on the plasmid and (ii) by locating on the restriction map a DNA insertion of known genetic position. A 600-base pair HincII-HaeII segment was shown to contain all or part of the promoters for malT and malP, which are known to be transcribed in opposite directions. Strains carrying gene malT on a plasmid synthesized a 94,000-dalton polypeptide which was not produced by identical strains carrying similar plasmids in which malT was partially deleted. Estimates of the size of the malT gene support the conclusion that the 94,000-dalton polypeptide is the malT product.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Fiandt M., Hass K. K., Twose P. A., Szybalski W. Deletions and insertions in the immunity region of coliphage lambda: revised measurement of the promoter-startpoint distance. Virology. 1974 Dec;62(2):458–471. doi: 10.1016/0042-6822(74)90407-3. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Cunin R., Glansdorff N. Cloning and endonuclease restriction analysis of argF and of the control region of the argECBH bipolar operon in Escherichia coli. Gene. 1979 Mar;5(3):207–231. doi: 10.1016/0378-1119(79)90079-9. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Debarbouille M., Schwartz M. The use of gene fusions to study the expression of malT the positive regulator gene of the maltose regulon. J Mol Biol. 1979 Aug 15;132(3):521–534. doi: 10.1016/0022-2836(79)90273-0. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Genetic analysis of the maltose A region in Escherichia coli. J Bacteriol. 1969 May;98(2):559–567. doi: 10.1128/jb.98.2.559-567.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Nonsense mutations in the maltose A region of the genetic map of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1311–1315. doi: 10.1128/jb.100.3.1311-1315.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M., Hatfield D. Complementation studies in the maltose-A region of the Escherichia coli K12 genetic map. J Mol Biol. 1971 Nov 14;61(3):681–694. doi: 10.1016/0022-2836(71)90072-6. [DOI] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M. Mutations allowing growth on maltose of Escherichia coli K 12 strains with a deleted malT gene. Mol Gen Genet. 1971;112(2):117–132. doi: 10.1007/BF00267490. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers T. D., Noti J., Umbarger H. E. Physical characterization of ilv-lac fusions. J Bacteriol. 1979 Oct;140(1):251–260. doi: 10.1128/jb.140.1.251-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C., Greenblatt J., Hofnung M. malB region in Escherichia coli K-12: specialized transducing bacteriophages and first restriction map. J Bacteriol. 1978 Dec;136(3):1109–1119. doi: 10.1128/jb.136.3.1109-1119.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P., Polisky B., Gelfand D. H. Regulated expression by readthrough translation from a plasmid-encoded beta-galactosidase. J Bacteriol. 1978 May;134(2):645–654. doi: 10.1128/jb.134.2.645-654.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. N., Ryman B. E., Whelan W. J. The action pattern of amylomaltase from Escherichia coli. Eur J Biochem. 1976 Oct 1;69(1):105–115. doi: 10.1111/j.1432-1033.1976.tb10863.x. [DOI] [PubMed] [Google Scholar]
- Pourcel C., Marchal C., Louise A., Fritsch A., Tiollais P. Bacteriophage lambda-E. coli K12 vector-host system for gene cloning and expression under lactose promoter control: I. DNA fragment insertion at the lacZ EcoRI restriction site. Mol Gen Genet. 1979 Feb 26;170(2):161–169. doi: 10.1007/BF00337792. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Clément J. M., Hofnung M. Structure of the malB region in Escherichia coli K12. III. Correlation of the genetic map with the restriction map. Mol Gen Genet. 1979 Jul 24;174(3):261–267. doi: 10.1007/BF00267798. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Roa M., Braun-Breton C., Schwartz M. Structure of the malB region in Escherichia coli K12. I. Genetic map of the malK-lamB operon. Mol Gen Genet. 1979 Jul 24;174(3):241–248. doi: 10.1007/BF00267796. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. L., West R. W., Heyneker H. L., Bolivar F., Boyer H. W. Characterizing wild-type and mutant promoters of the tetracycline resistance gene in pBR313. Nucleic Acids Res. 1979 Jul 25;6(10):3267–3287. doi: 10.1093/nar/6.10.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I., Margarita D. Fine structure analysis of the threonine operon in Escherichia coli K-12. Mol Gen Genet. 1978 Jun 1;162(1):101–107. doi: 10.1007/BF00333856. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Expression phénotypique et localisation génétique de mutations affectant le métabolisme du maltose chez Escherichia coli K 12. Ann Inst Pasteur (Paris) 1967 Jun;112(6):673–698. [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. The adsorption of coliphage lambda to its host: effect of variations in the surface density of receptor and in phage-receptor affinity. J Mol Biol. 1976 May 25;103(3):521–536. doi: 10.1016/0022-2836(76)90215-1. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]
- Thanner F., Palm D., Shaltiel S. Hydrophobic and biospecific chromatography in the purification of maltodextrin phosphorylase from E. coli. FEBS Lett. 1975 Jul 15;55(1):178–182. doi: 10.1016/0014-5793(75)80987-2. [DOI] [PubMed] [Google Scholar]
- Tikchonenko T. I., Faizullin L. Z., Kalinin V. N., Granovsky N. N., Naroditsky B. S. Construction and properties of hybrid plasmids carrying the E. coli gal operon. Gene. 1979 Oct;7(2):109–119. doi: 10.1016/0378-1119(79)90027-1. [DOI] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]